Abstract
Disorganisation and aggregation of proteins containing expanded polyglutamine (polyQ) repeats, or ectopic expression of α-synuclein, underlie neurodegenerative diseases including Alzheimer's, Parkinson, Huntington, Creutzfeldt diseases. Small heat-shock proteins, such as αB-crystallin, act as chaperones to prevent protein aggregation and play a key role in the prevention of such protein disorganisation diseases. In this study, we have explored the potential for chaperone activity of αB-crystallin to suppress the formation of protein aggregates. We tested the ability of αB-crystallin to suppress the aggregation of a polyQ protein and α-synuclein in Drosophila. We found that αB-crystallin suppresses both the compound eye degeneration induced by polyQ and the α-synuclein-induced rough eye phenotype. Furthermore, by using histochemical staining we have determined that αB-crystallin inhibits the aggregation of polyQ in vivo. These data provide a clue for the development of therapeutics for neurodegenerative diseases.
1. Introduction
Expansion of polyglutamine (polyQ) domains causes inherited neurodegenerative diseases, such as Huntingtons, disease, various types of spinocerebellar ataxia, and spinobulbar muscular atrophy (SBMA) [1, 2]. The formation of intranuclear inclusions of polyQ-containing proteins in the brain is one of the pathological features of these diseases. Inhibition of the aggregation of the expanded polyQ-containing proteins may therefore provide treatment options for polyQ diseases [3]; however, the relationship between polyQ aggregation and cellular toxicity is not well understood. It was demonstrated in earlier studies in human cells that targeted expression of proteins with an expanded polyQ repeat led to nuclear inclusion formation followed by late-onset cell degeneration. Similar observations have been made in Drosophila suggesting that the cellular mechanisms underlying human glutamine-repeat diseases are conserved in invertebrates [4]. From a therapeutic view point, Drosophila provides a unique system in which to search for novel genetic factors that suppress cellular toxicity. Previous studies have indicated that QBP1 (polyQ binding peptide 1) binds to the expanded polyQ stretch and inhibits aggregate formation of the expanded polyQ protein in vitro [5, 6]. Furthermore, Ter 94, the Drosophila homolog of the myeloid leukemia factor 1, and heat-shock-transcription-factor-1 (HSF1-) activating compounds are also reported to have a dominant role in suppressing polyQ aggregation [7–9].
Alpha-crystallins (α-crystallins) are major protein components of the vertebrate eye lens. The α-crystallins and especially αB-crystallin are also found outside the lens, and show a wide tissue distribution [10]. The αB-crystallin protein is found to be overexpressed in many neurological diseases, and mutations in αA- or αB-crystallin can cause cataracts and myopathy [11]. The αB-crystallin protein is a member of the small heat shock protein family, and it has a chaperone-like function [12–15]. Therefore, αB-crystallin can bind to the unfolded proteins and inhibit nonspecific aggregation [10]. Consistent with this it has been shown to prevent the fibrillar aggregation of proteins implicated in human diseases and limit the onset of misfolding diseases [16–18]. Furthermore, accumulation of αB-crystallin is an important mechanism for the prevention of cellular damage associated with heat shock and oxidative stress conditions in the trabecular meshwork [19, 20].
α-synuclein is a presynaptic protein whose major role in neurodegeneration was recognized in Parkinson disease where a mutation in the α-synuclein gene was associated with autosomal dominant inheritance of the disease [21]. It is reported that αB-crystallin interacts with α-synuclein to protect against amyloid aggregation and limit the onset of misfolding diseases in vitro [16].
In the present study, we investigate the ability of the molecular chaperone, αB-crystallin, to inhibit the aggregation of protein induced by ectopic expression of polyQ and α-synuclein in fly model. Our results indicate that αB-crystallin suppresses the neurodegenerative effects induced by overexpression of polyQ as well as α-synuclein.
2. Material and Methods
2.1. Fly Strains
Fly strains were maintained at 25°C on standard food. The transgenic fly line carrying GMR-GAL4 on X chromosome was described earlier [22] as was the transgenic fly line carrying gmr-Q92 [4, 7]. The UAS-LacZ flies used in this study were obtained from Bloomington Drosophila stock center. Wild-type flies: W; UAS-α syn; +, was obtained from. Feany and Bender [23].
