Abstract
Vaccine adjuvants are compounds that enhance host immune responses to co-administered antigens in vaccines. Vaxjo is a web-based central database and analysis system that curates, stores, and analyzes vaccine adjuvants and their usages in vaccine development. Basic information of a vaccine adjuvant stored in Vaxjo includes adjuvant name, components, structure, appearance, storage, preparation, function, safety, and vaccines that use this adjuvant. Reliable references are curated and cited. Bioinformatics scripts are developed and used to link vaccine adjuvants to different adjuvanted vaccines stored in the general VIOLIN vaccine database. Presently, 103 vaccine adjuvants have been curated in Vaxjo. Among these adjuvants, 98 have been used in 384 vaccines stored in VIOLIN against over 81 pathogens, cancers, or allergies. All these vaccine adjuvants are categorized and analyzed based on adjuvant types, pathogens used, and vaccine types. As a use case study of vaccine adjuvants in infectious disease vaccines, the adjuvants used in Brucella vaccines are specifically analyzed. A user-friendly web query and visualization interface is developed for interactive vaccine adjuvant search. To support data exchange, the information of vaccine adjuvants is stored in the Vaccine Ontology (VO) in the Web Ontology Language (OWL) format.
1. Introduction
Vaccines are one of the most effective preventative health tools available against infectious diseases, cancer, allergy, and autoimmune diseases. Vaccination aims to generate a strong immune response to the administrated antigen and provide long-term protection against a disease. Frequently, using only the antigen is not enough to stimulate that protective immunity. Vaccine adjuvants are compounds that enhance the specific immune responses against coadministered antigens in vaccines [1, 2]. The word adjuvant comes from the Latin word adjuvare, meaning to help or to enhance. The term “adjuvant” was coined by Ramon in the 1920s when they observed that the injection of the diphtheria toxoid along with unrelated substances in horses developed an abscess at the injection site and generated higher specific antibody titers [3, 4]. In 1926, Glenny et al. demonstrated the adjuvant activity of aluminum compounds with diphtheria toxoid absorbed to alum [5]. In 1936, Freund's complete adjuvant (FCA), an emulsion of water and mineral oil containing killed mycobacteria, was developed [6]. Freund's incomplete adjuvant (FIA) that does not have the mycobacteria is less toxic and has been used in many human vaccine formulations [7]. In the 1950s, lipopolysaccharides (LPSs) from Gram-negative bacteria were found to exhibit adjuvant activity [8]. In 1974, muramyldipeptide (MDP) was found to be a mycobacterial component with adjuvant activity in FCA [9]. There is a wide array of adjuvants that are being developed and used, from a variety of sources. Currently, several hundred natural and synthetic compounds are known to have adjuvant activity [1, 10]. However, the adjuvants that are permitted in licensed vaccines are limited. In the US, alum salts and AS04 (which is composed of aluminum salt and MPL) are licensed for use in humans [11].
Adjuvants can function as immunostimulants, vehicles, and carriers. Cox and Coulter summarize five modes of action of vaccine adjuvants: (1) immunomodulation: the ability of many adjuvants to modify the cytokine network. (2) Presentation: the ability of an adjuvant to preserve the conformational integrity of an antigen and to present the antigen to appropriate immune effector cells. (3) CTL induction: induction of CD8+ cytotoxic T-lymphocyte (CTL) responses. (4) Targeting: the ability of an adjuvant to deliver an immunogen to immune effector cells, generally via antigen presentation cells (APCs). (5) Depot generation: generation of a short-term or long-term depot to give a continuous or pulsed release [12]. The use of vaccine adjuvants allows for a reduction in the number of immunizations or the amount of antigen needed for immunization [13]. Vaccine adjuvants can be administered either parenterally or mucosally.
VIOLIN (http://www.violinet.org/), primarily developed by our group, is the first web-based comprehensive vaccine database and analysis system that targets for vaccine research [14]. Currently, VIOLIN has included more than 2,800 vaccines for over 170 infectious diseases and many noninfectious diseases (e.g., cancers and allergies). Here, a vaccine includes a licensed vaccine or a vaccine construct that is not licensed but has been shown to induce significant protection against a disease in at least one laboratory animal model. Many of these vaccines use vaccine adjuvants. Therefore, it is important to classify them and analyze their differential applications in vaccine development. However, the original VIOLIN database did not support classifications of various vaccine adjuvants or provide detailed vaccine adjuvant information.
While intensive research has been conducted and resulted in identification of many vaccine adjuvants, there is no web-based central resource that allows storage, annotation, comparison, and analysis of vaccine adjuvants and their usages in vaccine development. To address this challenge, we have developed Vaxjo (http://www.violinet.org/vaxjo/), a web-based vaccine adjuvant database and analysis system. Vaxjo stores the information of manually curated vaccine adjuvants and their applications in vaccine development. It also allows bioinformatics analysis and comparison of various vaccine adjuvants. Vaxjo is publically available at http://www.violinet.org/vaxjo/.
2. Methods
2.1. Vaxjo System and Database Design
Vaxjo is implemented using a three-tier architecture built on two HP ProLiant DL380 G6 servers, which run the Redhat Linux operating system (Redhat Enterprise Linux ES 4). Users can submit database or analysis queries through the web. These queries are then processed using PHP/SQL (middle-tier, application server based on Apache) against a MySQL (version 5.0) relational database (back-end, database server). The result of each query is then presented to the user in the web browser. In addition, Vaxjo data curators and reviewers can also submit and review curated vaccine adjuvant data through a web-based Vaxjo data curation system. Two servers are scheduled to regularly backup each other's data. Vaxjo is an integrated program of the VIOLIN vaccine database and analysis [14].
Figure 1 demonstrates the Vaxjo workflow and system design. For each specific vaccine adjuvant, the Vaxjo database contains the following information: (1) detailed reference citation information, for example, authors, journal, title, year, volume, issue, and pages. These references are typically obtained from PubMed (http://www.ncbi.nlm.nih.gov/pubmed) and used to obtain reliable information for vaccine adjuvants. (2) General information of vaccine adjuvants, for example, alternate names, description, stage of vaccine adjuvant development (e.g., preclinical, clinical stage, or licensed), components, preparation, safety, and so forth. This information is manually curated and not available in the general VIOLIN vaccine database originally developed [14]. The manual curation of peer-reviewed journal articles emphasizes the retrieval of vaccine adjuvant information and experimental data associated with vaccine adjuvants. Using an internal script, all the vaccines that are stored in VIOLIN and use a specific vaccine adjuvant can be retrieved and displayed in Vaxjo searching page. A unique Vaccine Ontology (VO) identifier has been assigned to each vaccine adjuvant. The VO IDs used in both the Vaxjo database and the general VIOLIN vaccine database are used as primary keys for linking these two databases. This program allows advanced query and comparison between different vaccine adjuvants and vaccines that use these adjuvants.
Figure 1.
