Abstract
Imaging has played a variety of roles in the study of Alzheimer disease (AD) over the past four decades. Initially, computed tomography (CT) and then magnetic resonance imaging (MRI) were used diagnostically to rule out other causes of dementia. More recently, a variety of imaging modalities including structural and functional MRI and positron emission tomography (PET) studies of cerebral metabolism with fluoro-deoxy-d-glucose (FDG) and amyloid tracers such as Pittsburgh Compound-B (PiB) have shown characteristic changes in the brains of patients with AD, and in prodromal and even presymptomatic states that can help rule-in the AD pathophysiological process. No one imaging modality can serve all purposes as each have unique strengths and weaknesses. These modalities and their particular utilities are discussed in this article. The challenge for the future will be to combine imaging biomarkers to most efficiently facilitate diagnosis, disease staging, and, most importantly, development of effective disease-modifying therapies.
Various neuroimaging modalities (e.g., MRI and PET) have shown characteristic changes in the brains of patients with Alzheimer disease. Identifying imaging biomarkers will facilitate diagnosis, disease staging, and drug development.
THE CHANGING ROLES AND SCOPE OF NEUROIMAGING IN ALZHEIMER DISEASE
There has been a transformation in the part played by neuroimaging in Alzheimer disease (AD) research and practice in the last decades. Diagnostically, imaging has moved from a minor exclusionary role to a central position. In research, imaging is helping address many of the scientific questions outlined in Selkow et al. (2011): providing insights into the effects of AD and its temporal and spatial evolution. Furthermore, imaging is an established tool in drug discovery, increasingly required in therapeutic trials as part of inclusion criteria, as a safety marker, and as an outcome measure.
Concomitantly the potential of brain imaging has expanded rapidly with new modalities and novel ways of acquiring images and of analysing them. This article cannot be comprehensive. Instead, it addresses broad categories of structural, functional, and molecular imaging in AD. The specific modalities included are magnetic resonance imaging (MRI; both structural and functional) and positron emission tomography (PET; for assessment of both cerebral metabolism and amyloid). These modalities have different strengths and limitations and as a result have different and often complementary roles and scope.
Imaging in the Diagnosis and Prognosis of AD
The uncertainty inherent in a clinical diagnosis of AD has driven a search for diagnostic imaging markers. A definitive diagnosis still requires histopathological confirmation and the inaccessibility of the brain means imaging has a key role as a “window on the brain.” Historically, imaging—first computed tomography (CT) and then MRI—was used only to exclude potentially surgically treatable causes of cognitive decline. Now its position in diagnosis also includes providing positive support for a clinical diagnosis of AD in symptomatic individuals by identifying characteristic patterns (signatures) of structural and functional cerebral alterations. We can now also visualize the specific molecular pathology of the disease—amyloid deposits—with amyloid imaging. Alongside this increasing specificity for AD, imaging also contributes to differential diagnosis in practice by identifying alternative and/or contributory pathologies. Imaging is central to identifying vascular and non-AD degenerative pathologies and has helped in the recognition of the prevalence of mixed pathology in dementia.
In the setting of mild cognitive impairment (MCI) (Petersen 2004), the determination of underlying pathology carries immediate prognostic importance. Only a fraction of patients with MCI progress to clinical AD over 5–10 years (Petersen et al. 1999; Ritchie et al. 2001; Visser et al. 2006) and a recent meta-analysis concluded that most people with MCI will not progress to dementia even after 10 years of follow-up (Mitchell and Shiri-Feshki 2009). Two community-based studies have shown over one-third of patients diagnosed with MCI at baseline may eventually return to normal cognition (Larrieu et al. 2002; Ganguli et al. 2004). Obviously, it would be of great value to be able to predict which MCI subjects were destined to progress to a clinical diagnosis of AD. This is true even in the absence of disease-modifying treatments, but will be especially critical when disease-modifying treatments become available.
Looking to the future, imaging has helped establish that there is a long preclinical and presymptomatic period where the pathological effects of AD are detectable. Although more data are needed, imaging is starting to provide prognostic information at this early preclinical stage. The need for an earlier and more certain diagnosis will only increase as disease-modifying therapies are identified. This will be particularly true if, as expected, these therapies work best (or only) when initiated at the preclinical stage.
Understanding the Biology of AD
Importantly, imaging has a major role to play in improving our understanding of this disease (or diseases). Uniquely, imaging is able to delineate in life the location within the brain of the effects of AD. Together with this topographical information imaging can quantify multiple different aspects of AD pathology and assess how they relate to each other and how they change over time. The clinical correlations of these changes and their relationships to other biomarkers and to prognosis can be studied. Ultimately the role of imaging in improving our understanding of the biology of AD underpins all its applications and is a theme that runs through the following sections of this article.
STRUCTURAL MRI IN AD
Basics of Structural MRI as Applied to AD
MRI utilizes the fact that protons have angular momentum which is polarized in a magnetic field. This means that a pulse of radiofrequency can alter the energy state of protons and, when the pulse is turned off, the protons will, on returning to their energy stage, emit a radiofrequency signal. By a combination of different gradients and pulses, “sequences” can be designed to be sensitive to different tissue characteristics. In broad terms structural MRI in AD can be divided into assessing atrophy (or volumes) and changes in tissue characteristics which cause signal alterations on certain sequences such as white matter hyperintensities on T2-weighted MRI as a result of vascular damage. A number of MR sequences that are sensitive to microstructural change (e.g., magnetization transfer or diffusion) have shown alterations in AD. These sequences are already important research tools; however, they have not yet found a place in routine clinical practice in AD and they will not be considered further here.
Utility of Structural MRI in the Study of AD
Atrophy in AD
Progressive cerebral atrophy is a characteristic feature of neurodegeneration that can be visualized in life with MRI (best with T1-weighted volumetric sequences; see Fig. 1). The major contributors to atrophy are thought to be dendritic and neuronal losses. Studies of regional (e.g., hippocampal) MRI volumes have shown these are closely related to neuronal counts at autopsy (Bobinski et al. 2000; Gosche et al. 2002; Jack et al. 2002). The pattern of loss differs between diseases reflecting selective neuronal vulnerability and/or regional disease expression. AD is characterized by an insidious onset and inexorable progression of atrophy that is first manifest in the medial temporal lobe (Scahill et al. 2002). The entorhinal cortex is typically the earliest site of atrophy, closely followed by the hippocampus, amygdala, and parahippocampus (Lehericy et al. 1994; Chan et al. 2001; Dickerson et al. 2001; Killiany et al. 2002). Other structures within the limbic lobe such as the posterior cingulate are also affected early on. These losses then spread to involve the temporal neocortex and then all neocortical association areas usually in a symmetrical fashion. This sequence of progression of atrophy on MRI most closely fits histopathological studies that have derived stages for the spread of neurofibrillary tangles (Braak and Braak 1991). Nonetheless, a significant minority of AD cases have atypical presentations and in these cases the pattern of atrophy accords with clinical phenotype: with language presentations particularly having left temporal atrophy and visual variants having posterior cortical atrophy.
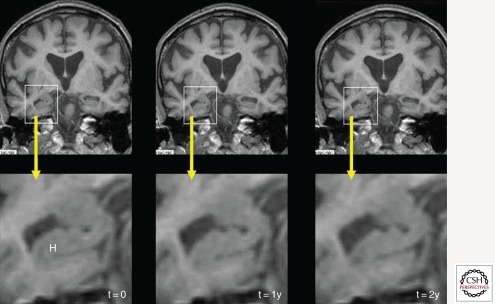
Figure 1.
This series of three coronal T1-weighted studies, from an individual with autopsy-proven Alzheimer disease (AD), were each acquired ∼1 yr apart and show progressive hippocampal (H) atrophy as the individual progressed from memory complaints (left column, t = 0) to MCI (center, t = 1y) and on to fulfill criteria for AD.
It is increasingly clear that by the time a typical AD patient comes to diagnosis atrophy is well established. Even in mildly affected individuals (e.g., mean MMSE of ∼24/30) entorhinal volumes are already reduced by ∼20–30% and hippocampal volumes by ∼15–25% (Chan et al. 2001; Dickerson et al. 2001; Schuff et al. 2009). Because rates of hippocampal atrophy in mild AD are ∼3–5% per year (Barnes et al. 2009) this suggests that there must have been a period of several years before diagnosis where medial temporal lobe atrophy was already in process. Longitudinal MRI studies of individuals who are initially asymptomatic but who subsequently develop AD support this suggestion and find that hippocampal volumes are already reduced by about 10% 3 years before receiving a diagnosis of dementia due to AD and that rates of hippocampal atrophy increase gradually some 5 years before diagnosis. By the time a clinical diagnosis is made, atrophy is also quite widespread with whole brain volumes down by ∼6%; rates of loss having gradually accelerated (at ∼0.3%/yr2) in the 2–4 years up to a diagnosis (Chan et al. 2003; Ridha et al. 2006; Jack et al. 2008b).
Assessment of medial temporal atrophy on MRI has been shown to have positive predictive value for AD. Visual assessment differentiates mild AD from normal aging with a sensitivity and specificity of ∼80–85% (Scheltens et al. 1992; Duara et al. 2008; Burton et al. 2009). Differentiating MCI subjects who will progress to AD in the near future from those who will not is a more difficult task: Medial temporal atrophy on MRI is still a very significant predictor of progression with sensitivity and specificity of ∼50–70% for distinguishing individuals who will progress to AD from those who will not (Korf et al. 2004; DeCarli et al. 2007). For these reasons medial temporal lobe atrophy now forms one of the biomarkers of AD included in proposed criteria for diagnosing (prodromal) AD at a pre-dementia stage (Dubois et al. 2007). The severity of hippocampal atrophy tends to be greater in AD than in dementia with Lewy bodies (DLB) or vascular dementia (VaD)—when matched for clinical severity. Nonetheless, hippocampal atrophy is a feature of DLB and VaD, and in frontotemporal dementia (FTD) can be more severe anteriorly than in AD (Barber et al. 2000; Chan et al. 2001; McKeith et al. 2005; Burton et al. 2009). The differential diagnosis of AD therefore needs to take into account the overall pattern of imaging (and other) features of these dementias: for instance, focal frontal/temporal lobar atrophy on MRI would point to a diagnosis of FTD, whereas marked signal changes in white matter may suggest VaD (Chan et al. 2001; Scheltens et al. 2002; Likeman et al. 2005; Rabinovici et al. 2007; Frisoni et al. 2010). The overall pattern of atrophy is used in clinical practice and there is interest in automated pattern classification of MRI to predict AD at an early stage and to distinguish it from other dementias (Kloppel et al. 2008; Misra et al. 2009; Vemuri et al. 2009).
Measuring Progression in AD with Structural MRI
The fact that pathologically increased cerebral atrophy starts early (even presymptomatically), continues relentlessly, at least until individuals are severely affected, and correlates with clinical decline has led to atrophy on MRI being suggested as a marker of disease progression and a potential outcome measure in trials. The amount, distribution, and rate of cerebral atrophy are all closely correlated with cognitive deficits (Hua et al. 2008; Ridha et al. 2008; Cardenas et al. 2009; Fox et al. 1999b). In the absence of an intervention cerebral volume loss in AD has clear, direct, and profound negative clinical consequences. Epidemiological-autopsy studies of individuals with and without dementia showed that, whereas plaques, tangles, and atrophy are all associated with dementia, atrophy was the factor that most strongly correlated with dementia at all ages (Savva et al. 2009). It appears that histopathological hallmarks of AD are markers of disease process whereas the clinical state is captured by the extent of neurodegeneration—for which atrophy may be considered an in vivo measure. Rates of regional and/or global atrophy on MRI have as a result been proposed as outcome measures in trials seeking to show a disease-modification effect in AD; the motivation for this is the potentially increased power to detect a disease-slowing effect. Sample size calculations based on natural history studies would support this with only ∼20% as many patients being expected to be needed for the same effect using MRI measures than if clinical scales were used (Fox et al. 2000; Jack et al. 2008a; Ridha et al. 2008; Schuff et al. 2009). Rates of hippocampal and whole brain atrophy on MRI have to date been the most widely included imaging measures in trials; however, other MRI measures show promise, including cortical thickness or composites of change (Lerch et al. 2005; Hua et al. 2008; Jack et al. 2008a; Vemuri et al. 2009). The validation of this approach, however, awaits the discovery of disease-modifying therapies particularly as therapies may have an effect on progression of volume loss through mechanisms other than reduced rates of neuronal loss (e.g., hydration, inflammatory, and anti-inflammatory effects) (Fox et al. 2005a). It is likely that multiple imaging and fluid biomarkers will be included in trials that seek to understand as well as measure effects on disease progression.
Availability and Utility of Structural MRI
An obvious strength of MRI is its availability. A testament to its value in diagnosis in dementia is the fact that European and U.S. guidelines recommend that all subjects with cognitive decline undergo structural imaging (MRI or CT) and that it is part of proposed diagnostic criteria for AD and for other dementias (Waldemar et al. 2000; Knopman et al. 2001; McKeith et al. 2005; Dubois et al. 2007). In most centers, MRI is regarded as an essential investigation in dementia—a marker of its utility. Although not as rapid as CT, a typical high-resolution volumetric sequence can be acquired in 5–10 min and more basic sequences in considerably less time. MRI is safe and as it does not involve ionizing radiation individuals can be imaged serially without concerns about carcinogenicity. MRI offers a range of different sequences that can probe different tissue characteristics providing multiple clinical and research measures in the same session. Atrophy as an outcome measure has strengths over clinical measures because it is not subject to practice effects or (realistically) to floor or ceiling effects, and it theoretically has a greater ability to detect disease slowing. MRI measures of atrophy reflect cumulative neuronal damage which in turn is directly responsible for clinical state. When compared with other imaging markers (and other biomarkers) cerebral atrophy has, as a strength, its strong correlation with cognitive decline.