2.2. Plasmid Construction and Establishment of Transgenic Flies
To construct the plasmid pUAS-αB-crystallin-HA, the full coding sequence of human αB-crystallin cDNA (accession number S45630) corresponding to amino acids (aa) 1 to aa175 was amplified by PCR with primers carrying BglII site and XhoI site (aB-crystallin-BglII: 5′-gca gat ctg aca tcg cca tcc acc acc, aB-crystallin-XhoI: 5′-cgc tcg agc tat ttc ttg ggg gct gc). The PCR products were digested with BglII and XhoI and inserted into the plasmid pUAST-HA (Lablife).
The P element-mediated germ line transformation with pUAS-αB-crystallin-HA was carried out as describe earlier [24], and F1 transformants were selected on the basis of white-eye color rescue [25]. The established transgenic fly lines and their chromosome linkages are listed in Table 1.
Table 1.
Transgenic fly lines established in this study and their chromosome linkages.
| P-element plasmid | Strains | Chromosome linkages |
|---|---|---|
| UAS-αB-crystallin-HA | 27 | II |
| 8 | II | |
| 3 | III | |
| 11 | III | |
| 12 | III |
2.3. Western Immunoblot Analysis
Proteins were extracted from eye imaginal discs of third-instar larvae, applied to a SDS-polyacrylamide gel containing 12% acrylamide and transferred to polyvinylidene difluoride membranes (Bio-Rad) in transfer buffer (50 mM borate-NaOH, pH 9.0, and 20% ethanol) by using iBlot system (Invitrogen). The blotted membranes were incubated with monoclonal antibodies to HA (HA124, Invitrogen) at 1 : 400 dilution or anti-αtubulin IgG (Sigma) at 1 : 2.000 for 16 h at 4°C. The bound antibodies were detected with peroxidase-conjugated goat anti-mouse IgG and the ECL system (GE healthcare) according to the manufacturer's recommendations. The images were analyzed with a Lumivision ProHSII image analyzer (Aisin Seiki, Aichi, Japan).
2.4. Scanning Electron Microscopy
Adult flies were anesthetized, mounted on stages, and observed under a VE-7800 scanning electron microscope (Keyence Inc.) in the high vacuum mode. In every experiment, the eye phenotype of at least five adult flies of each line was simultaneously examined by scanning electron microscopy, and these experiments were repeated 3 times. In the experiments, no significant variation in eye phenotype among the five individuals was observed.
2.5. Immunohistochemistry
Third instar larvae were dissected in PBS, and tissues were fixed in 3% paraformaldehyde/PBS for 30 min at 25°C. After washing with PBS/0.3% TritonX-100 (PBS-T), the samples were blocked with PBS-T containing 10% normal goat serum for 20 min at 25°C and incubated with an anti-HA (Invitrogen), anti-FLAG monoclonal antibody (Sigma), or anti-β-galactosidase monoclonal antibody (Developmental Studies Hybridoma Bank, DSHB) at a 1 : 200 dilution at 4°C for 16 h. After extensive washing with PBS-T, the tissues were incubated with an anti-mouse IgG conjugated with Alexa 594 or Alexa 488 (Molecular Probes) at a 1 : 400 dilution each at 37°C for 1 h. After extensive washing with PBS-T and PBS, samples were mounted in Fluor Guard Antifade Reagent (Bio-Rad) for microscopic observation under a fluorescence BX-40 microscope (Olympus, Tokyo, Japan) equipped with a cooled CCD camera (ORCA-ER; Hamamatsu Photonics K. K., Shizuoka, Japan).
3. Results and Discussion
3.1. Ectopic Expression of αB-Crystallin Results in Abnormal Eye Morphology
We first established lines of transgenic flies carrying the uas-human-αB-crystallin. In order to confirm that human-αB-crystallin is functional in Drosophila, we used the gmr-gal4 driver strain to provide ectopic expression of αB-crystallin-HA from the morphogenetic furrow to the posterior end of the Drosophila eye imaginal disc [26]. By inspection with the scanning electron microscope, the compound eyes of adult flies carrying the gmr-gal4 alone showed normal eye morphology (Figures 1(a) and 1(b)), while those of flies carrying both the gmr-gal4 and the uas-αB-crystallin exhibited a rough-eye phenotype (Figures 1(d) and 1(e)).
Figure 1.
