Abstract
Research has confirmed that peptides and larger protein molecules pass through the mucosal barrier of the gastrointestinal tract. Orally administered serine and cysteine proteases of plant and animal origin also reach blood and lymph as intact, high molecular weight and physiologically active protein molecules. Their absorption may be supported by a self-enhanced paracellular transport mechanism resulting in sub-nanomolar concentration of transiently free protease molecules or, in a complex with anti-proteases, at higher concentrations. Data from pharmacokinetic investigations reveals dose linearity for maximum plasma levels of free proteases not unusual for body proteases and a high inter-individual variability. There is no interference with each other after oral administration of protease combinations, and absorption follows an unusual invasion and elimination kinetic due to slow velocity of absorption and a fast 100% protein binding to anti-proteases. Oral application of proteases leads to increased proteolytic serum activity and increased plasma concentrations of the corresponding anti-proteases. Their biological activity is determined by their proteolytic activity as free proteases on soluble peptides/proteins or cell surface receptors (e.g. protease activated receptors) and their activity in the complex formed with their specific and/or unspecific anti-proteases. The anti-protease-complexes, during immune reaction and injuries often loaded with different cytokines, are cleared from body fluids and tissue by receptor mediated endocytosis on hepatocytes and/or blood cells. Oral administration of enteric coated tablets containing proteolytic enzymes of plant and animal origin may be a safe method to stabilize, positively influence or enhance physiological and immunological processes during disease processes and in healthy consumers.
Keywords: Gastrointestinal absorption, serine and cysteine protease, proteinase, proteolytic enzyme activity, anti-protease, protease activated receptors
Introduction
Single orally administered proteolytic enzymes of plant and animal origin are widely used as a medical treatment for a variety of digestive, absorption and pancreatic disorders. Historically, porcine and bovine pancreatic enzymes have been the preferred form of supplementation for exocrine pancreatic insufficiency. Plant-based enzymes, such as bromelain from pineapple, also serve as effective digestive aids in the breakdown of proteins [1].
However, orally administered proteolytic enzyme combinations often supplemented with rutosid are widely used as an alternative or a supplementary treatment of different disease conditions such as acute and post surgical trauma, phlebitis, rheumatoid and osteoarthritis, as well as in an adjunctive therapy in cancer treatment [2, 3]. The terms proteolytic enzymes, proteases or proteinases are often synonymously used and the medical treatment is often described as systemic enzyme therapy, which implies a gastrointestinal absorption for being active in the organism by distribution in body fluids.
In the United States and in Europe various proteolytic enzyme preparations are available on the market. Their marketing status is different and varies from prescription drug to OTC or food supplementation. They are marketed either as single proteolytic enzyme preparations or as combinations of various proteases of plant and/ or animal origin. Even combinations with vitamins and other food supplements like oligomeric proanthocyanidins (OPC), quercetin or selenium are on the market. Most often the plant cysteine proteases bromelain and papain and the animal serine proteases trypsin and chymotrypsin are used. Some preparations are supplemented with pancreatin, amylase, lipase and/or rutosid, a flavonoid that is known to be able to neutralize radicals and to exert immunological effects [4, 5].
Proteolytic enzyme combinations are generally provided as enteric coated tablets for oral administration at dosages varying from 1 to 200 mg per tablet. The amount of proteases provided in the preparation is described by their F.I.P. activity (F.I.P.-units of the Fédération Internationale Pharmaceutique). One F.I.P.-unit is the amount of enzyme which is able to turn over 1 μmol of substrate in 1 minute under standard conditions. Due to enteric coating, degradation of the protein structure to single amino acids or peptides in the acidic environment of the stomach is reduced. If at all, the absorption of proteases from the gastrointestinal tract may be favoured in the small intestine. Table 1 shows the characteristics of three typical representatives of proteolytic enzyme combinations.
Table 1.
Overview of active ingredients used in 3 different marketed preparations
| DrugSubstance | Preparation 1 | Preparation 2 | Preparation 3 |
|---|---|---|---|
| Bromelain | 90 mg = 900 F.I.P.-units | 45 mg = 450 F.I.P.-units | 133-178 mg = 800 units |
| Papain | - | 60 mg = 328 F.I.P.-units | - |
| Pancreatin | - | 100 mg = 300 Ph. Eur.-units proteinase | - |
| Chymotrypsin | - | 1 mg = 596 F.I.P.-units | - |
| Trypsin | 48 mg = 24 μkat* | 24mg = 12 μkat* | - |
| Rutosid | 100 mg | 50 mg | - |
*1μkat is the amount of enzyme which turns over 1 μM of substrate per second under standard conditions. It corresponds to 60 F.I.P - Units.
In general, proteases' side effects are few. Complications typically arise from either excessive dosing or allergic reaction to bovine or porcine substances, papaya or pineapple. In the case of excessive dosing, transient gastrointestinal upset may result. To avoid hypersensitivity reactions, it is best to confirm a patient is not allergic to the given proteases' source prior to use.
Hyperuricosuria (excess uric acid in the urine) and hyperuricemia (excess uric acid in the blood) are associated with extremely high doses of exogenous pancreatic enzymes [6].
The lack of unwanted drug interactions and major side-effects as well as overall safety aspects, make orally administered proteolytic enzyme combinations an interesting tool for the management of acute and chronic inflammatory processes. Nevertheless, the clinical benefit is subject to controversy as the biological rationale and the mechanism(s) of action are widely unknown and far from being understood. Additionally, clinical trials performed in the last century did not always come to an end with clinically relevant and statistically significant results. Often an insufficient patient number and organizational or other insufficiencies such as only mild to moderate disease activity at inclusion of patients were identified as a cause. As there is also doubt on the absorption at all or at least on sufficient amounts of larger protein molecules and missing proof of tissue distribution, clinically relevant systemic effects in the treatment of diseases are not readily accepted. Nevertheless, protease complexes with their counterpart anti-proteases (often as acute phase proteins in animals) [7], as well as the specific proteolytic activity of proteases on specific receptors (e.g. protease activated receptors, PAR) in the gastrointestinal tract [8] and, provided they are absorbed, in body fluids, may play a major role in many regulatory processes in humans [9, 10]. Thus, clinical effects and benefits are still a matter of controversy and clinical and pharmacological effects have to be further evaluated.
This article summarizes the existing data on gastrointestinal absorption of proteases after oral administration and makes a mechanism of absorption reasonable. The pharmacokinetic data are summarized as well as some unusual properties of proteases' pharmacokinetics and safety. Evidence of absorption as intact and biologically active molecules is provided and the concentration in the blood is shown to be sufficiently high to probably result in a variety of biological effects either by the different intrinsic proteolytic cleavage characteristics for proteins and certain surface receptors or their binding to specific (e.g. α1-anti-trypsin, α1-anti-chymotrypsin) or unspecific anti-proteases (α2-macroglobulin).
Gastrointestinal absorption of macromolecules
Even today, the doubt on gastrointestinal absorption of larger peptides and proteins is often based on the view that the degradation of macromolecules like starch, fatty acids and proteins contained in nutrients is thought to be important for the absorption processes in the gastrointestinal tract. Several reasons are responsible for this thinking. First, pancreatic and intestinal proteases or peptidases produce free amino acids within the intestinal lumen. Their activity leads to the measurement of an increased amount of free amino acids in blood following ingestion of protein. Second, the characterization of amino acid carrier systems in the brush-border membrane of the small intestine further detracted attention from the possibility that forms other than amino acids enter the body during assimilation of a protein. Third, there were substantial methodological difficulties in quantifying the amounts of intact peptides and proteins crossing the gastrointestinal tract under unequivocally physiological conditions. These difficulties also included ensuring of an adequate specificity of analytical methods, overcoming the lack of methods for estimation, clearing by tissue or hydrolysis before and during sampling and handling [11-13].
Absorption of peptides and proteins
Early investigations on absorption of high molecular weight proteins were usually performed by oral application of radio-labelled proteins in animals. This method does not alter the enzymatic activity of the proteases, and the purification of labelled material for use by enteric tubes in sedated animals is easily performed. Only radioactivity was measured, and as shown by Skogh [14], the absorption rates estimated were too high. This is caused by splitting off iodine from the protein molecule by intestinal deiodinases or dissociation by changes in pH-conditions. These results were confirmed by Bohe et al. [15] for human cationic trypsin provided into the duodenum of nine healthy individuals pre-treated with “cold”, i.e. non-radioactive iodine. The recovered radioactivity in plasma, characterized by dialysis and gel-filtration, was found to be in the form of free iodine131. Furthermore, it was shown that pre-treatment with “cold” iodine prevented isotope binding to circulating plasma proteins, and, after intra-gastric application, the radioactive markers had been eliminated, especially in probes containing enzymes, had been adsorbed anywhere (e.g. thyroid gland), or had been bound to blood proteins.
The latter aspect holds true for the proteolytic enzymes discussed, as their free forms are physiologically bound to either specific (α1-anti-chymotrypsin, α1-anti-trypsin) or unspecific (α2-macroglobulin) anti-proteases [16, 10]. This is a physiological necessity, as free, uncontrolled proteases, displaying their intrinsic proteolytic activity for prolonged times, may destroy biological material in body fluids after absorption. As shown later, this proteolytic activity may also be used to prove and quantify absorption across the gastrointestinal border into either blood or lymph.
