Abstract
Rationale
Exposure to stressors promotes ethanol (EtOH) consumption and enhances drug craving during abstinence. Corticotropin-releasing factor (CRF), and in particular, CRF actions via type 1 CRF receptors (CRF1) are critical in behavioral responses to stressors. CRF1 play a role in EtOH-induced behavioral neuroadaptation, in binge-like EtOH consumption, and in heightened EtOH consumption in dependent animals.
Objectives
We investigated the involvement of CRF1 in swim-stress-induced changes in EtOH consumption and in baseline consumption as a function of EtOH concentration. The role of CRF2 in adapting to effects of the stressor was also examined.
Methods
Wild-type mice and knockout mice lacking CRF1 were tested for two-bottle choice EtOH consumption at concentrations of 3–20%. Also, intake of 10% EtOH was examined in wild-type mice and knockout mice lacking CRF1, or lacking both CRF1 and CRF2, before and after acute or repeated swim stress exposures.
Results
EtOH intake was reduced in CRF1 compared with wild-type mice when presented at a concentration of 20% but not when presented at lower concentrations. No genotype-dependent effects were found for saccharin or quinine drinking. Acute swim stress had no effect, but repeated swim stress resulted in higher levels of EtOH consumption in wild-type mice, compared with both types of knockout mice. Stress effects on EtOH drinking were longer lasting in double knockout mice.
Conclusions
These data suggest a prominent role of CRF1 in stressor-induced changes in EtOH consumption, with involvement of CRF2 in recovery from stressor effects.
Keywords: CRF, Stress, Knockout, Alcohol, Drinking, Swim stress
Introduction
Preclinical research results indicate that the 41-amino acid polypeptide corticotropin-releasing factor (CRF) is a key neurobiological mediator of stress responses and stressor-induced effects on alcohol (ethanol) consumption (Heilig and Koob 2007; Lowery et al. 2008; Lowery and Thiele 2010; Martin-Fardon et al. 2010). Ethanol (EtOH) and classical stressors produce neuroendocrine and behavioral outcomes that are mediated by activation of CRF signaling (Bale and Vale 2004; Heinrichs and Koob 2004; Pastor et al. 2008; Pautassi et al. 2010; Rivier 1996). CRF interacts with two G-protein-coupled CRF receptors, type 1 (CRF1) and type 2 (CRF2), that are both positively coupled to adenylate cyclase through stimulatory G proteins (Binder and Nemeroff 2010; Hauger et al. 2009; Zorrilla and Koob 2004). CRF, which shows greater affinity for CRF1, has been described to exert actions at central and peripheral levels (Hauger et al. 2006; Heinrichs and Koob 2004). It is the main initiator of stressor-induced responses via activation of the hypothalamic–pituitary–adrenal (HPA) axis. Release of CRF from the hypothalamus produces CRF1 binding-induced secretion of adrenocorticotropic hormone, which subsequently stimulates glucocorticoid release from the adrenal gland cortices (Bale and Vale 2004). Extra-hypothalamic functions of CRF include CRF1-mediated cell activation at the level of structures such as the bed nucleus of the stria terminalis, the amygdala, and the ventral tegmental area (Chalmers et al. 1995; Lovenberg et al. 1995). Central CRF signaling, therefore, is critically involved in the neurobiology that mediates emotional responses and motivated behavior (Koob 2010; Rodaros et al. 2007; Rotzinger et al. 2010; Ungless et al. 2003).
In rodent research, various procedures have been used to demonstrate stressor-induced changes in EtOH intake. Increases in EtOH consumption have been seen subsequent to the application of restraint stress (Chester et al. 2004), swim stress (Lowery et al. 2008), and social defeat-induced stress (Croft et al. 2005). EtOH withdrawal, which induces an anxiogenic/stress response, also increased EtOH intake (Funk et al. 2007). Additionally, stressful stimuli such as foot-shock can promote reinstatement of an extinguished EtOH-seeking behavior (Liu and Weiss 2002). CRF1 antagonists or CRF1 genetic deletion attenuated stressor-induced increases in EtOH intake (Lowery et al. 2008), reduced EtOH withdrawal-dependent anxiogenic effects (Breese et al. 2004; Rassnick et al. 1993), blocked increased EtOH drinking in dependent animals (Chu et al. 2007; Funk et al. 2007), and prevented foot-shock-induced reinstatement of EtOH-seeking behavior (Liu and Weiss 2002). However, stressors have also been found to decrease EtOH intake (Brunell and Spear 2005; van Erp and Miczek 2001).
The influence of CRF-mediated systems on EtOH intake may be dependent upon level of intake. Most of the studies cited above involved application of a stressor to heighten intake. In addition, increased responding for EtOH, often seen after a period of alcohol deprivation, was reduced by administration of a CRF1 antagonist (Sparta et al. 2009), and high levels of EtOH drinking observed with “Drinking-in-the-Dark” (DID) procedures were reduced by CRF1 antagonists (Sparta et al. 2008). One characteristic of protocols used for induction of excessive or binge-like drinking is the use of high concentrations of EtOH (Crabbe et al. 2009; Rhodes et al. 2007). This factor might facilitate increased per-bout blood EtOH concentrations and, perhaps, the activation of CRF signaling.