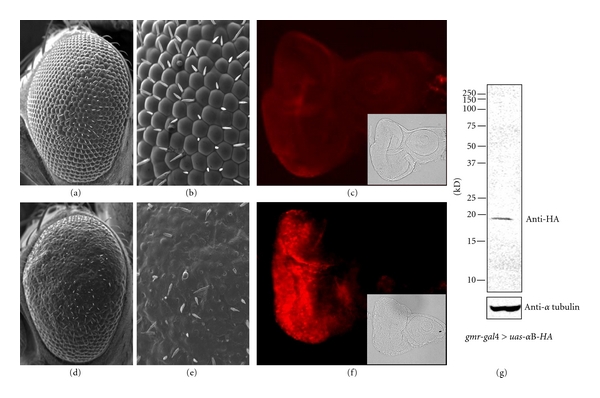
Scanning electron micrographs of adult compound eyes. (a, b) gmr-gal4; +; +. (d, e) gmr-gal4; uas-αB-crystallin-HA; +. (c, f) Immunostaining of the eye imaginal discs with anti-HA antibody. The eye disc from gmr-gal4;+; + flies (c) and gmr-gal4; uas-αB-crystallin-HA; + flies (f). The insets indicate Nomarski images. (g) The anti-HA antibody specifically recognized αB-crystallin-HA in the Western Blot of the eye imaginal discs of gmr-gal4; uas-αB-crystallin-HA; + flies.
To further confirm the expression of αB-crystallin carrying an HA tag in its C-terminal end in eye imaginal discs, we carried out immunostaining of eye imaginal discs from third instar larvae using anti-HA antibody. Only gmr-gal4; uas-αB-crystallin flies showed ectopic αB-crystallin-HA signals from the morphogenetic furrow to the posterior end of eye discs (Figure 1(f)). In contrast, the gmr-gal4 line that was used as the negative control showed no signal (Figure 1(c)). The expression of αB-crystallin-HA was further confirmed by the Western immunoblot analysis of eye imaginal disc extracts with anti-HA antibody, detecting a single αB-crystallin-HA band at around 20 kDa (Figure 1(g)).
3.2. Expression of αB-Crystallin Suppresses the Compound Eye Degeneration Induced by PolyQ
To examine the effect of αB-crystallin protein on polyQ-induced neurodegeneration in Drosophila, we crossed gmr-Q92 fly lines expressing a Q92 peptide in the eye discs under the direct control of the gmr promoter [5, 7], and gmr-gal4 > uas-αB-crystallin-HA (uas-αB-HA) flies in which αB-crystallin-HA expression is driven by gmr-gal4.
Expression of the FLAG-tagged 92-glutamine peptides induced compound eye degeneration such as rough eye and loss of red eye pigment (loss-of-pigment phenotype) as described (Figures 2(a) and 2(d)) [5, 7] and the eye phenotype, especially the loss-of-pigment phenotype, was effectively rescued by coexpression of αB-crystallin-HA (Figures 2(c) and 2(f)). In contrast, expression of the control lacZ protein did not affect the polyQ-induced phenotype (Figures 2(b) and 2(e)).
Figure 2.

Suppression of compound eye degeneration by coexpression of αB-crystallin in the Q92-expressing flies. Light microscopic images of the adult compound eyes of the polyQ lines expressing only one Q92 peptide (a) or 2xQ92 peptide (d) under control of the gmr promoter. (c, f) Transgenic flies coexpressing Q92 peptides and αB-crystallin (gmr-gal4; gmr-Q92/+; uas-αB-crystallin-HA). (b, e) Transgenic flies coexpressing Q92 peptides and control LacZ protein (gmr-gal4; gmr-Q92/+; uas-LacZ). (g) Transgenic flies expressing αB-crystallin alone (gmr-gal4; uas-αB-crystallin-HA). (h) Transgenic flies expressing lacZ alone (gmr-gal4; uas-LacZ). Magnification x 90.
3.3. Expression of αB-Crystallin Suppresses PolyQ Aggregate Formation in the Eye Imaginal Discs
In order to investigate the ability of αB-crystallin to inhibit polyQ aggregation in vivo, we examined polyQ aggregate formation in the eye imaginal discs of third instar larvae in the gmr-Q92 fly lines with and without expression the αB-crystallin-HA. PolyQ forms inclusion bodies in the region posterior to the morphogenetic furrow in eye discs of the gmr-Q92 flies and many of these aggregate formations could be detected by immunostaining with anti-FLAG antibody (Figures 3(A1) and 3(C1)).
Figure 3.