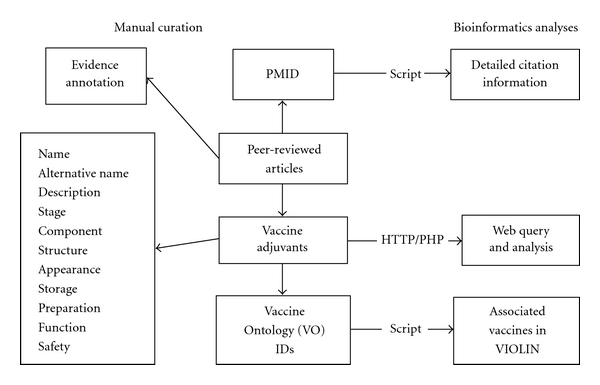
Vaxjo workflow and system design. Manual curation includes peer-reviewed publications from PubMed. A PubMed ID (PMID) is extracted and used by an internal script to retrieve detailed citation information (e.g., authors, journal, and date). The detailed information of a vaccine adjuvant is curated and cited from published studies. Each vaccine adjuvant is assigned with a Vaccine Ontology (VO) ID, which is further used in an internal computational script to obtain the information of relevant adjuvanted vaccines from the general VIOLIN relational database.
2.2. Semiautomatic Annotation of Vaccine Adjuvant Information
A semiautomatic Vaxjo annotation system is developed for vaccine adjuvant curation and analysis by modifying an in-house web-based literature mining and curation system called Limix [14–16] (Figure 2). The interactive Limix data submission and review system (a) allows a data curator to search literature, copy and edit text, and submit data to database and (b) provides user-friendly tools for a data reviewer to review, edit, and approve the curated data on a comprehensive web interface. Upon approval after the critical review by a reviewer, the curated data submitted by a curator will be posted publicly. Limix also features automated reference tracking and management using a version control mechanism similar to that used in Wikipedia (http://www.wikipedia.org/). The system stores all the history changes made to every vaccine adjuvant. A reviewer can compare any two of the history versions to trace the detailed changes made to a vaccine adjuvant. Figure 2 provides a more detailed view of the Limix curation process and reviewing features.
Figure 2.
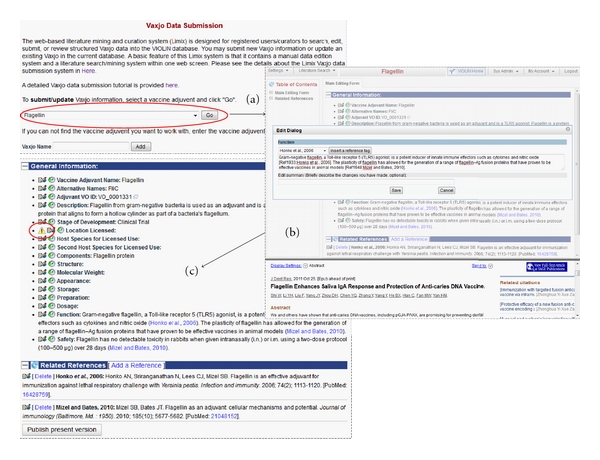
Submission and review processes of vaccine adjuvants into Vaxjo. (a) Selecting an adjuvant from a dropdown list or adding a new adjuvant by entering the name in the field below the dropdown list. (b) Vaxjo submission page, where each section is edited via a popup dialog box that also provides options to insert references when needed. The bottom section of the Vaxjo curation web page includes detailed reference information for a curator to review. (c) Review page. Vaxjo curators review information curated into Vaxjo and, after review, submit the page for publication in Vaxjo.
Vaxjo is updated automatically when additional vaccine adjuvants are added. All vaccine adjuvant updates can be found and queried in the Vaxjo website.
2.3. Vaxjo Data Exchange, Transfer, and Download
To facilitate data exchange and transfer, the information of vaccine adjuvants in Vaxjo is now stored in the Vaccine Ontology (VO; http://www.violinet.org/vaccineontology/) [17]. The vaccine adjuvant data stored in VO is machine-readable and can be used for various software programs for data exchange and transfer.
2.4. Vaxjo Data Query and Display
The programming languages PHP and HTML were primarily used to interact with the backend Vaxjo relational database to obtain vaccine adjuvant information. Since Vaxjo has been integrated with the general VIOLIN database system, the Vaxjo data query tool is also able to query the general VIOLIN vaccine database to obtain the information of vaccines that use specific vaccine adjuvants. Meanwhile, the vaccine adjuvant information in the Vaccine Ontology (VO) is queried using internal SPARQL scripts. The VO IDs of different vaccine adjuvants are used as the glue that links the vaccine adjuvants in the Vaxjo database and the general VIOLIN vaccine database.
2.5. Bioinformatics Analysis of the Vaxjo Vaccine Adjuvant Data
The vaccine adjuvants stored in Vaxjo and their applications in development of vaccines stored in the general VIOLIN database were obtained using the web-based Vaxjo query or through internal scripts. A systematic analysis was conducted to classify and compare different characteristics of vaccine adjuvants.
3. Results
3.1. Vaxjo Statistics
Vaxjo is a web-based relational database and analysis system based on a classical three-tier architecture design [15]. Currently, Vaxjo has included 103 vaccine adjuvants. In total, 384 vaccines have been detected to use these vaccine adjuvants. Occasionally, more than one vaccine adjuvant is used in a single vaccine. These 384 vaccines are developed against infections of 76 pathogens, such as Bacillus anthracis (anthrax), Brucella spp. (brucellosis), influenza virus, and Plasmodium spp. (malaria). Vaxjo also stores 15 vaccine adjuvants that have been used for vaccine development against many cancers and allergies.
3.2. Analyses of Vaxjo Vaccine Adjuvants Based on Vaccine Adjuvant Types
With the establishment of the Vaxjo vaccine adjuvant database, we were able to systematically analyze the vaccine adjuvants and their usages in vaccine development. Table 1 shows the types of vaccine adjuvants and lists those adjuvants which have five or more associated vaccines. The adjuvant types are based on the derivation of the adjuvants or the composition of the adjuvants. The largest numbers of vaccine adjuvants are found in microorganism-derived adjuvants (18), synthetic adjuvants (28), and mineral salt adjuvants (13) (Table 1). If the numbers of vaccines using different adjuvants are considered, vaccine adjuvants based on mineral salts, emulsions, and microorganism derivatives have been used in 151, 115, and 73 vaccines, respectively (Table 1). Aluminum hydroxide is the most common adjuvant found, with 62 associated vaccines collected in VIOLIN. Freund's complete and incomplete adjuvants are also commonly used with each being associated with 42 vaccines. Although 28 synthetic adjuvants are curated in Vaxjo, only 28 vaccines listed in VIOLIN use these synthetic adjuvants. Only two of these synthetic vaccine adjuvants collected in Vaxjo are not associated with any vaccines in VIOLIN.
Table 1.
Curated vaccine adjuvants used in at least five vaccines curated in VIOLIN.
| Adjuvant type (number of adjuvants) | Adjuvants with >5 vaccines in VIOLIN | Number of vaccines |
|---|---|---|
| Mineral salts (13) | 154 | |
| Alhydrogel | 13 | |
| Aluminum hydroxide vaccine adjuvant | 62 | |
| Aluminum phosphate vaccine adjuvant | 50 | |
| Aluminum potassium sulfate adjuvant | 6 | |
| Aluminum vaccine adjuvant* | 14 | |
| Emulsions (15) | 118 | |
| Freund's complete adjuvant | 42 | |
| Freund's incomplete adjuvant | 42 | |
| Montanide ISA 720 adjuvant | 6 | |
| Ribi vaccine adjuvant | 11 | |
| Microorganism-derived (18) | 73 | |
| Cholera toxin | 17 | |
| Cholera toxin B subunit | 6 | |
| CpG DNA vaccine adjuvant | 12 | |
| LTR192G vaccine adjuvant | 6 | |
| MPL adjuvant | 10 | |
| Synthetic adjuvant (28) | 28 | |
| Cytokines as adjuvants (9) | 20 | |
| IL-12 vaccine adjuvant | 9 | |
| Tensoactive compounds (5) | 12 | |
| Quil-A vaccine adjuvant | 5 | |
| Combination vaccine adjuvants (6) | 9 | |
| Particulate antigen delivery systems (9) | 11 | |
| Carbohydrates (2) | 1 |
Note: *shows those aluminum vaccine adjuvants that were used but without more specific details (e.g., using aluminum hydroxide).
