Abstract
Multiphoton ionization of thymine and uracil clusters generated by a supersonic molecular beam gave rise to a remarkable alternation of mass spectral intensities between even- and odd-numbered clusters. Such alternation was observed in clusters of up to 30 molecules. Excitation to the two lowest electronically excited states seemed to be a strong prerequisite. In view of the well known photodimerization reaction of thymine and uracil in the bulk phase, it is proposed that such alternation in the mass spectral intensity resulted from formation of photodimer units within the cluster on intense UV irradiation. Several analogues of thymine with no known propensity for photodimerization in the bulk phase did not exhibit any sign of such alternation in the cluster mass spectrum. The intrinsic UV window for photodimerization, and hence photoinduced mammalian mutagenesis, was estimated to be approximately 210–280 nm, significantly narrower than the previously reported bulk values of 150–300 nm.
Solar radiation is known to cause mutagenesis and carcinogenesis in mammalian cells (1–4). It is now widely accepted that DNA is the central target for such sunlight-induced lethality. In particular, pyrimidine base dimers are believed to play a key role. The adjacent bases of thymine in a DNA strand tend to undergo facile photodimerization on irradiation of UV light (1–5), and these photodimers cause a miscarriage of genetic information during replication of DNA. Despite many studies (6–11) and a recent heightened awareness of the ozone-hole problem, direct information on the intrinsic features of this process is lacking, mainly because it has never been investigated at an isolated molecular level. Here we generated molecular clusters of pyrimidine bases and found that strong UV irradiation of these cold clusters leads to a mass spectrum with a striking alternation of intensities between even- and odd-numbered clusters. Several derivatives of thymine and uracil with no known propensity for photodimerization did not yield such mass distribution when they were subject to the same irradiation condition. We therefore seemed to have found a strong correlation between the occurrence of the alternation pattern in cluster mass spectrum and the known tendency of the same molecule to undergo photodimerization in the bulk phase. By using the alternation as the criterion for photodimerization in an isolated molecular system, we estimated the intrinsic wavelength range of UV light causing photodimerization, a leading cause of photoinduced mammalian mutagenesis. It covers a range of 210–280 nm, which is significantly narrower than the previously reported bulk value of 150–300 nm.
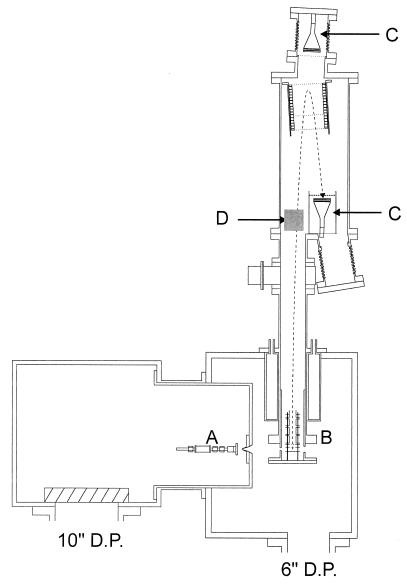
The experimental methods used in the present study have been described elsewhere (12, 13). Briefly, we used a molecular beam machine equipped with a photoionization time-of-flight (TOF) MS (Fig. 1). Clusters of thymine or uracil were generated by supersonic expansion of the sample with 2 atm (1 atm = 101.3 kPa) argon carrier gas through a high-temperature pulsed nozzle. The expanding gas was sampled by a skimmer and multiphoton-ionized by the third or fourth harmonic of an Nd-YAG laser or the frequency-doubled output of a dye laser. The ionized molecules were accelerated and guided into the TOF MS, which was run typically in linear mode for the present study with a mass resolution M/ΔM of 150.
Figure 1.
A schematic diagram for the experimental apparatus. The molecular beam machine consists of two vacuum chambers and a photoionization TOF MS, which are pumped by a 10-, 6-, and 2-inch (1 in = 2.54 cm) diffusion pump, respectively. The source chamber contains a high-temperature pulsed nozzle (A), and the photoionization TOF MS consists of an ion optics assembly (B), a set of microchannel plate detectors (C) for both linear and reflectron mode detection, and a deflector (D).
