Abstract
We evaluated levels of exercise-induced brain-derived neurotrophic factor (BDNF) messenger RNA (mRNA) within the hippocampal formation in rats selectively bred for 1) high intrinsic (i.e., untrained) aerobic capacity (High Capacity Runners, HCR), 2) low intrinsic aerobic capacity (Low Capacity Runners, LCR), and 3) unselected Sprague-Dawley (SD) rats with or without free access to running wheels for three weeks. The specific aim of the study was to determine whether a dose-response relationship exists between cumulative running distance and levels of BDNF mRNA. No additional treatments or behavioral manipulations were used. HCR, LCR, and SD rats were grouped by strain and randomly assigned to sedentary or activity (voluntary access to activity wheel) conditions. Animals were killed after 21 days of exposure to the assigned conditions. Daily running distances (mean ± standard deviation meters/d) during week three were: HCR (4726 ± 3220), SD (2293 ± 3461), LCR (672 ± 323). Regardless of strain, levels of BDNF mRNA in CA1 were elevated in wheel runners compared to sedentary rats and this difference persisted after adjustment for age (p=0.040). BDNF mRNA was not affected by intrinsic aerobic capacity and was not related to total running distance. The results support that BDNF mRNA expression is increased by unlimited access to activity wheel running for 3 weeks but is not dependent upon accumulated running distance.
Keywords: trophic factors, hippocampus, physical activity, intrinsic fitness, dose-response
1. Introduction
Brain-derived neurotrophic factor (BDNF) is a neurotrophin that exerts widespread effects throughout the central nervous system, including the support of neuronal survival, differentiation, and connectivity; it also plays a role in activity-dependent synaptic plasticity and is implicated in processes of learning, memory, and neurological disease (Binder and Scharfman, 2004; Johnson and Mitchell, 2003; Scharfman et al., 2005; Zhang and Ko, 2009).
BDNF protein and mRNA levels can be elevated following both forced and voluntary physical activity (Adlard and Cotman, 2004; Berchtold et al., 2001; Berchtold et al., 2005; Chen and Russo-Neustadt, 2005; Duman et al., 2008; Garza et al., 2004; Gomez-Pinilla et al., 2008; Kim et al., 2005; Kitamura et al., 2003; Klintsova et al., 2004; Neeper et al., 1996; Rasmussen et al., 2009; Rhodes et al., 2003; Russo-Neustadt et al., 1999; Russo-Neustadt et al., 2001; Russo-Neustadt et al., 2000; Russo-Neustadt et al., 2004; Scharfman et al., 2005; Soya et al., 2007; Tong et al., 2001; Van Hoomissen et al., 2003; Vaynman et al., 2004; Zheng et al., 2006). Investigators have concluded that responses by BDNF to running manipulations are not merely the result of an enriched environment but have a dose-response relation to running distance, with higher levels of BDNF mRNA expression observed in animals that run for a longer duration or farther distance (Adlard and Cotman, 2004; Bjornebekk et al., 2005; Griesbach et al., 2004; Griesbach et al., 2008; Johnson and Mitchell, 2003; Johnson et al., 2003; Oliff et al., 1998; Widenfalk et al., 1999).
However, the extant evidence regarding the correlation between running distance and BDNF protein or mRNA levels is not, as yet, clear. Past studies of physical activity and BDNF induction (protein and mRNA) used mice or different strains of rat with activity wheel exposures that varied widely from a few hours to several weeks and running distances that were either not reported (Oliff et al., 1998) or ranged from 250 meters to 7,000 meters per day in separate studies (Adlard and Cotman, 2004; Johnson and Mitchell, 2003; Johnson et al., 2003). Of 8 studies we know of that reported a correlation between distance run and BDNF levels (Adlard and Cotman, 2004; Bjornebekk et al., 2005; Griesbach et al., 2004; Griesbach et al., 2008; Johnson and Mitchell, 2003; Johnson et al., 2003; Oliff et al., 1998; Widenfalk et al., 1999) (Table 1), only 4 studies evaluated continuous running effects without the confounding of additional treatments or manipulations (Adlard and Cotman, 2004; Johnson and Mitchell, 2003; Johnson et al., 2003; Oliff et al., 1998). Our calculation of 95% confidence intervals included zero for nearly half the correlations reported in the studies. The weighted mean correlation (95% CI) (random effects model) computed from the studies reported in Table 1 is 0.68 (0.50 – 0.80). However, an additional null result from a single study of 12 or more animals would yield a non-significant overall estimate of the correlation (p > .05) (Rosenberg, 2005). Furthermore, the studies collectively did not discount that elevations in BDNF levels or mRNA associated with wheel running were confounded by intrinsic factors specific to animal strains that might influence both running and BDNF expression.
Table 1.
Running Distance in meters per day and BDNF protein/mRNA for studies included for comparison.
