Abstract
The renin-angiotensin system (RAS) and its active peptide angiotensin II (AngII) have major involvements not only in hypertension but also in mood and anxiety disorders. Substantial evidence supports the notion that AngII acts as a neuromodulator in the brain. In this review, we provide an overview of the link between the RAS and anxiety or mood disorders, and focus on recent advances in the understanding of AngII-linked, NADPH oxidase-derived oxidative stress in the central nervous system, which may underlie pathogenesis of mood and anxiety disorders.
Keywords: Renin-angiotensin system, angiotensin II, anxiety disorder, bipolar disorder, major depressive disorder, reactive oxygen species, NADPH oxidase
Introduction
The renin-angiotensin system (RAS) is essential for maintaining the balance between fluid intakes and blood pressure. Renin, typically produced in kidneys, cleaves an inactive peptide angiotensinogen into angiotensin I. Angiotensin I, a precursor of AngII with little physiological effects, is converted to AngII by the angiotensin I-converting enzyme (ACE) that is secreted by pulmonary and renal endothelial cells. AngII is known for its robust cardiovascular actions, not only increasing blood pressure by stimulating the Gq protein in arterial smooth muscle cells, but also stimulating kidneys to retain sodium and water via aldosterone released from the adrenal cortex. AngII is also widely expressed in the brain and plays important roles in the regulation of blood pressure. Injection of AngII into key brain nuclei produced hypertension, an effect blocked by the AngII antagonist saralasin or AngII type 1 receptor (AT1R) deletion [1]. Although peripheral AngII does not cross the blood-brain barrier (BBB), the links between peripheral and central RAS are established through circumventricular organs (CVOs) that sense circulating AngII via AngII receptors. Effects of AngII are usually mediated by two well-characterized subtypes of AngII receptors, AT1R and AT2R [2]. AT1Rs are highly expressed in the subfornical organs (SFO), paraventricular nucleus (PVN) [3], nucleus tractus solitarius (NTS) [4], hypothalamic-pituitary-adrenal axis (HPA) and amygdala nuclei [5]. Furthermore, two highly homologous AT1R isoforms, termed AT1AR and AT1BR, are expressed in the brain and may have different functions within the same region. In the mouse SFO, for example, AT1ARS are involved in blood pressure regulation, while AT1BRS mediate water drinking response [6]. AT1Rs contribute to most of the harmful effects induced by AngII, such as hypertension, heart failure and mood disorders [1, 6]. AT2Rs are significantly expressed during early development, but decline in the adulthood [2]. AT1Rs and AT2Rs are coupled with transduction signaling, including G proteins, phospholipases and NADPH oxidase [2, 4, 7-9]. However, pharmacological actions mediated by AT2Rs may functionally oppose those induced by AT1Rs. One example is that AT1R-induced reactive oxygen species (ROS) are scavenged by AT2R-induced nitric oxide (NO). As a result, an imbalance between the AT1R- and AT2R-triggered signals may lead to hypertension [7, 8].
Evidence for the link between AngII and mood or anxiety disorders
Mood disorders
Clinically, two groups of mood disorders are widely recognized: (1) Major depressive disorder, a mental disorder characterized by depressed mood accompanied by anhedonia, feeling of guilty or hopelessness, change of appetite and weight; low energy, poor concentration, and suicidal ideation. This mood must represent a change from a person's normal mood; social, occupational, educational or other important functioning is impaired by the depressive symptoms. (2) Bipolar disorder, characterized by intermittent episodes of mania or hypomania, usually interlaced with depressive episodes. It is also a serious mood disorder clinically presented as unusual shifts in mood, energy and cognitive levels, with or without depressive episodes. Symptoms are different from the normal ups and downs, and may seriously damage relationships, job or school performance, and even cause suicide [10].
Anxiety
Mild, brief anxiety caused by a stressful event (such as speaking in public) is a normal reaction to stress. But when anxiety becomes excessive, irrational and persistent, it becomes pathologic. Anxiety disorders commonly occur along with other mental or physical illnesses. Major types of anxiety disorders are: (1) Panic disorder is characterized by recurrent, unexpected attacks of terror, accompanied by a pounding heart, sweatiness, weakness, faintness, shakiness and dizziness. (2) Post-traumatic stress disorder presents as a cluster of symptoms such as re-experience, avoidance and hyperarousal developing after a person experiences or witnesses a traumatic event. Other anxiety disorders include obsessive-compulsive and generalized anxiety disorders [10].