The present study evaluated baseline EtOH drinking in CRF1-deficient mice as a function of EtOH concentration. This experiment was complemented with the measurement of preference for tastants (saccharin and quinine) to eliminate a role for CRF1 in taste reactivity/preference. CRF1 knockout (KO) and wild-type (WT) mice were also evaluated for sensitivity to the sedative/hypnotic effects of EtOH (measured by evaluation of loss of the righting reflex (LORR)). Finally, the effects of acute and repeated swim stress on EtOH consumption were investigated in CRF1 KO and WT mice, as well as in mice lacking both CRF type 1 and 2 receptors (CRF1/2). Single CRF2-deficient mice on a similar genetic background were not available for use in this study. However, the double KO mice were tested to answer the question of whether elimination of CRF2 would have a more profound effect than elimination of CRF1 alone, given evidence that CRF2 have a more significant role in stress adaptation, than in initiation of the stress response (Coste et al. 2001; de Kloet 2004).
Materials and methods
Animals
Male and female WT and KO mice (C57BL/6J × 129SV/J background) were used in the present experiments. Mice heterozygous for the mutant allele(s) were mated to generate the animals used here (a total of 125). Single gene mutant mice were generated using now standard gene inactivation methods (Smith et al. 1998; Timpl et al. 1998; Coste et al. 2000), and the double mutant was created by crossing the single gene mutants. Mutant mice were backcrossed onto the C57BL/6J (B6) strain for four (CRF1) or six (CRF1/2) generations to generate the mice for heterozygous mating. Mice were weaned at 21+2 days of age and housed in like-sex groups of two to four mice under standard laboratory conditions until assignment to experiments. Colony rooms were maintained at 21±1°C on a 12:12-h light/dark cycle (lights on at 0600 h). Sex-balanced groups of animals (68 to 127 days old) were used in these experiments. For drinking experiments, mice were individually housed 4 days before drinking experiment initiation in standard, acrylic plastic mouse cages with corncob bedding and food and water available ad libitum. Individual housing was necessary for measurement of individual drinking levels. All procedures were approved by the Portland VA and Oregon Health & Science University animal care and use committees and followed the NIH Guidelines for the Care and Use of Laboratory Animals.
Drugs
EtOH used for drinking (100%; Pharmco Products, Brookfield, CT) was diluted to a 3%, 6%, 10%, or 20% (v/v) solution in tap water. For intraperitoneal (ip) injection, 20% (v/v) EtOH was prepared in saline (0.9%; Baxter Healthcare, Deerfield, IL). Saccharin sodium salt (Sigma, St. Louis, MO) for drinking was prepared as a 0.033% or 0.066% (w/v) solution in tap water. Quinine hemisulfate salt (Sigma) was prepared as a 0.015 or 0.03 mM solution in tap water.
Twenty-four-hour, two-bottle choice baseline EtOH drinking
Procedures for measuring 24-h EtOH intake and preference have been described in detail previously (Palmer et al. 2004; Phillips et al. 1994). Mice were first acclimated to consuming tap water for four consecutive days from two glass graduated cylinders fitted with stoppers and sipper tubes. Food was dispersed around both tubes to avoid food-associated preference for one tube over the other. On day 1 of EtOH access, one water tube was replaced with a tube containing 3% EtOH. This EtOH concentration was offered for four consecutive days, and then increased to 6%, then 10% and finally 20% at 4-day intervals. The position of the water and EtOH tubes was alternated every 2 days. Mice were weighed every 4 days. The volume of fluid remaining in each tube (recorded at 24-h intervals and read to 0.2 ml) was adjusted for potential leakage and evaporation by using the average volume lost from identical tubes placed on two empty cages on the same racks as the experimental animals. Body weight was used to determine EtOH consumption in grams per kilogram per 24 h. EtOH consumption values from the second day after a tube position switch (days 2 and 4 for each concentration) were averaged to index intake. This allowed stabilization of drinking after the animals had identified the new location of the EtOH-containing tube.
Twenty-four-hour, two-bottle choice saccharin and quinine drinking
Saccharin and quinine consumption and preference were measured in the same mice, 1 week after measurement of baseline EtOH drinking. During the intervening week, the animals were left undisturbed with ad libitum water access. Methods were as described for EtOH drinking and two concentrations of each tastant were evaluated (0.033% and 0.066% for saccharin; 0.015 and 0.03 mM for quinine) in ascending and counterbalanced order, consistent with our previous similar studies (Palmer et al. 2004; Wheeler et al. 2009).
Loss of the righting reflex
EtOH-induced LORR was used to investigate potential differences in sensitivity to the sedative–hypnotic effects of EtOH in CRF1 KO and WT mice. Experimentally naïve mice were moved to the procedure room and weighed 1 h before testing began. At time zero, mice were injected ip with 3.6 g/kg EtOH, and then tested for latency to LORR and for LORR duration. Latency to LORR was defined as time from EtOH injection to time when the mouse was unable to right itself from a supine position in a V-shaped trough for at least 30 s (T1). Any mouse that did not lose the righting reflex within 5 min from the time of injection had a 20-μl periorbital blood sample taken. This sample was processed to determine blood EtOH concentration (BEC) and verify low BEC, which would suggest that absence of LORR was due to a misplaced EtOH injection. Mice that showed LORR remained undisturbed in a supine position until they could right themselves onto all four paws, twice within a 30-s period. Time of the first righting was scored as regain of the righting reflex (T2). Duration of LORR was calculated by subtraction (T2–T1). A 20-μl periorbital blood sample was taken at regain of the righting reflex and used to determine BEC at righting. BEC was determined using standard gas chromatographic methods, as described in Boehm et al. (2000), and these data were examined to identify neurosensitivity differences between genotypes.