| Study | Subjects (n) | Running Distance (m/day) | Other Manipulations | Dose-Response: BDNF mRNA/protein Expression in brain region (methods) |
|---|---|---|---|---|
| Adlard, Perreau, & Cotman (2004) | Male; C57 B16 mice; age range 2 mos. to 24 mos. (n=5/age/condition) | 7 to 28 days of running; range: Young = 5000m – 10,000m, Middle-age = 1800m – 4800m, Old = 2400m – 3800m | None | Hippocampal protein (ELISA), young group (2 mos.), 7 days only: r = 0.959 (n=5); 95% CI: 0.499 to 0.997 |
| Bjornebekk, Mathe, & Brene (2005) | Male; FSL and FRL rats; age not reported (n=8/strain/conditio n) | 35 days of running; range: FSL = 1600m – 2600m, FR = 2500m – 5600m | Forced swim test & BrdU administration | mRNA expression in DG (in situ hybridization), last week of running: r = 0.69 (n =14); 95% CI: 0.252 to 0.893 |
| Johnson & Mitchell (2003) | Male; 4 strains; age range 7–8 weeks; BN, DA, PVG, & SD (n = 8/strain/condition, 4 groups) | Voluntary; 1 and 7 night time points; Running distance: no distance differences among strains at 1 night (range ~750m to 1500m, including SE), strain differences found after 7 nights – PVG: 1000m to 7000m, DA: 500 to 3000m, SD: 500 to 1500m, BN: 2500 to 2200m | None | Hippocampal protein (ELISA) among all strains, 7 nights only p<0.001 r = .53 (n=32); 95% CI: 0.393 to 0.644, similar slopes between strains |
| Griesbach, Hovda, Molteni, Wu, & Gomez-Pinilla (2004) | Male; SD rats; age not reported (n=6–10/condition) | 7 days of running; acute and DSham-RW groups range: 500m – 3400m, acute FPI-RW range: 250m – 2100m, DFPI-RW range: 800m – 2300m | FPI vs. Sham & Water maze training | Hippocampal protein (ELISA); DSham-RW: r = 0.872 (n = 15), 95% CI: 0.65 to 0.956; DFPI-RW: r = 0.555 (n =8) (ipsilateral) (n = 8), 95% CI: −0.245 to 0.905, and r = 0.621 (contralateral), 95% CI = −0.148 to 0.922 |
| Oliff, Berchtold, Isackson, & Cotman (1998) | Male; Fisher-344 Rats; age range 3–4 mos. (n=7–8/condition) | 6 or 12 hrs of running following 3 day activity wheel training period and 10 day activity cessation period; distance not reported | None | mRNA expression in Hilus, DG, CA1, and CA3 (in situ hybridization), total distance for 6 h running: (Hilus) r = 0.866, (CA1) r = 0.894, (CA3) r = 0.704, (DG) not significant (n = 7); 95% CI: (Hilus) 0.325 to 0.979, (CA1) 0.432 to 0.984, (CA3) −0.104 to 0.952 |
| Griesbach, Hovda, Gomez-Pinilla, & Sutton (2008) | Male; SD rats; age not reported (n=6/condition) | 7 days of running; Sham-S-Amphetamine RW and Sham-AMPH-RW groups range = 1000m– 1800m, CCI-S-RW and CCI-AMPH-RW groups range = 20m to 1250m | CCI vs. Sham; RW: r = 0.74 (n = 6), 95% CI: −0.179 (AMPH) vs. Saline (S) | Hippocampal protein (ELISA); Sham-S- to 0.969; Sham-AMPH-RW: r = 0.47 (n = 6). 95% CI: −0.552 to 0.927 |
| Johnson, Rhodes, Jeffrey, Garland, & Mitchell (2003) | Male; gen. 25 mice selectively bred for high voluntary running wheel behavior; age 8 weeks (n=8–16/condition) | 1 or 7 nights of running; S mice range = 2900m – 8600m, C mice range = 1000m – 3400m | None | Hippocampal protein (ELISA), combined C and S mice (n = 64): p < 0.05, r-value estimated as .30 assuming a p-value between .05 and .01. |
Abbreviations for animals correspond to the following strain/treatment groups identifiers: FSL – Flinders Sensitive Line; FRL – Flinders Resistant Line; BN –Brown Norway; DA – Dark Agouti; SD – Sprague Dawley; DSham-RW – delayed sham-surgery, running wheel assignment; FPI-RW – fluid percussion injury, running wheel assignment; DFPI-RW – delayed fluid percussion injury, running wheel assignment; Sham-S-RW – sham surgery saline administration running wheel assignment; Sham-AMPH-RW – sham surgery amphetamine administration running wheel assignment; CCI-S-RW – controlled cortical impact, saline administration, running wheel assignment; CCI-AMPH-RW – controlled cortical impact, amphetamine administration, running wheel assignment; S mice – high voluntary runners; C mice – control
The purpose of this study, therefore, was to examine whether there is a dose-response relation between running distance after wheel exposure and BDNF mRNA expression in the hippocampus without confounding influences of additional manipulations or intrinsic traits. To provide a broad range of running exposure in a single study, we used rats that were widely heterogeneous in their intrinsic (i.e., untrained) running capacity. Three strains were used: Sprague-Dawley derived outbred rats (SD), high capacity runners (HCR) selectively bred for high intrinsic aerobic capacity, and low capacity runners (LCR) selectively bred for low intrinsic aerobic capacity (Koch and Britton, 2001; Koch and Britton, 2005; Koch and Britton, 2008). The HCR and LCR rats differ widely in their capacity to run on a treadmill to the point of exhaustion (Koch and Britton, 2001) and demonstrate a substantial divergence in running speed, duration, and maximal oxygen uptake (Høydal et al., 2007; Howlett et al., 2009). We expected that rats bred for higher-aerobic capacity would have greater advantage to obtain higher average voluntary running distances in activity wheels than the rats selected for low capacity or an unselected outbred strain. Hence, we used the HCR and LCR rats to optimize the range of wheel running exposure for the purpose of testing whether exercise-induced upregulation of BDNF follows a dose-response gradient.
Age also modifies wheel running exposures and outcomes in rats. Average running distances decrease with age; older rats maintain a constant or decreasing level of activity over time, whereas younger rats increase average running distance across time (Adlard et al., 2005). Because age and exercise can have differential effects on the expression of activity-related proteins in the brain, the present experiment included and analyzed age as a covariate.
We hypothesized that all animals (HCR, LCR, and SD rats) in the activity wheel groups would show higher levels of BDNF mRNA within the hippocampal structures analyzed compared to sedentary controls of the same strain. In addition, we hypothesized that running distance would be correlated with levels of exercise-induced BDNF mRNA, such that higher levels of running would result in higher levels of BDNF mRNA.
2. Results
2.1. Wheel Running Distance
Weekly running distance was highly reliable, ICC (2, 3) =0.96, 95% CI, 0.93–0.98, and increased over time, F(2,52)=7.497, ε = .694, p=0.005, χ2 = .24. There was a main effect of strain, F(2, 26)=4.426, p=0.022, χ2 = .25, whereby HCR rats ran more than LCR rats, p=0.022, but not more than SD rats, p=0.158. There was also a strain by quadratic trend effect of time, F(2, 26)=3.8, p=0.036, χ2 = .23 (Figure 1). Adjustment for an age by time effect (p=0.012) gave similar results for the time effect, F(2, 50)=12.459, ε = .768, p<0.001, χ2 = .33 and the strain by quadratic trend effect of time, F(2, 25)=3.67, p= 0.040, χ2 = .23. To further assess the strain by quadratic time effect, each strain was analyzed individually with age as a covariate. LCR rats daily running distances indicated a quadratic increase, F(1,7) = 6.24, p = .036, between the first (380 ± 193) and third (672 ± 323; p=0.017) weeks. SD rats increased daily running distances linearly, F(1,9)=4.86, p=.055, across all three weeks (mean ± standard deviation) (week 1: 1112 ± 945, week 2: 1695 ± 2404, week 3: 2293 ± 3461). There was a non-significant increase, F(1,7) = 4.10, p=.083, across weeks for HCR (week 1: 3152 ± 2155, week 2: 4502 ± 2374, week 3: 4726 ± 3220). The mean daily running distances were: LCR (468 ± 277), SD (1669 ± 2477), HCR (4076 ± 3810).