Limitations of Structural MRI in AD
Structural MRI lacks molecular specificity. It cannot directly detect the histopathological hallmarks of AD (amyloid plaques or neurofibrillary tangles) and as such it is downstream from the molecular pathology. Cerebral atrophy is a nonspecific result of neuronal damage and, whereas certain patterns of loss are characteristic of different diseases, they are not entirely specific. Atrophy patterns overlap with other diseases and unusual forms of AD have atypical patterns of atrophy too. In more severely affected individuals and those with claustrophobia, MRI may not be tolerated whereas a rapid CT scan may be more feasible. In terms of measuring progression, volume changes on MRI may be produced by factors other than the progression of neuronal loss and as such assessment of disease modification may be obscured, at least in the short term, by such spurious effects. As the name implies, structural MRI cannot assess function; this is provided with increasing sophistication by functional MRI and PET.
Overall the availability, ease of use, and multiple applications of structural MRI in AD mean it will play a central role in research and practice for some years to come. Increasingly, the other (complementary) modalities described in this article will address the weaknesses of MRI.
FUNCTIONAL MRI IN AD
Basics of Functional MRI as Applied to AD
Functional MRI (fMRI) is being increasingly used to probe the functional integrity of brain networks supporting memory and other cognitive domains in aging and early AD. fMRI is a noninvasive imaging technique which provides an indirect measure of neuronal activity, inferred from measuring changes in blood oxygen level–dependent (BOLD) MR signal (Ogawa et al. 1990; Kwong et al. 1992). Whereas fluoro-deoxy-d-glucose (FDG)-PET is thought to be primarily a measure of synaptic activity, BOLD fMRI is considered to reflect the integrated synaptic activity of neurons via MRI signal changes because of changes in blood flow, blood volume, and the blood oxyhemoglobin/deoxyhemoglobin ratio (Logothetis et al. 2001). fMRI can be acquired during cognitive tasks, typically comparing one condition (e.g., encoding new information) to a control condition (e.g., viewing familiar information or visual fixation on a cross-hair), or during the resting state to investigate the functional connectivity (fc-MRI) within specific brain networks. Fc-MRI techniques examine the correlation between the intrinsic oscillations or time course of BOLD signal between brain regions (Fox et al. 2005b), and have clearly documented the organization of the brain into multiple large-scale brain networks (Damoiseaux et al. 2006; Vincent et al. 2006). Both task-related and resting fMRI techniques have the potential to detect early brain dysfunction related to AD, and to monitor therapeutic response over relatively short time periods; however, the use of fMRI in aging, MCI, and AD populations thus far has been limited to a relatively small number of research groups.
Utility of Functional MRI in the Study of AD
Much of the early fMRI work in MCI and AD used episodic memory tasks, and was focused on the pattern of fMRI activation in hippo campus and related structures in the medial temporal lobe. In patients with clinically diagnosed AD, the results have been quite consistent, showing decreased hippocampal activity during the encoding of new information (Small et al. 1999; Rombouts et al. 2000; Kato et al. 2001; Gron et al. 2002; Machulda et al. 2003; Sperling et al. 2003; Remy et al. 2004; Golby et al. 2005; Hamalainen et al. 2007). Several studies have reported increased prefrontal cortical activity in AD patients (Grady et al. 2003; Sperling et al. 2003; Sole-Padulles et al. 2009), suggesting that other networks may increase activity as an attempted compensatory mechanism during hippocampal failure.
A relatively small number of fMRI studies have been published in subjects at risk for AD, including MCI subjects and genetic at-risk individuals yielding somewhat discrepant findings. Several studies have reported decreased mesial temporal lobe (MTL) activation in MCI (Small et al. 1999; Machulda et al. 2003; Johnson et al. 2006; Petrella et al. 2006) and genetic at-risk subjects (Smith et al. 1999; Lind et al. 2006a,b; Trivedi et al. 2006; Borghesani et al. 2007; Mondadori et al. 2007; Ringman et al. 2010). Interestingly, several fMRI studies have reported evidence of increased MTL activity in at-risk subjects, particularly among very mild MCI subjects (Dickerson et al. 2004, 2005; Celone et al. 2006; Hamalainen et al. 2006; Heun et al. 2007; Kircher et al. 2007; Lenzi et al. 2009), and cognitively intact individuals with genetic risk for AD (Bookheimer et al. 2000; Smith et al. 2002; Wishart et al. 2004; Bondi et al. 2005; Fleisher et al. 2005; Han et al. 2007; Filippini et al. 2009). It is likely that these discrepant results are related to specific paradigm demands, stage of impairment, and behavioral performance. A common feature of the studies reporting evidence of increased fMRI activity is that the at-risk subjects were able to perform the fMRI tasks reasonably well. In particular, the event-related fMRI studies have found that hyperactivity was observed specifically during successful memory trials, which suggested that hyperactivity might represent a compensatory mechanism in the setting of early AD pathology (Dickerson and Sperling 2008; Sperling et al. 2009).
Cross-sectional studies suggest that the hyperactivity may be present only at early stages of MCI, followed by a loss of activation in late stages of MCI, similar to the pattern seen in AD patients (Celone et al. 2006). Longitudinal studies furthermore suggest that the presence of hyperactivity at baseline is a predictor of rapid cognitive decline (Bookheimer et al. 2000; Dickerson et al. 2004; Miller et al. 2008a), and loss of hippocampal function on serial fMRI (O’Brien et al. 2010). The mechanistic underpinnings of MTL hyperactivation remain unclear; however, these new longitudinal data suggest that hyperactivity may be a marker of impending neuronal failure. This phenomena may reflect cholinergic or other neurotransmitter up-regulation (DeKosky et al. 2002), aberrant sprouting of cholinergic fibers (Masliah et al. 2003), inefficiency in synaptic transmission (Stern et al. 2004), increased calcium influx, and evidence of excitotoxicity (Palop et al. 2007; Busche et al. 2008).
Converging data suggest that memory function is subserved by a network of brain regions, which includes not only the MTL system, but also a set of cortical regions, including the precuneus, posterior cingulate, lateral parietal, lateral temporal, and medial prefrontal regions, collectively known as the “default network” which typically deactivate during memory encoding and other cognitively demanding tasks focused on the processing of external stimuli (Raichle et al. 2001; Buckner et al. 2008). Recent studies have also suggested that the default network shows markedly abnormal responses during memory tasks in clinical AD patients and in subjects at risk for AD (Lustig and Buckner 2004; Celone et al. 2006; Petrella et al. 2007a; Pihlajamaki et al. 2008, 2009). Interestingly, it is the same default network regions that typically show beneficial deactivations in healthy subjects, particularly, the posterior cingulate/precuneus (Daselaar et al. 2004; Miller et al. 2008b), which tend to manifest a paradoxical increase in fMRI activity (or loss of normal default network deactivation) in both at-risk groups and clinical AD patients (Petrella et al. 2007b; Pihlajamaki et al. 2008; Fleisher et al. 2009; Sperling et al. 2010).
There has been a recent emphasis on BOLD fMRI techniques to study spontaneous brain activity and the interregional correlations during the resting state. These studies have clearly documented the organization of the brain into multiple large-scale brain networks (Damoiseaux et al. 2006; Vincent et al. 2007). Interestingly, both independent component analyses and “seed-based” connectivity techniques have shown the robust intrinsic connectivity between the posteromedial nodes of the default network, in particular the posterior cingulate/precuneus, with the hippocampus. Multiple groups have confirmed impaired intrinsic functional connectivity in the default network during the resting state in MCI and AD (Greicius et al. 2004; Rombouts et al. 2005, 2009; Sorg et al. 2007; Bai et al. 2008; Koch et al. 2010) over and above more general age-related disruption of large-scale networks (Andrews-Hanna et al. 2007; Damoiseaux et al. 2008). One recent study suggests that these resting fMRI techniques may be more readily applied to at-risk clinical populations than task fMRI (Fleisher et al. 2009). Fc-MRI may be particularly advantageous for use in clinical trials, as no special equipment is required, subjects do not have to be able to perform a cognitive task, and a resting run could be added to the end of a safety or volumetric MRI protocol. Additional longitudinal work is needed to determine if longitudinal changes in fc-MRI will parallel clinical decline.
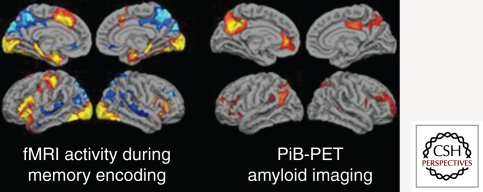
Interestingly, the default network regions showing aberrant task-related fMRI activity and dysconnectivity in MCI and AD also overlap the anatomy of regions with the highest amyloid burden in AD patients (Fig. 2; Klunk et al. 2004; Buckner et al. 2005, 2009; Sperling et al. 2009). Several recent studies in cognitively normal older individuals with evidence of amyloid deposition on PET imaging have shown evidence of disrupted default network activity during memory tasks and at rest (Hedden et al. 2009; Sheline et al. 2009; Sperling et al. 2009), suggesting these markers may be particularly useful to track response to antiamyloid therapies in preclinical trials.
Figure 2.
(Left) Group map of fMRI activity showing regions that increase activity (yellow/red) or decrease (blue) activity during successful encoding. (Right) Group map of 11C-PiB retention in a group of non-demented older individuals. Note the anatomic overlap of PiB retention to default network (regions in blue on left).
fMRI, either during cognitive paradigms or during resting state, may hold the greatest potential for the evaluation of novel pharmacological strategies to treat AD. Several studies in healthy young and older subjects suggest that fMRI can detect acute pharmacological effects on memory networks (Thiel et al. 2001; Sperling et al. 2002; Kukolja et al. 2009). To date, only a few small fMRI studies have shown enhanced brain activation after acute or prolonged treatment with cholinesterase inhibitors in MCI and AD, although these studies were not conducted as typical double-blind, placebo-controlled trials (Rombouts et al. 2002; Goekoop et al. 2004; Saykin et al. 2004; Shanks et al. 2007; Bokde et al. 2009; Venneri et al. 2009). fMRI is now being incorporated into a small number of investigator-initiated add-on studies to ongoing Phase II and Phase III trials, which should provide some valuable information regarding the potential utility of these techniques in clinical trials.
Limitations of fMRI in AD
There are multiple challenges in performing longitudinal fMRI studies in patients with neurodegenerative dementias. It is likely that fMRI will remain quite problematic in examining patients with more severe cognitive impairment, as these techniques are very sensitive to head motion. If the patients are not able to adequately perform the cognitive task, one of the major advantages of task fMRI activation studies is lost. Resting state fMRI may be more feasible in more severely impaired patients.
It is critical to complete further validation experiments. BOLD fMRI response is known to be variable across subjects, and very few studies examining the reproducibility of fMRI activation in older and cognitively impaired subjects have been published to date (Clement and Belleville 2009; Putcha et al. 2010). Longitudinal functional imaging studies are needed to track the evolution of alterations in the fMRI activation pattern over the course of the cognitive continuum from preclinical to prodromal to clinical AD. It is also important to evaluate the contribution of structural atrophy to changes observed with functional imaging techniques in neurodegenerative diseases. Finally, longitudinal multimodality studies, including structural MRI, fMRI, and FDG-PET and PET amyloid imaging techniques, are needed to understand the relationship between these markers, and the relative value of these techniques in tracking change along the clinical continuum of AD (Jack et al. 2010).
FLUORODEOXYGLUCOSE (FDG) PET IN AD
Basics of FDG PET as Applied to AD
Brain FDG PET primarily indicates synaptic activity. Because the brain relies almost exclusively on glucose as its source of energy, the glucose analog FDG is a suitable indicator of brain metabolism and, when labeled with Fluorine-18 (half-life 110 min) is conveniently detected with PET. The brain’s energy budget is overwhelmingly devoted to the maintenance of intrinsic, resting (task-independent) activity, which in cortex is largely maintained by glutamaturgic synaptic signaling (Sibson et al. 1997). FDG uptake strongly correlates at autopsy with levels of the synaptic vesicle protein synaptophysin (Rocher et al. 2003). Hence, FDG PET is widely accepted to be a valid biomarker of overall brain metabolism to which ionic gradient maintenance for synaptic activity is the principal contributor (Schwartz et al. 1979; Magistretti 2006). In this context, a single, specific AD-related alteration in FDG metabolism has not been identified and therefore the FDG-PET abnormalities described below are assumed to be the net result of some combination of processes putatively involved in the pathogenesis of AD including, but not limited to, expression of specific genes, mitochondrial dysfunction, oxidative stress, deranged plasticity, excitotoxicity, glial activation and inflammation, synapse loss, and cell death.