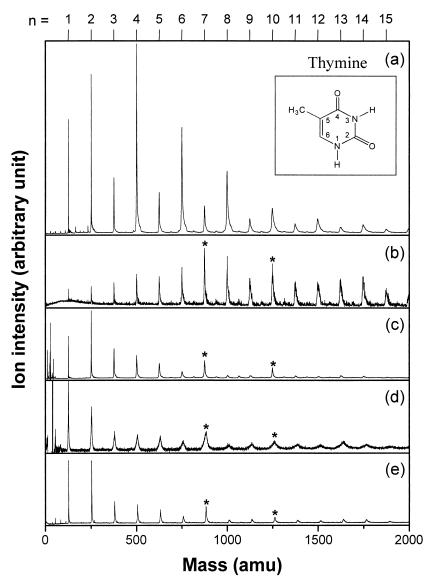
Fig. 2a shows a typical one-color, resonant two-photon ionization (R2PI) mass spectrum of thymine clusters at 274 nm under a high laser fluence condition. It is quite evident that every even-numbered cluster exhibits a conspicuously higher intensity than the neighboring odd-numbered ones. Such alternation of intensities was observed up to a cluster size of n ≈ 30. Uracil, the counterpart of thymine in RNA, showed a similar alternation pattern, but other bases of DNA did not. On lowering the laser fluence, the mass distribution changes drastically (Fig. 2b), and the alternation disappears completely.
Figure 2.
Mass spectra of thymine clusters obtained by linear TOF MS. (a) One-color R2PI spectrum using a 6-ns laser pulse at 274 nm at a fluence of 1.6 × 106 W/cm2. (Inset) The molecular structure of thymine. (b) The same spectrum as a obtained at a lower fluence of 5.3 × 105 W/cm2. (c) One-color (2 + 1) resonance-enhanced multiphoton ionization spectrum using a 6-ns, 4.0 × 108-W/cm2 laser pulse at 355 nm. (d) Electron impact ionization mass spectrum at a 30-eV electron energy. (e) The same spectrum as a obtained by using a 400-fs, 3.2 × 1011-W/cm2 laser pulse centered at 267 nm. Peaks denoted by * at n = 7 and 10 in b–e may indicate the stability of these clusters as neutrals.
We also examined under what irradiation conditions such even-odd alternation fails to emerge. First of all, the alternation was observed only at wavelengths between 210 and 280 nm, with the contrast in the alternation pattern becoming gradually reduced below approximately 250 nm. Even at 210 nm however, the relative intensities of the mass peaks for n = 2, 3, and 4 are 2.8, 1.0, and 1.6, respectively. This wavelength range covers the two lowest electronically excited states of thymine (ref. 14 and references therein), which are estimated to be located at 4.6 and 5.6 eV above the ground state. On the other hand, use of 355-nm photons in the (2 + 1) resonance-enhanced multiphoton ionization scheme did not reveal any such alternation features (Fig. 2c). We also failed to observe the alternation with electron impact ionization (Fig. 2d). Finally, even the same R2PI scheme as used for Fig. 2a failed to reproduce the alternation when a very short laser pulse (of approximately 400-fs pulsewidth) was used from a regeneratively amplified Ti:Sapphire femtosecond laser system (Fig. 2e). We conclude that the alternation in Fig. 2a does not reflect the stability of neutral clusters, because it so much depends on the method of ionization. Instead, it is likely to be a consequence of secondary processes such as photoexcitation followed by fragmentation, which somehow preferentially enhances relative population of the even-numbered clusters. Photoexcitation to the lowest electronically excited states at 4.6 and 5.6 eV seems to be a prerequisite, judging from the wavelength window of 210–280 nm for R2PI. The conditions for the emergence of alternation do not seem to be satisfied when the laser fluence is too low (Fig. 2b) or when the excitation energy is too high (2 photons at 355 nm ≅ 7 eV, Fig. 2c). Direct ionization without prior excitation to intermediate levels also fails to bring about the alternation (Fig. 2d). Finally, it seems the photoinduced process occurring at the excited electronic states that ultimately leads to the alternation takes place in time scales exceeding several hundred femtoseconds (Fig. 2e).