Effects of swim stress on two-bottle choice EtOH drinking
Experimentally naïve WT and KO animals were first introduced to 3%, 6%, and 10% EtOH drinking, following the same protocol described for 24-h baseline EtOH drinking. This phase was followed by periods of EtOH deprivation, periods when 10% EtOH was offered for 21 h/day, and periods when swim stress application occurred prior to the 21-h period of EtOH access. The temporal features of the study are detailed in Table 1. EtOH was available for 21 h instead of 24 h during later phases of the study to allow time needed for swim stress application. Independent non-stressed groups were not included in the within-groups design based on preliminary studies (data not shown) revealing no time- or strain-dependent changes between WT and CRF1 KO mice in EtOH consumption, and due to limited availability of animals.
Table 1.
Sequence of events for the experiment examining the effects of swim stress on two-bottle choice EtOH drinking in CRF1, CRF1/2, and WT mice
| Day | Swim stress exposure and solutions offered |
|---|---|
| 1–4 | 3% EtOH vs water; 24-h/day EtOH access |
| 5–8 | 6% EtOH vs water; 24-h/day EtOH access |
| 9–12 | 10% EtOH vs water; 24-h/day EtOH access |
| 13–16 | Water only |
| 17–19 | 10% EtOH vs water; 21-h/day EtOH access |
| 20–21 | Water only |
| 22–23 | 10% EtOH vs water; 21-h/day EtOH access |
| 24 | Swim stress (5 min) then 10% EtOH vs water; 21-h EtOH access |
| 25–26 | 10% EtOH vs water; 21-h/day EtOH access |
| 27–28 | Water only |
| 29–30 | 10% EtOH vs water; 21-h/day EtOH access |
| 31–33 | Daily swim stress (5 min) then 10% EtOH vs water; 21-h/day EtOH access |
| 34–35 | Water only |
| 36–40 | 10% EtOH vs water; 21-h/day EtOH access |
| 41–42 | Water only |
| 43–47 | 10% EtOH vs water; 21-h/d EtOH access |
Swim stress sessions
Mice were placed individually into 1,000 ml Pyrex beakers containing 800 ml of 25°C water. Forced swim stress sessions were 5-min long. After the 5-min period, mice were removed from the water and gently dried with a cloth towel before being returned to their home cages. It took approximately 2 h and 30 min to run all animals through the swim stress procedure and forced swim sessions were undertaken during the light cycle between 1400 and 1700 h. EtOH was placed on cages beginning at about 3 h after swim stress initiation. Genotypes were distributed equally across time for stress exposure so that differences in time between forced swim and EtOH availability were similar.
Statistical analysis
Baseline EtOH or tastant consumption (grams or milligrams per kilogram), preference (ratio of milliliters consumed from EtOH or tastant tube to total milliliters consumed) and total volume (milliliters) were analyzed using two-way analysis of variance (ANOVA) with repeated measures (genotype×EtOH/tastant concentration). LORR data (latency and duration) and BEC (milligrams per milliliter) were analyzed using one-way ANOVAs with genotype as the independent variable. Each phase (week) of the swim stress experiment was analyzed with a two-way ANOVA with repeated measures (genotype×day). Significant interactions were examined for simple main effects, and the Newman–Keuls test was used for individual mean comparisons. Statistica 6.1 software (StatSoft, Inc., Tulsa, OK) was used for all analyses.
Results
Sex was included as a factor in all initial analyses. Main effects of sex were found for sensitivity to the sedative–hypnotic effects of EtOH (latency to LORR; male>female; no difference for duration) and in some experiments, for amount of EtOH consumed (female>male). However, there were no significant interactions of sex and genotype; therefore, data were subsequently examined with the sexes combined.
Consumption of EtOH is reduced in CRF1 KO compared with WT mice when offered in a high concentration
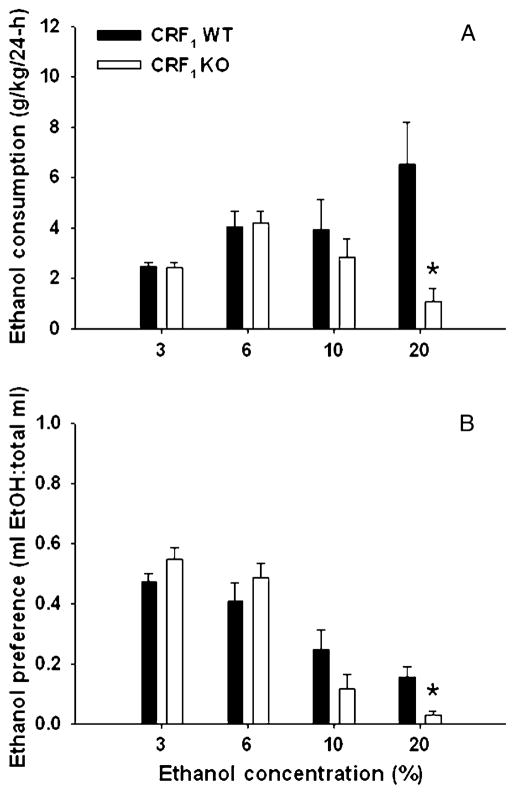
Figure 1 shows EtOH consumption (grams per kilogram; Fig. 1a) and preference (Fig. 1b) for CRF1 KO and WT mice across four different EtOH concentrations. A two-way ANOVA with repeated measures revealed a significant interaction of genotype and EtOH concentration for grams per kilogram EtOH consumed [F(3, 66)=8.3, p<0.01]. Simple main effects analyses showed that KO mice consumed less EtOH than WT mice only when it was offered as a 20% solution (p<0.01). A similar pattern of results was found for EtOH preference (Fig. 1b). Again, there was a significant interaction of genotype and EtOH concentration [F(3, 66)= 5.7, p<0.01] and KO mice showed reduced preference for the 20% EtOH concentration (p<0.01). WT and KO mice did not differ in their total fluid intake (data not shown).