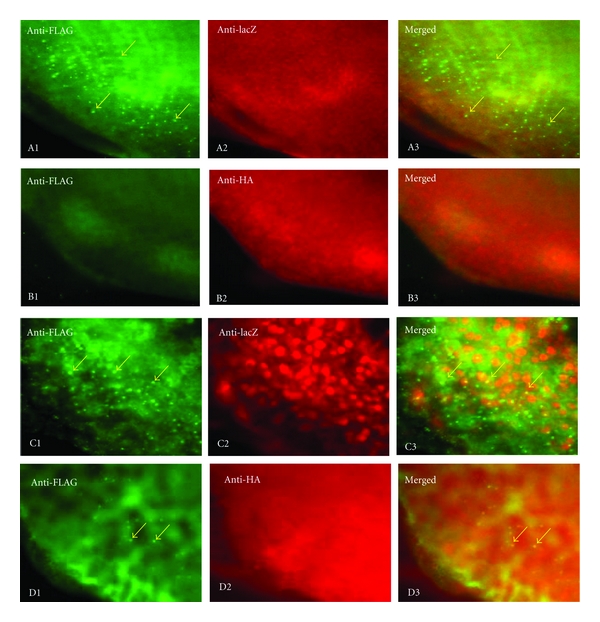
Suppression of Q92 aggregate formation by expression of αB-crystallin in the imaginal discs of the Q92 flies. The eye discs were immunostained with anti-FLAG antibody to detect Q92 tagged with FLAG (green) and anti-HA or anti-LacZ antibodies to detect αB-crystallin-HA (red) and lacZ, respectively (red). The arrows indicate examples of Q92 aggregates. (A1–A3) gmr-gal4; gmr-Q92/+; uas-lacZ. (B1–B3) gmr-gal4; gmr-Q92/+; uas-αB-crystallin-HA. (C1–C3) gmr-gal4; 2x(gmr-Q92)/+; uas-lacZ. (D1–D3) gmr-gal4; 2x(gmr-Q92-FLAG)/+; uas-αB-crystallin-HA.
Expression of αB-crystallin-HA completely suppressed formation of the polyQ aggregates in the line exhibiting a mild eye phenotype (gmr-Q92) (Figure 3(B1)) and dramatically suppressed it in the line exhibiting a severe eye phenotype (2xgmr-Q92) (Figure 3(D1)), while expression of control lacZ protein exerted no suppressing effect (Figures 3(A1) and 3(C1)). These results suggest correlation between formation of the polyQ aggregates and neurodegeneration.
3.4. Expression of αB-Crystallin Suppresses the Human α-Synuclein Induced Rrough-Eye Phenotype
A previous in vitro study indicated that αB-crystallin was very effective in suppressing fibrillization of both α-synuclein wild-type and A30P α-synuclein mutant [15]. Therefore, in this study, to evaluate ability of αB-crystallin to suppress the rough-eye phenotype induced by ectopic expression of α-synuclein, we performed coexpression of αB-crystallin and α-synuclein in fly eyes and compared the results with those of control flies coexpressing lacZ and α-synuclein. As noted previously, overexpression of wild-type α-synuclein induced rough eye phenotype (Figures 4(g) and 4(h)). The rough eye phenotype was significantly suppressed by coexpressing αB-crystallin (Figures 4(a) and 4(b)), while no effect on the rough eye was observed by coexpressing control lacZ (Figures 4(c) and 4(d)). These results indicate that αB-crystallin protein effectively suppresses the events induced by overexpression of α-synuclein.
Figure 4.
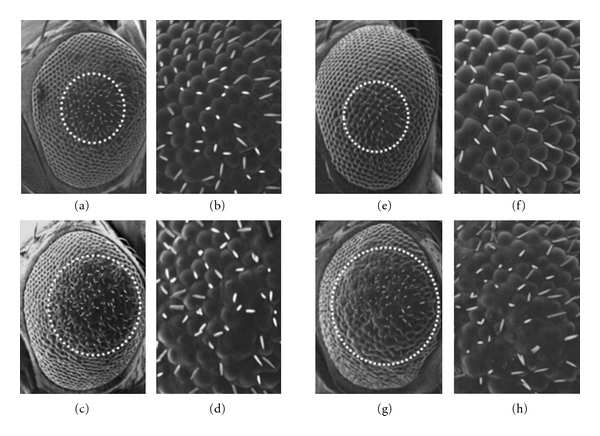
Suppression of compound eye degeneration by coexpression of αB-crystallin in the α-synuclein-expressing flies. Light microscope images of the adult compound eyes. (a, b) Transgenic flies coexpressing α-synuclein and αB-crystallin (gmr-gal4/+; uas-αB-crystallin-HA/+; uas-α-synuclein/+). (c, d) Transgenic flies coexpressing α-synuclein and control LacZ protein (gmr-gal4/+; uas-LacZ/+; uas-α-synuclein/+). (e, f) Control flies (gmr-gal4/+; +; +). (g, h) Transgenic flies expressing α-synuclein (gmr-gal4/+; uas-α-synuclein/+; +). (a, c, e, g ) Magnification x 200. (b, d, f, h) Magnification x 700.