Utility of FDG PET in the Study of AD
The Pattern of FDG Hypometabolism Is an Endophenotype of AD
A substantial body of work over many years has identified a FDG-PET endophenotype of AD (Fig. 3)—that is, a characteristic or signature ensemble of limbic and association regions that are typically hypometabolic in clinically established AD patients (Foster et al. 1983; Reiman et al. 1996; Minoshima et al. 1997; De Santi et al. 2001). The anatomy of the AD signature includes posterior midline cortices of the parietal (precuneus) and posterior cingulate gyri, the inferior parietal lobule, posterolateral portions of the temporal lobe, as well as the hippocampus and medial temporal cortices. Metabolic deficits in AD gradually worsen throughout the course of the disease. Bilateral asymmetry is common at early stages, more advanced disease usually involves prefrontal association areas, and in due course even primary cortices may be affected. Interestingly, the regions initially hypometabolic in AD are anatomically and functionally interconnected and form part of the large-scale distributed brain network known as the default mode network (Raichle et al. 2001). We now know in addition that these regions are highly vulnerable to amyloid-β (Aβ) deposition (Klunk et al. 2004; Buckner et al. 2005).
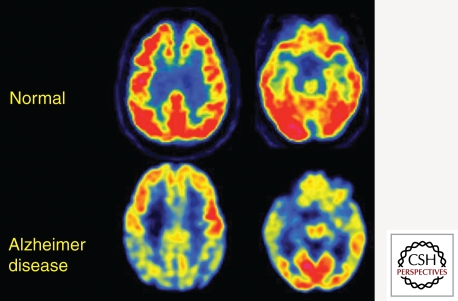
Figure 3.
Transaxial FDG-PET images of a normal control subject and a patient with mild AD. Note severe hypometabolism (yellow and blue cortical regions) in association and limbic cortex. These are the typically involved brain regions that define the FDG endophenotype of AD. They include posteriomedial parietal (precuneus), lateral parietal, lateral temporal, and medial temporal lobes. This pattern slowly worsens in parallel with symptoms and is well correlated at autopsy with AD pathologic diagnosis.
Less severe or consistent hypometabolism has been identified in MCI patients, some of whom were found on follow-up examination to have converted to AD (Arnaiz et al. 2001; de Leon et al. 2001; Jagust et al. 2002, 2007; Chetelat et al. 2003; Caselli et al. 2008; Langbaum et al. 2009; Landau et al. 2010). Differences in FDG between MCI and normal aging have not typically been large, but the control groups in most of these studies were likely contaminated with a number of individuals who, although clinically normal, were amyloid positive (see below) and possibly in earlier phases of preclinical AD. FDG hypometabolism parallels cognitive function along the trajectory of normal, preclinical, prodromal, and established AD (Minoshima et al. 1997; Furst et al. 2010); however, higher levels of brain and cognitive reserve are well known to attenuate the strength of these correlations and highly intelligent AD patients can be clinically mild, but severely hypometabolic (Stern et al. 1992; Alexander et al. 1997). Coexisting vascular disorders, including ischemia, amyloid angiopathy, and micro-hemorrhage, potentially confound the relation of FDG to clinical phenotype, but the classic AD FDG pattern is well correlated with histopathologic diagnosis of AD at autopsy (Hoffman et al. 2000; Jagust et al. 2007).
FDG Hypometabolism Is Related to Other AD Biomarkers and to Genes
The association between amyloid deposition and brain function in AD has been studied with FDG PET. Longitudinal data has shown that, once the stage of established AD is reached, amyloid deposition in most regions has plateaued (Engler et al. 2006; Jack et al. 2009), but FDG continues to decline along with cognitive function (Engler et al. 2006). Several groups have observed high amyloid deposition in parietal regions to be associated with co-localized FDG hypometabolism, possibly indicating a local toxicity (Klunk et al. 2004; Engler et al. 2006; Edison et al. 2007; Cohen et al. 2009). In other groups, this association was not statistically significant, possibly because the amyloid burden in these patients was already at its plateau (Kadir et al. 2008; Furst et al. 2010). An important clue to this relationship could lie in the observation that the relation is consistently weaker in frontal regions, where some of the highest amyloid burdens are found (Klunk et al. 2004; Edison et al. 2007). Interestingly, amyloid-positive MCI patients in one study had preserved FDG metabolism that was positively correlated with extensive Pittsburgh Compound-B (PiB) retention, possibly suggesting a mediating role for metabolism, perhaps either as a brain reserve factor or as an accelerant of deposition (Cohen et al. 2009). Additional longitudinal data will be required to clarify these relationships, but clearly FDG metabolism appears to be changing as amyloid is accumulating. It is possible that FDG data could signal an intermediate stage between the initiating pathologic event and the subsequent development of synaptic failure and neurodegeneration (Cohen et al. 2009).
Brain volume loss is also observed in AD hypometabolic areas, but the FDG findings have generally survived MRI-based corrections for cortical atrophy (Meltzer et al. 1996; Ibanez et al. 1998; Jagust et al. 2006; Cohen et al. 2009; Lowe et al. 2009; Rabinovici et al. 2010), suggesting that volume loss and function loss are separable phenomena in AD. Both domains of data are reported to have predictive power: FDG hypometabolism that predicts ultimate development of AD occurs before impairment (de Leon et al. 2001; Jagust et al. 2006) and brain volume loss has also been reported in cognitively normal individuals who go on to develop AD (Fox et al. 1999a; Jack et al. 2004). Systematic comparison of two imaging biomarkers requires caution because of rapidly evolving technology. For example, recently developed methods for subject-specific MRI segmentation have revealed subtle cortical thinning in a distribution similar to that seen with FDG (Walhovd et al. 2009; Karow et al. 2010). A continuing challenge is presented by the fact that FDG-PET data inherently contains volume information, and PET-based partial volume correction (e.g., with deconvolution [Tohka and Reilhac 2008]), may eventually be useful to disentangle FDG retention and structural loss.
Initial reports associating FDG hypometabolism and AD-related CSF measures have varied, likely due in part to image and fluid sample processing differences. FDG was associated with low CSF Aβ and increased CSF tau in amyloid-positive clinically normal older individuals (Petrie et al. 2009), but with CSF Aβ and not tau in an Alzheimer’s Disease Neuroimaging Initiative (ADNI) study of AD, MCI, and controls, adjusted for diagnosis (Jagust et al. 2009).
Carriers of the apolipoprotein-E (APOE) ε4 allele have a higher risk of developing AD, and the classic AD pattern of hypometabolism described above is seen in cognitively normal APOE ε4 carriers (Reiman et al. 1996, 2005). A relationship of this FDG pattern to serum cholesterol and to an aggregate cholesterol-related genetic score in middle age has also been reported (Reiman et al. 2008, 2010). Maternal history of dementia has recently been related both to increased PiB retention and to FDG hypometabolism in AD-related areas among asymptomatic individuals (Mosconi et al. 2009, 2010).
FDG PET Is a Valid AD Biomarker
Over the course of three decades of investigation, FDG PET has emerged as a robust marker of brain dysfunction in AD. Its principal value is twofold: first, clinical utility has been documented when confounding conditions (e.g., DLB or frontotemporal lobar degeneration [FTLD]), are in question. Thus, when frontotemporal rather than temporoparietal hypometabolism is prominent, a clinically uncertain AD diagnosis may be changed to FTLD (Foster et al. 2007); when prominent occipital hypometabolism is found in addition to temporoparietal, the data are highly suggestive of DLB (Albin et al. 1996; Mosconi et al. 2008).
Second, FDG has emerged as a robust biomarker of neurodegeneration with which hypometabolism can be observed to precede the appearance of cognitive symptoms and to predict the rate of progressive cognitive decline in individuals who are later found to have progressed to AD (de Leon et al. 2001; Jagust et al. 2006). FDG hypometabolism is also predictive of the rate of memory decline in APOE ε4 carriers with mild memory loss over 2 years (Small et al. 2000). Most importantly for AD treatment research, a recent analysis of ADNI FDG data found that AD and MCI groups each showed progression of AD-like hypometabolism over 1 year that paralleled changes in a standard clinical endpoint, the clinical dementia rating scale (CDR) sum-of-boxes (Chen et al. 2010). These authors calculated that the use of FDG PET in clinical trials of AD therapy could reduce sample sizes by approximately one order of magnitude.
The Limitations of FDG PET in AD
FDG PET is relatively expensive and, like all PET techniques, has more limited availability, although its use in oncology has dramatically increased availability in the USA over the past decade. It requires intravenous access and involves exposure to radioactivity, although at levels well below significant known risk. Brain FDG retention is a nonspecific indicator of metabolism that can be deranged for a variety of reasons (e.g., ischemia or inflammation) and may in certain individuals be irrelevant or only indirectly related to any AD-related process.
AMYLOID PET IN AD
Basics of Amyloid PET as It Is Applied to AD
An important “first principle” of amyloid imaging in the context of AD is that amyloid PET is intended first and foremost as an in vivo surrogate for Aβ pathology, and not necessarily as a surrogate for clinical diagnosis. As discussed below, there are diagnostic applications of amyloid imaging, but these share the same strengths and limitations as postmortem determinations of Aβ content. Another important principle of amyloid imaging is that the substrate for all currently known Aβ tracers is fibrillar Aβ in a beta-sheet conformation (Ikonomovic et al. 2008). When speaking of the binding substrates of amyloid tracers, it is preferable to think in terms of fibrillar and nonfibrillar Aβ rather than visual descriptions of plaques as fleecy, amorphous, diffuse, compact, cored, neuritic, etc., because there can be varying amounts of fibrillar Aβ in any of these plaque types. Compact, cored, and neuritic plaques typically have large amounts of fibrillar amyloid and fleecy and amorphous plaque deposits typically have very little (particularly in the cerebellum). However, diffuse plaques are not a precisely defined term and can have widely varying amounts of fibrillar Aβ from case to case. Along similar lines, cerebrovascular amyloid typically has a high degree of fibrillar Aβ and appears to be a very good substrate for amyloid tracer binding (Bacskai et al. 2007; Johnson et al. 2007; Lockhart et al. 2007; Ikonomovic et al. 2008). Increasing recognition has been given to the toxicity of oligomeric species of Aβ and this is described in Mucke and Selkow (2011). Although it is possible that currently available amyloid tracers could bind to oligomers of Aβ in a beta-sheet conformation once they reach a necessary size (probably at least a trimer or tetramer), the in vivo signal of amyloid tracers is not directly representative of these species because of their low concentration relative to insoluble Aβ fibrils. However, there may be a relationship between the amyloid PET signal and oligomer concentration based on the existence of an equilibrium between monomers, oligomers, and fibrillar Aβ. Although claims have been made that some tracers can image neurofibrillary tangles, there have been no validation studies in this regard. To the contrary, there is evidence that some amyloid tracers do not bind neurofibrillary pathology (Klunk et al. 2003; Ikonomovic et al. 2008).
With regard to specific amyloid imaging agents, this review will discuss “amyloid tracers” in general, while acknowledging that most of the statements are derived from data on the most widely evaluated PET tracer, PiB (Klunk et al. 2004). At the time of writing, there have been one or two, small published studies using each of the fluorine-18-labelled tracers, [F-18]florbetaben (18F-BAY94-9172 or AV-1; Rowe et al. 2008), [F-18]florbetapir (AV-45; Wong et al. 2010; Clark et al. 2011) and [F-18]flutemetamol (3′F-PiB or GE-067; Nelissen et al. 2009; Vandenberghe et al. 2010) in AD patients. Although the PiB PET findings may ultimately be found to extend to these F-18-labeled tracers as well, this cannot be assumed until appropriate studies have been repeated with each individual tracer or until pharmacological equivalency to PiB has been established by direct comparison in the same subjects.
Utility of Amyloid PET in the Study of AD
The obvious strength of amyloid imaging is that it has allowed the determination of brain Aβ content to be moved from the pathology laboratory into the clinic. Amyloid imaging can detect cerebral β-amyloidosis and appears specific for this type of amyloid pathology, giving negative signals in pathologically confirmed cases of prion amyloid (Villemagne et al. 2009), pathologically confirmed pure α-synucleinopathy (Burack et al. 2010), as well as in apparently pure cases of tauopathy in semantic dementia (Drzezga et al. 2008).
In the setting of clinical dementia, particularly in clinically atypical presentations, this has important diagnostic utility. Reviewing recent publications from 15 research groups who have performed amyloid PET on clinically diagnosed AD patients, 96% of AD patients were amyloid positive (Fig. 4; Kemppainen et al. 2006; Aizenstein et al. 2008; Edison et al. 2008; Shin et al. 2008; Drzezga et al. 2009; Hedden et al. 2009; Lowe et al. 2009; Maetzler et al. 2009; Wolk et al. 2009; Devanand et al. 2010; Forsberg et al. 2010; Jagust et al. 2010; Rabinovici et al. 2010; Roe et al. 2010; Rowe et al. 2010; Tolboom et al. 2010). One assumption is that amyloid-negative demented patients diagnosed as AD have been given an incorrect diagnosis. Another possibility is that amyloid imaging was simply not sensitive enough in some patients and these patients would become amyloid positive over time. One follow-up of three amyloid-negative subjects initially diagnosed as AD (Klunk et al. 2004), has shown that all three subjects have remained amyloid negative for 5 years (Kadir et al. 2010), suggesting that sensitivity was not the issue and that these patients are not likely to have AD as the cause of their cognitive deficits. On the other side of the coin are amyloid-positive patients who have been diagnosed with a dementia other than AD. In the case of FTD, it has been assumed that patients who present with a clinical FTD syndrome but have AD-like amyloid PET scans are really atypical presentations of AD (Rabinovici et al. 2007, 2008; Engler et al. 2008), but pathological verification remains to be done. These patients will be particularly important to identify when there are effective treatments for AD directed at Aβ deposition.