With the development of new experimental techniques in addition to radio-labelling, the evidence for absorption of intact proteins across the gastrointestinal barrier increased. The new approaches were: (i) the detection of immunoreactive or both enzymatic and immunologic active specific proteins in body fluids; (ii) detection of antibodies against specific dietary proteins after ingestion in body fluids; (iii) animal experiments in vitro to follow the transport by techniques as specific as enzyme-linked immunosorbent assay and with establishment of the size by gel-filtration chromatography; and (iv) cytochemical and, preferably, immunocytochemical visualization in vitro of macromolecules after luminal administration, preferably in vivo. The latter two approaches have also provided evidence defining routes and mechanisms of transport emphasizing the use of endocytotic trans-cellular processes. While each of these approaches has quite severe limitations, the fact that all approaches point to the same conclusion (i.e. transport of small, but potentially biologically significant amounts of biologically active macromolecules) greatly strengthens the validity of this conclusion [13].
Immunological investigations confirming the absorption of not degraded dietary proteins in healthy humans include those on human β-lactoglobulin by Jakobsson et al. [17], on oval-bumin by Husby et al. [18], on bovine serum albumin, beta-lactoglobulin and ovalbumin by Paganelli and Levinsky [19], and on horseradish peroxidase [20]. However, the majority of experimental studies on macromolecule transport have been undertaken on animal intestine. Bockmann and Winbom [21] visualized by electron microscopy intracellular vesicles containing absorbed ferritin in hamster intestine and Walker and Isselbacher [22] these containing horseradish peroxidase.
Studies on absorption of proteins across biopsies of human intestine, although generally from children or from initially abnormal (e.g. malnourished) intestine, have provided generally similar information (e.g. [20, 23] and other references in [12]). Therefore, there is no reason to suspect major intra-species differences in the gastrointestinal handling of intact proteins.
In animal experiments, generally in rats, similar developments in knowledge have occurred concerning absorption of intact (or incompletely digested) peptides: as for large protein molecules, it was firmly believed that peptides are wholly hydrolyzed before absorption. This dogma has also had to be revised to account for transport of small but significant amounts of intact, biologically active peptides. The mechanisms for their trans-intestinal transport have now been characterized (e.g. [11, 12]) as described below. For example, in rats, biologically effective amounts of luteinizing hormone releasing hormone can be absorbed [24, 25], in animals and man vasopressin and analogues [26-30], and responses to oral thyreotropinreleasing hormone have been recorded in man [31].
Within the last four decades the view on the absorption of high molecular weight molecules (e.g. proteins and peptides) across the gastrointestinal barrier has completely changed. It is now accepted beyond reasonable doubt that significant (albeit small) amounts of macromolecules can be absorbed in intact and biologically active form [13]. Further, the fact that actual mechanisms of transport have been defined without controversy adds further weight to this conclusion.
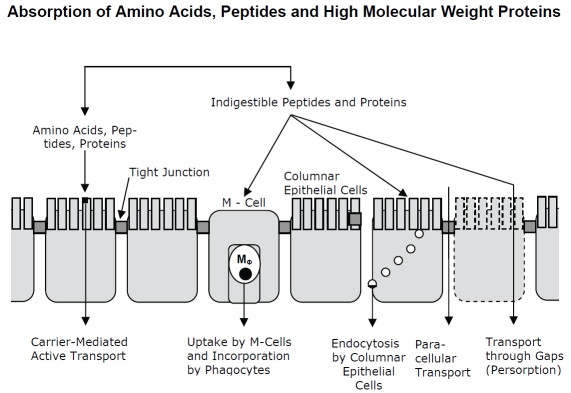
Research of the last 30 years led to the schematic view of absorption models provided in Figure 1. Proteins are digested by multiple hydrolases, associated with membranes of columnar epithelial cells and secreted into the gut lumen. Specific carrier molecules transport amino acids, peptides and small proteins. In contrast, proteins are incorporated by M-cells present in the follicle-associated envelope of Peyer's patches or through endocytosis by columnar epithelial cells. The incorporation of proteins by diffusion through intercellular spaces may be supported by intrinsic proteolytic activity of the administered proteases to solve the tight junctions or by reorganization processes of the epithelial layer (persorption). Detailed information on morphological and functional aspects of gastrointestinal absorption is outside the scope of this review and is described elsewhere.
Figure 1.
Schematic view on absorption mechanisms of the gastrointestinal tract for amino acids, peptides and protein molecules.
Absorption of proteases
In line with the ongoing efforts to characterize the absorption of macromolecules, the first results were also published on absorption of proteases. As described, early investigations on absorption of proteases were also performed by oral application of radio-labelled material in animals with the same implications concerning the measured amounts. Another method used was the quantitative determination in plasma by measuring proteases' intrinsic esterase activity on specific substrates e.g. N-benzoyl-L-arginine ethyl ester (BAEE) as substrate for trypsin and N -acetyl L-tyrosine ethyl ester (ATEE) as substrate for chymotrypsin, as well as haemoglobin for total proteolytic activity [32-34].
Evidence has accumulated that intestinal absorption is governed by several factors more important than molecular size. While many small molecules are not absorbed (for example, succinyl sulfathiazole, cathartics of the isatin group), ferritin with a molecular weight of 500,000 seems to be absorbed [35]. Martin and co-workers described absorption of I131-labeled trypsin from the buccal cavity and the intestine of experimental animals [36, 37], and Miller [38] reported similar studies in man. Bogner and colleagues obtained comparable results with I131-labelled trypsin in rats and I131-labeled chymotrypsin in man [39]. Kabacoff and co-workers found ATEE activity in the blood of rabbits after intestinal or rectal administration of chymotrypsin [40]. Using the same method, Avakian demonstrated oral chymotrypsin absorption in man [41]. The same conclusion was drawn by Kabacoff (quoted by Bodi [42] on the basis of comparative oral and parenteral toxicity studies in mice. In 1964, Megel et al. [43] developed a sensitive method for detecting trypsin -like activity in rat plasma. For the first time they were able to determine that a minimal effective dose of 500 mg/kg of orally fed trypsin significantly increased plasma trypsin in rats [43].
First investigations with isolated rat intestines started in 1972, when Faudemay et al. [44] studied the transport of trypsin across the intestinal wall, using pieces of isolated rat jejunum and ileum. The intestine was filled with trypsin solution and was incubated in buffer. Aliquots of the acceptor buffer (serosal fluid) were taken and trypsin activity was determined by enzymatic reaction. Trypsin was found in the external medium in amounts increasing with time.
Detailed studies concerning the oral bioavailability of chymotrypsin were carried out by Moriya et al. in 1967 [45]. Radio-iodinated chymotrypsin was administered into rat intestine and the tissue distribution was determined. As a control, I131-labeled potassium iodide was used, resulting in a different radioactive distribution. In addition, protein-bound radioactivity was proven as well as the stability of chymotrypsin after incubation with intestinal juices and increased serum esterase activity.
Absorption of the plant hydrolase bromelain (Ananas comosus) has been shown by Miller and Opher in 1964 [46]. They demonstrated an increased ability of the blood serum to digest casein after oral administration of enteric coated bromelain tablets. The bioavailability of bromelain has been studied in detail by White et al. in 1988 [47]. The total plasma radioactivity, TCA-precipitable I125-compounds and the molecular weight profile of I125-proteins, was measured after oral administration in rats. A maximum level, equivalent to 270 ng/ml bromelain, was found at 1 h after administration. Approximately 40% of the I125 in plasma could be precipitated by trichloroacetic acid. Electrophoresis analysis showed one major peak of radioactivity in the plasma samples, with a molecular weight of 26-32,000 Dalton. This is identical to the main molecular weight fraction in the bromelain mixture and corresponds to the molecular weight of the purified enzyme. In the 1 hour plasma sample this peak contained 0.003 per cent of the administered dose per millilitre.
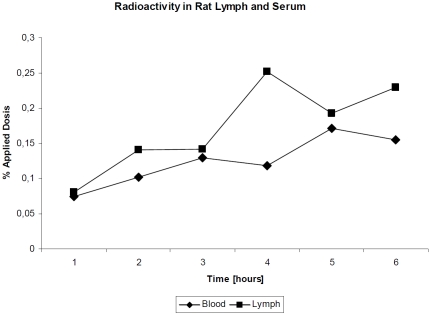
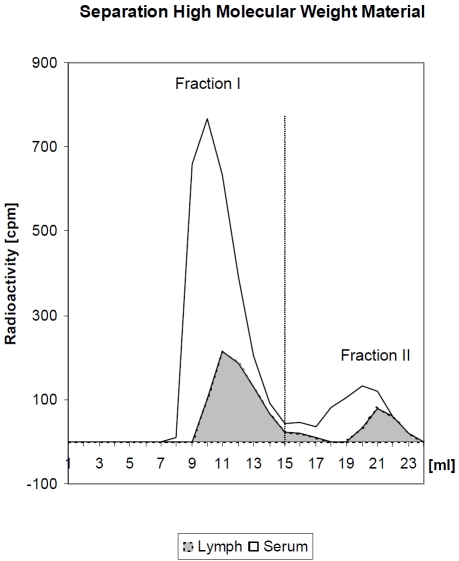
In contrast, Seifert [48], administered radio-labeled I125-bromelain intra-duodenal and took blood und lymph aliquots over a period of six hours, determined a bromelain absorption of about 50% of the administered dose after 6 hours (Figure 2). The samples were separated by radio-chromatography into high and low molecular weight labelled protein (Figure 3). About 80% of the radioactivity was found in high molecular weight fraction and its integrity was confirmed using Ouchterlony's agar-double diffusion technique.
Figure 2.