In view of the striking regularity in both the even- and odd-number progressions having a recurrence period of Δn = 2, we propose that these progressions, and hence the alternation, may result from formation of dimeric molecular units within the cluster. Under the condition of strong UV irradiation, a likely candidate for such dimeric units is a photodimer produced by photoinduced chemical reactions. In fact, we suggest that the remarkable even-odd alternation experimentally observed in the present cluster mass spectrum is related intimately to the well known phenomenon of photodimerization of thymine and uracil in the bulk phase (6–11). The [2 + 2]-cyclobutane-type adducts of thymine and uracil have been isolated and identified as the major photoproduct of these bases in aqueous solutions, frozen solutions, solid films, and even in bacteria. The dimerization occurs at the C5–C6 double bond of these bases (see Fig. 2a, Inset) as cycloaddition of α,β-unsaturated carbonyl compounds. The polarization of the double bond by the conjugated carbonyl group is believed to play a crucial role in this reaction (15). A striking parallel also has been established between the photodimerization yield and the total concentration of uncoupled electrons in this bond (16). The reaction is known to occur in the electronically excited states reached by UV light of 150- to 300-nm wavelength or in the triplet states (8).
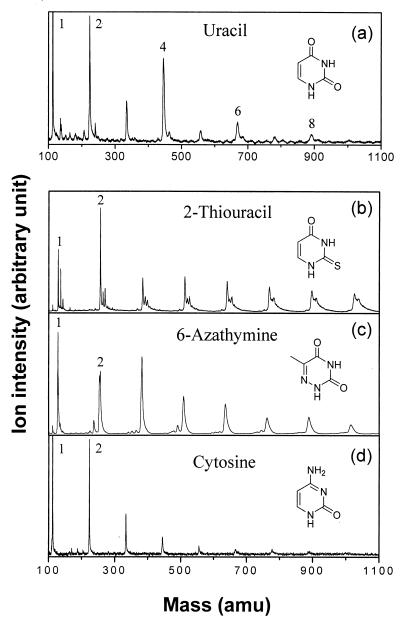
Because mass spectrometry cannot provide direct evidence of dimer formation, we attempted to indirectly relate the observed alternation of mass spectral intensities to intracluster photodimerization by testing whether certain derivatives of thymine or uracil show a similar alternation behavior. We chose 2-thiouracil, 6-azathymine, and cytosine, the photodimerization yields of which are known to be very small (cytosine) or nearly zero (2-thiouracil and 6-azathymine) in the bulk. This characteristic is due to the fact that they cannot undergo the cycloaddition reaction because of insufficient polarization or low electron density in the C5–C6 bond in the excited state (15, 16). In contrast to the uracil case (Fig. 3a) however, we failed to observe any sign of even-odd alternation for all of these molecules under any experimental conditions (Fig. 3 b–d). These observations exemplify the direct correlation between the known photodimerization yield of a given base in the bulk phase and the occurrence of intensity alternation in the mass spectrum observed in the present study.
Figure 3.
Cluster mass spectra of thymine analogues such as uracil (a), 2-thiouracil (b), 6-azathymine (c), and cytosine (d). Only uracil shows alternation of mass spectral intensity, as in the case of thymine. All other analogues are known not to undergo photodimerization in the bulk phase.
In view of the extensiveness of the alternation (well up to n ≈ 30) found throughout our study, we surmise that photodimerization is very efficient in the environment of cold clusters produced by a supersonic jet. It is to be noted from the bulk studies that the photodimerization yield is much higher in an ordered, constrained medium such as frozen solution or bacteria than in a disordered, labile environment such as aqueous solution (15). A quantum yield as high as approximately 100% has been reported in frozen solution, in contrast to a typical value of a few percent in a more labile medium (8). In addition, the photodimerization rate in frozen solution was estimated to be faster than the singlet excimer fluorescence lifetime (11). It has been suggested that certain crystal structures of thymine in ice favor photodimerization (9, 10), and in this regard, the jet-cooled clusters in the present study may provide a very efficient medium for photodimerization.