In this study, we investigated a role of αB-crystallin on polyQ aggregation in a Drosophila model to gain insight into pathways which could be useful in the treatment of neurodegenerative diseases. We used immunohistochemical methods to show that the GMR-GAL4/UAS-αB-crystallin flies expressed an abundant αB-crystallin protein in the region from the morphogenetic furrow to the posterior end of eye discs. Consequently, flies exhibit rough-eye phenotype in adults. These results indicate that the human αB-crystallin protein is functional in flies.
Previous studies showed that αB-crystallin can act as a molecular chaperone to prevent fibril formation and aggregation of α-synuclein, β2-microglobulin, and κ-casein in vitro [10, 12, 15–17, 27–29]. Moreover, chaperone activity sites of αB-crystallin were identified in the sequences from residues aa73-aa85 and aa101-aa110 [30–32], suggesting that expression of full length or a part of chaperone sequences of this protein is capable of suppressing polyglutamine-induced protein aggregation. In the present study, we found that the full-length αB-crystallin could inhibit aggregation of the 92-polyQ peptide in vivo. The results support the hypothesis that interactive domains in αB-crystallin play a role in prevention of protein aggregation. In contrast, the recent study shows that αB-crystallin does not inhibit or modify the fibrillar aggregation of SpAcQ52 in vitro [33]. Because glutamine is a hydrophilic amino acid, the surface hydrophobicity of polyQ may not be changed as aggregation progresses. Furthermore, the polyQ region is thought to be in a condensed disordered state in the native conditions [34]. Therefore, the physiochemical properties of the polyQ tract may lead it to interact with chaperonin differently in vitro compared with in vivo. The effectiveness of suppression of polyQ disorders may also depend on the other factors, such as the temperature, concentrations of chemical compounds, or the inhibitor proteins [33, 35, 36]. Here, our data indicate that inhibition of polyQ-aggregation by αB-crystallin in flies carrying one copy of the Q92 gene is complete, but flies carrying two copies of the Q92 gene show only partial suppression, suggesting the importance of optimization to determine the effective level of inhibitors for therapies for polyQ diseases. More interestingly, it is reported that αB-crystallin is a potent in vitro inhibitor of the fibrillization of α-synuclein, a protein involved in neurodegenerative diseases, both wild-type and the two mutant forms (A30P and A53T) [15, 16]. The data we present here suggests that this is achieved by the interaction of αB-crystallin and α-synuclein inhibiting further aggregation.
4. Conclusion
The present study has demonstrated an essential role of αB-crystallin in suppressing the neurodegeneration induced by polyQ expansion and ectopic expression of α-synuclein in vivo and suggest a possible target for the development of therapeutics for neurodegenerative diseases. Moreover, the assay system to suppress neurodegeneration using transgenic flies described in the present study should be a powerful tool to evaluate the in vivo effect of peptides carrying potential chaperone activities, and the αB-crystallin-overexpressing flies established in this study might provide an invaluable standard for the evaluation.