Radioactivity (percentage of the administered dose) in serum of rats after oral administration of radio-labelled enzymes (according to [50]).
Figure 3.
Radio-chromatography with blood and lymph of rats after intraduodenal application of radio-labelled bromelain. Fraction I corresponds to high molecular weight bromelain and fraction II to low molecular fragments (B) (cpm = counts per minute; ml = millilitre) (according to [50]).
The calculated total amount of absorbed bromelain resulted in about 40% of the applied dose. The distribution of radioactivity in the different organs of the rat is shown in Table 2. Except the high radioactivity in kidney and thyroid gland it remains low with about 1% of the applied dose per gram organ weight. In the kidney 0.4% and in thyroid gland even 3.6% of the originally apapplied dose was determined per gram organ weight.
Table 2.
Radioactivity in different compartments I125-labeled bromelain in rats of the intestine, absorption rate and distribution in different organs 6 hours after intra-duodenal application of 7.6 mg bromelain [48].
| Organ | % applied Dose |
|---|---|
| Small intestine | 49.6 ± 4.25 |
| Large intestine | 0.84 ± 0.26 |
| Rate of absorption | 49.6 ±4.4 |
| Lung | 0.14 ± 0.025 |
| Liver | 0.10 ± 0.015 |
| Spleen | 0.11 ±0.017 |
| Kidney | 0.42 ± 0.244 |
| Muscle | 0.10 ± 0.018 |
| Skin | 0.16 ± 0.032 |
| Thyroid gland | 3.62 ±0.561 |
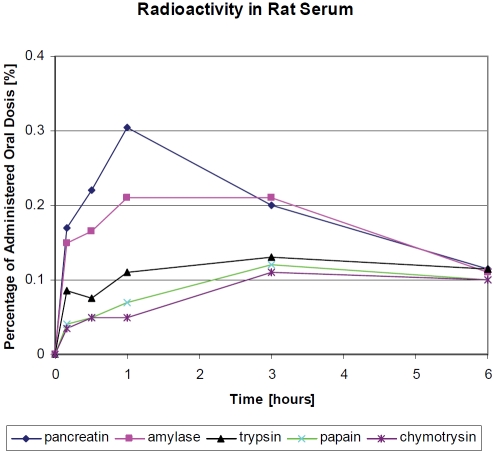
Further investigations on proteases used iodide125 radio-labelled material [49]. After purification, the proteases amylase, trypsin, chymotrypsin, papain, and pan-creatin were administered by enteric tubes to rats while sedated. At the time points indicated in Figure 4, blood samples were taken and radioactivity was measured over 6 hours. It was confirmed by radio-chromatography and immunological methods that the proteases are qualitatively absorbed in the high molecular form. As shown, an increase in blood radioactivity was found in each animal. The findings are shown as percentages of the administered dose for the purpose of standardization. Pan-was absorbed most readily, followed by and chymotrypsin. Amylase was also easily absorbed, but the absorption of papain was poorer. Measurements of radioactivity by lymph drainage up to six hours after administration led to equivalent amounts in the lymph for some enzymes and somewhat higher for others (data not shown).
Figure 4.
Radioactivity (percentage of the administered dose/gram blood) in serum of rats after oral administration of I123 radio-labeled proteases [50].
The radio-labelled material in the blood and lymph samples was precipitable after addition of the specific antibodies to the proteases by forming the corresponding antigen-antibody complexes (double diffusion test in agar gel) [49]. High molecular weight material was also found after separation by Sephadex G20 gel filtration. The amount of high molecular weight material corresponds to 77% of amylase, 37% of chymotrypsin, 50% of pancreatin, 24% of papain and 54% of trypsin with reference to serum. The absorption rate of high molecular weight proteases from the originally applied material was calculated to be as about 45% for amylase, 14-21% for chymotrypsin, 18-20% for pancreatin, 6-15% for papain and 26-34% for trypsin as shown in Table 3.
Table 3.
Quantitative analysis of absorption of proteases from rat intestine into serum and lymph [49].
| Protease | Applied amount of protease (mg) | Total rate of absorption (%)* | Total rate of absorption (mg) | High molecular part (%) | Low molecular part (mg) | High molecular part (%) | |
|---|---|---|---|---|---|---|---|
| Serum | Lymph | ||||||
| Amylase | 10 | 59 | 5.9 | 77.1 | 73.7 | 4.35 - 4.55 | 4.35 - 4.55 |
| Chymotrypsin | 5 | 38.1 | 1.91 | 37.3 | 57.3** | 0.71 - 1.09 | 14.2 - 21.9 |
| Pancreatin | 2.25 | 36.6 | 0.82 | 50.3 | 55.7 | 0.41 - 0.46 | 18.4 - 20.3 |
| Papain | 5 | 26 | 1.3 | 24.1 | 60.6** | 0.31 - 0.79 | 6.27 - 15.8 |
| Trypsin | 5 | 49.6 | 2.48 | 54.2 | 68.7** | 1.34 - 1.7 | 26.9 - 34,1 |
*Total rate of absorption was calculated as radioactivity applied by oral administration reduced by the part of residual activity of the intestine without differentiation into high or low molecular weight material;
**Due to differences between concentration of high molecular weight proteases in serum and lymph, the calculated total amount of absorbed high molecular material is lower.
However, it must be realized that in this case the radioactive portion of the radio-labeled molecules could be broken off during the process of absorption. The measured radioactivity may be quantitatively incorrect.
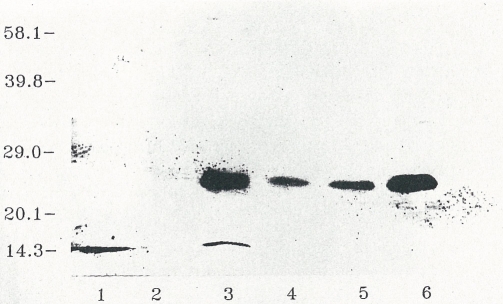
The presence of high molecular weight, obviously full size immune reactive bromelain, was confirmed in human blood samples after oral application [51, 52]. Plasma samples of the volunteers were drawn at the time of the maximum concentration after oral application and were immune precipitated with specific antibodies. The immune complexes were affinity isolated with Sepharose-bound goat IgG anti-rabbit IgG and dissociated with sodium dodecyl sulfate (SDS) and dithiothreitol (DTT). After separation of the plasma proteins by SDS gel electrophoresis, the protein bands were blotted from the gel to a PVDF-membrane. It was successively incubated with biotinylated anti-bromelain antibodies and extravidin-bound alkaline phosphatase. The color reaction catalyzed by the avidin-bound phosphatase revealed a protein band located at a molecular weight of 24 kDa (Figure 5, lanes 3-5). This value corresponds to the full-size bromelain molecule (lane 6). In the plasma of control individuals without bromelain application this protein band is not observed (lane 2).
Figure 5.
Presence of full-size bromelain in human plasma after oral intake of enteric-coated tablets in healthy male volunteers (figure 12 in [51]).
Until this point, we had concerned ourselves with the immunological intactness and the dimensions of the molecules. The next question to be answered was whether proteases may retain their original activity after absorption, and whether this activity could be measured. Activity of amylase could not be found in the serum, but it could be detected by its activity in the lymph of the test animals [52]. As the activity of horse radish peroxidase has also been detected in serum [13], these two examples provide sufficient evidence that proteins may be absorbed as physiologically intact molecules.
It is expected that the amount of free proteases is small in body fluids due to their binding to specific or unspecific anti-proteases. To prove this binding, radioactive (I125) bromelain was incubated with human citrated blood. A sample containing radioactive bromelain was subjected to high performance gel permeation chromatography. Individual fractions were examined by fuse rocket immune-electrophoresis (Figure 6B) on an agar layer that contained antibodies against α2-macroglobulin, α1-anti-trypsin and α1-anti-chymotrypsin. Subsequently the gel was exposed to a photographic film (Figure 6A). Most of the radioactivity was associated with the α2-macroglobulin precipitation line and, to a lesser extent with that of α1-anti-chymotrypsin [51].
Figure 6.
Binding of bromelain to plasma proteins. (A) Photographic film of radioactivity associated with plasma proteins. (B) Analysis of different fractions of separated plasma proteins with antibodies against α2-macroglobulin, α1-anti-trypsin and α1-anti-chymotrypsin (figure 14 in [51]).
So far, we can conclude that proteolytic enzymes are absorbed as intact, high molecular weight molecules, retaining their activity as either free proteases or anti-protease bound complexes.
Pharmacokinetics of proteolytic enzymes
For pharmacokinetic investigations it was necessary to develop a test system to identify the intact, active protease molecules in the plasma of healthy volunteers after oral application [51, 52]. It was supposed, due to earlier experience, that the amount of molecules to be estimated in plasma is low. Therefore, sufficient amounts of proteolytic enzymes had to be administered orally and a low level of detection was necessary. Bromelain was chosen to start pharmacokinetic characterization of orally applied proteolytic enzymes in humans. The first strategy using the purified 2 major fractions of the original purchased bromelain product to raise antibodies in rabbits by an evaluated immunization cocktail and schedules of administration of the antigen failed due to the fact that the sensitivity (0.2 ng/well) was too low to allow for detection of small amounts of bromelain in the plasma of volunteers after oral application.