A study of photodimerization yield in an amorphous solid film over the wavelength range of 150–290 nm showed that photodimerization occurs with a nearly constant quantum yield (approximately 0.8%) above 200 nm, but this decreases linearly to about 0.3% at 150 nm (17). By comparison, in the free isolated molecular state of our experiment with no bulk interference, the wavelength range of UV light for photodimerization, deduced from the occurrence of the alternation pattern, spans a much narrower range of approximately 210–280 nm. The blue shift of the absorption edge (from approximately 300 to 280 nm) is quite typical for a system going through a transition from a bulk phase to an isolated molecular state. On the other hand, the apparent red shift of the upper bound in energy (from 150 to 210 nm) seems to be due to the fact that only a few low-lying electronic states can lead directly to photodimerization in the isolated molecular state. As noted earlier, the present UV window of 210–280 nm covers only the two lowest excited states (ref. 14 and references therein) accessible by π − π* transitions at 270 and 220 nm. It is quite likely that the higher-lying excited states above 210 nm are intrinsically incapable of bringing about the photodimerization reaction because of an unfavorable electron distribution. In fact, withdrawal of electronic charge from the C5-C6 bond in such high-lying excited states has been predicted theoretically (ref. 14 and references therein). In the bulk phase, however, the energy in these high-lying states becomes dissipated quickly to the medium, resulting in the relaxation of the system to lower excited states in which dimerization can occur readily. In fact, the monotonic decrease in the photodimerization quantum yield in solid film below approximately 200 mm (17) may well reflect the increasingly insufficient relaxation at higher levels of initial excitation. In all of the previous bulk studies, these higher-lying states have seemed to induce photodimerization directly, but our study finds it to be because of the rapid relaxation of electronic energy in the bulk.
Acknowledgments
This work was supported by the National Creative Research Initiatives Program (99-C-CT-01-C-50) of the Ministry of Science and Technology.
Abbreviations
- TOF
time-of-flight
- R2PI
resonant two-photon ionization
References
- 1.Hollaender A, Emmons C W. Cold Spring Harbor Symp Quant Biol. 1946;11:78–84. [Google Scholar]
- 2.Blum H F. Carcinogenesis by Ultraviolet Light. Princeton: Princeton Univ. Press; 1959. [Google Scholar]
- 3.Urbach F. The Biological Effects of Ultraviolet Radiation. New York: Pergamon; 1969. [Google Scholar]
- 4.Epstein J H. Photophysiology. 1970;5:235–273. [PubMed] [Google Scholar]
- 5.Kantor G J. Photochem Photobiol. 1985;41:741–746. doi: 10.1111/j.1751-1097.1985.tb03628.x. [DOI] [PubMed] [Google Scholar]
- 6.Beukers R, Berends W. Biochim Biophys Acta. 1960;41:550–551. doi: 10.1016/0006-3002(60)90063-9. [DOI] [PubMed] [Google Scholar]
- 7.Eisinger J, Shulman R G. Proc Natl Acad Sci USA. 1967;58:895–900. doi: 10.1073/pnas.58.3.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner P J, Bucheck D J. J Am Chem Soc. 1970;92:181–185. [Google Scholar]
- 9.Beukers R, Berends W. Biochim Biophys Acta. 1961;49:181–189. [Google Scholar]
- 10.Füchtbauer W, Mazur P. Photochem Photobiol. 1966;5:323–335. [Google Scholar]
- 11.Lamola A A, Eisinger J. Proc Natl Acad Sci USA. 1968;59:46–51. doi: 10.1073/pnas.59.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim N J, Kang H, Jeong G, Kim Y S, Lee K T, Kim S K. J Phys Chem A. 2000;104:6552–6557. [Google Scholar]
- 13.Kim N J, Jeong G, Kim Y S, Sung J, Kim S K, Park Y D. J Chem Phys. 2000;113:10051–10055. [Google Scholar]
- 14.Lorentzon J, Fülscher M P, Roos B O. J Am Chem Soc. 1995;117:9265–9273. [Google Scholar]
- 15.Wacker A. Prog Nucleic Acid Res Mol Biol. 1963;1:369–399. [Google Scholar]
- 16.Pullman B. Photochem Photobiol. 1968;7:525–530. [Google Scholar]
- 17.Yamada H, Hieda K. Photochem Photobiol. 1992;55:541–548. doi: 10.1111/j.1751-1097.1992.tb04276.x. [DOI] [PubMed] [Google Scholar]