Figure 1.
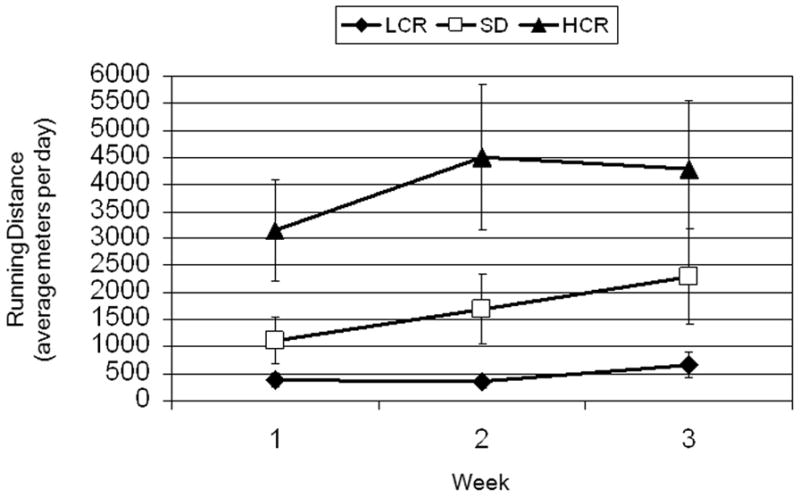
Average (means and standard error bars) meters run per day each week according to strain: LCR (low-capacity runners), SD (Sprague-Dawley), HCR (high-capacity runners).
2.2. Hippocampal BDNF mRNA Expression
2.2.1. CA1
There was a significant effect of exposure to the wheel running condition on BDNF mRNA in CA1, F(1, 61)=14.9, p=0.05, χ2 = .87 (Figure 2). The effect remained F(1, 61)=17.0, p=0.04, χ2 = .88 after ANCOVA adjustment for effects of age F(1, 61)=3.93, p=0.052 and strain F(2, 61)=5.359, p=0.089. There was no interaction between strain and condition, F(2, 61)=.164, p= 0.849.
Figure 2.
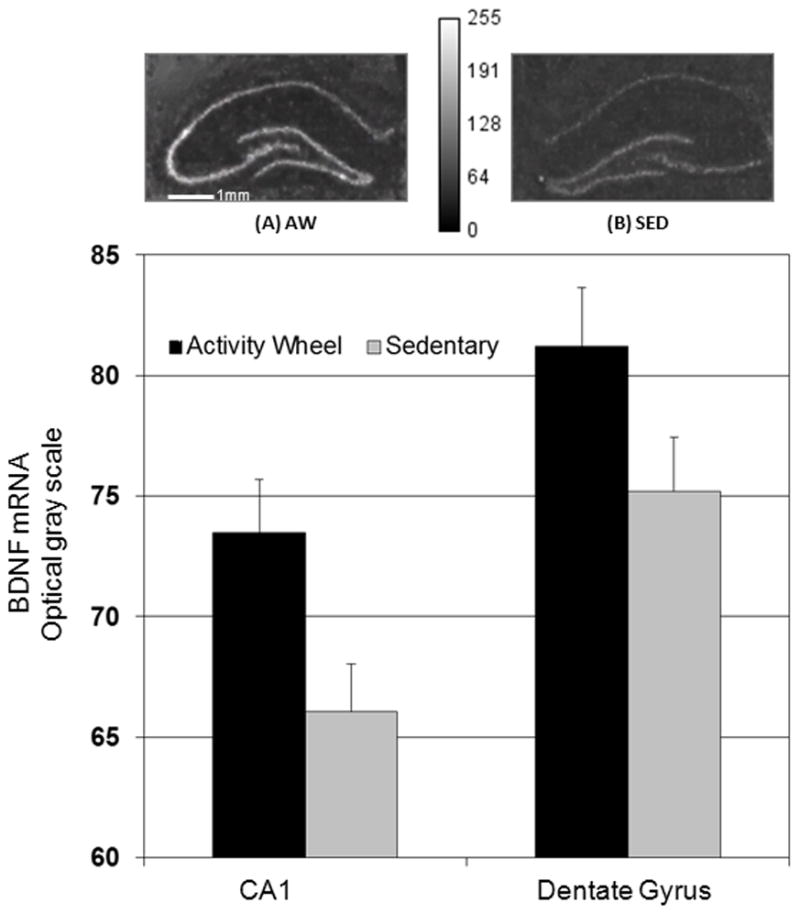
BDNF mRNA optical grayscale values for regions CA1 and DG (means with standard error bars) for activity wheel and sedentary conditions. Representative autoradiographic images are shown for dorsal hippocampal section of rat assigned to activity wheel condition (A) or rat assigned to sedentary condition (B).
Regression analysis revealed no significant relation between total distance run and BDNF mRNA expression within the CA1 region, R=0.101, p=0.615 (Figure 3), and the relation remained non-significant (β=0.157, p=0.582) after controlling for non-significant effects (p-values ≥ .419) of age and strain. Also, non-significant interaction effects of running distance with strain or age (p-values ≥ 0.483) indicated that the relation between running distance and BDNF mRNA did not differ according to strain or age. The correlations between running distance and BDNF mRNA was .22 (p=.443) in 16 young rats determined by median age split (ages 60 to 159 days, mean=111 standard deviation=32) and −.484 (p=.111) in 13 old rats (ages 160 to 341 days, mean=256 standard deviation=74) (Figure 4). Correlations between total distance run and BDNF within each strain and according to those age groups were: LCR = −.23 (p=.560); −.085 (p=.873) in 5 young and .40 (p=.737) in 4 old, HCR = .50 (p=.209); −.24 (p=.700) in 4 young and −.83 (p=.375) in 4 old.
Figure 3.