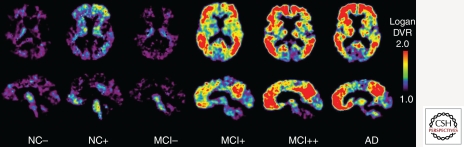
Figure 4.
PiB PET Images of normal control, MCI, and AD subjects showing a range of amyloid-β deposition. Most controls show no evidence of amyloid-β deposition (NC−), but a substantial portion (∼25%) do (NC+). Most patients with MCI show moderate (MCI+) or severe amyloid-β deposition (MCI++), but as many as 40%–50% show no evidence of amyloid-β pathology (MCI−). The vast majority of clinically diagnosed AD patients show heavy amyloid-β deposition (AD).
In the setting of MCI, combined data from nine amyloid PET studies show that 161 of 272 MCI patients were amyloid positive (59%) (Fig. 4; Forsberg et al. 2008; Koivunen et al. 2008; Lowe et al. 2009; Okello et al. 2009; Tolboom et al. 2009; Wolk et al. 2009; Devanand et al. 2010; Jagust et al. 2010; Rowe et al. 2010). Five of these studies included longitudinal clinical follow-up for 1–3 years on 155 MCI patients and showed that 57 of these 155 progressed to clinical AD (37%) and 53 of these 57 were amyloid positive at baseline (93%); only four of 54 amyloid-negative MCI patients progressed to clinical AD in these studies (7%) (Forsberg et al. 2008; Koivunen et al. 2008; Okello et al. 2009; Wolk et al. 2009; Jagust et al. 2010).
The most substantial contribution of amyloid imaging may come in the setting of the cognitively normal elderly. It is at this clinically “invisible” stage that detection of underlying cerebral β-amyloidosis (the sine qua non of AD pathology) may give us the greatest insights into the very beginnings of this disease. Furthermore, it may be at this asymptomatic stage that our chances are greatest of discovering truly effective treatments. In a series of studies from 13 sites, 155 of 651 (24%) of cognitively normal controls showed evidence of cerebral Aβ deposition (Fig. 4; Kemppainen et al. 2006; Mintun et al. 2006; Edison et al. 2008; Shin et al. 2008; Hedden et al. 2009; Lowe et al. 2009; Maetzler et al. 2009; Wolk et al. 2009; Devanand et al. 2010; Jagust et al. 2010; Rabinovici et al. 2010; Roe et al. 2010; Rowe et al. 2010; Tolboom et al. 2010). In most cases, the degree of amyloid deposition was fairly easy to distinguish from that typically seen in AD (Aizenstein et al. 2008), but this is not always the case. The prevalence of amyloid positivity is related closely to age and apolipoprotein-E allele status (Morris et al. 2010; Rowe et al. 2010). Although some subtle cognitive effects of PET amyloid positivity may be discernable in this population (Rentz et al. 2010), in most cases the overriding conclusion is that there is no tight, direct relationship between amyloid PET and cognition at these earliest stages of Aβ deposition. As discussed above, other protective or vulnerability factors must be invoked to fully explain the connection between early PET amyloid positivity and cognitive impairment. It is possible that the failure to directly assess oligomeric Aβ concentration could preclude the demonstration of amyloid PET-related cognitive effects, but vulnerability factors (such as subclinical cerebrovascular disease) and brain/cognitive reserve factors are likely to play a role as well (Kemppainen et al. 2008; Roe et al. 2008, 2010; Cohen et al. 2009; Rentz et al. 2010).
In Blennow et al. (2011), CSF biomarkers are discussed. There is clearly a large overlap in the information available from CSF Aβ42 levels and amyloid PET, but each technique has its advantages and limitations (see below). The advantages of amyloid PET center around the regional information and in the continuously variable nature of the biological changes. The latter refers to the fact that decreases in CSF Aβ42 appear to occur early (at least as early as changes in amyloid PET) and precipitously—achieving its final level very early in the course of the pathophysiological spectrum of AD—probably presymptomatically (Blennow and Hampel 2003; Hansson et al. 2006; Fagan et al. 2007, 2009). That is, the change in CSF appears to be a step-function and longitudinal studies have not shown a progressive decrease in CSF Aβ42 over time (Buchhave et al. 2009). This is not surprising given that typical concentrations of Aβ found in insoluble deposits in AD cortex are approximately 5000 µg/L (∼1 µm), while typical CSF Aβ42 concentrations are around 0.5 µg/L—or 0.01% of insoluble cortical Aβ. Thus, it is not surprising that relatively little cortical Aβ would need to deposit before a new equilibrium would be established with CSF. This has an important implication for clinical trials: As an outcome measure, CSF Aβ42 is not likely to normalize until the vast majority of cortical Aβ deposits are removed. Thus, CSF Aβ42 and amyloid PET are likely to be equivalent as screening tools for clinical trials, but the more dynamic nature of amyloid PET and the fact that amyloid tracer retention correlates directly with Aβ load (Ikonomovic et al. 2008) makes this a more suitable outcome measure when the goal is to detect changes in brain Aβ load. In support of this statement, the ability of amyloid PET to show an amyloid-lowering effect of passive immunotherapy in humans has already been reported (Rinne et al. 2010).
A unique strength of amyloid PET across the entire clinical spectrum is the regionally specific nature of the quantitative data. Although we often reduce imaging data to a single number (e.g., mean cortical retention), we must remember that a strength of any imaging technique is the wealth of regional information that is supplied. Although amyloid PET can quantify amyloid load throughout the brain, it is not clear what pool of brain Aβ is represented by changes in CSF Aβ. One study has suggested that CSF Aβ is most tightly correlated with amyloid retention in brain regions adjacent to CSF spaces (Grimmer et al. 2009).
The Limitations of Amyloid PET in AD
Major deterrents to the widespread use of amyloid PET remain cost and availability. Availability has been improved by the development of F-18-labeled agents that can be distributed to PET scanners not associated with a cyclotron. Cost remains an issue, especially where CSF measurement of Aβ42 can provide very similar information when the question is simply the presence or absence of brain Aβ deposition. Being an early event in the pathogenesis of AD, amyloid PET is not a good surrogate marker of progression during the clinical stage of the disease (Engler et al. 2006; Kadir et al. 2010). This role is filled much better by structural MRI and FDG PET (Jack et al. 2010). Similarly, amyloid imaging gives much more of a binary diagnostic readout than techniques such as MRI and FDG PET. That is, amyloid imaging has a certain specificity for the pathology of AD, but when that pathology is absent, a negative amyloid PET scan will be identical regardless of the non-AD etiology of the dementia. In contrast, MRI and FDG PET may give an indication of a frontotemporal or vascular pathology when an amyloid PET scan would be ambiguously negative in both cases. The threshold of sensitivity of amyloid PET has yet to be precisely determined, but it is clear that some level of amyloid deposition is histologically detectable prior to the in vivo signal becoming “positive” (Cairns et al. 2009).
SUMMARY
State-of-the-(Imaging)-Art
In this chapter we briefly reviewed the most commonly used imaging technologies: structural and functional MRI and FDG and amyloid PET. Other MRI techniques such as diffusion tensor imaging (DTI) and associated tractography technologies, arterial spin labeling measures of cerebral blood flow and PET tracers targeted at the cholinergic system, microglial activation and other tracers in development are also contributing to our basic understanding of AD. A particularly exciting pursuit is PET ligands targeting the other major AD pathologic hallmark, the neurofibrillary tangle. Biomarkers of tau have been a particular challenge because of the need to target binding to something other than the β-sheet fibril dominated by Aβ deposits and the relatively smaller total mass of tau deposits, but steady progress is being made to achieve sufficient ligand affinity and selectivity. It should be clear from the above discussions that no single imaging technique can provide all of the answers. Fortunately, the strengths and weakness of the available imaging technologies are largely complementary. This has led to a variety of “multi-modal” imaging studies in which several techniques are simultaneously or sequentially applied to the same subjects for the same period of time. These direct comparisons have contributed greatly to our understanding of AD and the strengths and limitations of each technique.
Looking to the Future: The Role of Imaging in the Treatment of AD
The search for therapies that can modify the course of AD—to slow, delay, or prevent it—is clearly our most important challenge. That search has in turn led to a search for imaging markers that can be used as outcomes in drug discovery and trials. The value of any imaging technology will ultimately be determined by its contribution to meeting the challenge of finding and using effective therapies. This value includes contributions toward diagnosis. The large variability, intrinsic to clinical outcomes in AD, means that studies relying purely on clinical measures are necessarily large and consequently very costly. Using clinical outcomes to power studies to establish meaningful disease-slowing effects may require complicated designs and thousands of subjects. A major aim in academia and industry has been to find biomarkers that could identify disease-slowing effects earlier and/or with significantly fewer subjects exposed to treatment. Imaging is being increasingly incorporated into trial designs to measure the effects of a therapy on fibrillary amyloid (with amyloid imaging) on atrophy (with MRI) and on metabolism (PET and fMRI).
As increasingly biologically active therapies are studied, so too have side effects increased. Imaging is emerging as a means of detecting potential adverse effects that can initially be clinically silent or go unrecognized because of a patient’s level of cognitive impairment and confusion (Salloway et al. 2009). Particularly with more biologically active therapies, regular monitoring, or so-called safety scans, are now a prerequisite in such trials.
The recognition that it may be necessary to intervene at a very early stage to effect disease modification has led to interest in “prevention” studies. Preclinical intervention studies, almost by definition, are difficult to power on clinical outcomes. Imaging and other biomarkers are likely to be needed to select subjects for these studies and to provide outcome measures that can assess whether therapies are having a disease-modifying effect that could potentially translate into a delay in clinical onset.
Footnotes
Editors: Dennis J. Selkoe, Eckhard Mandelkow, and David M. Holtzman
Additional Perspectives on The Biology of Alzheimer Disease available at www.perspectivesinmedicine.org
REFERENCES
*Reference is also in this collection.
- Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR, et al. 2008. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol 65: 1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Minoshima S, D’Amato CJ, Frey KA, Kuhl DA, Sima AA 1996. Fluoro-deoxyglucose positron emission tomography in diffuse Lewy body disease. Neurology 47: 462–466 [DOI] [PubMed] [Google Scholar]
- Alexander GE, Furey ML, Grady CL, Pietrini P, Brady DR, Mentis MJ, Schapiro MB 1997. Association of premorbid intellectual function with cerebral metabolism in Alzheimer’s disease: Implications for the cognitive reserve hypothesis. Am J Psychiatry 154: 165–172 [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL 2007. Disruption of large-scale brain systems in advanced aging. Neuron 56: 924–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaiz E, Jelic V, Almkvist O, Wahlund LO, Winblad B, Valind S, Nordberg A 2001. Impaired cerebral glucose metabolism and cognitive functioning predict deterioration in mild cognitive impairment. Neuroreport 12: 851–855 [DOI] [PubMed] [Google Scholar]
- Bacskai BJ, Frosch MP, Freeman SH, Raymond SB, Augustinack JC, Johnson KA, Irizarry MC, Klunk WE, Mathis CA, Dekosky ST, et al. 2007. Molecular imaging with Pittsburgh Compound B confirmed at autopsy: A case report. Arch Neurol 64: 431–434 [DOI] [PubMed] [Google Scholar]
- Bai F, Zhang Z, Yu H, Shi Y, Yuan Y, Zhu W, Zhang X, Qian Y 2008. Default-mode network activity distinguishes amnestic type mild cognitive impairment from healthy aging: A combined structural and resting-state functional MRI study. Neurosci Lett 438: 111–115 [DOI] [PubMed] [Google Scholar]
- Barber R, Ballard C, McKeith IG, Gholkar A, O’Brien JT 2000. MRI volumetric study of dementia with Lewy bodies: A comparison with AD and vascular dementia. Neurology 54: 1304–1309 [DOI] [PubMed] [Google Scholar]
- Barnes J, Bartlett JW, van de Pol LA, Loy CT, Scahill RI, Frost C, Thompson P, Fox NC 2009. A meta-analysis of hippocampal atrophy rates in Alzheimer’s disease. Neurobiol Aging 30: 1711–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, Hampel H 2003. CSF markers for incipient Alzheimer’s disease. Lancet Neurol 2: 605–613 [DOI] [PubMed] [Google Scholar]
- *.Blennow K, Zetterberg H, Fagan AM 2011. Fluid biomarkers in Alzheimer disease. Cold Spring Harb Perspect Med 10.1101/cshperspect.a006221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobinski M, de Leon MJ, Wegiel J, Desanti S, Convit A, Saint Louis LA, Rusinek H, Wisniewski HM 2000. The histological validation of post mortem magnetic resonance imaging-determined hippocampal volume in Alzheimer’s disease. Neuroscience 95: 721–725 [DOI] [PubMed] [Google Scholar]
- Bokde AL, Karmann M, Teipel SJ, Born C, Lieb M, Reiser MF, Moller HJ, Hampel H 2009. Decreased activation along the dorsal visual pathway after a 3-month treatment with galantamine in mild Alzheimer disease: A functional magnetic resonance imaging study. J Clin Psychopharmacol 29: 147–156 [DOI] [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, Brown GG 2005. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology 64: 501–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW 2000. Patterns of brain activation in people at risk for Alzheimer’s disease. New Engl J Med 343: 450–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghesani PR, Johnson LC, Shelton AL, Peskind ER, Aylward EH, Schellenberg GD, Cherrier MM 2007. Altered medial temporal lobe responses during visuospatial encoding in healthy APOE*4 carriers. Neurobiol Aging 29: 981–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E 1991. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol (Berl) 82: 239–259 [DOI] [PubMed] [Google Scholar]
- Buchhave P, Blennow K, Zetterberg H, Stomrud E, Londos E, Andreasen N, Minthon L, Hansson O 2009. Longitudinal study of CSF biomarkers in patients with Alzheimer’s disease. PLoS One 4: e6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, et al. 2005. Molecular, structural, and functional characterization of Alzheimer’s disease: Evidence for a relationship between default activity, amyloid, and memory. J Neurosci 25: 7709–7717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL 2008. The brain’s default network: Anatomy, function, and relevance to disease. Ann NY Acad Sci 1124: 1–38 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA 2009. Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci 29: 1860–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burack MA, Hartlein J, Flores HP, Taylor-Reinwald L, Perlmutter JS, Cairns NJ 2010. In vivo amyloid imaging in autopsy-confirmed Parkinson disease with dementia. Neurology 74: 77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton EJ, Barber R, Mukaetova-Ladinska EB, Robson J, Perry RH, Jaros E, Kalaria RN, O’Brien JT 2009. Medial temporal lobe atrophy on MRI differentiates Alzheimer’s disease from dementia with Lewy bodies and vascular cognitive impairment: A prospective study with pathological verification of diagnosis. Brain 132: 195–203 [DOI] [PubMed] [Google Scholar]
- Busche MA, Eichhoff G, Adelsberger H, Abramowski D, Wiederhold KH, Haass C, Staufenbiel M, Konnerth A, Garaschuk O 2008. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science 321: 1686–1689 [DOI] [PubMed] [Google Scholar]
- Cairns NJ, Ikonomovic MD, Benzinger T, Storandt M, Fagan AM, Shah A, Schmidt RE, Perry A, Reinwald LT, Carter D, et al. 2009. Absence of Pittsburgh Compound B detection of cerebral amyloid β in a patient with clinical, cognitive, and cerebrospinal fluid markers of Alzheimer disease. Arch Neurol 66: 1557–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Chao LL, Studholme C, Yaffe K, Miller BL, Madison C, Buckley ST, Mungas D, Schuff N, Weiner MW 2009. Brain atrophy associated with baseline and longitudinal measures of cognition. Neurobiol Aging 32: 572–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Chen K, Lee W, Alexander GE, Reiman EM 2008. Correlating cerebral hypometabolism with future memory decline in subsequent converters to amnestic pre-mild cognitive impairment. Arch Neurol 65: 1231–1236 [DOI] [PubMed] [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, DePeau K, Rentz DM, Selkoe DJ, Blacker D, et al. 2006. Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: An independent component analysis. J Neurosci 26: 10222–10231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan D, Fox NC, Scahill RI, Crum WR, Whitwell JL, Leschziner G, Rossor AM, Stevens JM, Cipolotti L, Rossor MN 2001. Patterns of temporal lobe atrophy in semantic dementia and Alzheimer’s disease. Ann Neurol 49: 433–442 [PubMed] [Google Scholar]
- Chan D, Janssen JC, Whitwell JL, Watt HC, Jenkins R, Frost C, Rossor MN, Fox NC 2003. Change in rates of cerebral atrophy over time in early-onset Alzheimer’s disease: Longitudinal MRI study. Lancet 362: 1121–1122 [DOI] [PubMed] [Google Scholar]
- Chen K, Langbaum JB, Fleisher AS, Ayutyanont N, Reschke C, Lee W, Liu X, Bandy D, Alexander GE, Thompson PM, et al. 2010. Twelve-month metabolic declines in probable Alzheimer’s disease and amnestic mild cognitive impairment assessed using an empirically pre-defined statistical region-of-interest: Findings from the Alzheimer’s Disease Neuroimaging Initiative. Neuroimage 51: 654–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetelat G, Desgranges B, de la Sayette V, Viader F, Eustache F, Baron JC 2003. Mild cognitive impairment: Can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology 60: 1374–1377 [DOI] [PubMed] [Google Scholar]
- Clark CM, Schneider JA, Bedell BJ, Beach TG, Bilker WB, Mintun MA, Pontecorvo MJ, Hefti F, Carpenter AP, Flitter ML, et al. 2011. AV45-A07 Study Group. Use of florbetapir-PET for imaging β-amyloid pathology. J Am Med Assoc 305: 275–283 Erratum in: J Am Med Assoc 305: 1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement F, Belleville S 2009. Test-retest reliability of fMRI verbal episodic memory paradigms in healthy older adults and in persons with mild cognitive impairment. Hum Brain Mapp 30: 4033–4047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AD, Price JC, Weissfeld LA, James J, Rosario BL, Bi W, Nebes RD, Saxton JA, Snitz BE, Aizenstein HA, et al. 2009. Basal cerebral metabolism may modulate the cognitive effects of Ab in mild cognitive impairment: An example of brain reserve. J Neurosci 29: 14770–14778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF 2006. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci 103: 13848–13853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA 2008. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex 18: 1856–1864 [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Cabeza R 2004. When less means more: Deactivations during encoding that predict subsequent memory. Neuroimage 23: 921–927 [DOI] [PubMed] [Google Scholar]
- DeCarli C, Frisoni GB, Clark CM, Harvey D, Grundman M, Petersen RC, Thal LJ, Jin S, Jack CR Jr, Scheltens P 2007. Qualitative estimates of medial temporal atrophy as a predictor of progression from mild cognitive impairment to dementia. Arch Neurol 64: 108–115 [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Ikonomovic MD, Styren SD, Beckett L, Wisniewski S, Bennett DA, Cochran EJ, Kordower JH, Mufson EJ 2002. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol 51: 145–155 [DOI] [PubMed] [Google Scholar]
- de Leon MJ, Convit A, Wolf OT, Tarshish CY, DeSanti S, Rusinek H, Tsui W, Kandil E, Scherer AJ, Roche A, et al. 2001. Prediction of cognitive decline in normal elderly subjects with 2-[(18)F]fluoro-2-deoxy-D-glucose/poitron-emission tomography (FDG/PET). Proc Natl Acad Sci 98: 10966–10971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santi S, de Leon MJ, Rusinek H, Convit A, Tarshish CY, Roche A, Tsui WH, Kandil E, Boppana M, Daisley K, et al. 2001. Hippocampal formation glucose metabolism and volume losses in MCI and AD. Neurobiol Aging 22: 529–539 [DOI] [PubMed] [Google Scholar]
- Devanand DP, Mikhno A, Pelton GH, Cuasay K, Pradhaban G, Kumar JS, Upton N, Lai R, Gunn RN, Libri V, et al. 2010. Pittsburgh Compound B (11C-PIB) and Fluorodeoxyglucose (18F-FDG) PET in patients with Alzheimer disease, mild cognitive impairment, and healthy controls. J Geriatr Psychiatry Neurol 23: 185–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Sperling RA 2008. Functional abnormalities of the medial temporal lobe memory system in mild cognitive impairment and Alzheimer’s disease: Insights from functional MRI studies. Neuropsychologia 46: 1624–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Goncharova I, Sullivan MP, Forchetti C, Wilson RS, Bennett DA, Beckett LA, deToledo-Morrell L 2001. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer’s disease. Neurobiol Aging 22: 747–754 [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, Dale AM, Stern CE, Blacker D, Albert MS, et al. 2004. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol 56: 27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Salat D, Greve D, Chua E, Rand-Giovannetti E, Rentz D, Bertram L, Mullin K, Tanzi R, Blacker D, et al. 2005. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology 65: 404–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzezga A, Grimmer T, Henriksen G, Stangier I, Perneczky R, Diehl-Schmid J, Mathis CA, Klunk WE, Price J, Dekosky S, et al. 2008. Imaging of amyloid plaques and cerebral glucose metabolism in semantic dementia and Alzheimer’s disease. Neuroimage 39: 619–633 [DOI] [PubMed] [Google Scholar]
- Drzezga A, Grimmer T, Henriksen G, Muhlau M, Perneczky R, Miederer I, Praus C, Sorg C, Wohlschlager A, Riemenschneider M, et al. 2009. Effect of APOE genotype on amyloid plaque load and gray matter volume in Alzheimer disease. Neurology 72: 1487–1494 [DOI] [PubMed] [Google Scholar]
- Duara R, Loewenstein DA, Potter E, Appel J, Greig MT, Urs R, Shen Q, Raj A, Small B, Barker W, et al. 2008. Medial temporal lobe atrophy on MRI scans and the diagnosis of Alzheimer disease. Neurology 71: 1986–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, et al. 2007. Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS-ADRDA criteria. Lancet Neurol 6: 734–746 [DOI] [PubMed] [Google Scholar]
- Edison P, Archer HA, Hinz R, Hammers A, Pavese N, Tai YF, Hotton G, Cutler D, Fox N, Kennedy A, et al. 2007. Amyloid, hypometabolism, and cognition in Alzheimer disease: An [11C]PIB and [18F]FDG PET study. Neurology 68: 501–508 [DOI] [PubMed] [Google Scholar]
- Edison P, Archer HA, Gerhard A, Hinz R, Pavese N, Turkheimer FE, Hammers A, Tai YF, Fox N, Kennedy A, et al. 2008. Microglia, amyloid, and cognition in Alzheimer’s disease: An [11C](R)PK11195-PET and [11C]PIB-PET study. Neurobiol Dis 32: 412–419 [DOI] [PubMed] [Google Scholar]
- Engler H, Forsberg A, Almkvist O, Blomquist G, Larsson E, Savitcheva I, Wall A, Ringheim A, Langstrom B, Nordberg A 2006. Two-year follow-up of amyloid deposition in patients with Alzheimer’s disease. Brain 129: 2856–2866 [DOI] [PubMed] [Google Scholar]
- Engler H, Santillo AF, Wang SX, Lindau M, Savitcheva I, Nordberg A, Lannfelt L, Langstrom B, Kilander L 2008. In vivo amyloid imaging with PET in frontotemporal dementia. Eur J Nucl Med Mol Imaging 35: 100–106 [DOI] [PubMed] [Google Scholar]
- Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM 2007. Cerebrospinal fluid tau/β-amyloid42 ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol 64: 343–349 [DOI] [PubMed] [Google Scholar]