Pharmacological and genetic links between AngII and mood disorders or anxiety
The hypothesis that the RAS is linked to mood disorders is based on early observations that the patient with hypertension and major depression was successfully treated for both conditions with the ACE inhibitor captopril [1, 11-13]. Captopril improved mood status and attenuated depressive symptoms, which was not associated with its antihypertensive action because other anti-hypertensive medications such as α-methyldopa and prazosin did not exert this mood elevating effect [11]. Antidepressant effects by other ACE inhibitors were also reported in patients with major depression or the depressive phase of bipolar disorder. Since plasma AngII does not cross BBB into the brain but the ACE inhibitor does, it is therefore implicated that the brain AngII plays a critical role in the ACE-sensitive mood disorders [12]. ACE expression is under control of ACE gene variants, which are due to an insertion/deletion (I/D) polymorphism resulting from the presence or absence of ∼250-basepair fragments in the 16th intron of the ACE gene located on chromosome 17q23. Subjects with homozygous genotype DD display higher ACE activity, while those with homozygous genotype of II show decreased ACE activity. Significant associations of the DD allele with major depression and bipolar disorder were reported [1, 14-16]. Significant associations of two SNPs (rs4291, rs4295) located in the promoter area within the ACE gene were also identified in patients with major depression [17]. Moreover, the risk of suicidal behavior was higher for the subjects bearing the DD genotype than other variants [18]. In addition, the DD genotype was also linked with psychotic symptoms in bipolar and schizophrenic patients [19, 20]. For anxiety disorders, an association of another two SNPs (rs4311, rs4333) within the ACE gene was identified in patients with panic attacks [21]. However, the II allele was only associated with panic disorder in male patients, suggesting a gender-specific effect in the ACE I/D polymorphism [22]. More interestingly, the DD allele was associated with both high plasma AngII and NADPH oxidase-generated ROS in phagocytes in hypertensive patients [23]. In addition, variant genes for AT1Rs are involved in major depression and anxiety disorders. AT1R A1166C polymorphism CC gene was significantly associated with major depression [24]. Differences in AT1Rs gene expression between strains were associated with their anxiety phenotypes [25]. Taken collectively, these results indicate that AngII and AT1Rs are involved in mood and anxiety disorders.
AngII-induced emotional stress and anxiety disorders
Substantial evidence indicates the involvement of AngII in anxiety disorder mediated by the HPA and sympatho-adrenal axis [26]. AngII in the brain was associated with higher HPA axis activity, enhanced responses to stress and anxiety. Peripheral AngII also participated in emotional stress responses via the AT1R activation within the forebrain, because peripheral administration of the AT1R antagonist candesartan prevented peripheral and central sympathetic activations characteristic of isolation stress and abolishes the HPA activation during isolation [27]. In addition, AT1Rs were expressed in the stress-sensitive brain structures including the dorsomedial hypothalamus (DMH) and amygdala [28]. The DMH plays a functional role in initiation of panic-like responses induced by lactate or AngII via the osmosensitive periven-tricular pathway that relays the signals to the forebrain limbic structures mediating anxiety responses. While injection of lactate or AngII into the DMH produced panic-like responses in panic-prone rats, co-injection with the AT1R antagonist losartan into the DMH blocked these anxiety-and panic-like responses in panic-prone models [29, 30]. The amygdala, a region implicated in the conditioned fear, is rich in AT1Rs. The AngII antagonist saralasin antagonized behavioral and physiological responses to the panicogen sodium lactate in rat panic models, indicating that AT1Rs mediate panic attacks [31]. Therefore, these results led to the suggestion that both central and peripheral AngII are involved in the AT1R-mediated stress or anxiety disorders.