Fig. 1.
Twenty-four-hour, two-bottle choice EtOH consumption (a; mean ± SEM) and preference (b; mean ± SEM) in CRF1 KO and WT mice (n=12 per genotype). Means for each concentration of EtOH represent the average of days 2 and 4. Increasing concentrations of EtOH (3% to 20%) were offered at 4-day intervals. *p<0.01 for the difference between WT and KO mice at the 20% concentration
CRF1 KO and WT mice do not show differences in tastant intake and preference
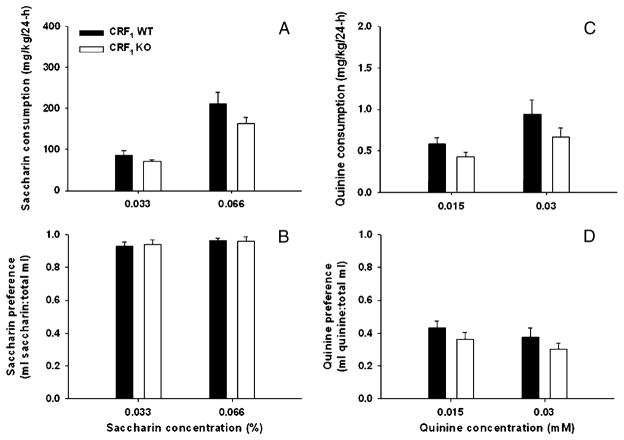
Figure 2 shows consumption (milligrams per kilogram) and preference for saccharin (Fig. 2a, b) and quinine (Fig. 2c, d) in CRF1 KO and WT mice. Two-way ANOVAs with repeated measures identified significant effects of concentration for both saccharin [F(1, 22)= 12.6, p<0.01] and quinine consumption [F(1, 22)=14.3, p < 0.01], but no effect of genotype or significant interaction of genotype and concentration. There were no differences between genotypes in preference for either saccharin or quinine or in total volume of fluid consumed during the tastant study.
Fig. 2.
Twenty-four-hour, two-bottle choice saccharin and quinine intake (a, c; mean ± SEM) and preference (b, d; mean ± SEM) in CRF1 KO and WT mice (n=12 per genotype). Each bar represents average intake and preference for days 2 and 4 of each 4-day period
CRF1 KO and WT mice do not show differences in EtOH-induced LORR or in neurosensitivity to EtOH
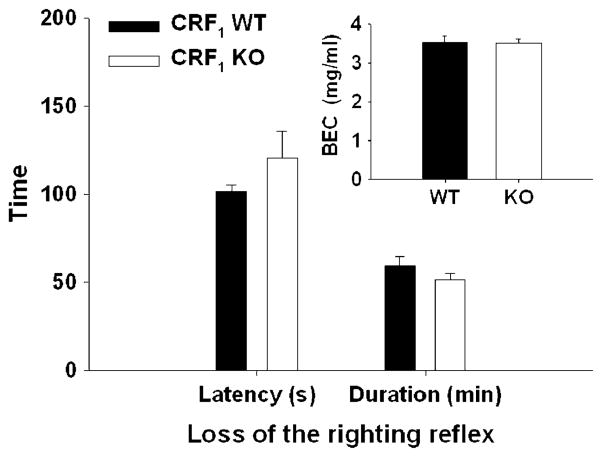
One KO and one WT mouse did not lose the righting reflex within 5 min and low BEC values were found, suggesting that EtOH injections may have been misplaced (e.g., into the intestine or bladder, rather than intraperitoneal cavity). Latency to and duration of LORR (Fig. 3) were not significantly different between CRF1 KO and WT mice. There were also no differences in BEC at the time of regain of the righting reflex. This indicates similar neurosensitivity to EtOH for the two genotypes.
Fig. 3.