The next method developed used a non competitive one-species antibody ELISA. Two rabbit antibodies were raised against different bromelain epitopes and one of them was biotinylated. The first antibody was absorbed to the surface of the well, incubated with plasma probes containing bromelain and then incubated with the second biotinylated antibody. This method reached a limit of detection of about 100 pg/ml and a range of linearity of 20-2.000 pg/assay. No interference with plasma proteins was detected and the accuracy of inter-assay variation ranged from 95% to 103%. This antibody was used to confirm full-size bromelain in plasma (Figure 5).
The best method to solve the problem of determination of low levels of free proteases in serum from volunteers was the measurement of the intrinsic proteolytic activity of bromelain [53] by an antibody-capture method. Bromelain was first enriched by using the antibody to concentrate the enzyme from the plasma of volunteers and a specific, sensitive fluorogenic substrate was selected. Plasma samples were incubated first in wells previously coated with antibodies. This allowed bromelain to be captured by plate-bound antibody, facilitating the subsequent proteolytic activity measurement. A specific fluorogenic substrate, Z-Arg-coumarin, was used. The enzymatic hydrolysis of this non-fluorescent compound results in formation of a highly fluorescent coumarin. By combination of the two methods, a practical limit of detection of 1 ng bromelain/ml plasma could be reached.
A randomized, controlled, double-blind pharmacokinetic clinical trial has been performed in 19 healthy white males, aged 18-45 years. Fifteen volunteers received enteric-coated film tablets, each one containing 200 mg Bromelain. Four were administered placebo tablets. During the first day of the clinical trial, 3 tablets were administered at 0800 (time zero of the investigation), 1100, 1400, 1700, and 2000, followed by 5 tablets at 2300. The same pattern was followed on the second day. On the third day, only 3 tablets were administered at 0800. The volunteers received standard meals at 0900, 1200, 1530, and 1830. Blood samples (12 ml) were drawn each time before bromelain administration. Plasma was assayed for the presence of immunoreactive bromelain.
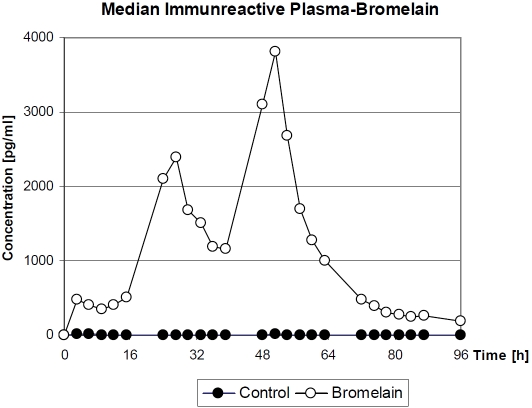
The subjects in the clinical trial showed significant variability in their plasma concentration profiles as well as in the profiles of individual proteolytic activity which matched the bromelain concentration profile (data not shown) and the median of immunoreactive bromelain shown in Figure 7. Consequently, the pharmacokinetic parameters peak plasma concentration (Cmax), timing of peak plasma concentration (Tmax), half-life (t½), and area under the curve (AUC) were calculated independently for each experiment (Table 4), assuming a mono-compartmental model of distribution. For most volunteers, Cmax was reached at ∼48 hours with an average of 5 ng/ml. To estimate the t½ of intestinally absorbed bromelain, the data of the decay phase of the second peak of the plasma concentration curve was used. Results from all volunteers show quite similar t½ values of bromelain in blood (average value, 6.07 hours). The AUC for the period of 3-51 hours was calculated from the available experimental data as 82.2 ng/h/ ml. The average blood concentration in this period, in individuals administered the protein orally, was 10.28 μg. The identity of bromelain was confirmed.
Figure 7.
Time course of plasma concentration of bromelain after oral administration expressed as medians of immunoreactive bromelain in plasma of recipients (n = 15) and in controls (n=4) [52].
Table 4.
Pharmacokinetic constants measured in plasma of healthy male volunteers after oral administration of enteric-coated tablets containing Bromelain [51], [52].
| Cmax (ng/ml) | Tmax (h) | t1/2 (h) | AUC (ng/h × ml) | |
|---|---|---|---|---|
| Volunteer 13 (Minimum) | 1.83 | 51 | 6.2 | 31.2 |
| Volunteer 08 (Maximum) | 9.47 | 24 | 7.1 | 215.1 |
| Mean | 4.93 | 44 | 6.1 | 82.2 |
| Standard Deviation | 2.40 | 11 | 1.2 | 42.0 |
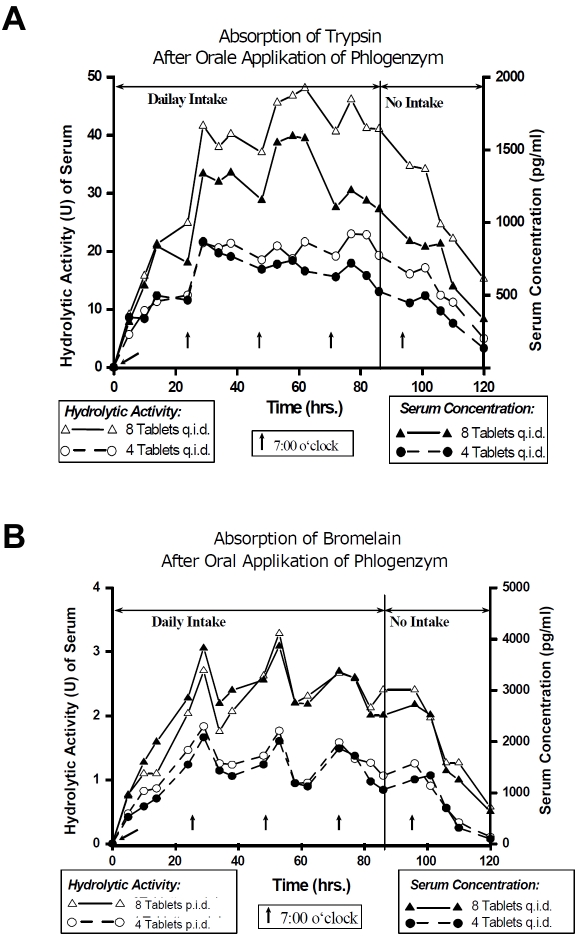
Using the new technique, a proteolytic enzyme combination consisting of trypsin, bromelain and rutosid was investigated in 20 healthy volunteers using two different doses (4 and 8 tablets four times a day) for 4 days in a crossover design. Plasma concentration curves and those for hydrolytic activity of trypsin and bromelain are shown in Figure 8 [54-56].
Figure 8.
Course of mean plasma concentration and hydrolytic activity of trypsin (A) and bromelain (B) in healthy volunteers after oral application of 2 dosages of an enzyme-rutoside combination product over 4 days (n =20) [54-56].
As a result, a dose-dependent linear increase of the maximum plasma levels was detected in the plasma of the volunteers (Table 5) [57] and the course of the proteolytic serum activity paralleled that of the plasma concentration (Figure 8A and 8B). This means that active bromelain and trypsin were detectable in significant (physiological) albeit small concentrations as free proteases. Additionally, the information for papain is presented in Table 5.
Table 5.
Linear dose dependency of maximum plasma levels depending on different daily doses in pharmacokinetic investigations.
| No. | Bromelain | Trypsin | Papain | ||||
|---|---|---|---|---|---|---|---|
| daily doses [g] | max. plasma concentration [ng/ml] | daily doses [g] | max. plasma concentration [ng/ml] | daily doses [g] | max. plasma concentration [ng/ml] | ||
| 1 | 1.08 | 2.5 | 0.38 | 1.2 | 1.44 | 2.5 | |
| 2 | 1.44 | 3.8 | 0.72 | 2.0 | 1.80 | 2.7 | |
| 3 | 2.16 | 4.8 | 0.77 | 2.2 | 2.88 | 6.0 | |
| 4 | 2.88 | 5.6 | 1.44 | 3.7 | 3.60 | 5.2 | |
To summarize, data from pharmacokinetic investigations show a dose-depending linearity of maximum plasma levels, despite a high inter-individual variability. The detected concentrations are comparable to the concentration of body's own proteases, and the administration of protease-combinations does not interfere. The kinetic shows an unusual invasion and elimination kinetic (slow velocity of absorption, fast and 100% protein binding to anti-proteases) [57].
Mechanism of absorption of proteases
To further investigate the absorption mechanisms of proteases through the natural barrier of the gastrointestinal surface, the ex vivo, native tissue model using the human colon carcinoma cell line Caco-2 was used. The cells of a post-confluent culture of a Caco-2 mono-layer are similar to normal small intestinal enterocytes [58] and may be used for the measurement of selectivity as well as confluence and permeability by measuring the trans-epithelial electrical resistance (TEER) [59]. Transport of fluorescent markers normally not transported across the intact mono-layers is used as a measure for the leakage of the monolayer.