Those commonly used vaccine adjuvants and their associated vaccines are introduced below.
3.2.1. Salt-Based Vaccine Adjuvants
Mineral salt adjuvants are commonly used as aluminum adjuvants that are approved for use in licensed vaccines. Table 2 lists the 13 mineral salt adjuvants in Vaxjo and displays their properties, including the number of associated vaccines to each adjuvant, mechanism of action, route of administration, and whether or not the adjuvant is used in licensed vaccines. Of the mineral salt adjuvants in Vaxjo, 10 are licensed for use in vaccines either in Europe or the United States. Most mineral salt adjuvants act as vehicles for antigens rather than as immunostimulants (Table 2).
Table 2.
Mineral salt adjuvants.
| Adjuvant name | Number of Vaccines | Mechanism | Route | Licensed |
|---|---|---|---|---|
| Adjumer | 1 | Immunostimulant | Either* | No |
| Aluminum hydroxide vaccine adjuvant | 62 | Vehicle/immunostimulant | Parenteral | Yes** |
| Aluminum phosphate vaccine adjuvant | 50 | Vehicle/immunostimulant | Parenteral | Yes |
| Alhydrogel | 13 | Vehicle/immunostimulant | Either | Yes |
| Aluminum vaccine adjuvant*** | 14 | Vehicle/immunostimulant | Parenteral | Yes |
| Aluminum potassium sulfate adjuvant | 6 | Vehicle/immunostimulant | Parenteral | Yes |
| Amorphous aluminum hydroxyphosphate sulfate adjuvant (AAHSA) | 2 | Vehicle/immunostimulant | Parenteral | Yes |
| Adjumer | 1 | Immunostimulant | Either | No |
| Calcium phosphate gel | 1 | Vehicle | Parenteral | In Europe |
| Calcium phosphate vaccine adjuvant | 1 | Vehicle | Parenteral | In Europe |
| DOC/Alum complex | 1 | Vehicle/immunostimulant | Parenteral | No |
| Rehydragel HPA | 1 | Vehicle | Parenteral | Yes |
| Rehydragel LV | 1 | Vehicle | Parenteral | Yes |
Notes: *the term “either” means either mucosal or parenteral route. **the term “yes” means that this adjuvant is licensed in the USA. ***shows those aluminum vaccine adjuvants that were used but without more specific details (e.g., using aluminum hydroxide).
Mineral salts, specifically aluminum salts, have been used as adjuvants for decades and are therefore common in vaccine formulations [3, 13]. Alum salts have a depot effect, allowing the antigen to persist in the body so the immune system can react to the antigen and facilitate uptake into antigen-presenting cells (APCs). Aluminum also enhances the antigenicity of some vaccines such as diphtheria and tetanus toxoids [18]. Aluminum-adsorbed diphtheria and tetanus toxoids are more effective than plain fluid toxoids in primary immunization of children. However, there is little difference between plain and adsorbed toxoids for booster immunization [19]. Immune responses to proteins adjuvanted with alum tend to be a T helper cell type 2 (Th2) response [20]. The Chapter 21 of the US Code of Federal Regulations (610.15(a)) limits the amount of aluminum in human vaccines to 0.85 mg/dose. The majority are parenterally used, rather than mucosally. The amount of an aluminum adjuvant in vaccines currently licensed in the US ranges from 0.125 to 0.85 mg/dose [19]. In more than six decades, aluminum vaccine adjuvants usually do not induce serious adverse effects. However, they often induce local reactions such as redness, swelling, and tenderness at the injection site. Occasionally, aluminum adjuvants were found to be associated with severe local reactions such as erythema, subcutaneous nodules, and contact hypersensitivity [19].
3.2.2. Emulsion-Based Vaccine Adjuvants
Emulsion based adjuvants are oil-in-water or water-in-oil emulsions, which work to enable slow release of an antigen at the injection site. Fourteen emulsion-based vaccine adjuvants have been curated and stored in Vaxjo (Table 3). With the exception of the nanoemulsion vaccine adjuvant [21], all the emulsion adjuvants in Vaxjo are delivered via the parenteral route. Nanoemulsion vaccine adjuvant is delivered mucosally [21]. Water-in-oil emulsion adjuvants turn to cause high levels of reactogenicity. Oil-in-water emulsion adjuvants turn to have a low reactogenicity profile. MF59 and AS03 are two licensed oil-in-water emulsion adjuvant used in humans. MF59 is not toxic and prepared with a low content of squalene (4.3% w/w), a biodegradable oil naturally found in plants and animals including humans. In humans, squalene is an intermediate organic compound in the steroid hormone biosynthetic pathway and is a direct synthetic precursor to cholesterol. MF59 induces low reactogenicity at the site of injection. MF59 is able to induce fast priming of antigen-specific CD4+ T-cell responses and to induce strong and long-lasting memory T- and B-cell responses [22]. MF59 has been licensed in more than 20 countries for more than 14 years. It has been used in many licensed vaccines such as Fluad, Focetria, and Aflunov. AS03 (for “Adjuvant System 03”) is another squalene-based immunologic adjuvant used in various vaccine products by GlaxoSmithKline (GSK). AS03 is used, for example, in GSK's A/H1N1 pandemic flu vaccine Arepanrix H1N1.
Table 3.
Emulsion adjuvants.
| Adjuvant name | Number of vaccines | Mechanism | Route | Licensed |
|---|---|---|---|---|
| Freund's complete adjuvant | 42 | Immunostimulant | Parenteral | No |
| Freund's incomplete adjuvant | 42 | Vehicle | Parenteral | No |
| Ribi vaccine adjuvant | 11 | Immunostimulant | Parenteral | No |
| Montanide ISA 720 adjuvant | 6 | Vehicle | Parenteral | No |
| Nanoemulsion vaccine adjuvant | 3 | Immunostimulant | Mucosal | No |
| TiterMax gold adjuvant | 3 | Immunostimulant | Parenteral | No |
| AF03 | 1 | Immunostimulant | Parenteral | No |
| AS03 | 1 | Immunostimulant | Parenteral | Licensed |
| MF59 | 1 | Vehicle | Parenteral | In Europe |
| Montanide incomplete seppic adjuvant | 1 | Vehicle | Parenteral | No |
| Montanide ISA 51 | 1 | Vehicle | Parenteral | No |
| Specol | 1 | Immunostimulant | Parenteral | No |
| SPT (antigen formulation) | 1 | Vehicle | Parenteral | No |
| Squalene-based adjuvants | 1 | Vehicle | Parenteral | No |
| TiterMax gold adjuvant | 3 | Immunostimulant | Parenteral | No |
3.2.3. Microorganism-Based Vaccine Adjuvants
Microorganism-based vaccine adjuvants are adjuvants that are derived from microorganisms. Bacteria have an abundance of capacity for stimulating the immune system, and the adjuvants derived from bacteria are abundant. As whole bacteria are generally too toxic to use as an adjuvant, many bacterium-derived vaccine adjuvants are derived from parts of bacteria [1]. Table 4 displays the properties of the 18 microorganism-based adjuvants in Vaxjo. Only two of the microorganism-based adjuvants are licensed: MPL licensed in the United States as part of the AS04 adjuvant formulation [23], and Bordetella pertussis component vaccine adjuvant [24] that is used in several licensed vaccines. Microorganism-derived adjuvants cholera toxin and MPL are used in 17 and 10 vaccines, respectively. Many microorganism-derived adjuvants are based on microbial nucleic acids. For example, CpG DNA is the unmethylated CpG dinucleotides in what is called motifs, which are abundant in microbial DNA, but not in the DNA of vertebrates [25]. CpG DNA is immunostimulants that have the ability to activate Th1 immunity [26].