EtOH-induced LORR latency (in seconds (s); mean ± SEM), duration (in minutes (min); mean ± SEM), and BEC (milligrams per milliliter (mg/ml); inset) at recovery of the righting reflex in CRF1 and WT mice (n=11 per genotype). The EtOH dose was 3.6 g/kg. No genotype differences were seen for any of the measures using the same animals shown in Fig. 1 (EtOH baseline experiment). For saccharin and quinine intake (a, c), there was a main effect of concentration (p<0.01); however, no effects of genotype were found for either consumption or preference
EtOH consumption is differentially affected by swim stress exposure in CRF1 and CRF1/2-deficient, compared with WT, mice
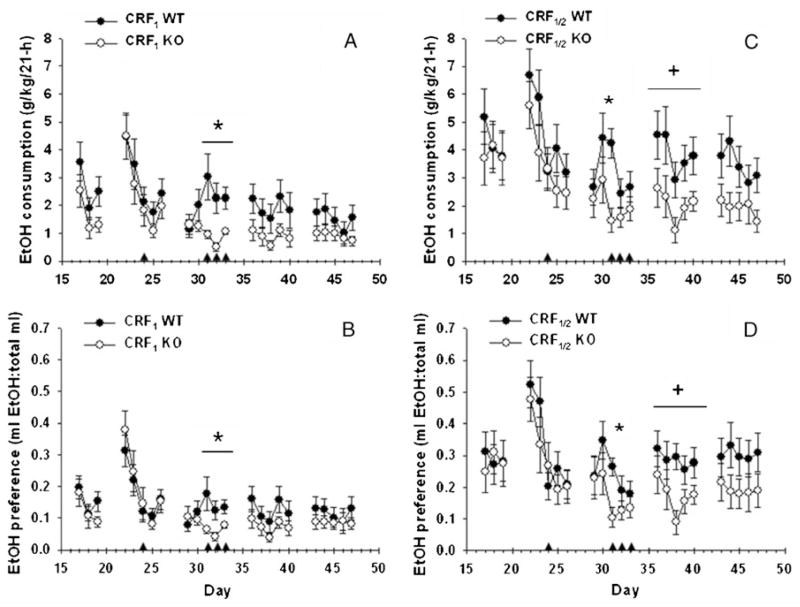
Figure 4 shows EtOH intake and preference, and the effects of swim stress, in CRF1 (Fig. 4a, b) and CRF1/2 (Fig. 4c, d) mice. Consistent with data shown in Fig. 1, no baseline genotype effects were found for CRF1 versus WT mice for EtOH consumption, preference or total fluid intake (data not shown). When drinking was assessed in 21-h periods prior to stress exposure, no effects of genotype were found (Fig. 4, days 17–19). Analysis of data from this phase revealed an effect of day for grams per kilogram, preference and total fluid consumed [F(2, 80)=20.3, 19.3 and 22.6, respectively; all p<0.01], but no genotype or interaction effects. At the beginning of week 1, increased EtOH consumption was apparent after 2 days of EtOH deprivation (see day 22 of the 22–26-day period). This increased EtOH consumption was not seen after additional periods of deprivation. For week 1, a significant effect of day was found for grams per kilogram, preference and total fluid consumed [F(4, 160)=61.4, 71.5 and 77.9, respectively; all p<0.01]; however, no genotype or interaction effects were found. An acute swim stress session did not significantly alter EtOH consumption or preference, but a different conclusion was reached after exposure to repeated episodes of swim stress in week 2 (days 29–33). Analysis of these data identified a significant genotype×day interaction for grams per kilogram and preference [F(4, 160)=8.2 and 9.9, respectively; both p<0.01]. Further analysis of these interactions showed significant differences between CRF1 KO and WT mice on days 31, 32, and 33 after forced swim stress (all p<0.01). No effects of genotype or day were found on total fluid consumed in week 2. Although there was an effect of day on grams per kilogram and preference (but not total fluid intake) for week 3 [F(4, 160)=3.8 and 5.4 for grams per kilogram and preference, respectively; both p<0.01] and week 4 [F(4, 160)=3.6 and 4.4 for grams per kilogram and preference, respectively; both p<0.01], there were no significant genotype or interaction effects.
Fig. 4.
Twenty-one-hour, two-bottle choice EtOH consumption (a, b; mean ± SEM) and preference (b, d; mean ± SEM) in CRF1 KO and WT mice (n=21–22 per genotype) and CRF1/2 KO and WT mice (n = 15–19 per genotype). Days 17–19 represent baseline values, and days 22–26, 29–33, 36–40, and 43–47 represent post-baseline weeks 1–4, respectively. Arrows indicate forced swim exposure. *p<0.01 WT different from KO mice on the indicated days. +p<0.05 for the main effect of genotype for post-baseline week 3 (days 36–40)
Similar results were found for the double KO mice, although the effects of stress were more protracted (Fig. 4c, d). There were no differences between CRF1/2 KO and WT mice in grams per kilogram EtOH consumed or EtOH preference during the introduction to EtOH (data not shown) or during baseline assessment of 21-h 10% EtOH drinking (days 17–19). At the beginning of week 1 (days 22–26), after 2 days of EtOH deprivation, increased EtOH consumption was seen. An effect of day was found for week 1 for grams per kilogram, preference and total fluid consumed [F (4, 128)=20.1, 25.5, and 26.6, respectively; all p<0.01]. However, there was no significant effect of acute swim stress exposure. There was no EtOH deprivation effect seen between weeks 1 and 2. As a whole, analysis of week 2 data (the repeated stress week) did not identify a main effect of genotype or a genotype by day interaction. However, we had a priori reason to examine differences specifically on stressor exposure days. Mean comparisons identified significant differences in both EtOH consumption and preference between WT and CRF1/2 KO mice only on day 31, after the first of the three swim stress exposures (both p<0.01). EtOH consumption was elevated in the initial part of week 3, after 2 days of EtOH deprivation. Also, different from what was found with CRF1 KO versus WT mice, an effect of genotype was found in week 3 for CRF1/2 KO versus WT mice for EtOH consumption and preference [F(1, 32)=4.3 and 4.5, respectively; both p< 0.05] but not for total fluid consumed. A trend for relatively increased EtOH consumption (p=0.08) and preference (p=0.09) remained in WT mice during week 4.
Discussion
The present data suggest that CRF1 play a role in voluntary, 24-h, two-bottle choice EtOH drinking, but only when EtOH is offered at a high concentration. Mice lacking CRF1 showed reduced 20% EtOH consumption and preference, compared with WT mice. Saccharin and quinine consumption and preference were not affected by CRF1 deficiency. These data, together with results showing comparable EtOH neurosensitivity, indicate that the difference between CRF1 KO and WT mice in EtOH consumption and preference cannot be explained by differential taste reactivity/preference or differential sensitivity to the sedative–hypnotic effects of EtOH.