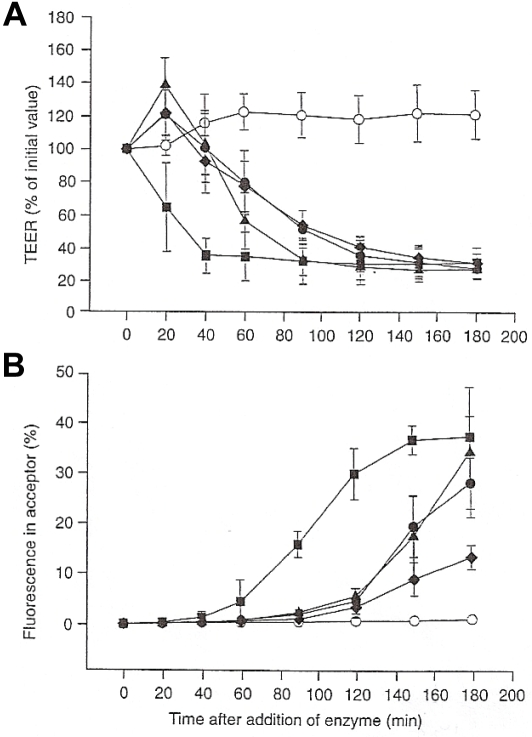
The effects of proteases on the confluence and permeability of the differentiated Caco-2 monolayer were examined using fluorescein (MW 400) as a transport marker and by measuring the TEER [60]. A time and concentration dependent effect of trypsin, chymotrypsin, papain and bromelain both on TEER (Figure 9A) and on the transport of fluorescein across the monolayer was observed (Figure 9B). Proteases showed different effect sizes with the plant proteases being more active than the animal ones: (papain > bromelain > trypsin > chymotrypsin). Both effects, decrease of TEER simultaneously with the increase of marker transport, are indicative for a loosening of the tight junction between epithelial monolayer of Caco-2 cells. Further experiments confirmed that even fluorescent transport markers with a molecular weight of up to 600,000 Dalton are transported across the Caco-2 monolayer, and reversibility experiments revealed that the barrier function of the Caco-2 cell mono-layer was altered after incubation with proteases, but cells completely recovered after a few days. It seemed that some of the cells were removed from the monolayer, whereas cell death, caused by the enzymes, was unlikely.
Figure 9.
Influence of different proteases on Caco-2 mono-layer integrity [modified according to Kolac et al., 1996 in reference 60]. (A) Influence of proteases on the Trans-Epithelial Electrical Resistance (TEER) of confluent Caco-2 mono-layer (o) control with fluorescein; (•) 17.75 F.I.P.-units/well trypsin in KRB; (▴) 1.23 F.I.P.-units/well bromelain in KRB; (▪) 1.75 F.I.P.-units/well papain in KRB; (♦) 745 F.I.P.-units/ well chymotrypsin in KRB. (B) Influence on transport of the transport marker substrate fluorescein (legend as before). FITC = Fluoroisothiocyanate; DMEM = Dulbecco's Modified Eagle Medium; kDA = Kilo-Dalton; KRB = Krebs Ringer buffer.
The Caco-2 cell model is lacking some physiological factors with influence on the absorption of macromolecules, e.g. the unstirred water layer, the presence of mucus, food, and components of blood plasma (albumin, antiproteases). The addition of mucin and albumin to the Caco-2 mono-layer had different effects on the activity of proteases on TEER and fluorescein transport, suggesting different mechanisms of action of proteases. The activity of trypsin and chymotrypsin was widely unaffected, that of papain was decreased by mucin and that of bromelain was increased by albumin. Albumin did not have any inhibitory effect on the proteases.
The absorption of proteases across the intestinal mucosa could be interpreted as self-enhanced paracellular diffusion through locally widened intercellular junctions. This hypothesis is further supported by the already known strong mucolytic activity of bromelain, trypsin and papain, based upon cleavage of amino acid binding sequence of mucus glycoproteins. Proteases are widely used in cell isolation for their ability to degrade extra-cellular matrix components. As shown above, the tightness of intra-cellular junctions is affected, opening the paracellular route across epithelium for non absorbable compounds. Within some tissues, papain has proved to be less damaging and more effective than other proteases. Enhancement of further low molecular weight compounds through the small intestine mucosa could also be shown [61].
Orally applied proteolytic enzymes reach concentration in the gastrointestinal tract several folds higher than in the used in vitro experiment, making the mechanism of paracellular transport even more reasonable, and the regeneration of the effects further support the safety of orally applied proteolytic enzyme in clinical use.
During the last decade, a possible role of proteases as signalling molecules has been emphasized with the discovery of a novel class of G-protein coupled receptors located on cell membranes that may be activated by proteolytic cleavage of their N-terminal extra cellular domain. Type 2 protease-activated receptors (PAR-2) are cleaved by serine-proteases such as trypsin and tryptase. PAR-2 is present in many intestinal cell types and particularly on epithelial cells. Multiple functions have been demonstrated in the gut for PAR-2, including epithelial permeability, mainly the intercellular permeability that is of paramount importance in the equilibrium between the external milieu (digestive contents) and the submucosal immune system. Alterations of both tissue and luminal levels of proteases or serine-protease activity may affect gut permeability and subsequently the immune status of the mucosa. Activation of PAR-2 on epithelial cells may directly affect cytoskeleton contraction by triggering phosphorylation of myosin light chain with subsequent changes in tight junction permeability. Thus, not only absorption of proteases is modified but also influences on changes in permeability occur. This opens a new field for further clinical evaluations [8,9].
Safety aspects for oral application of proteases
As described, the single components of the orally applied proteolytic enzyme combination are absorbed in the gut, probably supported by a self-enhanced paracellular transport mechanism. They reach plasma concentrations of free proteases from customarily applied doses in the same order of magnitude of naturally occurring body's own proteases. The amount may be sufficient for their effects in the body.
The proteases are able to be detected in the serum after oral application in sub molecular concentrations, as it has been described for other physiologically active protease (e.g. proteases of the coagulation system). According to Klimmek [57] the maximum concentrations reached in plasma are a function of the daily dose of the single proteases and are independent from the combination of proteases applied (see Table 5). From a statistical point of view the correlation may be expressed by either (y = a · x + b) or by geometric regression (y = a · xb). Initially, the linear regression is valid only for the dose intervals used. They only allow drawing a conclusion concerning the maximum concentration of free protease in the plasma reached in the investigated dose range. The amount of maximum concentrations below and above the investigated dose ranges may not be determined by a simple linear extrapolation due to the fact that the regression slopes are not going through zero. Despite that, maximum protease concentrations may be estimated by the help of the geometric regression for different daily doses of bromelain, trypsin and papain (Table 6). An increase of dose may, despite initial or continued dosing, not contribute to a maintained increase of the concentration of free proteases in serum. Therefore, the risk of a strong increase of the concentration of free proteases is low.
Table 6.
Point estimates for the plasma concentrations y [ng/ml] of free soluble bromelain, trypsin and papain dependent from daily dose x [g] in humans deriving from corresponding geometric regression equation [57].
| Daily dose × [g] | Bromelain | Trypsin | Papain |
|---|---|---|---|
| y = 2,5588×0.7863 | 2.7061×0.8466 | 1.6916×0.9867 | |
| 0 | 0 | 0 | 0 |
| 0.1 | 0.41854 | 0.38525 | 0.17442 |
| 0.2 | 0.72183 | 0.69278 | 0.34564 |
| 0.3 | 0.99288 | 0.97651 | 0.51567 |
| 0.4 | 1.24491 | 1.24580 | 0.68494 |
| 0.5 | 1.48367 | 1.50485 | 0.85363 |
| 0.6 | 1.71237 | 1.75601 | 1.02188 |
| 0.8 | 2.14702 | 2.24027 | 1.35730 |
| 1.0 | 2.55880 | 2.70610 | 1.69160 |
| 1.5 | 3.51963 | 3.81437 | 2.52375 |
| 2.0 | 4.41302 | 4.86627 | 3.35215 |
| 3.0 | 6.07011 | 6.85922 | 5.00119 |
| 4.0 | 7.61090 | 8.75080 | 6.64279 |
The safety of orally applied protease combinations is also documented by calculation. The occurrence of free proteases in the blood is limited by time and derives from a rapid complex formation with anti-proteases (e.g. α2-macroglobulin). The complex formation follows the law of mass action and reaches for the absorbed protease 100% with time. Not only the foreign protease but also body's own proteases verifiably have only a tiny chance to escape the catching by α2-macroglobulin.
The amount and the occurrence of α2-macroglobulin have been excessively investigated without detecting a complete deficiency of this macroglobulin. It indicates that α2-macroglobulin is of vital importance in man. Depending on the velocity, about 0.2% (being very rational from a practical point of view) and after ingestion of 800 mg protease about 16% (practically very improbable) of the naturally occurring α2-macroglobulin reservoir might be irreversibly and cumulatively occupied and made available for a rapid elimination in the liver. A complete occupation of the body's α2-macroglobulin reserve by orally applied proteases is more than unlikely. Therefore, the components of the protease combinations are safe even in high doses due to these pharmacological mechanisms.
Biological activity of proteolytic enzymes
The biological activity of proteases is deriving either from their site-specific hydrolytic cleavage activity or their binding to anti-proteases.
The hydrolytic activity is essential in biological processes, and thousands of different proteases are already known. Their activity can be differentiated by their substrate specificity, pH-dependency, confirmation and modification (e.g. anti-protease binding). After oral administration and absorption, free proteases may be active in body fluids and even damaged tissue despite their low concentrations (pmol - nmol) as long as the complex with anti-proteases is not formed [13, 50, 62].
The binding to anti-proteases is required to protect the organism from self destruction. If proteases' concentration, from whatever source, increases locally, their activity must be controlled. Mostly, proteases are activated as a response to changes in the environment e.g. metabolic changes, injury, invading pathogens or negative chronic effects like oxidative stress or inflammation, either acute or chronic by auto reactive antibodies. This makes them an ideal signal molecule to participate in regulatory processes. As all free proteases are harmful to cells, tissues and even organisms, they are all controlled by unique mechanisms such as inactive precursor generation or binding to anti-proteases. Obviously the organism does not differentiate proteases according to their origin (foreign plant or animal as well as body's own proteases). The anti-proteases can bind a variety of proteases [63, 7].