Table 4.
Microorganism-derived adjuvants.
| Adjuvant name | Number of Vaccines | Mechanism | Route | Licensed |
|---|---|---|---|---|
| Cholera toxin | 17 | Immunostimulant | Mucosal | No |
| CpG DNA vaccine adjuvant | 12 | Immunostimulant | Either * | No |
| MPL adjuvant | 10 | Immunostimulant | Either | As part of AS04 |
| Cholera toxin B subunit | 6 | Immunostimulant | Mucosal | No |
| LTR192G vaccine adjuvant | 6 | Immunostimulant | Mucosal | No |
| Bordetella pertussis component vaccine adjuvant | 3 | Immunostimulant | Parenteral | Yes |
| E. coli heat-labile toxin, LT | 3 | Immunostimulant | Mucosal | No |
| CTA1-DD gene fusion protein | 2 | Immunostimulant | Either | No |
| Etx B subunit adjuvant | 2 | Immunostimulant | Either | No |
| Killed Corynebacterium parvum vaccine adjuvant | 2 | Immunostimulant | Parenteral | No |
| Lipopolysaccharide vaccine adjuvant | 2 | Immunostimulant | Either | No |
| LTK63 vaccine mutant adjuvant | 2 | Immunostimulant | Mucosal | No |
| Corynebacterium-derived P40 vaccine adjuvant | 1 | Immunostimulant | Parenteral | No |
| Flagellin vaccine adjuvant | 1 | Immunostimulant | Either | No |
| LTK72 vaccine adjuvant | 1 | Immunostimulant | Mucosal | No |
| MPL-SE vaccine adjuvant | 1 | Immunostimulant | Parenteral | No |
| Non-toxic mutant E112K of Cholera Toxin mCT-E112K | 1 | Immunostimulant | Mucosal | No |
| Ty particles vaccine adjuvant | 1 | Vehicle | Parenteral | No |
Note: *either means either mucosal or parenteral route.
All but one microorganism-derived adjuvants act as immunostimulants as their mechanism of action. Adjuvants derived from the toxins of bacteria are used to stimulate mucosal immunity, which is important for protecting against many pathogens that are contracted mucosally [27]. The route of administration is able to reveal what type of immunity the adjuvant can stimulate, with eight microorganism-derived adjuvants being mucosally administered (generally intranasally). These are adjuvants which can help to stimulate mucosal immunity which is often critical in inducing protection from pathogens that are contracted mucosally. Six of the adjuvants are administered both parenterally and mucosally, and five are parenterally administered (Table 4).
3.2.4. Other Vaccine Adjuvants
The other six adjuvant types are synthetic adjuvants, cytokine adjuvants, particulate antigen delivery systems, carbohydrate vaccine adjuvants, tensoactive compounds, and combination vaccine adjuvants (Table 1).
Synthetic adjuvants are synthetically made compounds that mostly are utilized as immunostimulants and occasionally act as carriers. There are 28 synthetic adjuvants in Vaxjo. Some are derived to mimic bacterial components (Table 1). For example, muramyl dipeptide (MDP) is formulated to imitate a component found in the cell walls of mycobacteria used in Freund's complete adjuvant [28]. In addition, many synthetic MDP analogs are used as vaccine adjuvants. Administration routes are diverse, as there are parenterally, mucosally, and also topically administered synthetic vaccine adjuvants.
Cytokines, signaling molecules of the immune system, are also used as adjuvants to elicit a specific immune response [23]. There are nine cytokines as adjuvants in Vaxjo (Table 1). For example, granulocyte-macrophage colony-stimulating factor (GM-CSF), a cytokine that activates mature granulocytes and macrophages, is being used as an adjuvant for Hepatitis B in immunocompromised patients [29]. The GM-CSF adjuvant is also used in vaccines for HIV and cancer [30, 31]. Currently, none of cytokine-based adjuvants are licensed.
Particulate antigen delivery systems are adjuvants that have a depot effect for the antigen and include liposomes, polymeric microspheres, virus-like particles, and immunostimulating complexes (ISCOMs) [13]. There are nine particulate antigen delivery systems in Vaxjo. These adjuvants act as vehicles for the antigen, while often also containing immunostimulating compounds. They are parenteral adjuvants. Liposome-based adjuvants consist of spheres formed by lipid layers that can encapsulate antigens and thus act both as a vehicle and an immunostimulant [1]. Six liposome-based vaccine adjuvants are listed in Vaxjo. MTP-PE liposomes are liposomes that contain a synthetic analog of MDP, MTP-PE. It is used in vaccines for hepatitis B and herpes simplex virus [32, 33].
Carbohydrate adjuvants are adjuvants composed of complex carbohydrates of natural origin that stimulate the immune system. The main sources of these carbohydrates are plants and fungi [1]. For example, gamma inulin is a carbohydrate derived from the root of plants of the Compositae family. Gamma inulin is an activator of humoral and cellular immunity as well as the alternate complement pathway [1]. Two carbohydrate adjuvants are curated in Vaxjo (Table 1). They are generally parenterally administered and are immunostimulating adjuvants.
Tensoactive compounds are surfactants or active surface agents. They are immunostimulating adjuvants generally derived from plants, namely, Quillaja saponaria. There are five tensoactive adjuvants in Vaxjo (Table 1). Most saponins are considered too toxic for human use, although Quil A has been used in animals [1]. The saponins act as a vehicle/immunostimulant and are parenterally administered.
Combination vaccine adjuvants include two or more different adjuvants that are used in combination. Examples of combination adjuvants include adjuvants such as AS04 and Algammulin. There are six such adjuvants in Vaxjo (Table 1). These adjuvants combine different mechanisms of action from two or more adjuvants [13]. For example, AS04, a licensed vaccine in the United States, is a combination of aluminum and MPL [34]. Alum and MPL complement one another: Alum provides signals required for generating long-lived memory cells, whereas MPL enhances the differentiation of cytotoxic T lymphocytes (CTL) [35].
3.3. Analysis of Vaccine Adjuvants Based on the Pathogen Types
Table 5 displays adjuvanted vaccines by pathogen/disease types and vaccine types. Of the pathogens/diseases in VIOLIN, 46.2% have vaccines that use adjuvants. Gram-positive pathogens and parasites are associated with a high percentage of adjuvanted vaccines, with 79% and 71% of species containing adjuvanted vaccines, respectively. Meanwhile, 45.8% of Gram-negative pathogen vaccines use adjuvants. Of 1,982 viral vaccines in VIOLIN, only 4% are adjuvanted. For the cancer and allergy vaccines in VIOLIN, 20.83% use adjuvants. 13% of all the vaccines in VIOLIN are adjuvanted.