- Fagan AM, Mintun MA, Shah AR, Aldea P, Roe CM, Mach RH, Marcus D, Morris JC, Holtzman DM 2009. Cerebrospinal fluid tau and ptau181 increase with cortical amyloid deposition in cognitively normal individuals: Implications for future clinical trials of Alzheimer’s disease. EMBO Mol Med 1: 371–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE 2009. Distinct patterns of brain activity in young carriers of the APOE-ε4 allele. Proc Natl Acad Sci 106: 7209–7214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher AS, Houston WS, Eyler LT, Frye S, Jenkins C, Thal LJ, Bondi MW 2005. Identification of Alzheimer disease risk by functional magnetic resonance imaging. Arch Neurol 62: 1881–1888 [DOI] [PubMed] [Google Scholar]
- Fleisher AS, Sherzai A, Taylor C, Langbaum JB, Chen K, Buxton RB 2009. Resting-state BOLD networks versus task-associated functional MRI for distinguishing Alzheimer’s disease risk groups. Neuroimage 47: 1678–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg A, Engler H, Almkvist O, Blomquist G, Hagman G, Wall A, Ringheim A, Langstrom B, Nordberg A 2008. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol Aging 29: 1456–1465 [DOI] [PubMed] [Google Scholar]
- Forsberg A, Almkvist O, Engler H, Wall A, Langstrom B, Nordberg A 2010. High PIB retention in Alzheimer’s disease is an early event with complex relationship with CSF biomarkers and functional parameters. Curr Alzheimer Res 7: 56–66 [DOI] [PubMed] [Google Scholar]
- Foster NL, Chase TN, Fedio P, Patronas NJ, Brooks RA, Di Chiro G 1983. Alzheimer’s disease: Focal cortical changes shown by positron emission tomography. Neurology 33: 961–965 [DOI] [PubMed] [Google Scholar]
- Foster NL, Heidebrink JL, Clark CM, Jagust WJ, Arnold SE, Barbas NR, DeCarli CS, Turner RS, Koeppe RA, Higdon R, et al. 2007. FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer’s disease. Brain 130: 2616–2635 [DOI] [PubMed] [Google Scholar]
- Fox NC, Warrington EK, Rossor MN 1999a. Serial magnetic resonance imaging of cerebral atrophy in preclinical Alzheimer’s disease. Lancet 353: 2125. [DOI] [PubMed] [Google Scholar]
- Fox NC, Scahill RI, Crum WR, Rossor MN 1999b. Correlation between rates of brain atrophy and cognitive decline in AD. Neurology 52: 1687–1689 [DOI] [PubMed] [Google Scholar]
- Fox NC, Cousens S, Scahill R, Harvey RJ, Rossor MN 2000. Using serial registered brain magnetic resonance imaging to measure disease progression in Alzheimer disease: Power calculations and estimates of sample size to detect treatment effects. Arch Neurol 57: 339–344 [DOI] [PubMed] [Google Scholar]
- Fox NC, Black RS, Gilman S, Rossor MN, Griffith SG, Jenkins L, Koller M 2005a. Effects of Aβ immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology 64: 1563–1572 [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME 2005b. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci 102: 9673–9678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisoni GB, Fox NC, Jack CR Jr, Scheltens P, Thompson PM 2010. The clinical use of structural MRI in Alzheimer disease. Nat Rev Neurol 6: 67–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furst AJ, Rabinovici GD, Rostomian AH, Steed T, Alkalay A, Racine C, Miller BL, Jagust WJ 2010. Cognition, glucose metabolism and amyloid burden in Alzheimer’s disease. Neurobiol Aging (in press) 10.1016/j.neurobiolaging.2010.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguli M, Dodge HH, Shen C, DeKosky ST 2004. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology 63: 115–121 [DOI] [PubMed] [Google Scholar]
- Goekoop R, Rombouts SA, Jonker C, Hibbel A, Knol DL, Truyen L, Barkhof F, Scheltens P 2004. Challenging the cholinergic system in mild cognitive impairment: A pharmacological fMRI study. Neuroimage 23: 1450–1459 [DOI] [PubMed] [Google Scholar]
- Golby A, Silverberg G, Race E, Gabrieli S, O’Shea J, Knierim K, Stebbins G, Gabrieli J 2005. Memory encoding in Alzheimer’s disease: An fMRI study of explicit and implicit memory. Brain 128: 773–787 [DOI] [PubMed] [Google Scholar]
- Gosche KM, Mortimer JA, Smith CD, Markesbery WR, Snowdon DA 2002. Hippocampal volume as an index of Alzheimer neuropathology: Findings from the Nun Study. Neurology 58: 1476–1482 [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Beig S, Keightley ML, Burian H, Black SE 2003. Evidence from functional neuroimaging of a compensatory prefrontal network in Alzheimer’s disease. J Neurosci 23: 986–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V 2004. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proc Natl Acad Sci 101: 4637–4642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimmer T, Riemenschneider M, Forstl H, Henriksen G, Klunk WE, Mathis CA, Shiga T, Wester HJ, Kurz A, Drzezga A 2009. Beta amyloid in Alzheimer’s Disease: Increased deposition in brain is reflected in reduced concentration in cerebrospinal fluid. Biol Psychiatry 65: 927–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gron G, Bittner D, Schmitz B, Wunderlich AP, Riepe MW 2002. Subjective memory complaints: Objective neural markers in patients with Alzheimer’s disease and major depressive disorder. Ann Neurol 51: 491–498 [DOI] [PubMed] [Google Scholar]
- Hamalainen A, Pihlajamaki M, Tanila H, Hanninen T, Niskanen E, Tervo S, Karjalainen PA, Vanninen RL, Soininen H 2006. Increased fMRI responses during encoding in mild cognitive impairment. Neurobiol Aging 28: 1889–1903 [DOI] [PubMed] [Google Scholar]
- Hamalainen A, Pihlajamaki M, Tanila H, Hanninen T, Niskanen E, Tervo S, Karjalainen PA, Vanninen RL, Soininen H 2007. Increased fMRI responses during encoding in mild cognitive impairment. Neurobiol Aging 28: 1889–1903 [DOI] [PubMed] [Google Scholar]
- Han SD, Houston WS, Jak AJ, Eyler LT, Nagel BJ, Fleisher AS, Brown GG, Corey-Bloom J, Salmon DP, Thal LJ, et al. 2007. Verbal paired-associate learning by APOE genotype in non-demented older adults: fMRI evidence of a right hemispheric compensatory response. Neurobiol Aging 28: 238–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L 2006. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: A follow-up study. Lancet Neurol 5: 228–234 [DOI] [PubMed] [Google Scholar]
- Hedden T, Van Dijk KR, Becker JA, Mehta A, Sperling RA, Johnson KA, Buckner RL 2009. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci 29: 12686–12694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heun R, Freymann K, Erb M, Leube DT, Jessen F, Kircher TT, Grodd W 2007. Mild cognitive impairment (MCI) and actual retrieval performance affect cerebral activation in the elderly. Neurobiol Aging 28: 404–413 [DOI] [PubMed] [Google Scholar]
- Hoffman JM, Welsh-Bohmer KA, Hanson M, Crain B, Hulette C, Earl N, Coleman RE 2000. FDG PET imaging in patients with pathologically verified dementia. J Nucl Med 41: 1920–1928 [PubMed] [Google Scholar]
- Hua X, Leow AD, Parikshak N, Lee S, Chiang MC, Toga AW, Jack CR Jr, Weiner MW, Thompson PM 2008. Tensor-based morphometry as a neuroimaging biomarker for Alzheimer’s disease: An MRI study of 676 AD, MCI, and normal subjects. Neuroimage 43: 458–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez V, Pietrini P, Alexander GE, Furey ML, Teichberg D, Rajapakse JC, Rapoport SI, Schapiro MB, Horwitz B 1998. Regional glucose metabolic abnormalities are not the result of atrophy in Alzheimer’s disease. Neurology 50: 1585–1593 [DOI] [PubMed] [Google Scholar]
- Ikonomovic MD, Klunk WE, Abrahamson EE, Mathis CA, Price JC, Tsopelas ND, Lopresti BJ, Ziolko S, Bi W, Paljug WR, et al. 2008. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain 131: 1630–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr, Dickson DW, Parisi JE, Xu YC, Cha RH, O’Brien PC, Edland SD, Smith GE, Boeve BF, Tangalos EG, et al. 2002. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology 58: 750–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr, Shiung MM, Gunter JL, O’Brien PC, Weigand SD, Knopman DS, Boeve BF, Ivnik RJ, Smith GE, Cha RH, et al. 2004. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology 62: 591–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr, Petersen RC, Grundman M, Jin S, Gamst A, Ward CP, Sencakova D, Doody RS, Thal LJ 2008a. Longitudinal MRI findings from the vitamin E and donepezil treatment study for MCI. Neurobiol Aging 29: 1285–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr, Weigand SD, Shiung MM, Przybelski SA, O’Brien PC, Gunter JL, Knopman DS, Boeve BF, Smith GE, Petersen RC 2008b. Atrophy rates accelerate in amnestic mild cognitive impairment. Neurology 70: 1740–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr, Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, Shiung MM, Gunter JL, Boeve BF, Kemp BJ, et al. 2009. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: Implications for sequence of pathological events in Alzheimer’s disease. Brain 132: 1355–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ 2010. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 9: 119–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust WJ, Eberling JL, Wu CC, Finkbeiner A, Mungas D, Valk PE, Haan MN 2002. Brain function and cognition in a community sample of elderly Latinos. Neurology 59: 378–383 [DOI] [PubMed] [Google Scholar]
- Jagust W, Gitcho A, Sun F, Kuczynski B, Mungas D, Haan M 2006. Brain imaging evidence of preclinical Alzheimer’s disease in normal aging. Ann Neurol 59: 673–681 [DOI] [PubMed] [Google Scholar]
- Jagust W, Reed B, Mungas D, Ellis W, Decarli C 2007. What does fluorodeoxyglucose PET imaging add to a clinical diagnosis of dementia? Neurology 69: 871–877 [DOI] [PubMed] [Google Scholar]
- Jagust WJ, Landau SM, Shaw LM, Trojanowski JQ, Koeppe RA, Reiman EM, Foster NL, Petersen RC, Weiner MW, Price JC, et al. 2009. Relationships between biomarkers in aging and dementia. Neurology 73: 1193–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust WJ, Bandy D, Chen K, Foster NL, Landau SM, Mathis CA, Price JC, Reiman EM, Skovronsky D, Koeppe RA 2010. The Alzheimer’s Disease neuroimaging initiative positron emission tomography core. Alzheimers Dement 6: 221–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Moritz CH, Meyerand ME, Rowley HA, Alexander AL, Hansen KW, Gleason CE, Carlsson CM, Ries ML, et al. 2006. Activation of brain regions vulnerable to Alzheimer’s disease: The effect of mild cognitive impairment. Neurobiol Aging 27: 1604–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Gregas M, Becker JA, Kinnecom C, Salat DH, Moran EK, Smith EE, Rosand J, Rentz DM, Klunk WE, et al. 2007. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol 62: 229–234 [DOI] [PubMed] [Google Scholar]
- Kadir A, Andreasen N, Almkvist O, Wall A, Forsberg A, Engler H, Hagman G, Larksater M, Winblad B, Zetterberg H, et al. 2008. Effect of phenserine treatment on brain functional activity and amyloid in Alzheimer’s disease. Ann Neurol 63: 621–631 [DOI] [PubMed] [Google Scholar]
- Kadir A, Almkvist O, Forsberg A, Wall A, Engler H, Langstrom B, Nordberg A 2010. Dynamic changes in PET amyloid and FDG imaging at different stages of Alzheimer’s disease. Neurobiol Aging (in press) 10.1016/j.neurobiolaging.2010.06.015 [DOI] [PubMed] [Google Scholar]
- Karow DS, McEvoy LK, Fennema-Notestine C, Hagler DJ Jr, Jennings RG, Brewer JB, Hoh CK, Dale AM 2010. Relative capability of MR imaging and FDG PET to depict changes associated with prodromal and early Alzheimer disease. Radiology 256: 932–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Knopman D, Liu H 2001. Dissociation of regional activation in mild AD during visual encoding: A functional MRI study. Neurology 57: 812–816 [DOI] [PubMed] [Google Scholar]
- Kemppainen NM, Aalto S, Wilson IA, Nagren K, Helin S, Bruck A, Oikonen V, Kailajarvi M, Scheinin M, Viitanen M, et al. 2006. Voxel-based analysis of PET amyloid ligand [11C]PIB uptake in Alzheimer disease. Neurology 67: 1575–1580 [DOI] [PubMed] [Google Scholar]
- Kemppainen NM, Aalto S, Karrasch M, Nagren K, Savisto N, Oikonen V, Viitanen M, Parkkola R, Rinne JO 2008. Cognitive reserve hypothesis: Pittsburgh Compound B and fluorodeoxyglucose positron emission tomography in relation to education in mild Alzheimer’s disease. Ann Neurol 63: 112–118 [DOI] [PubMed] [Google Scholar]
- Killiany RJ, Hyman BT, Gomez-Isla T, Moss MB, Kikinis R, Jolesz F, Tanzi R, Jones K, Albert MS 2002. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology 58: 1188–1196 [DOI] [PubMed] [Google Scholar]
- Kircher TT, Weis S, Freymann K, Erb M, Jessen F, Grodd W, Heun R, Leube DT 2007. Hippocampal activation in patients with mild cognitive impairment is necessary for successful memory encoding. J Neurol Neurosurg Psychiatry 78: 812–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloppel S, Stonnington CM, Chu C, Draganski B, Scahill RI, Rohrer JD, Fox NC, Jack CR Jr, Ashburner J, Frackowiak RS 2008. Automatic classification of MR scans in Alzheimer’s disease. Brain 131: 681–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk WE, Wang Y, Huang GF, Debnath ML, Holt DP, Shao L, Hamilton RL, Ikonomovic MD, DeKosky ST, Mathis CA 2003. The binding of 2-(4′-methylaminophenyl)benzothiazole to postmortem brain homogenates is dominated by the amyloid component. J Neurosci 23: 2086–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergström M, Savitcheva I, Huang GF, Estrada S, et al. 2004. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol 55: 306–319 [DOI] [PubMed] [Google Scholar]
- Knopman DS, DeKosky ST, Cummings JL, Chui H, Corey-Bloom J, Relkin N, Small GW, Miller B, Stevens JC 2001. Practice parameter: Diagnosis of dementia (an evidence-based review). Report of the quality standards subcommittee of the American Academy of Neurology. Neurology 56: 1143–1153 [DOI] [PubMed] [Google Scholar]