Acknowledgments
The authors thank Dr. Mel B. Feany for supplying fly strains carrying the α-synuclein, Dr. M. Moore and S. Cotterill for comments on the English usage in the paper, and all members in our laboratory for helpful discussions. This study was partially supported by Grants-in-Aid from KIT, JST, JSPS, and the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Annual Review of Neuroscience. 2007;30:575–621. doi: 10.1146/annurev.neuro.29.051605.113042. [DOI] [PubMed] [Google Scholar]
- 2.Shao J, Diamond MI. Polyglutamine diseases: emerging concepts in pathogenesis and therapy. Human Molecular Genetics. 2007;16:R115–R123. doi: 10.1093/hmg/ddm213. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka M, Machida Y, Nukina N. A novel therapeutic strategy for polyglutamine diseases by stabilizing aggregation-prone proteins with small molecules. Journal of Molecular Medicine. 2005;83(5):343–352. doi: 10.1007/s00109-004-0632-2. [DOI] [PubMed] [Google Scholar]
- 4.Warrick JM, Paulson HL, Gray-Board GL, et al. Expanded polyglutamine protein forms nuclear inclusions and causes neural degeneration in Drosophila. Cell. 1998;93(6):939–949. doi: 10.1016/s0092-8674(00)81200-3. [DOI] [PubMed] [Google Scholar]
- 5.Nagai Y, Fujikake N, Ohno K, et al. Prevention of polyglutamine oligomerization and neurodegeneration by the peptide inhibitor QBP1 in Drosophila. Human Molecular Genetics. 2003;12(11):1253–1259. doi: 10.1093/hmg/ddg144. [DOI] [PubMed] [Google Scholar]
- 6.Nagai Y, Inui T, Popiel HA, et al. A toxic monomeric conformer of the polyglutamine protein. Nature Structural and Molecular Biology. 2007;14(4):332–340. doi: 10.1038/nsmb1215. [DOI] [PubMed] [Google Scholar]
- 7.Higashiyama H, Hirose F, Yamaguchi M, et al. Identification of ter94, Drosophila VCP, as a modulator of polyglutamine-induced neurodegeneration. Cell Death and Differentiation. 2002;9(3):264–273. doi: 10.1038/sj.cdd.4400955. [DOI] [PubMed] [Google Scholar]
- 8.Fujikake N, Nagai Y, Popiel HA, Okamoto Y, Yamaguchi M, Toda T. Heat shock transcription factor 1-activating compounds suppress polyglutamine-induced neurodegeneration through induction of multiple molecular chaperones. Journal of Biological Chemistry. 2008;283(38):26188–26197. doi: 10.1074/jbc.M710521200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kazemi-Esfarjani P, Benzer S. Suppression of polyglutamine toxicity by a Drosophila homolog of myeloid leukemia factor 1. Human Molecular Genetics. 2002;11(21):2657–2672. doi: 10.1093/hmg/11.21.2657. [DOI] [PubMed] [Google Scholar]
- 10.Horwitz J. The function of alpha-crystallin in vision. Seminars in Cell and Developmental Biology. 2000;11(1):53–60. doi: 10.1006/scdb.1999.0351. [DOI] [PubMed] [Google Scholar]
- 11.Horwitz J. Alpha-crystallin. Experimental Eye Research. 2003;76(2):145–153. doi: 10.1016/s0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- 12.Reddy GB, Kumar PA, Kumar MS. Chaperone-like activity and hydrophobicity of α-crystallin. IUBMB Life. 2006;58(11):632–641. doi: 10.1080/15216540601010096. [DOI] [PubMed] [Google Scholar]
- 13.Datta SA, Rao CM. Differential temperature-dependent chaperone-like activity of αA- and αB-crystallin homoaggregates. Journal of Biological Chemistry. 1999;274(49):34773–34778. doi: 10.1074/jbc.274.49.34773. [DOI] [PubMed] [Google Scholar]
- 14.Sun TX, Das BK, Liang JJN. Conformational and functional differences between recombinant human lens αA and αB-crystallin. Journal of Biological Chemistry. 1997;272(10):6220–6225. doi: 10.1074/jbc.272.10.6220. [DOI] [PubMed] [Google Scholar]
- 15.Rekas A, Adda CG, Andrew Aquilina J, et al. Interaction of the molecular chaperone αB-crystallin with α-Synuclein: effects on amyloid fibril formation and chaperone activity. Journal of Molecular Biology. 2004;340(5):1167–1183. doi: 10.1016/j.jmb.2004.05.054. [DOI] [PubMed] [Google Scholar]
- 16.Waudby CA, Knowles TPJ, Devlin GL, et al. The interaction of αB-crystallin with mature α-synuclein amyloid fibrils inhibits their elongation. Biophysical Journal. 2010;98(5):843–851. doi: 10.1016/j.bpj.2009.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rekas A, Jankova L, Thorn DC, Cappai R, Carver JA. Monitoring the prevention of amyloid fibril formation by α-crystallin: temperature dependence and the nature of the aggregating species. FEBS Journal. 2007;274(24):6290–6304. doi: 10.1111/j.1742-4658.2007.06144.x. [DOI] [PubMed] [Google Scholar]