Biological activity of anti-protease complexes
It is not known which residual hydrolytic activity is expressed by anti-protease bound proteases. Low molecular weight substrates and probably peptides may be hydrolysed.
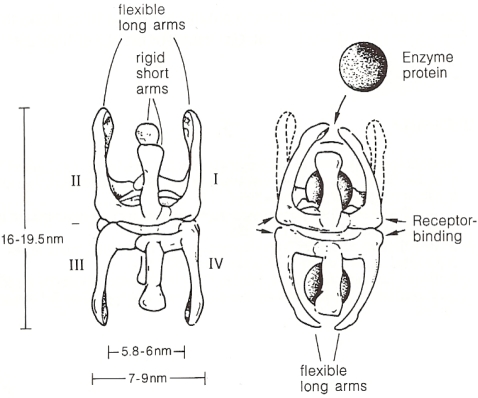
Figure 10 shows protease molecules entrapped by α2-macroglobulin [63]. The resulting complexes are recognized by low density lipoprotein receptor-related proteins on the surface of blood cells and hepatocytes for rapid elimination and activating the production of anti-proteases; a necessity, if their consumption due to acute or chronic processes is increased.
Figure 10.
The structural changes in the α2-macroglobulin molecule are identical through the binding of one or two enzyme molecules. In both cases the “fast” form is created from the slow eliminated form relatively quickly, phagocytosed by the cells of the mononuclear phagocytotic system or by hepatocytes (figure 12 in [63]).
Anti-proteases have a half life of several days. Structural changes of the α2-macroglobulin molecules after binding of 1 or 2 protease molecules in both cases generate a “fast”-form, which is, in contrast to the “slow”-form, eliminated within minutes by macrophages and hepatocytes [64, 65].
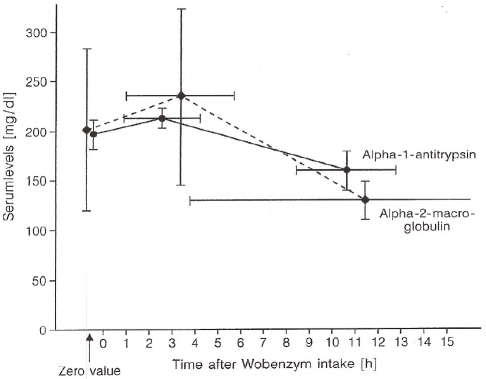
As shown in Figure 11, there is a significant increase of plasma anti-protease levels after oral administration of an enzyme-rutoside combination (Wobenzym®) [50]. The advantages of such increases of hydrolytic activity and anti-protease levels need to be further investigated. Hydrolytic serum activity is reduced with increasing age and is under diurnal control. Several chronic (arthritis) [66] or acute diseases (heavy burns) are accompanied by reduced anti-protease levels (high consumption by free proteases) [67]. Beneficial effects from consumption of food additives or therapeutics might arise by increase of anti-protease levels after oral administration of protease combinations.
Figure 11.
Plasma concentration curves of α2-macroglobulin and α1-anti-trypsin in healthy volunteers after oral application of 12.5 g Wobenzym® hydrolase mixture (figure 11 in [63]).
The activated anti-protease-complex with α2-macroglobulin has an essential function in the course of acute or chronic inflammation. It is able to bind different cytokines and growth factors. Obviously, this mechanism is essential for the elimination of such mediators from the inflammatory tissue [68-71].
The activated anti-protease-complex is subject to further modifications. Once formed, it may be oxidized depending on the local conditions (e.g. pro-inflammatory radical formation). The oxidized form preferentially binds cytokines that stimulate the immune process (TH-1-cytokines). If the oxidizing activity is reduced during ongoing inflammation/repair process, the preference is changed to preferential binding of TH-2-cytokines and growth factors. This process might be a trigger mechanism in the course of the immune reaction [72, 73].
The central role of the activated anti-protease-complex is further substantiated by the knowledge that it induces G-protein-receptor-coupled signal transduction and second messenger production. A serpine-enzyme complex receptor [74, 75] as well as an activated α2-macroglobulin-signalling receptor different from lipoprotein-related protein receptor involved in degradation of the complex have been described [76].
Biological activity based on hydrolytic cleavage
In addition to the activities mediated by the complex with an anti-protease, orally provided free proteolytic enzymes may influence cell activity by different hydrolytic mechanisms. First, cells may be (in-)activated or desensitized by proteolytic cleavage of cell surface receptors generating soluble peptides or proteins. As an example the interaction of trypsin and chymotrypsin on protease activated receptors (PAR) is described [77]. Actually four different subtypes have been described with about 30% homology but less sequence homology. They owe diverse functions, are participating in many biological processes, and span across the membrane (7 loops) with an intracellular binding site for G-protein(s) [78]. The presently known biological effectors are shown in Table 7.
Table 7.
(De-)Activators and desensitizer of protease-activated-receptor (PAR) 1 to 4 (modified according to [78])
| Activators | Deactivators |
|---|---|
| Thrombin (+++); [Trypsin, Granzyme A, Plasmin (-)] | Cathepsin G, Elastase, Plasmin, Proteinase 3, Chymotrypsin (Sensitivity, down regulation) |
| Trypsin (+++), Tryptase, Factor Xa, Proconvertin | Cathepsin G, Plasmin, Proteinase 3 |
| Thrombin, Trypsin, Factor X | Cathepsin G, Elastase |
| Thrombin, Trypsin, Cathepsin G | ? |
The different proteases cleave the PAR receptor at a particular site, unmasking a previously cryptic N-terminal sequence of the receptor, defined as “tethered ligand”. This tethered ligand sequence interacts with the conserved second extra cellular loop and activates the same receptor. If no tethered ligand is formed, but a respective protease is bound, the receptor is desensitized. If a tethered ligand is bound though no cleavage of the tethered ligand sequences occurred, the receptor is inactivated.
Trypsin is an agonist of PAR-1 (weak) and PAR-2 (strong), whereas chymotrypsin is an antagonist by desensitizing the receptor's activity. Steinhoff et al. described biologic activity of trypsin and chymotrypsin on signal transduction cascades and production of second messengers leading to apoptosis, supporting host defense, regulating immune modulation, inflammation and others [78].
Summary
Orally administered proteolytic enzymes can be detected transiently as intact, high molecular weight, physiologically active protein molecules, either free (nanomolar concentrations) or in a complex with anti-proteases in plasma, lymph, or injured tissue. Data from pharmacokinetic investigations reveal a dose-depending linearity of maximum plasma levels, a high inter-individual variability, plasma concentrations comparable to body's own proteases, no interference by administration of protease-combinations, an unusual invasion and elimination kinetic (slow velocity of absorption, fast and 100% protein binding to anti-proteases). Oral application of proteases leads to increased proteolytic serum activity and increased plasma concentrations of the corresponding anti-proteases. Biological activity of orally administered proteases is determined by their proteolytic activity as free proteases on cell surface receptors (e.g. protease activated receptors) or soluble peptides/proteins and their activity in a complex formed with anti-proteases. The complex of proteolytic enzyme and anti-protease induces increased plasma concentrations of anti-proteases and elimination of anti-protease-complexes and cytokines.
Oral administration of enteric coated tablets containing proteolytic enzymes of plant and animal origin may stabilize or probably enhance a variety of physiological and immunological processes even in healthy consumers.
Acknowledgments
Figures 5, 6, 10, 11 have been kindly authorized by Springer Science and Business Media and the authors. The author wishes to thank Sonja Raum, Munich, for critical reading of the manuscript.