Table 5.
Vaccines with adjuvants by vaccine-targeted pathogens or diseases.
| Pathogen or disease | Number of pathogen species | Subunit vaccines | Toxoid vaccines | DNA vaccines | Conjugate vaccines | Live vaccines | Killed vaccines | RCV vaccine** | VDP*** | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Gram+ bacteria | 11/14* | 39 | 26 | 2 | 6 | 0 | 4 | 1 | 18 | 96 |
| Gram− bacteria | 22/48 | 96 | 6 | 2 | 7 | 0 | 2 | 5 | 13 | 130 |
| Viruses | 27/87 | 43 | 0 | 5 | 0 | 2 | 19 | 1 | 13 | 83 |
| Fungi | 1/1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Parasite | 15/21 | 50 | 0 | 1 | 0 | 1 | 1 | 2 | 0 | 55 |
| Cancer | — | 11 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 13 |
| Allergy | — | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 |
|
| ||||||||||
| Total | 80/173 | 245 | 32 | 14 | 13 | 4 | 26 | 9 | 44 | 384 |
*11/14 means 11 out of 14 spp. that have adjuvanted vaccines. Other numbers in this column use the same representation.
**RCV stands for recombinant vector vaccine.
*** VDP stands for vaccine made with different preparation methods, including (1) vaccine containing subunit component and inactivated whole organism, (2) vaccine containing DNA vaccine for priming and subunit vaccine for boosting, and (3) vaccine containing DNA vaccine for priming and Ad vector vaccine for boosting.
To develop vaccines for a specific pathogen, various strategies, including different vaccine adjuvants, may have been used. The choice of using different vaccine adjuvants may be dependent on the type of protective immunity required for the specific pathogen. As an example of illustrating these points, here we summarize and compare all vaccine adjuvants used in development of Brucella vaccines. Brucella, a Gram-negative intracellular pathogen that causes brucellosis in humans, is a disease for which there is no licensed human vaccine. Although humoral immune response against Brucella lipopolysaccharides (LPS) provides partial protection, the cell-mediated immunity plays a major role in protection against virulent Brucella challenge [36, 37]. In VIOLIN, 45 Brucella vaccines are curated. Of these 45 Brucella vaccines, nine use adjuvants (Table 6). Among these nine vaccines, six are subunit vaccines [38–42]. This phenomenon follows the trend of subunit vaccines being the majority of vaccines that use adjuvants. Among three of these six subunit vaccines that use Freund's adjuvants, two of them use complete Freund's adjuvant for the primary immunization and incomplete Freund's adjuvant for the boost vaccination [40, 41]. Three other vaccines are not categorized as subunit vaccines [43–45]. Among them is a vaccine that uses a DNA vaccine for priming and uses a recombinant protein adjuvanted by incomplete Freund's adjuvant for boosting [45]. The NPAP Brucella vaccine is a killed vaccine that uses Freund's incomplete adjuvant [43]. The recombinant O. anthropi expressing Brucella Cu/Zn SOD is live vaccine that uses with CpG DNA adjuvant [44]. The CpG DNA adjuvant is able to bias the immune response to the Brucella antigen towards a cell-mediated immunity. The recombinant O. anthropi expressing Brucella Cu/Zn SOD alone without the CpG DNA adjuvant is not able to induce protection, indicating the importance of the cell-mediated immunity in the protection induction [44].
Table 6.
Brucella vaccines with adjuvants in VIOLIN.
| Vaccine name | Vaccine type | Adjuvant name | Reference |
|---|---|---|---|
| B. melitensis DNA vaccine encoding Omp31 boosted with Omp31 | DNA vaccine with recombinant protein | Incomplete Freund's adjuvant | [45] |
| B. melitensis P39 protein vaccine | Subunit vaccine | CpG DNA vaccine adjuvant | [38] |
| Brucella ovis microparticle subunit vaccine | Subunit vaccine | Lipopolysaccharide adjuvant | [39] |
| Brucella recombinant SurA protein vaccine | Subunit vaccine | Incomplete Freund's adjuvant | |
| Complete Freund's adjuvant | [40] | ||
| E. coli Escheriosome-mediated cytosolic delivery of recombinant Brucella rL7/L12 protein | Subunit vaccine | Complete Freund's adjuvant | [41] |
| NPAP Brucella vaccine | Inactivated or “killed” vaccine | Incomplete Freund's adjuvant | [43] |
| Porin and S-LPS extracted from virulent Brucella abortus 2308 | Subunit vaccine | MPL vaccine adjuvant | [42] |
| Recombinant Brucella DnaK protein vaccine | Subunit vaccine | Incomplete Freund's adjuvant | |
| Complete Freund's adjuvant | [40] | ||
| Recombinant O. anthropi 49237SOD | Recombinant vector vaccine | CpG DNA vaccine adjuvant | [44] |
Although most subunit vaccines use adjuvants, some subunit vaccines do not use adjuvants. For example, two of these Brucella subunit vaccines contain Brucella lipopolysaccharide (LPS) as a primary antigen that can also perform as an adjuvant-like function by inducing cell-mediated immunity [46, 47]. The recombinant chimera vaccine BLSOmp31 includes a known Brucella Omp31-derived protective epitope fused to Brucella lumazine synthase (BLS) [48]. The BLS is highly immunogenic and functions as a scaffold protein and a carrier for the foreign peptide [49].
3.4. Analysis of Vaccine Adjuvants Based on Vaccine Types
The majority of adjuvanted vaccines, with 245 out of 384 vaccines (63.8%), are subunit vaccines. Other types of adjuvanted vaccines stored in VIOLIN include toxoid vaccines (8%), killed vaccines (7%), DNA vaccines (4%), and conjugate vaccines (3%). Forty-three vaccines in VIOLIN contain more than one adjuvant (http://www.violinet.org/vaxjo/stat2.php). In many cases, a primary vaccination uses one adjuvant, and then a boost immunization uses another adjuvant. Alternatively, one vaccine includes more than one adjuvant for one vaccination.
A subunit vaccine is composed of a purified protein(s) or other antigenic determinant(s) from a disease-causing organism. A subunit vaccine does not include pathogen nucleic acids. The subunits have less risk of causing adverse reactions. Subunit vaccines usually consist of specific proteins targeted to generate protection; however, they are rarely immunogenic on their own to trigger a response that will generate protection [50]. The very nature of subunit vaccines generates a need for adjuvantation. The vast majority of vaccines using adjuvants are subunit vaccines, representing 63.8% of the adjuvanted vaccines in VIOLIN (Table 5). In Vaxjo, 53 of the vaccine adjuvants are used in subunit vaccines. Most of these adjuvants have not frequently been used and occur only in one or two vaccines. A few are used more frequently, such as aluminum hydroxide, aluminum phosphate, Alhydrogel, Freund's complete and incomplete adjuvants, and cholera toxin.
The most frequently adjuvanted killed vaccines, with 19 out of 26, are viral vaccines (Table 5). Twelve adjuvants have been used in killed vaccines, with the most frequently used being aluminum hydroxide. The list of the US licensed vaccines that contain aluminum adjuvants include Anthrax vaccine, Hepatitis A and B vaccines, Rabies vaccine, Human papillomavirus vaccine, Pneumococcal conjugate vaccine, DTP (diphtheria-tetanus-pertussis vaccine) [19]. However, licensed human inactivated Polio Virus (IPV) vaccine, measles, mumps and rubella vaccine (MMR), varicella vaccine, and influenza vaccines do not contain any aluminum salt vaccine adjuvant [50]. Some of the other adjuvants used in killed vaccines in research are QuilA, aluminum phosphate, AS03, AS04, AF03, and Ribi vaccine adjuvant.