There is considerable consensus that CRF, via CRF1, plays a pivotal role in voluntary EtOH drinking only when there is a history of stress or EtOH dependency (Funk et al. 2007; Gehlert et al. 2007; Heilig and Koob 2007). Nonselective CRF receptor antagonists and CRF1 antagonism or genetic deletion reduced EtOH intake (Chu et al. 2007; Finn et al. 2009; Funk et al. 2006; Lowery et al. 2008; Valdez et al. 2002), when intake was measured after stressor exposure or in EtOH-dependent animals. However, recently, a CRF1 antagonist was also found to reduce EtOH drinking, when measured after a period of EtOH deprivation (Sparta et al. 2009). Also, DID EtOH drinking, which has been previously shown to be sufficient to induce intoxication (Crabbe et al. 2009), was reduced by CRF1 antagonism (Sparta et al. 2008). This effect, moreover, appears to be mediated by central, HPA-independent, CRF1 mechanisms (Lowery et al. 2010). Our present results are consistent with the hypothesis that CRF1 play a role in EtOH drinking when experimental conditions facilitate higher levels of EtOH intake. EtOH concentration is among those facilitating conditions. Previous research using EtOH DID procedures in B6 mice found that CRF1 antagonism reduced EtOH intake in a 4-h, but not 2-h, period of restricted 20% EtOH availability (Sparta et al. 2008). Therefore, duration of access to a high concentration of EtOH may also be an important factor. One speculation about why this may be the case is that a longer period of time may be needed for a sufficient amount of EtOH to be consumed to stimulate stress-relevant pathways. Similar to CRF1 KO mice, CRF1/2 double KO mice did not differ from WT in consumption of EtOH, when EtOH was offered in concentrations of up to 10%. At the time we performed the study examining EtOH intake for concentrations up to 20%, the double KO mice were not available; thus, we do not have data for the high concentration in the double KO mice.
Our data are consistent for the single and double KO mice in showing that an acute exposure to forced swim stress did not induce genotype-dependent changes in EtOH intake. Repeated swim stress, however, resulted in higher levels of EtOH consumption and preference in WT, compared with CRF1 KO, mice. This genotype-dependent difference associated with repeated stress exposure was also present in CRF1/2 mice. Although in their case an effect was seen only in the first of the three stress-exposure sessions, longer lasting residual effects were present when EtOH intake was measured during the 2 weeks following stress exposure. Data shown here (Fig. 1), as well as data in a preliminary study of longer duration (data not shown), determined that WT and CRF1 KO mice, after a 3%, 6%, then 10% introduction to EtOH did not differ in their 10% EtOH drinking across a 50-day period in which no stress was applied. This suggests that genotype-dependent differences in EtOH consumption over time are unlikely to have contributed to the genotype-specific effects we observed following swim stress. Our data are consistent with previous results showing delayed effects on EtOH consumption after stress exposure (Chester et al. 2004; Croft et al. 2005; Lowery et al. 2008; Sillaber et al. 2002). In addition, the results of several studies have suggested that CRF2 play a role in stress coping and recovery (Coste et al. 2000; 2001), compared with the primary function of CRF1 in stress response. Our data, showing a prolonged effect of swim stress exposure in animals with the combined CRF1/2 deletion, are consistent with this suggestion.
A previous study using a CRF1 antagonist also indicated that swim stress-induced effects in EtOH drinking involve CRF1 (Lowery et al. 2008). This convergence is important as it suggests that potential developmental compensations in our constitutive CRF1 KO mice may not explain the present results. However, our data, and those of Lowery et al. (2008) are not consistent with a previous report (Sillaber et al. 2002). In that study, CRF1 KO mice on a mixed B6×129 background, not backcrossed to the EtOH-preferring B6 strain, exhibited a stress-induced increase in EtOH drinking that was higher than in WT mice. Levels of EtOH intake were lower in mice of their mixed genetic background compared with those used in our studies, and this could have played a role in the discrepant findings. The double CRF1/2 KO and WT mice used in our study were backcrossed to B6 for two more generations than our CRF1 KO and WT mice. The higher basal levels of EtOH intake in our double KO and associated WT are likely due to increased alleles from the preferring B6 strain in these mice, due to the extra generations of backcrossing. Evidence suggests that the effects of stress on EtOH consumption may depend on basal EtOH preference (Chester et al. 2004; Lowery et al. 2008). Increases in EtOH intake in response to swim stress have been seen in BALB/cJ mice, which have a lower EtOH preference, but not in the higher preference strain, C57BL/6N (Lowery et al. 2008). Level of initial EtOH consumption could be one explanation for variation in results across studies.
In conclusion, the present data are consistent with the current literature indicating that voluntary EtOH intake can be mediated by CRF signaling. CRF1 play an important role in acquisition of EtOH drinking when higher levels of intake are achieved. A previous study showed no difference between CRF2 and WT mice in EtOH consumption or preference for 3%, 6%, 10%, and 20% EtOH, using the acquisition methods used here (Sharpe et al. 2005). EtOH concentration can be an important factor in facilitating elevated intake, as can exposure to stressful events. The present results are consistent with pharmacological data indicating that the enhancing effects of stress on EtOH drinking are mediated by CRF1. Clinical data suggest that exposure to stressors has an important impact on alcohol abuse and relapse. Thus, pharmacological tools aimed at blocking CRF1 might be helpful for preventing stress-induced relapse or excessive drinking. Studies in CRF1 and CRF1/2 KO and WT mice examining stress-induced reinstatement of drinking or the effects of repeated withdrawal in dependent animals on EtOH drinking might further elucidate the roles of these receptors.