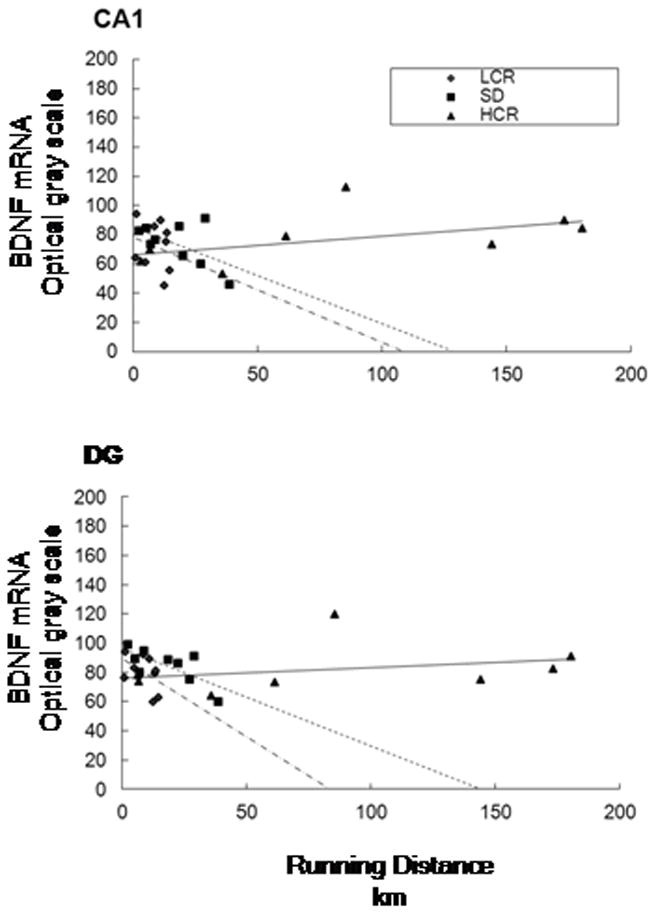
Scattergram of total running distance and BDNF mRNA optical grayscale values for region CA1 of the hippocampus (top) and region DG of the hippocampus (bottom) Strain abbreviations: HCR (high-capacity runners), SD (Sprague-Dawley), LCR (low-capacity runners).
Figure 4.
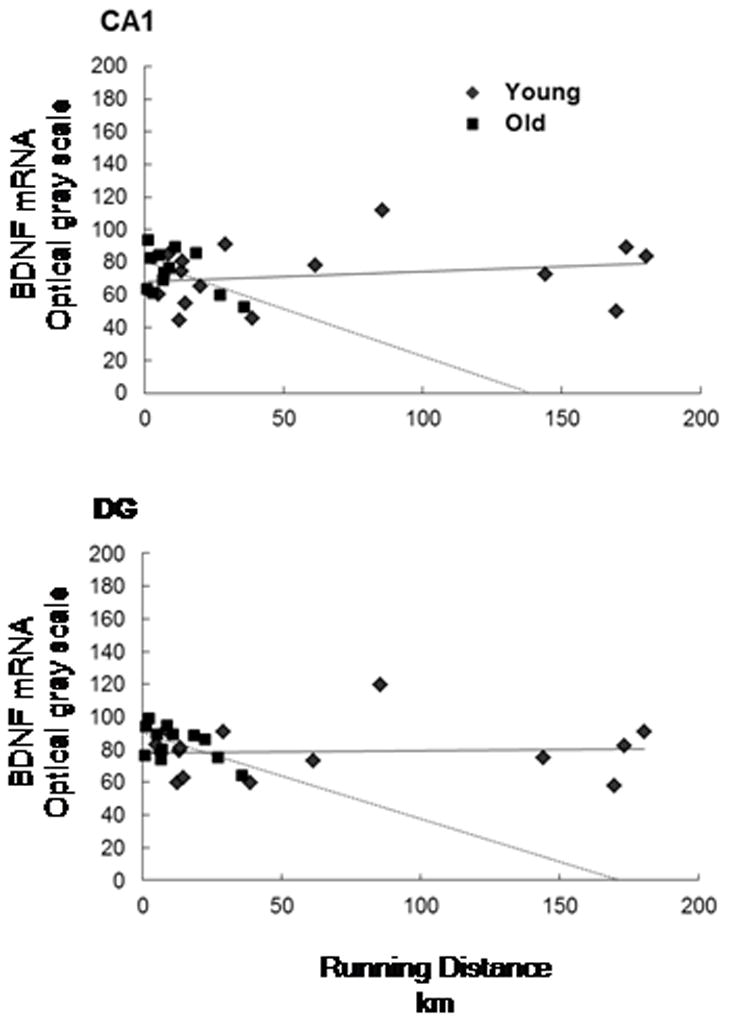
Scattergram of total running distance and BDNF mRNA optical grayscale values for region CA1 of the hippocampus (top) region DG of the hippocampus (bottom) according young and old age groups.
An additional regression analysis using running distance during the last week also revealed no relation with mRNA expression, p=0.925, and this finding remained after controlling for age and strain, p=0.950.
2.2.2. Dentate Gyrus, CA2, and CA3
There were no effects of exposure to the wheel running condition, F(1, 62)=1.412, p=0.355, χ2 = .41 or strain, F(2, 62)=1.615, p=0.382, χ2 = .62 on BDNF mRNA in the dentate gyrus (Figure 2). There also was no interaction between strain and condition, F(2, 61)= 1.98, p= 0.146, χ2 = .06. Null effects of condition F(1, 60)=1.152, p=0.394, χ2 = .36, strain F(1, 60)=1.848, p=0.340, χ2 = .63, and condition by strain F(2, 60)=1.825, p=0.170, χ2 = .06 remained after controlling for a significant effect for age on BDNF mRNA, F(1, 60)=13.095, p=0.001. A regression analysis for the DG was also conducted, and similarly revealed no significant relation between mRNA expression and total running distance across or within strains or ages (Figure 3).
Results for BDNF mRNA expression within regions CA2 and CA3 were also found to be non–significant and were similar to the levels obtained for the DG analysis (data not reported).
3. Discussion
We observed no dose-response relation between accumulated running distance or strain and BDNF mRNA expression in the hippocampus, despite daily running distances for HCR rats that were 7 times higher than LCR rats, and 2 times higher than SD rats, by the third week of wheel access. BDNF mRNA within the hippocampus was elevated by access to an activity wheel regardless of strain; although a significant increase was limited to CA1. The increase in BDNF mRNA after wheel running exposure remained significant after adjusting for age, which was inversely correlated with running distance and mRNA expression. Effects of activity wheel running in the DG and regions CA2 and CA3 remained non–significant after adjustment for age.
Previous research also found an age effect for both running distance and BDNF mRNA expression (Adlard et al., 2005), similar to our present findings. Adlard, et al. (2005) reported an increase in BDNF levels after only one week of exercise that decreased to baseline levels following the second week of activity wheel exposure. The authors also reported that younger animals tended to have higher levels of BDNF expression in conjunction with linear increases in running behavior across the three week study duration (Adlard et al., 2005). Running distances in the present study were significantly different among strains and increased over time, both with and without adjusting for age.