A toxoid vaccine consists of a toxoid (also called inactivated toxin), which is a processed toxin that has been treated by chemical means, heat, or irradiation and is no longer capable of causing disease. Toxoid vaccines, which are similar to subunit vaccines, are also usually adjuvanted, typically with aluminum. There are nine adjuvants associated with 32 toxoid vaccines (Table 5), which include aluminum adjuvants, squalene, and nanoemulsion vaccine adjuvant. The most frequently used is aluminum phosphate, which is used in nine toxoid vaccines.
A conjugate vaccine is vaccine that conjugates/links antigens to the molecules that form the outer coat of disease-causing bacteria to promote an immune response. Conjugate vaccines are not often adjuvanted, as they are usually immunogenic enough to not need an adjuvant. However, there are seven adjuvants in Vaxjo that are used in 13 conjugate vaccines (Table 5), including aluminum hydroxide, DDA, Ribi vaccine adjuvant, and MPL.
Live vaccines generally do not need adjuvants as they are rather immunogenic on their own. However, there are five vaccine adjuvants in Vaxjo that are used in four live attenuated vaccines (Table 5). These include aluminum hydroxide, Freund's complete and incomplete adjuvants, DHEA vaccine adjuvant, and Arlacel A. These three vaccines are for protection against Venezuelan equine encephalitis virus (VEE), cancer, smallpox, and E. maxima. For these vaccines, the adjuvant was given prior to immunization with the live attenuated vaccine.
Similar to live attenuated vaccines, DNA vaccines are not often adjuvanted. As for DNA vaccines, there are also eight adjuvants used that are in Vaxjo. There are 14 DNA vaccines that use adjuvants use GM-CSF, Resiquimod, and DDA among others (Table 5).
3.5. Ontology-Based Vaccine Adjuvant Representation for Exchange, Transfer, and Download of Vaccine Adjuvant Data
The basic information of vaccine adjuvants in Vaxjo is now stored in the Vaccine Ontology (VO; http://www.violinet.org/vaccineontology/) [17]. A biomedical ontology is a set of terms and relations that represent entities in a biomedical domain and how they relate to each other. Ontology terms are associated with documentation and definitions, which are, ideally, expressed in formal logic in order to support automated reasoning [51]. VO is a community-based ontology in the vaccine domain. It is developed based on the Web Ontology Language (OWL) format (http://www.w3.org/TR/owl-ref/), which can be processed by many existing software programs and used for Semantic Web applications. The storage of vaccine adjuvants in VO allows development of new software programs to integrate the vaccine adjuvant data with other biomedical data and support further bioinformatics analyses.
Figure 3 provides an example of how a VO vaccine adjuvant term “aluminum vaccine adjuvant” is shown in VO. The top level terms “entity → continuant → independent continuant → material entity” come from the Basic Formal Ontology (BFO: http://www.ifomis.org/bfo/), which is a top ontology that covers the universal entities. A continuant (e.g., a person or a quality) is an entity that exists in full at any time. An independent continuant (e.g., a person which is a material entity, but not a quality) is a continuant that bears its quality and is independent of other entities. All the other terms in the hierarchy shown in Figure 3(a) are VO-specific terms. As a part of a vaccine, a vaccine additive (e.g., preservative and adjuvant) is a material entity that is added to the immunogen of a vaccine. An aluminum vaccine adjuvant is a mineral salt vaccine adjuvant, a common vaccine adjuvant type (Figure 3(a)). Figure 3(b) indicates all vaccines that use this aluminum vaccine adjuvant (including all it child terms). One advantage of this OWL format is that it can be parsed and read by many computer programs. The machine readable feature supports automated reasoning.
Figure 3.

Modeling and application of vaccine adjuvants in the Vaccine Ontology (VO). (a) The hierarchical structure of vaccine adjuvants in VO. (b) All the vaccines that use this adjuvant. The usage information is obtained by a SPARQL query based on the logical definitions of vaccines. The website that contains this information is http://purl.obolibrary.org/obo/VO_0000884.
3.6. Vaxjo Data Query and Display
The manually curated and precomputed Vaxjo data can be efficiently queried and visualized as demonstrated in Figure 4. The vaccine adjuvant can be queried by specifying one or multiple criteria: (1) a characteristic of a vaccine adjuvant, including name, alternative name, adjuvant VO ID, description, components, and storage, (2) vaccine type, and (3) pathogen (Figure 4(a)). All query hits are first displayed on a web table containing basic adjuvant information (Figure 4(b)). Individual vaccine adjuvant can further be selected by a user to show more detailed information (Figure 4(c)). The associated vaccines are viewed by clicking on the number of associated vaccines (Figure 4(d)).
Figure 4.
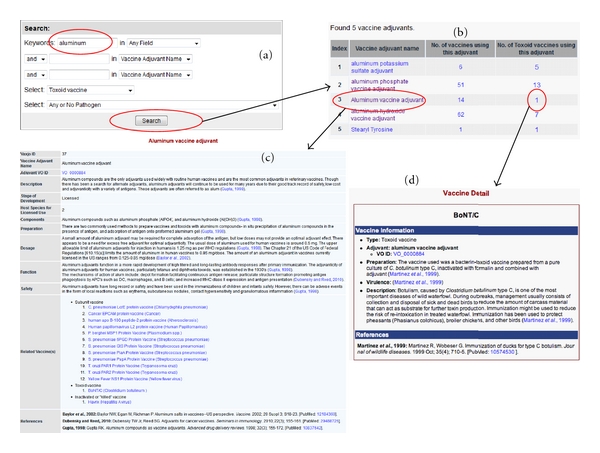
An example of searching a vaccine adjuvant and its related information in Vaxjo. (a) The keyword “aluminum” is queried in Vaxjo. (b) The adjuvants resulting from the Vaxjo keyword search. (c) The Vaxjo page obtained by clicking on “Aluminum vaccine adjuvant” in (b). The Vaxjo page for the adjuvant has information about the adjuvant (alternate names, components, function, etc.) and lists vaccines in which the adjuvant is used, as well as references. (d) The vaccine page obtained by clicking on the vaccine number shown in (b). This web page shows the details about this vaccine.
4. Discussion
Vaxjo contains manually curated vaccine adjuvants that have been used in a wide array of vaccines for a large variety of infectious diseases, as well as diseases such as cancer and allergies. To the best of our knowledge, Vaxjo is the first web-based, publically available database and analysis system that targets for the curation and analysis of vaccine adjuvants. There is a database of adjuvants/stimulants that is part of a nonhuman primate HIV/SIV vaccine trials database (http://www.hiv.lanl.gov/content/vaccine/adjuvants-stimulants.html). However, this database focuses on adjuvants used in HIV/SIV vaccine trials, which is a much narrower focus than Vaxjo. While many adjuvant review papers also exist [1, 2, 12, 13, 19, 50, 52], the information of vaccine adjuvants introduced in these review papers cannot be queried online and updated. The original VIOLIN database also includes many vaccines using different adjuvants. However, the general VIOLIN database does not include classification and details about each vaccine adjuvant. The Vaxjo database and query system is unique in that Vaxjo integrates the information of individual adjuvants and their uses in development of different vaccines stored in the general VIOLIN database. Therefore, Vaxjo is not just one simple extension of the VIOLIN vaccine database. The Vaxjo database is a new and relatively independent program.