Acknowledgments
This work was supported by a grant from the Department of Veterans Affairs and NIH grants from the National Institute of Alcohol Abuse and Alcoholism (R01AA13331 and P60AA010760) and from Mental Health (R01MH65689).
Contributor Information
Raúl Pastor, Behavioral Neuroscience Department and Portland Alcohol Research Center, Oregon Health & Science University, Portland, OR, USA. Area de Psicobiología, Universitat Jaume I, Castellón, Spain.
Cheryl Reed, Behavioral Neuroscience Department and Portland Alcohol Research Center, Oregon Health & Science University, Portland, OR, USA.
Sue Burkhart-Kasch, Behavioral Neuroscience Department and Portland Alcohol Research Center, Oregon Health & Science University, Portland, OR, USA.
Na Li, Behavioral Neuroscience Department and Portland Alcohol Research Center, Oregon Health & Science University, Portland, OR, USA.
Amanda L. Sharpe, Behavioral Neuroscience Department and Portland Alcohol Research Center, Oregon Health & Science University, Portland, OR, USA
Sarah C. Coste, Molecular Microbiology and Immunology Department, Oregon Health & Science University, Portland, OR, USA
Mary P. Stenzel-Poore, Molecular Microbiology and Immunology Department, Oregon Health & Science University, Portland, OR, USA
Tamara J. Phillips, Email: phillipt@ohsu.edu, Behavioral Neuroscience Department and Portland Alcohol Research Center, Oregon Health & Science University, Portland, OR, USA. Portland VA Medical Center, Research Service, R&D32, 3710 SW US Veterans Hospital Road, Portland, OR 97239, USA
References
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Binder EB, Nemeroff CB. The CRF system, stress, depression and anxiety-insights from human genetic studies. Mol Psychiatry. 2010;15:574–588. doi: 10.1038/mp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm SL, 2nd, Schafer GL, Phillips TJ, Browman KE, Crabbe JC. Sensitivity to ethanol-induced motor incoordination in 5-HT(1B) receptor null mutant mice is task-dependent: implications for behavioral assessment of genetically altered mice. Behav Neurosci. 2000;114:401–409. [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH. Stress sensitization of ethanol withdrawal-induced reduction in social interaction: inhibition by CRF-1 and benzodiazepine receptor antagonists and a 5-HT1A-receptor agonist. Neuropsychopharmacology. 2004;29:470–482. doi: 10.1038/sj.npp.1300282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunell SC, Spear LP. Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcohol Clin Exp Res. 2005;29:1641–1653. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, Blose AM, Zweifel M, Froehlich JC. Effects of stress on alcohol consumption in rats selectively bred for high or low alcohol drinking. Alcohol Clin Exp Res. 2004;28:385–393. doi: 10.1097/01.alc.0000117830.54371.7a. [DOI] [PubMed] [Google Scholar]
- Chu K, Koob GF, Cole M, Zorrilla EP, Roberts AJ. Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF1 receptor antagonist antalarmin and by CRF1 receptor knockout. Pharmacol Biochem Behav. 2007;86:813–821. doi: 10.1016/j.pbb.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste SC, Kesterson RA, Heldwein KA, Stevens SL, Heard AD, Hollis JH, Murray SE, Hill JK, Pantely GA, Hohimer AR, Hatton DC, Phillips TJ, Finn DA, Low MJ, Rittenberg MB, Stenzel P, Stenzel-Poore MP. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24:403–409. doi: 10.1038/74255. [DOI] [PubMed] [Google Scholar]
- Coste SC, Murray SE, Stenzel-Poore MP. Animal models of CRH excess and CRH receptor deficiency display altered adaptations to stress. Peptides. 2001;22:733–741. doi: 10.1016/s0196-9781(01)00386-2. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Rhodes JS, Yu CH, Brown LL, Phillips TJ, Finn DA. A line of mice selected for high blood ethanol concentrations shows drinking in the dark to intoxication. Biol Psychiatry. 2009;65:662–670. doi: 10.1016/j.biopsych.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft AP, Brooks SP, Cole J, Little HJ. Social defeat increases alcohol preference of C57BL/10 strain mice; effect prevented by a CCKB antagonist. Psychopharmacology. 2005;183:163–170. doi: 10.1007/s00213-005-0165-6. [DOI] [PubMed] [Google Scholar]
- De Kloet ER. Hormones and the stressed brain. Ann NY Acad Sci. 2004;1018:1–15. doi: 10.1196/annals.1296.001. [DOI] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AL. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12–41) Alcohol Clin Exp Res. 2009;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert DR, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Ciccocioppo R, Heilig M. 3-(4-Chloro-2-morpholin-4yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazol[1,2-b] pyridazine:a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007;27:2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS Neurol Disord Drug Targets. 2006;5:453–479. doi: 10.2174/187152706777950684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Oakley RH, Olivares-Reyes JA, Dautzenberg FM. Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Ann N Y Acad Sci. 2009;1179:120–143. doi: 10.1111/j.1749-6632.2009.05011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J Pharmacol Exp Ther. 2004;311:427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, Oltersdorf T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci USA. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery EG, Thiele TE. Pre-clinical evidence that corticotropin-releasing factor (CRF) receptor antagonists are promising targets for pharmacological treatment of alcoholism. CNS Neurol Disord Drug Targets. 2010;9:77–86. doi: 10.2174/187152710790966605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery EG, Sparrow AM, Breese GR, Knapp DJ, Thiele TE. The CRF-1 receptor antagonist, CP-154,526, attenuates stress-induced increases in ethanol consumption by BALB/cJ mice. Alcohol Clin Exp Res. 2008;32:240–248. doi: 10.1111/j.1530-0277.2007.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery EG, Spanos M, Navarro M, Lyons AM, Hodge CW, Thiele TE. CRF-1 antagonist and CRF-2 agonist decrease binge-like ethanol drinking in C57BL/6J mice independent of the HPA axis. Neuropsychopharmacology. 2010;35:1241–1252. doi: 10.1038/npp.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Zorrilla EP, Ciccocioppo R, Weiss F. Role of innate and drug-induced dysregulation of brain stress and arousal systems in addiction: focus on corticotropin-releasing factor, nociceptin/orphanin FQ, and orexin/hypocretin. Brain Res. 2010;1314:145–161. doi: 10.1016/j.brainres.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AA, Sharpe AL, Burkhart-Kasch S, McKinnon CS, Coste SC, Stenzel-Poore MP, Phillips TJ. Corticotropin-releasing factor overexpression decreases ethanol drinking and increases sensitivity to the sedative effects of ethanol. Psychopharmacology. 2004;176:386–397. doi: 10.1007/s00213-004-1896-5. [DOI] [PubMed] [Google Scholar]
- Pastor R, McKinnon CS, Scibelli AC, Burkhart-Kasch S, Reed C, Ryabinin AE, Coste SC, Stenzel-Poore MP, Phillips TJ. Corticotropin-releasing factor-1 receptor involvement in behavioral neuroadaptation to ethanol: a urocortin1-independent mechanism. Proc Natl Acad Sci USA. 2008;105:9070–9075. doi: 10.1073/pnas.0710181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Camarini R, Quadros IM, Miczek KA, Israel Y. Genetic and environmental influences on ethanol consumption: perspectives from preclinical research. Alcohol Clin Exp Res. 2010;34:976–987. doi: 10.1111/j.1530-0277.2010.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TJ, Crabbe JC, Metten P, Belknap JK. Localization of genes affecting alcohol drinking in mice. Alcohol Clin Exp Res. 1994;18:931–941. doi: 10.1111/j.1530-0277.1994.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Rivier C. Alcohol stimulates ACTH secretion in the rat: mechanism of action and interactions with other stimuli. Alcoholism Clin Exp Res. 1996;20:240–254. doi: 10.1111/j.1530-0277.1996.tb01636.x. [DOI] [PubMed] [Google Scholar]
- Rodaros D, Caruana DA, Amir S, Stewart J. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 2007;150:8–13. doi: 10.1016/j.neuroscience.2007.09.043. [DOI] [PubMed] [Google Scholar]
- Rotzinger S, Lovejoy DA, Tan LA. Behavioral effects of neuropeptides in rodent models of depression and anxiety. Peptides. 2010;31:736–756. doi: 10.1016/j.peptides.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Sharpe AL, Coste SC, Burkhart-Kasch S, Li N, Stenzel-Poore MP, Phillips TJ. Mice deficient in corticotropin-releasing factor receptor type 2 exhibit normal ethanol-associated behaviors. Alcohol Clin Exp Res. 2005;29:1601–1609. doi: 10.1097/01.alc.0000179371.46716.5e. [DOI] [PubMed] [Google Scholar]
- Sillaber I, Rammes G, Zimmermann S, Mahal B, Zieglgänsberger W, Wurst W, Holsboer F, Spanagel R. Enhanced and delayed stress-induced alcohol drinking in mice lacking functional CRH1 receptors. Science. 2002;296:931–933. doi: 10.1126/science.1069836. [DOI] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Sparta DR, Sparrow AM, Lowery EG, Fee JR, Knapp DJ, Thiele TE. Blockade of the corticotropin releasing factor type 1 receptor attenuates elevated ethanol drinking associated with drinking in the dark procedures. Alcohol Clin Exp Res. 2008;32:259–265. doi: 10.1111/j.1530-0277.2007.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparta DR, Ferraro FM, 3rd, Fee JR, Knapp DJ, Breese GR, Thiele TE. The alcohol deprivation effect in C57BL/6J mice is observed using operant self-administration procedures and is modulated by CRF-1 receptor signaling. Alcohol Clin Exp Res. 2009;33:31–42. doi: 10.1111/j.1530-0277.2008.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JM, Stalla GK, Blanquet V, Steckle T, Holsboer F, Wurst W. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- van Erp AM, Miczek KA. Persistent suppression of ethanol self-administration by brief social stress in rats and increased startle response as index of withdrawal. Physiol Behav. 2001;73:301–311. doi: 10.1016/s0031-9384(01)00458-9. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Wheeler JM, Reed C, Burkhart-Kasch S, Li N, Cunningham CL, Janowsky A, Franken FH, Wiren KM, Hashimoto JG, Scibelli AC, Phillips TJ. Genetically correlated effects of selective breeding for high and low methamphetamine consumption. Genes Brain Behav. 2009;8:758–771. doi: 10.1111/j.1601-183X.2009.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. The therapeutic potential of CRF1 antagonists for anxiety. Expert Opin Investig Drugs. 2004;13:799–828. doi: 10.1517/13543784.13.7.799. [DOI] [PubMed] [Google Scholar]