- Koch W, Teipel S, Mueller S, Benninghoff J, Wagner M, Bokde AL, Hampel H, Coates U, Reiser M, Meindl T 2010. Diagnostic power of default mode network resting state fMRI in the detection of Alzheimer’s disease. Neurobiol Aging (in press) 10.1016/j.neurobiolaging2010.04.013 [DOI] [PubMed] [Google Scholar]
- Koivunen J, Pirttila T, Kemppainen N, Aalto S, Herukka SK, Jauhianen AM, Hanninen T, Hallikainen M, Nagren K, Rinne JO, et al. 2008. PET amyloid ligand [11C]PIB uptake and cerebrospinal fluid β-amyloid in mild cognitive impairment. Dement Geriatr Cogn Disord 26: 378–383 [DOI] [PubMed] [Google Scholar]
- Korf ES, Wahlund LO, Visser PJ, Scheltens P 2004. Medial temporal lobe atrophy on MRI predicts dementia in patients with mild cognitive impairment. Neurology 63: 94–100 [DOI] [PubMed] [Google Scholar]
- Kukolja J, Thiel CM, Fink GR 2009. Cholinergic stimulation enhances neural activity associated with encoding but reduces neural activity associated with retrieval in humans. J Neurosci 29: 8119–8128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R, et al. 1992. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci 89: 5675–5679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Harvey D, Madison CM, Reiman EM, Foster NL, Aisen PS, Petersen RC, Shaw LM, Trojanowski JQ, Jack CR Jr, et al. 2010. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology 75: 230–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langbaum JB, Chen K, Lee W, Reschke C, Bandy D, Fleisher AS, Alexander GE, Foster NL, Weiner MW, Koeppe RA, et al. 2009. Categorical and correlational analyses of baseline fluorodeoxyglucose positron emission tomography images from the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Neuroimage 45: 1107–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrieu S, Letenneur L, Orgogozo JM, Fabrigoule C, Amieva H, Le Carret N, Barberger-Gateau P, Dartigues JF 2002. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology 59: 1594–1599 [DOI] [PubMed] [Google Scholar]
- Lehericy S, Baulac M, Chiras J, Pierot L, Martin N, Pillon B, Deweer B, Dubois B, Marsault C 1994. Amygdalohippocampal MR volume measurements in the early stages of Alzheimer disease. Am J Neuroradiol 15: 929–937 [PMC free article] [PubMed] [Google Scholar]
- Lenzi D, Serra L, Perri R, Pantano P, Lenzi GL, Paulesu E, Caltagirone C, Bozzali M, Macaluso E 2009. Single domain amnestic MCI: A multiple cognitive domains fMRI investigation. Neurobiol Aging 32: 1542–1557 [DOI] [PubMed] [Google Scholar]
- Lerch JP, Pruessner JC, Zijdenbos A, Hampel H, Teipel SJ, Evans AC 2005. Focal decline of cortical thickness in Alzheimer’s disease identified by computational neuroanatomy. Cereb Cortex 15: 995–1001 [DOI] [PubMed] [Google Scholar]
- Likeman M, Anderson VM, Stevens JM, Waldman AD, Godbolt AK, Frost C, Rossor MN, Fox NC 2005. Visual assessment of atrophy on magnetic resonance imaging in the diagnosis of pathologically confirmed young-onset dementias. Arch Neurol 62: 1410–1415 [DOI] [PubMed] [Google Scholar]
- Lind J, Ingvar M, Persson J, Sleegers K, Van Broeckhoven C, Adolfsson R, Nilsson LG, Nyberg L 2006a. Parietal cortex activation predicts memory decline in apolipoprotein E-ε4 carriers. Neuroreport 17: 1683–1686 [DOI] [PubMed] [Google Scholar]
- Lind J, Larsson A, Persson J, Ingvar M, Nilsson LG, Backman L, Adolfsson R, Cruts M, Sleegers K, Van Broeckhoven C, et al. 2006b. Reduced hippocampal volume in non-demented carriers of the apolipoprotein E ε4: Relation to chronological age and recognition memory. Neurosci Lett 396: 23–27 [DOI] [PubMed] [Google Scholar]
- Lockhart A, Lamb JR, Osredkar T, Sue LI, Joyce JN, Ye L, Libri V, Leppert D, Beach TG 2007. PIB is a non-specific imaging marker of amyloid-β (Aβ) peptide-related cerebral amyloidosis. Brain 130: 2607–2615 [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A 2001. Neurophysiological investigation of the basis of the fMRI signal. Nature 412: 150–157 [DOI] [PubMed] [Google Scholar]
- Lowe VJ, Kemp BJ, Jack CR Jr, Senjem M, Weigand S, Shiung M, Smith G, Knopman D, Boeve B, Mullan B, et al. 2009. Comparison of 18F-FDG and PiB PET in cognitive impairment. J Nucl Med 50: 878–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C, Buckner RL 2004. Preserved neural correlates of priming in old age and dementia. Neuron 42: 865–875 [DOI] [PubMed] [Google Scholar]
- Machulda MM, Ward HA, Borowski B, Gunter JL, Cha RH, O’Brien PC, Petersen RC, Boeve BF, Knopman D, Tang-Wai DF, et al. 2003. Comparison of memory fMRI response among normal, MCI, and Alzheimer’s patients. Neurology 61: 500–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maetzler W, Liepelt I, Reimold M, Reischl G, Solbach C, Becker C, Schulte C, Leyhe T, Keller S, Melms A, et al. 2009. Cortical PIB binding in Lewy body disease is associated with Alzheimer-like characteristics. Neurobiol Dis 34: 107–112 [DOI] [PubMed] [Google Scholar]
- Magistretti PJ 2006. Neuron-glia metabolic coupling and plasticity. J Exp Biol 209: 2304–2311 [DOI] [PubMed] [Google Scholar]
- Masliah E, Alford M, Adame A, Rockenstein E, Galasko D, Salmon D, Hansen LA, Thal LJ 2003. Aβ1–42 promotes cholinergic sprouting in patients with AD and Lewy body variant of AD. Neurology 61: 206–211 [DOI] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, et al. 2005. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium. Neurology 65: 1863–1872 [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Zubieta JK, Brandt J, Tune LE, Mayberg HS, Frost JJ 1996. Regional hypometabolism in Alzheimer’s disease as measured by positron emission tomography after correction for effects of partial volume averaging. Neurology 47: 454–461 [DOI] [PubMed] [Google Scholar]
- Miller SL, Fenstermacher E, Bates J, Blacker D, Sperling RA, Dickerson BC 2008a. Hippocampal activation in adults with mild cognitive impairment predicts subsequent cognitive decline. J Neurol Neurosurg Psychiatry 79: 630–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamaki M, Sperling RA 2008b. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci 105: 2181–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE 1997. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol 42: 85–94 [DOI] [PubMed] [Google Scholar]
- Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC, et al. 2006. [11C]PIB in a nondemented population: Potential antecedent marker of Alzheimer disease. Neurology 67: 446–52 [DOI] [PubMed] [Google Scholar]
- Misra C, Fan Y, Davatzikos C 2009. Baseline and longitudinal patterns of brain atrophy in MCI patients, and their use in prediction of short-term conversion to AD: Results from ADNI. Neuroimage 44: 1415–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AJ, Shiri-Feshki M 2009. Rate of progression of mild cognitive impairment to dementia—Meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand 119: 252–265 [DOI] [PubMed] [Google Scholar]
- Mondadori CR, de Quervain DJ, Buchmann A, Mustovic H, Wollmer MA, Schmidt CF, Boesiger P, Hock C, Nitsch RM, Papassotiropoulos A, et al. 2007. Better memory and neural efficiency in young apolipoprotein E ε4 carriers. Cereb Cortex 17: 1934–1947 [DOI] [PubMed] [Google Scholar]
- Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, Mintun MA 2010. APOE predicts amyloid-β but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol 67: 122–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Tsui WH, Herholz K, Pupi A, Drzezga A, Lucignani G, Reiman EM, Holthoff V, Kalbe E, Sorbi S, et al. 2008. Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer’s disease, and other dementias. J Nucl Med 49: 390–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Mistur R, Switalski R, Brys M, Glodzik L, Rich K, Pirraglia E, Tsui W, De Santi S, de Leon MJ 2009. Declining brain glucose metabolism in normal individuals with a maternal history of Alzheimer disease. Neurology 72: 513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Rinne JO, Tsui WH, Berti V, Li Y, Wang H, Murray J, Scheinin N, Nagren K, Williams S, et al. 2010. Increased fibrillar amyloid-β burden in normal individuals with a family history of late-onset Alzheimer’s. Proc Natl Acad Sci 107: 5949–5954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Mucke L, Selkoe DJ 2011. Neurotoxicity of amyloid β–protein: Synaptic and network dysfunction. Cold Spring Harb Perspect Med 10.1101/cshperspect.a006338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen N, Van Laere K, Thurfjell L, Owenius R, Vandenbulcke M, Koole M, Bormans G, Brooks DJ, Vandenberghe R 2009. Phase 1 study of the Pittsburgh Compound B derivative 18F-Flutemetamol in healthy volunteers and patients with probable Alzheimer disease. J Nucl Med 50: 1251–1259 [DOI] [PubMed] [Google Scholar]
- O’Brien JL, O’Keefe KM, LaViolette PS, DeLuca AN, Blacker D, Dickerson BC, Sperling RA 2010. Longitudinal fMRI in elderly reveals loss of hippocampal activation with clinical decline. Neurology 74: 1969–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Nayak AS, Glynn P 1990. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med 14: 68–78 [DOI] [PubMed] [Google Scholar]
- Okello A, Koivunen J, Edison P, Archer HA, Turkheimer FE, Nagren K, Bullock R, Walker Z, Kennedy A, Fox NC, et al. 2009. Conversion of amyloid positive and negative MCI to AD over 3 years: An 11C-PIB PET study. Neurology 73: 754–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, et al. 2007. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron 55: 697–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC 2004. Mild cognitive impairment as a diagnostic entity. J Intern Med 256: 183–194 [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos E, Kokmen E 1999. Mild cognitive impairment. Clinical characterization and outcome. Arch Neurol 56: 303–308 [DOI] [PubMed] [Google Scholar]
- Petrella J, Krishnan S, Slavin M, Tran T-T, Murty L, Doraiswamy P 2006. Mild cognitive impairment: Evaluation with 4-T functional MR imaging. Radiology 240: 177–186 [DOI] [PubMed] [Google Scholar]
- Petrella JR, Prince SE, Wang L, Hellegers C, Doraiswamy PM 2007a. Prognostic value of posteromedial cortex deactivation in mild cognitive impairment. PLoS One 2: e1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrella JR, Wang L, Krishnan S, Slavin MJ, Prince SE, Tran TT, Doraiswamy PM 2007b. Cortical deactivation in mild cognitive impairment: High-field-strength functional MR imaging. Radiology 245: 224–235 [DOI] [PubMed] [Google Scholar]
- Petrie EC, Cross DJ, Galasko D, Schellenberg GD, Raskind MA, Peskind ER, Minoshima S 2009. Preclinical evidence of Alzheimer changes: Convergent cerebrospinal fluid biomarker and fluorodeoxyglucose positron emission tomography findings. Arch Neurol 66: 632–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajamaki M, Depeau KM, Blacker D, Sperling RA 2008. Impaired medial temporal repetition suppression is related to failure of parietal deactivation in Alzheimer disease. Am J Geriatr Psychiatry 16: 283–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajamaki M, O’Keefe K, Bertram L, Tanzi R, Dickerson B, Blacker D, Albert M, Sperling R 2009. Evidence of altered posteromedial cortical fMRI activity in subjects at risk for Alzheimer disease. Alzheimer Dis Assoc Disord 24: 28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putcha D, O’Keefe K, LaViolette P, O’Brien J, Greve D, Rentz D, Locascio JJ, Atri A, Sperling R 2010. Reliability of fMRI associative encoding memory paradigm in non-demented elderly adults. Human Brain Mapping (in press) 10.1002/hbm.21166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovici GD, Furst AJ, O’Neil JP, Racine CA, Mormino EC, Baker SL, Chetty S, Patel P, Pagliaro TA, Klunk WE, et al. 2007. 11C-PIB PET imaging in Alzheimer disease and frontotemporal lobar degeneration. Neurology 68: 1205–1212 [DOI] [PubMed] [Google Scholar]
- Rabinovici GD, Jagust WJ, Furst AJ, Ogar JM, Racine CA, Mormino EC, O’Neil JP, Lal RA, Dronkers NF, Miller BL, et al. 2008. Aβ amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol 64: 388–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovici GD, Furst AJ, Alkalay A, Racine CA, O’Neil JP, Janabi M, Baker SL, Agarwal N, Bonasera SJ, Mormino EC, et al. 2010. Increased metabolic vulnerability in early-onset Alzheimer’s disease is not related to amyloid burden. Brain 133: 512–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL 2001. A default mode of brain function. Proc Natl Acad Sci 98: 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D 1996. Preclinical evidence of Alzheimer’s disease in persons homozygous for the ε4 allele for apolipoprotein E. New Engl J Med 334: 752–758 [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J 2005. Correlations between apolipoprotein E ε4 gene dose and brain-imaging measurements of regional hypometabolism. Proc Natl Acad Sci 102: 8299–8302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Caselli RJ, Alexander GE, Bandy D, Adamson JL, Lee W, Cannon A, Stephan EA, Stephan DA, et al. 2008. Cholesterol-related genetic risk scores are associated with hypometabolism in Alzheimer’s-affected brain regions. Neuroimage 40: 1214–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Langbaum JB, Lee W, Reschke C, Bandy D, Alexander GE, Caselli RJ 2010. Higher serum total cholesterol levels in late middle age are associated with glucose hypometabolism in brain regions affected by Alzheimer’s disease and normal aging. Neuroimage 49: 169–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy F, Mirrashed F, Campbell B, Richter W 2004. Mental calculation impairment in Alzheimer’s disease: A functional magnetic resonance imaging study. Neurosci Lett 358: 25–28 [DOI] [PubMed] [Google Scholar]