A few challenges have been identified throughout the vaccine adjuvant curation process. One challenge is that many adjuvants are proprietary, and therefore there is little information available about them. There are many licensed animal vaccines that reside in VIOLIN, that lack adjuvant information. Because there is little information available on these vaccines, it is possible that some of these vaccines are in fact adjuvanted.
The Vaxjo database and analysis system can be used to facilitate rational vaccine design. Many diseases are complex in their interactions with the host immune system, thus making vaccine design difficult. To induce a protective immunity against a specific disease, an appropriate vaccine adjuvant is often required. Due to the large number of adjuvants in the market, it is often difficult to decide which vaccine adjuvant to use. The Vaxjo database provides details for each adjuvant and how they have been used in development of effective vaccines against different types of diseases. Therefore, an efficient query, comparison, and analysis of the data stored in Vaxjo allows a researcher to identify a small tailored list of vaccine adjuvants that can be used for enhancing induction of desired immune responses and having the highest possibility of success.
As a central and vital source of vaccine adjuvants, Vaxjo is a timely repository and is expected to have a significant impact for vaccine research and development.
Acknowledgment
This research was supported by NIH-NIAID Grant R01AI081062.
References
- 1.Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunology and Cell Biology. 2004;82(5):488–496. doi: 10.1111/j.0818-9641.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- 2.Marciani DJ. Vaccine adjuvants: role and mechanisms of action in vaccine immunogenicity. Drug Discovery Today. 2003;8(20):934–943. doi: 10.1016/s1359-6446(03)02864-2. [DOI] [PubMed] [Google Scholar]
- 3.Ramon G. Sur l'augmentation anormale de l'antitoxine chez les chevaux producteurs de serum antidiphterique. Bulletin de la Société Centrale de Médecine Vétérinaire. 1925;101:227–234. [Google Scholar]
- 4.Ramon G. Procedes pour accroïtre la production des antitoxins. Annales de l'Institut Pasteur. 1926;40:1–10. [Google Scholar]
- 5.Glenny AT, Pope CG, Waddington H, Wallace V. The antigenic value of toxoid precipitated by potassium-alum. Journal of Pathology & Bacteriology. 1926;29:38–45. [Google Scholar]
- 6.Freund J, Casals J, Hosmer EP. Sensitization and antibody formation after injection of tubercle bacili and parafin oil. Proceedings of the Society for Experimental Biology and Medicine. 1937;37:509–513. [Google Scholar]
- 7.Stuart-Harris CH. Adjuvant influenza vaccines. Bulletin of the World Health Organization. 1969;41(3):617–621. [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson AG, Gaines S, Landy M. Studies on the O antigen of Salmonella typhosa. V. Enhancement of antibody response to protein antigens by the purified lipopolysaccharide. The Journal of Experimental Medicine. 1956;103(2):225–246. doi: 10.1084/jem.103.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellouz F, Adam A, Ciorbaru R, Lederer E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochemical and Biophysical Research Communications. 1974;59(4):1317–1325. doi: 10.1016/0006-291x(74)90458-6. [DOI] [PubMed] [Google Scholar]
- 10.Vogel FR, Powell MF. A compendium of vaccine adjuvants and excipients. In: Powell MF, Newman MJ, editors. Vaccine Design: The Subunit and Adjuvant Approach. New York, NY, USA: Plenum; 1995. pp. 141–228. [DOI] [PubMed] [Google Scholar]
- 11.Giannini SL, Hanon E, Moris P, et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine. 2006;24(33-34):5937–5949. doi: 10.1016/j.vaccine.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Cox JC, Coulter AR. Adjuvants—a classification and review of their modes of action. Vaccine. 1997;15(3):248–256. doi: 10.1016/s0264-410x(96)00183-1. [DOI] [PubMed] [Google Scholar]
- 13.Aguilar JC, Rodríguez EG. Vaccine adjuvants revisited. Vaccine. 2007;25(19):3752–3762. doi: 10.1016/j.vaccine.2007.01.111. [DOI] [PubMed] [Google Scholar]
- 14.Xiang Z, Todd T, Ku KP, et al. VIOLIN: vaccine investigation and online information network. Nucleic Acids Research. 2008;36(1):D923–D928. doi: 10.1093/nar/gkm1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiang Z, Zheng W, He Y. BBP: brucella genome annotation with literature mining and curation. BMC Bioinformatics. 2006;7, article 347 doi: 10.1186/1471-2105-7-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang B, Sayers S, Xiang Z, He Y. Protegen: a web-based protective antigen database and analysis system. Nucleic Acids Research. 2011;39(supplement 1):D1073–D1078. doi: 10.1093/nar/gkq944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He Y, Cowell L, Diehl AD. VO: Vaccine Ontology. In: Proceedings of the 1st International Conference on Biomedical Ontology (ICBO '09); July 2009; Buffalo, NY, USA. [Google Scholar]
- 18.Gupta RK. Aluminum compounds as vaccine adjuvants. Advanced Drug Delivery Reviews. 1998;32(3):155–172. doi: 10.1016/s0169-409x(98)00008-8. [DOI] [PubMed] [Google Scholar]
- 19.Baylor NW, Egan W, Richman P. Aluminum salts in vaccines—US perspective. Vaccine. 2002;20(supplement 3):S18–S23. doi: 10.1016/s0264-410x(02)00166-4. [DOI] [PubMed] [Google Scholar]
- 20.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33(4):492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bielinska AU, Janczak KW, Landers JJ, Markovitz DM, Montefiori DC, Baker JR., Jr. Nasal immunization with a recombinant HIV gp120 and nanoemulsion adjuvant produces Th1 polarized responses and neutralizing antibodies to primary HIV type 1 isolates. AIDS Research and Human Retroviruses. 2008;24(2):271–281. doi: 10.1089/aid.2007.0148. [DOI] [PubMed] [Google Scholar]
- 22.O'Hagan DT, Rappuoli R, De Gregorio E, Tsai T, Del Giudice G. MF59 adjuvant: the best insurance against influenza strain diversity. Expert Review of Vaccines. 2011;10(4):447–462. doi: 10.1586/erv.11.23. [DOI] [PubMed] [Google Scholar]
- 23.Nicholls EF, Madera L, Hancock REW. Immunomodulators as adjuvants for vaccines and antimicrobial therapy. Annals of the New York Academy of Sciences. 2010;1213(1):46–61. doi: 10.1111/j.1749-6632.2010.05787.x. [DOI] [PubMed] [Google Scholar]
- 24.Athanassiades TJ. Adjuvant effect of Bordetella pertussis vaccine to sheep erythrocytes in mice: enhancement of cell-mediated immunity by subcutaneous administration of adjuvant and antigen. Infection and Immunity. 1977;18(2):416–423. doi: 10.1128/iai.18.2.416-423.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bauer S, Wagner H. Bacterial CpG-DNA licenses TLR9. Current Topics in Microbiology and Immunology. 2002;270:145–154. doi: 10.1007/978-3-642-59430-4_9. [DOI] [PubMed] [Google Scholar]