Here, BDNF mRNA expression was significantly increased in only one region of the hippocampal formation evaluated, CA1. Previous research has reported that this region is a key component in the consolidation of long-term memories, matching sensory input recall to contextual memory, and order of recall for visual stimuli (Farovik et al., 2010; Hoge and Kesner, 2007; Remondes and Schuman, 2004). These findings pair well with a study that reported improved contextual memory consolidation after activity wheel running, which was apparently mediated by neurochemical and trophic changes observed within the hippocampus (i.e. increased BDNF mRNA expression) (Greenwood et al., 2009). The role of BDNF within the hippocampus is clearly established as being involved in cognitive and behavioral improvements, neuronal outgrowth, and learning and memory; but there remains a debate as to which is more influenced by physical activity. The current experiment did not include behavioral or cognitive evaluations, so as to reduce the influence of additional manipulations on running behavior and BDNF mRNA expression.
The aim of the present experiment was to focus on the relationship between running distance and hippocampal BDNF expression. The experiments did not attempt to dissociate the effects of wheel running and the environmental enrichment that is inherent in this activity on BDNF gene expression. Activity wheel running is comprised of both an environmental enrichment component and an exercise component, and both of these conditions influence BDNF expression in the hippocampus (Olson et al., 2006). The selection of an appropriate control condition is therefore problematic. The presence of a locked wheel allows subjects to actively explore and climb around the wheel. This activity thus represents a form of exercise, and this behavior has been consistently observed in our laboratory in previous experiments. Absence of the running wheel eliminates this confound. The disadvantage of this design is that it does not permit the evaluation of the relative contributions of exercise and environmental enrichment on the expression of BDNF. Nonetheless, running distance was unrelated to BDNF expression among animals exposed to the wheel enriched environment.
The present study is not the first to indicate changes in only one region of the hippocampus. In a study conducted in 1998 by Oliff and colleagues, BDNF mRNA expression increased after only 6 hours of activity wheel exposure and only within CA1 (Oliff et al., 1998). A 2004 study by Farmer and colleagues reported an increase in BDNF mRNA after 10 days of activity wheel exposure, but only within the dentate gyrus (Farmer et al., 2004). Finally, a 2009 study found that 6 weeks of activity wheel running increased BDNF mRNA within the dentate gyrus and CA1 (Greenwood et al., 2009). With such an array of exposure times and differenences in regions that express BDNF mRNA, it is evident that current literature remains uncertain as to the required amount of running exposure necessary for induction, and which hippocampal area is primarily influenced by exercise. Nonetheless, one parsimonius explanation for the discrepancy between the present results and some previous reports may be the sensitivity of the hybridization assay employed. As a semi-quantitative technique, the results of in situ hybridization experiments may under-represent the degree of changes in gene expression if assay conditons are not optimal. The effect sizes (Hedge’s d) we report here for CA1 (d= 0.414) and DG (d=0.350), both adjusted for age, were lower than reported in several of the past studies (Adlard and Cotman, 2004; Bjornebekk et al., 2005; Griesbach et al., 2004; Griesbach et al., 2008; Oliff et al., 1998) of the dose-response relation between wheel running and BDNF expression but are within the 95% confidence intervals (our calculation using random effects model) of several of those studies (Adlard and Cotman, 2004; Bjornebekk et al., 2005; Griesbach et al., 2004; Griesbach et al., 2008). The differences may therefore reflect normal variations in assay sensitivity. Nonetheless, there is no reason to expect that measurement error accounted for the absence of a correlation between BDNF expression and running distance. Random measurement error will inflate the standard error of the sample correlation and reduce the power of the statistical test of whether a sample slope is different from zero, but it will not change the slope. For measurement error to explain the flat slope shown in Figure 3, the BDNF response would have to be systematically underestimated in high runners and similarly overestimated in the low runners. Because the autoradiographic analysis was blinded to running distance, there is no reason to believe that occurred. Also, LCR rats are naturally less active than HCR rats when housed in the absence of a wheel (Novak et al., 2010), so it is unlikely that BDNF mRNA was differentially augmented in LCR despite their low wheel running.
The primary goal of this investigation was to evaluate the putative dose-response relation between running distance and BDNF mRNA expression within the hippocampus. We obtained the expected differences in daily running distances between the strains (HCR ranged from 3700m to 4200m, SD ranged from 1100m to 4300m, and LCR ranged from 400m to 700m) across the three-week exposure, but there was no dose-response relation with distance or strain differences among runners at any region of the hippocampus. Previous dose-response evaluations (Table 1) reported up to a 3-fold difference in running distances between the lowest and highest reported distances (Adlard et al., 2005; Bjornebekk et al., 2005; Griesbach et al., 2004; Griesbach et al., 2008; Johnson and Mitchell, 2003; Johnson et al., 2003) or did not report running distance (Oliff et al., 1998). We report here a 7-fold difference in running distances between the selectively bred HCR and LCR lines, with SD rats running about half the distance of the HCR running group. During week 3, the mean daily running distance per animal ranged from 57 meters to 13,800 meters. This wider range in wheel running distance permitted a fuller evaluation of the dose-response relation than provided by prior reports.
Additionally, we report here an increase in BDNF mRNA expression after a minimum of about 500 meters/day of wheel running in the LCR group. Some earlier studies also reported increased BDNF mRNA with similarly small exposures to activity wheel running, but a minimum distance has yet to be established for BDNF mRNA induction. Many studies have not reported running distances, so in addition to duration of exposure, future studies are needed to determine the threshold of activity required for mRNA induction and to distinguish the effects of running distance from other features of activity wheel exposure.