- Rentz DM, Locascio JJ, Becker JA, Moran EK, Eng E, Buckner RL, Sperling RA, Johnson KA 2010. Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol 67: 353–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridha BH, Barnes J, Bartlett JW, Godbolt A, Pepple T, Rossor MN, Fox NC 2006. Tracking atrophy progression in familial Alzheimer’s disease: A serial MRI study. Lancet Neurol 5: 828–834 [DOI] [PubMed] [Google Scholar]
- Ridha BH, Anderson VM, Barnes J, Boyes RG, Price SL, Rossor MN, Whitwell JL, Jenkins L, Black RS, Grundman M, et al. 2008. Volumetric MRI and cognitive measures in Alzheimer disease: Comparison of markers of progression. J Neurol 255: 567–574 [DOI] [PubMed] [Google Scholar]
- Ringman JM, Medina LD, Braskie M, Rodriguez-Agudelo Y, Geschwind DH, Macias-Islas MA, Cummings JL, Bookheimer S 2010. Effects of risk genes on BOLD activation in presymptomatic carriers of familial Alzheimer’s disease mutations during a novelty encoding task. Cereb Cortex 21: 877–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne JO, Brooks DJ, Rossor MN, Fox NC, Bullock R, Klunk WE, Mathis CA, Blennow K, Barakos J, Okello AA, et al. 2010. (11)C-PiB PET assessment of change in fibrillar amyloid-β load in patients with Alzheimer’s disease treated with bapineuzumab: A phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol 9: 363–372 [DOI] [PubMed] [Google Scholar]
- Ritchie K, Artero S, Touchon J 2001. Classification criteria for mild cognitive impairment: A population-based validation study. Neurology 56: 37–42 [DOI] [PubMed] [Google Scholar]
- Rocher AB, Chapon F, Blaizot X, Baron JC, Chavoix C 2003. Resting-state brain glucose utilization as measured by PET is directly related to regional synaptophysin levels: A study in baboons. Neuroimage 20: 1894–1898 [DOI] [PubMed] [Google Scholar]
- Roe CM, Mintun MA, D’Angelo G, Xiong C, Grant EA, Morris JC 2008. Alzheimer disease and cognitive reserve: Variation of education effect with carbon 11-labeled Pittsburgh Compound B uptake. Arch Neurol 65: 1467–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe CM, Mintun MA, Ghoshal N, Williams MM, Grant EA, Marcus DS, Morris JC 2010. Alzheimer disease identification using amyloid imaging and reserve variables: Proof of concept. Neurology 75: 42–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts SA, Barkhof F, Veltman DJ, Machielsen WC, Witter MP, Bierlaagh MA, Lazeron RH, Valk J, Scheltens P 2000. Functional MR imaging in Alzheimer’s disease during memory encoding. Am J Neuroradiol 21: 1869–1875 [PMC free article] [PubMed] [Google Scholar]
- Rombouts SA, Barkhof F, Van Meel CS, Scheltens P 2002. Alterations in brain activation during cholinergic enhancement with rivastigmine in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 73: 665–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts SA, Barkhof F, Goekoop R, Stam CJ, Scheltens P 2005. Altered resting state networks in mild cognitive impairment and mild Alzheimer’s disease: An fMRI study. Hum Brain Mapp 26: 231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts SA, Damoiseaux JS, Goekoop R, Barkhof F, Scheltens P, Smith SM, Beckmann CF 2009. Model-free group analysis shows altered BOLD FMRI networks in dementia. Hum Brain Mapp 30: 256–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe CC, Ackerman U, Browne W, Mulligan R, Pike KL, O’Keefe G, Tochon-Danguy H, Chan G, Berlangieri SU, Jones G, et al. 2008. Imaging of amyloid-β in Alzheimer’s disease with (18)F-BAY94–9172, a novel PET tracer: Proof of mechanism. Lancet Neurol 7: 129–135 [DOI] [PubMed] [Google Scholar]
- Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, Fripp J, Tochon-Danguy H, Morandeau L, O’Keefe G, et al. 2010. Amyloid imaging results from the Australian imaging, biomarkers and lifestyle (AIBL) study of aging. Neurobiol Aging 31: 1275–1283 [DOI] [PubMed] [Google Scholar]
- Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, Sabbagh M, Honig LS, Doody R, van Dyck CH, et al. 2009. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology 73: 2061–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C 2009. Age, neuropathology, and dementia. New Engl J Med 360: 2302–2309 [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Wishart HA, Rabin LA, Flashman LA, McHugh TL, Mamourian AC, Santulli RB 2004. Cholinergic enhancement of frontal lobe activity in mild cognitive impairment. Brain 127: 1574–1583 [DOI] [PubMed] [Google Scholar]
- Scahill RI, Schott JM, Stevens JM, Rossor MN, Fox NC 2002. Mapping the evolution of regional atrophy in Alzheimer’s disease: Unbiased analysis of fluid-registered serial MRI. Proc Natl Acad Sci 99: 4703–4707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheltens P, Leys D, Barkhof F, Huglo D, Weinstein HC, Vermersch P, Kuiper M, Steinling M, Wolters EC, Valk J 1992. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: Diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry 55: 967–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheltens P, Fox N, Barkhof F, De Carli C 2002. Structural magnetic resonance imaging in the practical assessment of dementia: Beyond exclusion. Lancet Neurol 1: 13–21 [DOI] [PubMed] [Google Scholar]
- Schuff N, Woerner N, Boreta L, Kornfield T, Shaw LM, Trojanowski JQ, Thompson PM, Jack CR Jr, Weiner MW 2009. MRI of hippocampal volume loss in early Alzheimer’s disease in relation to ApoE genotype and biomarkers. Brain 132: 1067–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz WJ, Smith CB, Davidsen L, Savaki H, Sokoloff L, Mata M, Fink DJ, Gainer H 1979. Metabolic mapping of functional activity in the hypothalamo-neurohypophysial system of the rat. Science 205: 723–725 [DOI] [PubMed] [Google Scholar]
- *.Selkoe D, Mandelkow E, Holtzman D 2011. Deciphering Alzheimer disease. Cold Spring Harb Perspect Med 10.1101/cshperspect.a011460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks MF, McGeown WJ, Forbes-McKay KE, Waiter GD, Ries M, Venneri A 2007. Regional brain activity after prolonged cholinergic enhancement in early Alzheimer’s disease. Magn Reson Imaging 25: 848–859 [DOI] [PubMed] [Google Scholar]
- Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, Mintun MA 2009. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry 67: 587–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Lee SY, Kim SH, Kim YB, Cho SJ 2008. Multitracer PET imaging of amyloid plaques and neurofibrillary tangles in Alzheimer’s disease. Neuroimage 43: 236–244 [DOI] [PubMed] [Google Scholar]
- Sibson NR, Dhankhar A, Mason GF, Behar KL, Rothman DL, Shulman RG 1997. In vivo 13C NMR measurements of cerebral glutamine synthesis as evidence for glutamate-glutamine cycling. Proc Natl Acad Sci 94: 2699–2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Perera GM, DeLaPaz R, Mayeux R, Stern Y 1999. Differential regional dysfunction of the hippocampal formation among elderly with memory decline and Alzheimer’s disease. Ann Neurol 45: 466–472 [DOI] [PubMed] [Google Scholar]
- Small GW, Ercoli LM, Silverman DH, Huang SC, Komo S, Bookheimer SY, Lavretsky H, Miller K, Siddarth P, Rasgon NL, et al. 2000. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer’s disease. Proc Natl Acad Sci 97: 6037–6042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Andersen AH, Kryscio RJ, Schmitt FA, Kindy MS, Blonder LX, Avison MJ 1999. Altered brain activation in cognitively intact individuals at high risk for Alzheimer’s disease. Neurology 53: 1391–1396 [DOI] [PubMed] [Google Scholar]
- Smith CD, Andersen AH, Kryscio RJ, Schmitt FA, Kindy MS, Blonder LX, Avison MJ 2002. Women at risk for AD show increased parietal activation during a fluency task. Neurology 58: 1197–1202 [DOI] [PubMed] [Google Scholar]
- Sole-Padulles C, Bartres-Faz D, Junque C, Vendrell P, Rami L, Clemente IC, Bosch B, Villar A, Bargallo N, Jurado MA, et al. 2009. Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging 30: 1114–1124 [DOI] [PubMed] [Google Scholar]
- Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, Laer L, Drzezga A, Forstl H, Kurz A, Zimmer C, et al. 2007. Selective changes of resting-state networks in individuals at risk for Alzheimer’s disease. Proc Natl Acad Sci 104: 18760–18765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Greve D, Dale A, Killiany R, Rosen B, Holmes J, Rosas HD, Cocchiarella A, Firth P, Lake S, et al. 2002. fMRI detection of pharmacologically induced memory impairment. Proc Natl Acad Sci 99: 455–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R, Bates J, Chua E, Cocchiarella A, Schacter DL, Rosen B, Albert M 2003. fMRI studies of associative encoding in young and elderly controls and mild AD patients. J Neurol Neurosurg Psychiatry 74: 44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Laviolette PS, O’Keefe K, O’Brien J, Rentz DM, Pihlajamaki M, Marshall G, Hyman BT, Selkoe DJ, Hedden T, et al. 2009. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron 63: 178–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, LaViolette PS, Vitolo OV, Hedden T, Becker JA, Rentz DM, Selkoe DJ, et al. 2010. Functional alterations in memory networks in early Alzheimer’s disease. Neuromolecular Med 12: 27–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Alexander GE, Prohovnik I, Mayeux R 1992. Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer’s disease. Ann Neurol 32: 371–375 [DOI] [PubMed] [Google Scholar]
- Stern EA, Bacskai BJ, Hickey GA, Attenello FJ, Lombardo JA, Hyman BT 2004. Cortical synaptic integration in vivo is disrupted by amyloid-β plaques. J Neurosci 24: 4535–4540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel CM, Henson RN, Morris JS, Friston KJ, Dolan RJ 2001. Pharmacological modulation of behavioral and neuronal correlates of repetition priming. J Neurosci 21: 6846–6852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohka J, Reilhac A 2008. Deconvolution-based partial volume correction in Raclopride-PET and Monte Carlo comparison to MR-based method. Neuroimage 39: 1570–1584 [DOI] [PubMed] [Google Scholar]
- Tolboom N, van der Flier WM, Yaqub M, Boellaard R, Verwey NA, Blankenstein MA, Windhorst AD, Scheltens P, Lammertsma AA, van Berckel BN 2009. Relationship of cerebrospinal fluid markers to 11C-PiB and 18F-FDDNP binding. J Nucl Med 50: 1464–1470 [DOI] [PubMed] [Google Scholar]
- Tolboom N, van der Flier WM, Boverhoff J, Yaqub M, Wattjes MP, Raijmakers PG, Barkhof F, Scheltens P, Herholz K, Lammertsma AA, et al. 2010. Molecular imaging in the diagnosis of Alzheimer’s disease: Visual assessment of [11C]PIB and [18F]FDDNP PET images. J Neurol Neurosurg Psychiatry 81: 882–884 [DOI] [PubMed] [Google Scholar]
- Trivedi MA, Schmitz TW, Ries ML, Torgerson BM, Sager MA, Hermann BP, Asthana S, Johnson SC 2006. Reduced hippocampal activation during episodic encoding in middle-aged individuals at genetic risk of Alzheimer’s disease: A cross-sectional study. BMC Med 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe R, Van Laere K, Ivanoiu A, Salmon E, Bastin C, Triau E, Hasselbalch S, Law I, Andersen A, Korner A, et al. 2010. (18)F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: A phase 2 trial. Ann Neurol 68: 319–329 [DOI] [PubMed] [Google Scholar]
- Vemuri P, Wiste HJ, Weigand SD, Shaw LM, Trojanowski JQ, Weiner MW, Knopman DS, Petersen RC, Jack CR Jr 2009. MRI and CSF biomarkers in normal, MCI, and AD subjects: Predicting future clinical change. Neurology 73: 294–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneri A, McGeown WJ, Shanks MF 2009. Responders to ChEI treatment of Alzheimer’s disease show restitution of normal regional cortical activation. Curr Alzheimer Res 6: 97–111 [DOI] [PubMed] [Google Scholar]
- Villemagne VL, McLean CA, Reardon K, Boyd A, Lewis V, Klug G, Jones G, Baxendale D, Masters CL, Rowe CC, et al. 2009. 11C-PiB PET studies in typical sporadic Creutzfeldt–Jakob disease. J Neurol Neurosurg Psychiatry 80: 998–1001 [DOI] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL 2006. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol 96: 3517–3531 [DOI] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME 2007. Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447: 83–86 [DOI] [PubMed] [Google Scholar]
- Visser PJ, Kester A, Jolles J, Verhey F 2006. Ten-year risk of dementia in subjects with mild cognitive impairment. Neurology 67: 1201–1207 [DOI] [PubMed] [Google Scholar]
- Waldemar G, Dubois B, Emre M, Scheltens P, Tariska P, Rossor M 2000. Diagnosis and management of Alzheimer’s disease and other disorders associated with dementia. The role of neurologists in Europe. European Federation of Neurological Societies. Eur J Neurol 7: 133–144 [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Amlien I, Grambaite R, Stenset V, Bjornerud A, Reinvang I, Gjerstad L, Cappelen T, Due-Tonnessen P, et al. 2009. Multimodal imaging in mild cognitive impairment: Metabolism, morphometry and diffusion of the temporal-parietal memory network. Neuroimage 45: 215–223 [DOI] [PubMed] [Google Scholar]
- Wishart HA, Saykin AJ, McDonald BC, Mamourian AC, Flashman LA, Schuschu KR, Ryan KA, Fadul CE, Kasper LH 2004. Brain activation patterns associated with working memory in relapsing-remitting MS. Neurology 62: 234–238 [DOI] [PubMed] [Google Scholar]
- Wolk DA, Price JC, Saxton JA, Snitz BE, James JA, Lopez OL, Aizenstein HJ, Cohen AD, Weissfeld LA, Mathis CA, et al. 2009. Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol 65: 557–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DF, Rosenberg PB, Zhou Y, Kumar A, Raymont V, Ravert HT, Dannals RF, Nandi A, Brasic JR, Ye W, et al. 2010. In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F-AV-45 (flobetapir F 18). J Nucl Med 51: 913–920 [DOI] [PMC free article] [PubMed] [Google Scholar]