References
- 1.Roxas M. The role of enzyme supplementation in digestive disorders. Alt Med Rev. 2008;13:307–314. [PubMed] [Google Scholar]
- 2.Leipner J, Iten F, Saller R. Therapy with proteolytic enzymes in rheumatic disorders. Biodrugs. 2001;15:779–789. doi: 10.2165/00063030-200115120-00001. [DOI] [PubMed] [Google Scholar]
- 3.Leipner J, Saller R. Therapy with proteolytic enzymes in oncology. Drugs. 2001;59:769–780. [Google Scholar]
- 4.Middleton E, Kandaswami C. The impact of plant flavonoids on mammalian biology: implications for immunity, inflammation and cancer. In: Harborne JB, editor. The flavonoids: advances in research since 1986. London: Chapman & Hall; 1993. pp. 619–652. [Google Scholar]
- 5.Boots AW, Haenen GRMM, Bast A. Health effects of Quercetin: From antioxidant to neutraceutical. Europ J Pharmacol. 2008;585:325–337. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Cichoke AJ. Pancreatic enzymes. In: Pizzorno J, Murray M, editors. Textbook of Natural Medicine. St Louis, MO: Churchill Livingstone; 2006. pp. 1131–1146. [Google Scholar]
- 7.Barrett AJ, Starkey PM. The Interaction of α2-macroglobulin with proteinases. Biochem J. 1973;133:709–724. doi: 10.1042/bj1330709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bueno L, Fiomonti J. Protease-activated receptor 2 and gut permeability: a review. Neurogastroenterol Motil. 2008;20:580–587. doi: 10.1111/j.1365-2982.2008.01139.x. [DOI] [PubMed] [Google Scholar]
- 9.Hollenberg MD, Houle S. Proteinases as hormone-like signal messengers - proteinase-activated receptors and the pathophysiology of inflammation, pain, cardiovascular disease and cancer. Swiss Med Wkly. 2005;135:425–436. doi: 10.4414/smw.2005.11037. [DOI] [PubMed] [Google Scholar]
- 10.Borth W. α2-macroglobulin, a multifunctional binding protein with targeting characteristics. FASEB. 1994;6:3345–3353. doi: 10.1096/fasebj.6.15.1281457. [DOI] [PubMed] [Google Scholar]
- 11.Gardner MLG. Intestinal assimilation of intact peptides and proteins - a neglected field? Biol Rev. 1984;59:289–331. doi: 10.1111/j.1469-185x.1984.tb00708.x. [DOI] [PubMed] [Google Scholar]
- 12.Gardner MLG. Absorption of intact proteins and peptides. In: Johnson LR, editor. The physiology of the gastrointestinal tract. New York: Raven Press; 1994. pp. 1795–1820. [Google Scholar]
- 13.Gardner MLG. A review of current knowledge of gastrointestinal absorption of intact proteins including medicinal preparations of proteolytic enzymes. In: Gardner MLG, Steffens KJ, editors. Absorption of orally administered Enzymes. Berlin: Springer Verlag; 1995. pp. 1–7. [Google Scholar]
- 14.Skogh T. Overestimate of 125J-protein uptake from adult mouse gut. Gut. 1982;23:1077–1080. doi: 10.1136/gut.23.12.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bohe M, Borgström A, Genell S, Ohlsson K. Characterization of radioactivity in plasma after intraduodenal administration of 131I-labelled human cationic trypsin. Scand J Gastroenterol Suppl. 1986;126:21–24. doi: 10.3109/00365528609091887. [DOI] [PubMed] [Google Scholar]
- 16.Silverman GA, Bird PI, Carrell RW, Church FC, Coughlin PB, Gettins PG, Irving JA, Lomas DA, Luke CJ, Moyer RW, Pemberton PA, Remold-O'Donnell E, Salvesen GS, Travis J, Whisstock JC. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J Biol Chem. 2001;276:33293–33296. doi: 10.1074/jbc.R100016200. [DOI] [PubMed] [Google Scholar]
- 17.Jakobsson I, Lindberg T, Lothe L, Axelson I, Benediktsson B. Human ß-Iactoglobulin as a marker of macromolecular absorption. Gut. 1986;27:1029–1034. doi: 10.1136/gut.27.9.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Husby S, Jensenius SC, Svehag SE. Passage of undegraded dietary antigen into the blood of healthy adults. Further characterisation of the kinetics of uptake and the size distribution of the antigen. Scand J Imrnunol. 1986;24:447–455. doi: 10.1111/j.1365-3083.1986.tb02133.x. [DOI] [PubMed] [Google Scholar]
- 19.Paganelli R, Levinsky RI. Solid phase radioimmunoassay for detection of circulating food protein antigen in human serum. I Immunol Methods. 1980;37:333–341. doi: 10.1016/0022-1759(80)90319-1. [DOI] [PubMed] [Google Scholar]
- 20.Heyman M, Boudraa G, Sarrut S, Giraud M, Evans L, Touhami M, Desjeux JF. Macromolecular transport in jejunal mucosa of children with severe malnutrition: a quantitative study. J Pediatr Gastroenterol Nutr. 1984;3:357–363. doi: 10.1097/00005176-198406000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Bockman DE, Winbom WB. Light and electron microscopy of intestinal ferritin absorption. Observations in sensitized and non-sensitized hamsters (Mesocricetus auratus) Anat Rec. 1966;155:603–662. [Google Scholar]
- 22.Walker WA, Isselbacher KJ. Uptake and transport of macromolecules by the intestine. Possible role in clinical disorders. Gastroenterol. 1974;67:531–550. [PubMed] [Google Scholar]
- 23.Heyman M, Desjeux JF. Significance of intestinal food protein transport. J Pediatr Gastroenterol Nutr. 1992;15:48–57. [PubMed] [Google Scholar]
- 24.Nishi N, Arimura A, Coy DH, Vilchez-Martinez JA, Schally AV. The effect of oral and vaginal administration of synthetic LHRH and D-ALA6, DES GLY-10-NH2]-LH-RH ethylamide on serum LH levels in ovariectomized, steroid blocked rats. Proc Soc Exp Biol Med. 1975;148:1009–1012. doi: 10.3181/00379727-148-38678. [DOI] [PubMed] [Google Scholar]
- 25.Amoss M, Rivier J, Guillemin R. Release of gonadotrophins by oral administration of synthetic LRF or a tripeptide fragment of LRF. Clin Endocrinol Metab. 1972;35:175–177. doi: 10.1210/jcem-35-1-175. [DOI] [PubMed] [Google Scholar]
- 26.Lundin S, Vilhardt H. Absorption of 1-deamino-8-D-arginine vasopressin from different regions of the gastrointestinal tract in rabbits. Acta Endocrinol (Copenh) 1986;112:457–460. doi: 10.1530/acta.0.1120457. [DOI] [PubMed] [Google Scholar]
- 27.Lundin S, Vilhardt H. Absorption of intragastrically administered DDAVP in conscious dogs. Life Sci. 1986;38:703–709. doi: 10.1016/0024-3205(86)90584-9. [DOI] [PubMed] [Google Scholar]
- 28.Vilhardt H, Lundin S. In vitro intestinal transport of vasopressin and its analogues. Acta Physiol Scand. 1986;126:601–607. doi: 10.1111/j.1748-1716.1986.tb07861.x. [DOI] [PubMed] [Google Scholar]
- 29.Vilhardt H, Lundin S. Biological effect and plasma concentrations of DDAVP after intranasal and peroral administration to humans. Gen Pharmacol. 1986;17:481–483. doi: 10.1016/0306-3623(86)90198-9. [DOI] [PubMed] [Google Scholar]
- 30.Williams TDM, Dunger DB, Lyon CC, Lewis RJ, Taylor F, Lightman SL. Antidiuretic effect and pharmacokinetics of oral l-desamino-8-D-arginine vasopressin. 1. Studies in adults and children. J Clin Endocrinol Metab. 1986;63:129–132. doi: 10.1210/jcem-63-1-129. [DOI] [PubMed] [Google Scholar]
- 31.Ormiston RJ. Clinical effects of TRH on TSH after i.v. and oral administration in normal volunteers and patients with thyroid disease. In: Hall H, Wemer I, Holgate H, editors. Thyreotropin releasing hormone. Basel: Karger; 1972. pp. 45–52. [Google Scholar]
- 32.Schwert GW, Takenaka Y. A spectrophotometrie determination of trypsin and chymotrypsin. Biochim Biophys Acta. 1955;16:570–575. doi: 10.1016/0006-3002(55)90280-8. [DOI] [PubMed] [Google Scholar]
- 33.Kabacoff BL, Umkey M, Wohlman A, Avakian S. Sensitive und reproducible assay method for chymotrypsin. J Pharm Sci. 1963;52:1188–1190. doi: 10.1002/jps.2600521221. [DOI] [PubMed] [Google Scholar]
- 34.Pryty B, Kabacoff L, Umhey M, Wohlman A, Avakian S. Abstracts of papers presented at the Cincinnati, Ohio, Meeting of the American Chemical Society Jan. 1963. Assay of chymotrypsin; pp. 13–17. [Google Scholar]
- 35.Ambrus JL, Lassman HB, DeMarchi JJ. Absorption of exogenous and endogenous proteolytic enzymes. Clin Pharmacol Ther. 1967;8:362–368. doi: 10.1002/cpt196783362. [DOI] [PubMed] [Google Scholar]
- 36.Martin GJ, Brendel R, Beiler M. Absorption of enzymes from the intestinal tract. Am J Pharm. 1957;127:194–197. [PubMed] [Google Scholar]
- 37.Martin GJ, Bogner RL, Edelman A. Further in vivo observations with radioactive trypsin. Am J Pharm. 1957;129:386–392. [PubMed] [Google Scholar]
- 38.Miller JM, Williard RF, Polachek A. An investigation of trypsin I-131 in patients. Exp Med Surg. 1960;18:352–370. [PubMed] [Google Scholar]
- 39.Bogner R, Snyder C. High dosage oral chymotrypsin as an adjunct in plastic surgery. J Internat Coll Surgeons. 1962;37:289–295. [PubMed] [Google Scholar]
- 40.Kabacoff BL, Wohlman A, Umhey M, Avakian S. Absorption of Chymotrypsin from the intestinal tract. Nature. 1963;199:815–817. doi: 10.1038/199815a0. [DOI] [PubMed] [Google Scholar]