- 18.Ecroyd H, Carver JA. Crystallin proteins and amyloid fibrils. Cellular and Molecular Life Sciences. 2009;66(1):62–81. doi: 10.1007/s00018-008-8327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamm ER, Russell P, Johnson DH, Piatigorsky J. Human and monkey trabecular meshwork accumulate αB-crystallin in response to heat shock and oxidative stress. Investigative Ophthalmology and Visual Science. 1996;37(12):2402–2413. [PubMed] [Google Scholar]
- 20.Klemenz R, Frohli E, Steiger RH, Schafer R, Aoyama A. αB-crystallin is a small heat shock protein. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(9):3652–3656. doi: 10.1073/pnas.88.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi Y, Hirose F, Matsukage A, Yamaguchi M. Identification of three conserved regions in the DREF transcription factors from Drosophila melanogaster and Drosophila virilis. Nucleic Acids Research. 1999;27(2):510–516. doi: 10.1093/nar/27.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feany MB, Bender WW. A Drosophila model of Parkinson’s disease. Nature. 2000;404(6776):394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- 24.Spradling A. P-element-mediated transformation. In: Roberts DB, editor. Drosophila: A Practical Approach. Oxford, UK: IRL Press; 1986 . [Google Scholar]
- 25.Robertson HM, Preston CR, Phillis RW, Johnson-Schlitz DM, Benz WK, Engels WR. A stable genomic source of P element transposase in Drosophila melanogaster. Genetics. 1988;118(3):461–470. doi: 10.1093/genetics/118.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirose F, Ohshima N, Shiraki M, et al. Ectopic expression of DREF induces DNA synthesis, apoptosis, and unusual morphogenesis in the Drosophila eye imaginal disc: possible interaction with Polycomb and trithorax group proteins. Molecular and Cellular Biology. 2001;21(21):7231–7242. doi: 10.1128/MCB.21.21.7231-7242.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raman B, Ban T, Sakai M, et al. αB-crystallin, a small heat-shock protein, prevents the amyloid fibril growth of an amyloid β-peptide and β2-microglobulin. Biochemical Journal. 2005;392(3):573–581. doi: 10.1042/BJ20050339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ecroyd H, Carver JA. The effect of small molecules in modulating the chaperone activity of αB-crystallin against ordered and disordered protein aggregation. FEBS Journal. 2008;275(5):935–947. doi: 10.1111/j.1742-4658.2008.06257.x. [DOI] [PubMed] [Google Scholar]
- 29.Horwitz J. α-crystallin can function as a molecular chaperone. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(21):10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghosh JG, Estrada MR, Clark JI. Interactive domains for chaperone activity in the small heat shock protein, human αB crystallin. Biochemistry. 2005;44(45):14854–14869. doi: 10.1021/bi0503910. [DOI] [PubMed] [Google Scholar]
- 31.Bhattacharyya J, Udupa EGP, Wang J, Sharma KK. Mini-αB-crystallin: a functional element of αB-crystallin with chaperone-like activity. Biochemistry. 2006;45(9):3069–3076. doi: 10.1021/bi0518141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghosh JG, Houck SA, Clark JI. Interactive sequences in the molecular chaperone, human αB crystallin modulate the fibrillation of amyloidogenic proteins. International Journal of Biochemistry and Cell Biology. 2008;40(5):954–967. doi: 10.1016/j.biocel.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robertson AL, Headey SJ, Saunders HM, et al. Small heat-shock proteins interact with a flanking domain to suppress polyglutamine aggregation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(23):10424–10429. doi: 10.1073/pnas.0914773107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dougan L, Li J, Badilla CL, Berne BJ, Fernandez JM. Single homopolypeptide chains collapse into mechanically rigid conformations. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(31):12605–12610. doi: 10.1073/pnas.0900678106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghosh JG, Houck SA, Clark JI. Interactive sequences in the stress protein and molecular chaperone human αB crystallin recognize and modulate the assembly of filaments. International Journal of Biochemistry and Cell Biology. 2007;39(10):1804–1815. doi: 10.1016/j.biocel.2007.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X, Smith DL, Meriin AB, et al. A potent small molecule inhibits polyglutamine aggregation in Huntington’s disease neurons and suppresses neurodegeneration in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(3):892–897. doi: 10.1073/pnas.0408936102. [DOI] [PMC free article] [PubMed] [Google Scholar]