- 26.Leroux-Roels G. Unmet needs in modern vaccinology. Adjuvants to improve the immune response. Vaccine. 2010;28(supplement 3):C25–C36. doi: 10.1016/j.vaccine.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 27.Chen K, Cerutti A. Vaccination strategies to promote mucosal antibody responses. Immunity. 2010;33(4):479–491. doi: 10.1016/j.immuni.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oppenheim JJ, Togawa A, Chedid L, Mizel S. Components of mycobacteria and muramyl dipeptide with adjuvant activity induce lymphocyte activating factor. Cellular Immunology. 1980;50(1):71–81. doi: 10.1016/0008-8749(80)90007-6. [DOI] [PubMed] [Google Scholar]
- 29.Pichichero ME. Improving vaccine delivery using novel adjuvant systems. Human Vaccines. 2008;4(4):262–270. doi: 10.4161/hv.4.4.5742. [DOI] [PubMed] [Google Scholar]
- 30.Bernhardt SL, Gjertsen MK, Trachsel S, et al. Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: a dose escalating phase I/II study. British Journal of Cancer. 2006;95(11):1474–1482. doi: 10.1038/sj.bjc.6603437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song R, Liu S, Adams RJ, Leong KW. Enhancing efficacy of HIV Gag DNA vaccine by local delivery of GM-CSF in murine and macaque models. Journal of Interferon and Cytokine Research. 2006;26(6):380–389. doi: 10.1089/jir.2006.26.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jain V, Vyas SP, Kohli DV. Well-defined and potent liposomal hepatitis B vaccines adjuvanted with lipophilic MDP derivatives. Nanomedicine. 2009;5(3):334–344. doi: 10.1016/j.nano.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Burke RL, Goldbeck C, Ng P, Stanberry L, Ott G, van Nest G. The influence of adjuvant on the therapeutic efficacy of a recombinant genital herpes vaccine. The Journal of Infectious Diseases. 1994;170(5):1110–1119. doi: 10.1093/infdis/170.5.1110. [DOI] [PubMed] [Google Scholar]
- 34.Garçon N, Segal L, Tavares F, van Mechelen M. The safety evaluation of adjuvants during vaccine development: the AS04 experience. Vaccine. 2011;29(27):4453–4459. doi: 10.1016/j.vaccine.2011.04.046. [DOI] [PubMed] [Google Scholar]
- 35.MacLeod MK, McKee AS, David A, et al. Vaccine adjuvants aluminum and monophosphoryl lipid A provide distinct signals to generate protective cytotoxic memory CD8 T cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(19):7914–7919. doi: 10.1073/pnas.1104588108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He Y, Vemulapalli R, Schurig GG. Recombinant Ochrobactrum anthropi expressing Brucella abortus Cu,Zn superoxide dismutase protects mice against B. abortus infection only after switching of immune responses to Th1 type. Infection and Immunity. 2002;70(5):2535–2543. doi: 10.1128/IAI.70.5.2535-2543.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He Y, Vemulapalli R, Zeytun A, Schurig GG. Induction of specific cytotoxic lymphocytes in mice vaccinated with Brucella abortus RB51. Infection and Immunity. 2001;69(9):5502–5508. doi: 10.1128/IAI.69.9.5502-5508.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Mariri A, Tibor A, Mertens P, et al. Protection of BALB/c mice against Brucella abortus 544 challenge by vaccination with bacterioferritin or P39 recombinant proteins with CpG oligodeoxynucleotides as adjuvant. Infection and Immunity. 2001;69(8):4816–4822. doi: 10.1128/IAI.69.8.4816-4822.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Estevan M, Gamazo C, Grilló MJ, del Barrio GG, Blasco JM, Irache JM. Experiments on a sub-unit vaccine encapsulated in microparticles and its efficacy against Brucella melitensis in mice. Vaccine. 2006;24(19):4179–4187. doi: 10.1016/j.vaccine.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 40.Delpino MV, Estein SM, Fossati CA, Baldi PC, Cassataro J. Vaccination with Brucella recombinant DnaK and SurA proteins induces protection against Brucella abortus infection in BALB/c mice. Vaccine. 2007;25(37-38):6721–6729. doi: 10.1016/j.vaccine.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Mallick AI, Singha H, Khan S, et al. Escheriosome-mediated delivery of recombinant ribosomal L7/L12 protein confers protection against murine brucellosis. Vaccine. 2007;25(46):7873–7884. doi: 10.1016/j.vaccine.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 42.Winter AJ, Rowe GE, Duncan JR, et al. Effectiveness of natural and synthetic complexes of porine and O polysaccharide as vaccines against Brucella abortus in mice. Infection and Immunity. 1988;56(11):2808–2817. doi: 10.1128/iai.56.11.2808-2817.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delpino MV, Estein SM, Fossati CA, Baldi PC. Partial protection against Brucella infection in mice by immunization with nonpathogenic alphaproteobacteria. Clinical and Vaccine Immunology. 2007;14(10):1296–1301. doi: 10.1128/CVI.00459-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He Y, Reichow S, Ramamoorthy S, et al. Brucella melitensis triggers time-dependent modulation of apoptosis and down-regulation of mitochondrion-associated gene expression in mouse macrophages. Infection and Immunity. 2006;74(9):5035–5046. doi: 10.1128/IAI.01998-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cassataro J, Velikovsky CA, Bruno L, et al. Improved immunogenicity of a vaccination regimen combining a DNA vaccine encoding Brucella melitensis outer membrane protein 31 (Omp31) and recombinant Omp31 boosting. Clinical and Vaccine Immunology. 2007;14(7):869–874. doi: 10.1128/CVI.00472-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhattacharjee AK, Izadjoo MJ, Zollinger WD, Nikolich MP, Hoover DL. Comparison of protective efficacy of subcutaneous versus intranasal immunization of mice with a Brucella melitensis lipopolysaccharide subunit vaccine. Infection and Immunity. 2006;74(10):5820–5825. doi: 10.1128/IAI.00331-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhattacharjee AK, van de Verg L, Izadjoo MJ, et al. Protection of mice against brucellosis by intranasal immunization with Brucella melitensis lipopolysaccharide as a noncovalent complex with Neisseria meningitidis group B outer membrane protein. Infection and Immunity. 2002;70(7):3324–3329. doi: 10.1128/IAI.70.7.3324-3329.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cassataro J, Pasquevich KA, Estein SM, et al. A recombinant subunit vaccine based on the insertion of 27 amino acids from Omp31 to the N-terminus of BLS induced a similar degree of protection against B. ovis than Rev.1 vaccination. Vaccine. 2007;25(22):4437–4446. doi: 10.1016/j.vaccine.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 49.Laplagne DA, Zylberman V, Ainciart N, et al. Engineering of a polymeric bacterial protein as a scaffold for the multiple display of peptides. Proteins. 2004;57(4):820–828. doi: 10.1002/prot.20248. [DOI] [PubMed] [Google Scholar]
- 50.Hunter RL. Overview of vaccine adjuvants: present and future. Vaccine. 2002;20(supplement 3):S7–S12. doi: 10.1016/s0264-410x(02)00164-0. [DOI] [PubMed] [Google Scholar]
- 51.Xiang Z, Courtot M, Brinkman RR, Ruttenberg A, He Y. OntoFox: web-based support for ontology reuse. BMC Research Notes. 2010;3, article 175 doi: 10.1186/1756-0500-3-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vogel FR. Improving vaccine performance with adjuvants. Clinical Infectious Diseases. 2000;30(supplement 3):S266–S270. doi: 10.1086/313883. [DOI] [PubMed] [Google Scholar]