- 41.Avakian S. Further studies on the absorption of Chymotrypsin. Clin Pharmacol Ther. 1964;5:712–715. doi: 10.1002/cpt196456part1712. [DOI] [PubMed] [Google Scholar]
- 42.Kabacoff BL. In Bodi T. Modifications of tissue permeability by orally administered proteolytic enzymes in man. Exp Med Surg. 1965;23:51–62. [Google Scholar]
- 43.Megel H, Strauss R, Ho R, Beiler M. Detection of trypsin-like activity in the plasma of rats after oral administration of trypsin. Arch Biochem Biophys. 1964;108:193–199. doi: 10.1016/0003-9861(64)90375-3. [DOI] [PubMed] [Google Scholar]
- 44.Faudemay F, Laporte JC, Trémolières J. Passage de la trypsine à travers la paroi intestinale de rat in vitro. Nutr Metab. 1973;15:207–212. [PubMed] [Google Scholar]
- 45.Moriya H, Moriwaki C, Akimoto S, Yamaguchi K, Iwadare M. Studies on the passage of α-chymotrypsin across the intestine. Chem Pharm Bull. 1967;15:1662–1668. doi: 10.1248/cpb.15.1662. [DOI] [PubMed] [Google Scholar]
- 46.Miller JM, Opher AW. The increased proteolytic activity of human blood serum after the oral administration of bromelain. Exp Med Surg. 1964;22:277–280. [Google Scholar]
- 47.White RR, Crawley FEA, Vellini M, Rovati LA. Bioavailability of 125I-bromelain after oral administration to rats. Biopharmaceut Drug Dispos. 1988;9:397–403. doi: 10.1002/bod.2510090408. [DOI] [PubMed] [Google Scholar]
- 48.Seifert J, Ganser R, Brendel W. Absorption of a proteolytic enzyme originating from plants out of the gastro-intestinal tract into blood and lymph of rats. Z Gastroenterol. 1979;17:1–8. [PubMed] [Google Scholar]
- 49.Seifert J, Siebrecht P, Lange JP. Quantitative Untersuchungen zur Resorption von Trypsin, Chymotrypsin, Amylase, Papain und Pankreatin aus dem Magen-Darm-Trakt nach oraler Applikation. Allgemeinmedizin. 1990;19:132–137. [Google Scholar]
- 50.Seifert J, Siebrecht D, Lange JP, Axt G, Bambas FB. The quantitative absorption or orally administered proteins and histological evidence of enzymes in the wound. In: Gardner MLG, Steffens KJ, editors. Absorption of orally administered Enzymes. Berlin: Springer Verlag; 1995. pp. 29–38. [Google Scholar]
- 51.Castell JV. Intestinal absorption of undegraded Bromelain in humans. In: Gardner MLG, Steffens KJ, editors. Absorption of orally administered Enzymes. Berlin: Springer Verlag; 1995. pp. 47–60. [Google Scholar]
- 52.Castell JV, Friedrich G, Kuhn CS, Poppe GE. Intestinal absorption of undegraded proteins in men: presence of bromelain in plasma after oral intake. Am J Physiol. 1997;273:G139–146. doi: 10.1152/ajpgi.1997.273.1.G139. [DOI] [PubMed] [Google Scholar]
- 53.Rowan AD, Buttle DJ, Barrett AJ. The cysteine proteinases of the pineapple plant. Biochem J. 1990;266:869–875. [PMC free article] [PubMed] [Google Scholar]
- 54.Roots I, Donath F, Rex A, Mai I. Institut für Klinische Pharmakologie. Berlin: 1995. Pilotstudie zur Untersuchung der relativen Bioverfügbarkeit von Trypsin aus zwei Peroralia. [Google Scholar]
- 55.Roots I. Fakultät Humboldt-Universität Berlin. Germany: 1997. Bioverfügbarkeit von Trypsin, Bromelain und Rutin-Metaboliten nach oraler Gabe von Phlogenzym® bei gesunden Probanden. Randomisierte doppelblinde Crossover-Studie gemäß GCP. Study No MU-695 427. Institut für Klinische Pharmakologie der Med. [Google Scholar]
- 56.Donath F, Roots I, Mai I, Maurer A, Wood G, Kuhn CS, Friedrich G. Dose-related bioavailability of bromelain and trypsin after repeated oral administration. Eur J Pharmacol. 1997;52:A146. [Google Scholar]
- 57.Klimmek R. Expert Report on Wobenzym N. 2006. [Google Scholar]
- 58.Pinto M. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol Cell. 1983;47:323–330. [Google Scholar]
- 59.Powell DW. Barrier function of epithelia. Am J Physiol. 1981;241:G275–G288. doi: 10.1152/ajpgi.1981.241.4.G275. [DOI] [PubMed] [Google Scholar]
- 60.Kolac C, Streichhan P, Lehr CM. Oral bioavail-ability of proteolytic enzymes. Europ J Pharma Biopharm. 1996;42:222–232. [Google Scholar]
- 61.Grabovac V, Schmitz T, Föger F, Bernkop-Schnürch A. Papaan: an effective permeation enhancer for orally administered low molecular weight heparin. Pharm Res. 2007;24:1001–1006. doi: 10.1007/s11095-006-9226-8. [DOI] [PubMed] [Google Scholar]
- 62.Siebrecht D, Lange PJ, Seifert J. The absorption of enzymes from the gut and their effects on tissue repair. Eur Surg Res. 1994;26:37. [Google Scholar]
- 63.Streichhan W, van Schaik W, Stauder G. Bioavailability of therapeutically used hydrolytic enzymes. In: Gardner MLG, Steffens KJ, editors. Absorption of orally administered Enzymes. Berlin: Springer Verlag; 1995. pp. 83–94. [Google Scholar]
- 64.Birkenmeier G, Usbeck E, Schaffer A, Otto A, Glander HJ. Prostate-specific antigen triggers transformation of seminal alpha-2-macroglobulin (alpha-2-M) and its binding to alpha-2-macroglobulin receptor/low-density lipoprotein receptor-related protein (α2-M-R/ LRP) on human spermatozoa. The Prostate. 1998;36:219–225. doi: 10.1002/(sici)1097-0045(19980901)36:4<219::aid-pros2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 65.Kounnas MZ, Church F, Argraves WS, Strickland DK. Cellular internalization and degradation of antithrombin III-thrombin, heparin cofactor II-thrombin, and α1-antitrypsin-trypsin complexes is mediated by the low density lipoprotein receptor-related Protein. J Biol Chem. 1996;271:6523–5429. doi: 10.1074/jbc.271.11.6523. [DOI] [PubMed] [Google Scholar]
- 66.Okonski M, Gregosiewicz A, Gil L. The protease-antiprotease system in the pathogenesis of transient arthritis of a single joint in children. Chir Narzadow Ruchu Ortop Pol. 1990;55:205–208. [PubMed] [Google Scholar]
- 67.Faymonville ME, Micheels J, Bodson L, Jacquemin D, Lamy N, Adam J, Duchateau J. Biochemical investigations after burning injury: complement system, protease-antiprotease balance and acute-phase reactants. Burns including thermal injury. 1987;13:26–33. doi: 10.1016/0305-4179(87)90252-x. [DOI] [PubMed] [Google Scholar]
- 68.Garber TR, Gonias SL, Webb DJ. Interleukin-4 and IL-10 bind covalently to activated human α2-macroglobulin by a mechanism that requires Cys949. J Interferon Cytokine Res. 2000;20:125–131. doi: 10.1089/107999000312522. [DOI] [PubMed] [Google Scholar]
- 69.LaMarre J, Wollenberg GK, Gonias SL, Hayes MA. Biology of disease. Cytokine binding and clearance properties of proteinase-activated α2-macroglobulins. Lab Invest. 1991;65:3–14. [PubMed] [Google Scholar]
- 70.LaMarre J, Hayes MA, Wollenberg GK, Hussaini I, Hall SW, Gonias SL. A α2-macroglobulin receptor-dependent mechanism for the plasma clearance of transforming growth factor β1 in mice. J Clin Invest. 1991;87:39–44. doi: 10.1172/JCI114998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bhattacharjee G, Asplin AR, Wu SM, Gawdi G, Pizzo SV. The conformation-dependent interaction of α2-macroglobulin with vascular endothelial growth factor. J Biol Chem. 2000;275:26806–26811. doi: 10.1074/jbc.M000156200. [DOI] [PubMed] [Google Scholar]
- 72.Wu SM, Boyer CM, Pizzo SV. The binding of receptor-recognized α2-macroglobulin to the low density lipoprotein receptor-related protein and the α2M signaling receptor is decoupled by oxidation. J Biol Chem. 1997;272:20627–20635. doi: 10.1074/jbc.272.33.20627. [DOI] [PubMed] [Google Scholar]
- 73.Wu SM, Dhavalkumar DP, Pizzo SV. Oxidized α2-macroglobulin (α2-M) differentially regulates receptor binding by cytokines/growth factors: Implications for tissue injury and repair mechanisms in inflammation. J Immunol. 1998;161:4356–4365. [PubMed] [Google Scholar]
- 74.Perlmutter DH, Glover GI, Rivetna M, Schasteen CS, Fallon RJ. Identification of a serpin-enzyme complex receptor on human hepatoma cells and human monocytes. PNAS. 1990;87:3753–3757. doi: 10.1073/pnas.87.10.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moraga F, Lindgren S, Janciauskiene S. Effects of noninhibitory α1-antitrypsin on primary human monocyte activation in vitro. Arch Biochem Biophys. 2001;386:221–226. doi: 10.1006/abbi.2000.2211. [DOI] [PubMed] [Google Scholar]
- 76.Misra UK, Chu CT, Gawdi G, Pizzo SV. Evidence for a second alpha-2-macroglobulin receptor. J Biol Chem. 1994;269:12541–12547. [PubMed] [Google Scholar]
- 77.Déry O, Bunnett NW. Proteinase-activated Receptors: A Growing Family of Heptahelical Receptors for Thrombin Trypsin Tryptase. Biochem Soc Trans. 1999;27:246–254. doi: 10.1042/bst0270246. [DOI] [PubMed] [Google Scholar]
- 78.Steinhoff M, Buddenkotte J, Shpacovitch V, Rattenholl A, Moormann C, Vergnolle N, Luger TA, Hollenberg MD. Proteinase-activated receptors: Transducers of Proteinase-mediated signaling in inflammation and immune response. Endocrine Rev. 2005;26:1–43. doi: 10.1210/er.2003-0025. [DOI] [PubMed] [Google Scholar]