The studies described in Table 1 varied in activity wheel exposure times from 6 or 12 hours to 28 and 35 days (Adlard et al., 2005; Bjornebekk et al., 2005; Oliff et al., 1998). The majority of studies, however, provided 1 to 7 days of activity wheel access (Griesbach et al., 2004; Griesbach et al., 2008; Johnson and Mitchell, 2003; Johnson et al., 2003). In our experience, such short durations of exposure do not allow full consolidation of wheel running behavior, which we have observed previously after 2–3 weeks (Holmes et al., 2006; Reiss et al., 2009; Van Hoomissen et al., 2003). Further evaluation of running exposure in past studies revealed that many ‘acute’ studies included a pre-exposure novelty check, followed by a 10-day activity cessation period. This protocol, presented in both Oliff, et al. (1998) and Neeper, et al. (1996) involves exposing animals to 3 days of activity wheel access to control for novelty effects. All animals were then removed from the activity wheel for 10 days, and subsequently divided into sedentary or activity conditions for a set time point of exposure. This would appear to account for any influence of novel environmental stimuli on BDNF expression. However, Oliff and colleagues reported that BDNF levels were increased immediately after the 10-day cessation period in animals that had received the 3 days of pre-training when compared to controls that had not received pre-training (Oliff et al., 1998). This suggests that the BDNF effects observed in this study after 6 hours of activity wheel exposure might have been the result of the previous training or, conversely, stimuli exposure effects rather than the result of acute running.
In additon to a wider range in running behavior and activity wheel exposure, the present study included 70 animals of three strains for comparison, with 9 to 11 animals from each strain in the activity wheel condition. Most of the studies presented in Table 1 included only 5 to 8 animals per running group. Finally, this experiment was free of confounding manipulations which allowed for a specific evaluation of running behavior and mRNA induction due to exercise manipulation only.
The findings from this experiment confirm an effect of exercise on BDNF mRNA in region CA1 of the rat hippocampus that is independent of cumulative running distance. The absence of a dose-dependent effect was observed despite daily running distances for HCR rats that were 7 times higher than LCR rats, and 2 times higher than SD rats, by the third week of wheel access. The conflicting prior evidence of dose-response can be partly explained by lack of uniformity in the amount or timing of running wheel exposure, regions of the brain that were examined, and the confounding of wheel exposure with other manipulations in several studies. Future research is needed to better clarify the threshold of running wheel exposure and its features (e.g., distance, timing, or novelty) sufficient to elicit BDNF transcription in the hippocampus and to determine the molecular mechanisms that explain the effect of wheel running on BDNF expression.
4. Experimental procedures
4.1. Animals and Experimental Design
Adult, male rats of 3 strains were housed individually throughout the experiments in 42Lx22Wx20H cm polycarbonate cages (HCR n=22, LCR n=18, SD n=30) in a temperature and humidity-controlled environment on a 12-hour light/dark schedule. Food and water were available ad libitum and animals were weighed weekly. Selectively bred HCR and LCR rats were obtained from the University of Michigan. The intrinsic running capacities estimated by a maximal running test to exhaustion were as follows: HCR rats ran 64 ± 3 minutes (mean ± standard deviation) and a corresponding distance of 1,642 ± 137 meters, compared to 18 ± 1 minutes and distance of 251 ± 31 meters for LCR rats. Ages for the LCR and HCR rats ranged from 121 to 218 days. For comparison, out bred Sprague-Dawley rats (SD; aged 60 to 321 days) supplied by Harlan were also studied;. Rats were grouped by strain and then randomly allocated within each strain to the activity wheel (AW) condition or to sedentary (SED) housing without access to a wheel using Research Randomizer (www.randomizer.org). To increase statistical power and because of wheel availability, SD rats were assigned at 2:1 ratio of sedentary to wheel running groups. Wheel running groups consisted of 9 HCR, 9 LCR, and 11 SD rats. The sample size was large enough to detect a correlation of .68 (the mean of published studies) or higher between BDNF expression and running distance in each strain and across age at a power of .80 and α = .05 (Erdfelder et al., 1996).
All animals underwent a 2 week quarantine and facility adaptation period prior to assignment to activity wheel or sedentary conditions. All procedures were conducted in accordance with NIH Guide for Care and Use of Laboratory Animals.
4.2. Activity Wheel Protocol
Activity wheels (MiniMitter) with a circumference of 105 cm were placed in the home cages and attached to a magnetic revolution counter. Home cages of sedentary rats did not contain an activity wheel. AW rats were given unlimited access to activity wheels for 21 days. Wheel revolutions were recorded and daily distances determined by multiplying the circumference (105 cm) of the activity wheel by the number of revolutions. Previous evidence regarding enriched environment versus activity wheel access and BDNF induction indicates that benefits derived from activity wheel access approximate or exceed enriched conditions (Gobbo and O’Mara, 2005; Olson et al., 2006); therefore we did not include an enriched environment control condition.
4.3. In Situ Hybridization Histochemistry
Animals were killed by rapid decapitation at the cessation of the 21 day exercise or control exposure immediately after the 12-hour light cycle. Brains were extracted and stored at −80°C. Brains were sliced into 12 μm sections at the level of the dorsal hippocampal formation (HF) using a Microm cryostat (Carl Zeiss, Waldorff, Germany) and thaw-mounted to gelatin coated microscope slides. In situ hybridization methods used are reported in detail elsewhere (Van Hoomissen et al., 2003). Briefly, slides were fixed in 4% formaldehyde in 0.12M sodium phosphate-buffered saline, rinsed in PBS, and placed in 0.25% acetic anhydride. Sections were then dehydrated through a series of ethanol washes, delipidated in chloroform, rinsed again in ethanol, and allowed to dry.
Oligonucleotide probes were obtained from Oligos Etc. (Wilsonville, OR). The BDNF oligonucleotide probe is complimentary to bases 650–699 of the mouse BDNF mRNA as previously described by Van Hoomissen, et al. (2003). Column separation was utilized to separate unincorporated nucleotides from the probes. Sections were hybridized with radiolabeled probes in solution containing formamide, NaCL, Tris-HCl, EDTA, sodium pyrophosphate, sodium dodecyl sulfate, and dextran sulfate. Sections were incubated in hybridization solution, followed by a series of washes to reduce nonspecific binding. Microscope slides were rinsed in deionized water and allowed to dry. Hybridized brain sections were exposed to autoradiographic film for a period of 3 weeks and then developed.
4.4. Film Analysis
Autoradiographic film analysis was conducted blind to wheel running distance using ImageJ (Rasband, 1997–2011), a computerized analysis system. Background was measured for each section to control for any excess, non-specific binding and remained constant across the experiments. The average optical density (OD) of ten 5 × 5-pixel circles placed randomly throughout the brain regions of interest within the HF; specifically the dentate gyrus (DG) and regions CA1, CA2, and CA3.
4.5. Data Analysis
Wheel running distance over time was analyzed with a 3 group (HCR vs. LCR vs. SD) × 3 time (weeks 1–3) mixed-model repeated measures analysis of variance (RM-ANOVA that was also adjusted for age (RM-ANCOVA). The Huynh-Feldt ε correction for sphericity violation was used. Optical densities from the in situ hybridization analyses for each brain region were compared using 3 group (HCR vs. LCR vs. SD) × 2 condition (activity wheel vs. sedentary) ANOVA that was also adjusted for age by ANCOVA. Effect sizes were estimated using χ2. Linear regression analysis adjusted for age was utilized to assess the effects of daily running distance on mRNA for BDNF. All analyses were conducted using SPSS Windows version 17.0 (SPSS, Inc., Chicago, IL).
Research Highlights.
Exposure to wheel running increases Hippocampal BDNF mRNA.
Whether that increase is directly related to dose of exposure had not been established.
Across a weekly range of running wheel activity from 400m to 4300m, no dose-relation was observed.
There were also no intrinsic differences in BDNF mRNA in rats selectively bred for high running capacity compared to lower running capacity group.
References
- Adlard PA, Cotman CW. Voluntary exercise protects against stress-induced decreases in brain-derived neurotrophic factor protein expression. Neuroscience. 2004;124:985. doi: 10.1016/j.neuroscience.2003.12.039. [DOI] [PubMed] [Google Scholar]
- Adlard PA, Perreau VM, Cotman CW. The exercise-induced expression of BDNF within the hippocampus varies across life-span. Neurobiol Aging. 2005;26:511–20. doi: 10.1016/j.neurobiolaging.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Kesslak JP, Pike CJ, Adlard PA, Cotman CW. Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. Eur J Neurosci. 2001;14:1992–2002. doi: 10.1046/j.0953-816x.2001.01825.x. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133:853–61. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22:123–31. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornebekk A, Mathe AA, Brene S. The antidepressant effect of running is associated with increased hippocampal cell proliferation. Int J Neuropsychopharmacol. 2005;8:357–68. doi: 10.1017/S1461145705005122. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Russo-Neustadt AA. Exercise activates the phosphatidylinositol 3-kinase pathway. Brain Res Mol Brain Res. 2005;135:181–93. doi: 10.1016/j.molbrainres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, Gandevia SC, Gomez-Pinilla F, Greenwood BN, Hillman CH, Kramer AF, Levin BE, Moran TH, Russo-Neustadt AA, Salamone JD, Van Hoomissen JD, Wade CE, York DA, Zigmond MJ. Neurobiology of exercise. Obesity (Silver Spring) 2006;14:345–56. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Russell DS, Duman RS. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008;1199:148–58. doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdfelder E, Faul F, Buchner A. GPOWER: A general power analysis program. Beh Res Meth. 1996;28:1–11. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–9. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Farovik A, Dupont LM, Eichenbaum H. Distinct roles for dorsal CA3 and CA1 in memory for sequential nonspatial events. Learn Mem. 2010;17:12–17. doi: 10.1101/lm.1616209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza AA, Ha TG, Garcia C, Chen MJ, Russo-Neustadt AA. Exercise, antidepressant treatment, and BDNF mRNA expression in the aging brain. Pharmacol Biochem Beh. 2004;77:209–20. doi: 10.1016/j.pbb.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Gobbo OL, O’Mara SM. Exercise, but not environmental enrichment, improves learning after kainic acid-induced hippocampal neurodegeneration in association with an increase in brain-derived neurotrophic factor. Behav Brain Res. 2005;159:21–6. doi: 10.1016/j.bbr.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Vaynman S, Ying Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur J Neuroscience. 2008;28:2278–2287. doi: 10.1111/j.1460-9568.2008.06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Foley TE, Fleshner M. A behavioral analysis of the impact of voluntary physical activity on hippocampus-dependent contextual conditioning. Hippocampus. 2009;19:988–1001. doi: 10.1002/hipo.20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesbach GS, Hovda DA, Molteni R, Wu A, Gomez-Pinilla F. Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience. 2004;125:129–39. doi: 10.1016/j.neuroscience.2004.01.030. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Hovda DA, Gomez-Pinilla F, Sutton RL. Voluntary exercise or amphetamine treatment, but not the combination, increases hippocampal brain-derived neurotrophic factor and synapsin I following cortical contusion injury in rats. Neuroscience. 2008;154:530–540. doi: 10.1016/j.neuroscience.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge J, Kesner RP. Role of CA3 and CA1 subregions of the dorsal hippocampus on temporal processing of objects. Neurobiol Learn Mem. 2007;88:225–31. doi: 10.1016/j.nlm.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes PV, Yoo HS, Dishman RK. Voluntary exercise and clomipramine treatment elevate prepro-galanin mRNA levels in the locus coeruleus in rats. Neurosci Lett. 2006;408:1–4. doi: 10.1016/j.neulet.2006.04.057. [DOI] [PubMed] [Google Scholar]
- Howlett RA, Kirkton SD, Gonzalez NC, Wagner HE, Britton SL, Koch LG, Wagner PD. Peripheral oxygen transport and utilization in rats following continued selective breeding for endurance running capacity. J Appl Physiol. 2009;106:1819–25. doi: 10.1152/japplphysiol.00914.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høydal MA, Wisloff U, Kemi OJ, Ellingsen O. Running speed and maximal oxygen uptake in rats and mice: practical implications for exercise training. Eur J Cardiovasc Prev Rehabil. 2007;14:753–60. doi: 10.1097/HJR.0b013e3281eacef1. [DOI] [PubMed] [Google Scholar]
- Johnson RA, Mitchell GS. Exercise-induced changes in hippocampal brain-derived neurotrophic factor and neurotrophin-3: effects of rat strain. Brain Res. 2003;983:108–14. doi: 10.1016/s0006-8993(03)03039-7. [DOI] [PubMed] [Google Scholar]
- Johnson RA, Rhodes JS, Jeffrey SL, Garland T, Jr, Mitchell GS. Hippocampal brain-derived neurotrophic factor but not neurotrophin-3 increases more in mice selected for increased voluntary wheel running. Neuroscience. 2003;121:1–7. doi: 10.1016/s0306-4522(03)00422-6. [DOI] [PubMed] [Google Scholar]
- Kim MW, Bang MS, Han TR, Ko YJ, Yoon BW, Kim JH, Kang LM, Lee KM, Kim MH. Exercise increased BDNF and trkB in the contralateral hemisphere of the ischemic rat brain. Brain Res. 2005;1052:16–21. doi: 10.1016/j.brainres.2005.05.070. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Mishina M, Sugiyama H. Enhancement of neurogenesis by running wheel exercises is suppressed in mice lacking NMDA receptor epsilon 1 subunit. Neuroscience Res. 2003;47:55–63. doi: 10.1016/s0168-0102(03)00171-8. [DOI] [PubMed] [Google Scholar]
- Klintsova AY, Dickson E, Yoshida R, Greenough WT. Altered expression of BDNF and its high-affinity receptor TrkB in response to complex motor learning and moderate exercise. Brain Res. 2004;1028:92–104. doi: 10.1016/j.brainres.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics. 2001;5:45–52. doi: 10.1152/physiolgenomics.2001.5.1.45. [DOI] [PubMed] [Google Scholar]
- Koch LG, Britton SL. Divergent selection for aerobic capacity in rats as a model for complex disease. Integr Comp Biol. 2005;45:405. doi: 10.1093/icb/45.3.405. [DOI] [PubMed] [Google Scholar]
- Koch LG, Britton SL. Development of animal models to test the fundamental basis of gene-environment interactions. Obesity (Silver Spring) 2008;16(Suppl 3):S28–32. doi: 10.1038/oby.2008.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- Novak CM, Escande C, Burghardt PR, Zhang M, Barbosa MT, Chini EN, Britton SL, Koch LG, Akil H, Levine JA. Spontaneous activity, economy of activity, and resistance to diet-induced obesity in rats bred for high intrinsic aerobic capacity. Hormones and Behavior. 2010;58(3):355–367. doi: 10.1016/j.yhbeh.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliff HS, Berchtold NC, Isackson P, Cotman CW. Exercise-induced regulation of brain-derived neurotrophic factor (BDNF) transcripts in the rat hippocampus. Brain Res Mol Brain Res. 1998;61:147–53. doi: 10.1016/s0169-328x(98)00222-8. [DOI] [PubMed] [Google Scholar]
- Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16:250–60. doi: 10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- Rasband WS. ImageJ. National Institutes of Health; Bethesda, MA, USA: 1997–2011. [Google Scholar]
- Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E, Secher NH, Pedersen BK, Pilegaard H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol. 2009;94:1062–1069. doi: 10.1113/expphysiol.2009.048512. [DOI] [PubMed] [Google Scholar]
- Reiss JI, Dishman RK, Boyd HE, Robinson JK, Holmes PV. Chronic activity wheel running reduces the severity of kainic acid-induced seizures in the rat: possible role of galanin. Brain Res. 2009;1266:54–63. doi: 10.1016/j.brainres.2009.02.030. [DOI] [PubMed] [Google Scholar]
- Remondes M, Schuman EM. Role for a cortical input to hippocampal area CA1 in the consolidation of a long-term memory. Nature. 2004;431:699–703. doi: 10.1038/nature02965. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, van Praag H, Jeffrey S, Girard I, Mitchell GS, Garland T, Jr, Gage FH. Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel running. Beh Neuroscience. 2003;117:1006–16. doi: 10.1037/0735-7044.117.5.1006. [DOI] [PubMed] [Google Scholar]
- Rosenberg MS. The file-drawer problem revisited: a general weighted method for calculating fail-safe numbers in meta-analysis. Evolution. 2005;59:464–8. [PubMed] [Google Scholar]
- Russo-Neustadt A, Beard RC, Cotman CW. Exercise, antidepressant medications, and enhanced brain derived neurotrophic factor expression. Neuropsychopharmacology. 1999;21:679–82. doi: 10.1016/S0893-133X(99)00059-7. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt A, Ha T, Ramirez R, Kesslak JP. Physical activity-antidepressant treatment combination: impact on brain-derived neurotrophic factor and behavior in an animal model. Beh Brain Res. 2001;120:87–95. doi: 10.1016/s0166-4328(00)00364-8. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt AA, Beard RC, Huang YM, Cotman CW. Physical activity and antidepressant treatment potentiate the expression of specific brain-derived neurotrophic factor transcripts in the rat hippocampus. Neuroscience. 2000;101:305–12. doi: 10.1016/s0306-4522(00)00349-3. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt AA, Alejandre H, Garcia C, Ivy AS, Chen MJ. Hippocampal brain-derived neurotrophic factor expression following treatment with reboxetine, citalopram, and physical exercise. Neuropsychopharmacology. 2004;29:2189–99. doi: 10.1038/sj.npp.1300514. [DOI] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Experimental Neurology. 2005;192:348–56. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Soya H, Nakamura T, Deocaris CC, Kimpara A, Iimura M, Fujikawa T, Chang H, McEwen BS, Nishijima T. BDNF induction with mild exercise in the rat hippocampus. Biochem Biophys Res Commun. 2007;358:961–7. doi: 10.1016/j.bbrc.2007.04.173. [DOI] [PubMed] [Google Scholar]
- Tong L, Shen H, Perreau VM, Balazs R, Cotman CW. Effects of exercise on gene-expression profile in the rat hippocampus. Neurobiol Dis. 2001;8:1046–56. doi: 10.1006/nbdi.2001.0427. [DOI] [PubMed] [Google Scholar]
- Van Hoomissen JD, Chambliss HO, Holmes PV, Dishman RK. Effects of chronic exercise and imipramine on mRNA for BDNF after olfactory bulbectomy in rat. Brain Res. 2003;974:228–35. doi: 10.1016/s0006-8993(03)02584-8. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Exercise induces BDNF and synapsin I to specific hippocampal subfields. J Neurosci Res. 2004;76:356–362. doi: 10.1002/jnr.20077. [DOI] [PubMed] [Google Scholar]
- Waters RP, Renner KJ, Pringle RB, Summers CH, Britton SL, Koch LG, Swallow JG. Selection for aerobic capacity affects corticosterone, monoamines and wheel-running activity. Physiol Behav. 2008;93:1044–54. doi: 10.1016/j.physbeh.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widenfalk J, Olson L, Thoren P. Deprived of habitual running, rats downregulate BDNF and TrkB messages in the brain. Neurosci Res. 1999;34:125–32. doi: 10.1016/s0168-0102(99)00051-6. [DOI] [PubMed] [Google Scholar]
- Zhang HN, Ko MC. Seizure activity involved in the up-regulation of BDNF mRNA expression by activation of central mu opioid receptors. Neuroscience. 2009;161:301–10. doi: 10.1016/j.neuroscience.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Liu Y, Li W, Yang B, Chen D, Wang X, Jiang Z, Wang H, Wang Z, Cornelisson G, Halberg F. Beneficial effects of exercise and its molecular mechanisms on depression in rats. Behav Brain Res. 2006;168:47–55. doi: 10.1016/j.bbr.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