NADPH oxidase (NOX)-triggered oxidative stress
NOX
NOX is originally identified as a key component of innate host defense systems. The primary function of the phagocyte NOX is production of O2- and its secondary metabolite H2O2 to induce oxidative burst in phagocytes that enables the digestion of engulfed bacteria. NOX is also widely distributed in nonphagocyte cells [32, 33]. Normal tissues and cells defend themselves against ROS-induced damages through their scavenger systems such as superoxide dismutase (SOD), catalase and peroxidases to eliminate excessive ROS. However, under the circumstance of high ACE activities or continuous activation of AT1Rs, ROS generation may exceed the antioxidant capacity, a condition termed oxidative stress occurs [2, 32-36].
NOX, along with other enzymes including nitric oxide synthase, xanthine oxidase or cytochrome P450, produce ROS as natural byproducts of the normal metabolism of oxygen [36-40]. ROS are small molecule metabolites of oxygen that participate in redox reactions via their high reactivity. Typical ROS include superoxide anion (O2-), H2O2, hydroxyl (HO-), peroxynitrite (ONOO-) and lipid peroxides (LOOH). O2- is considered as the “primary” ROS. One of the key endogenous anti-oxidative agents is the enzyme superoxide dismutase (SOD) that catalyzes O2- into H2O2. Other relevant endogenous enzymes involved in the metabolism of H2O2 are catalases and glutathione peroxidase (GPX) with glutathione (GSH) [37, 40]. Exogenous transition metal chelators, such as metal ions and EDTA complexes may also serve as one of the major anti-oxidant mechanisms in biological systems.
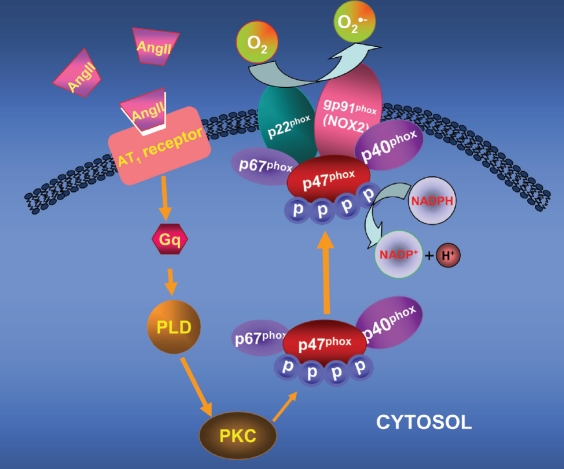
The structure of NOX2 includes two essential plasma membrane-bound subunits of NOX, gp91phox and p22phox, forming a heterodimer termed flavocytochrome b558, and cytoplasmic subunits of NOX including p47phox, p40phox and p67phox, translocating to the membrane upon stimulations and binding to flavocytochrome b558 during gp91phox activation. Complete complex assembly is necessary for complete NADPH oxidase activity. Electrons from NADPH are transferred through the enzyme to molecular oxygen to generate O2- [37, 40] (Figure 1). The catalytic subunit gp91phox, also termed NOX2, has several homologues, NOX1, NOX3 through 5. NOX expressed in the brain is also region-specific [40, 41]. Several NOX isoforms are expressed in neurons. NOX2 is located in the SFO, PVN, NTS, RVLM, amygdala, striatum, thalamus, hippocampus, and cortex [4, 42-47]. ROS play important roles in the normal cellular signaling, which includes delivery of electrons across membranes and oxidative modification of proteins or DNA. Under environmental stresses or pathological circumstances, however, intracellular ROS levels increase dramatically, eliciting oxidative stress. Oxidative stress exerts deleterious effects on lipids, proteins or nucleic acids. Peroxynitrite (ONOO-), for instance, one of ROS products due to reaction between O2- and NO, elicits tyrosine nitration in proteins. ROS are also able to oxidize many signaling proteins such as ion channels or neurotransmitter transporters [4, 33, 48], causing neurophysiological consequences.
Figure 1.
A schimatic signaling pathway for the AT1-mediated, NOX2-derived ROS production. The AT1R activation results in activation of phospholipase D (PLD) and protein kinase C (PKC), which then results in phosphorylation of p47phox, the cytosolic regulatory subunit of NOX2. The phosphorylated p47phox, along with other NOX2 subunits p67phox and p40phox, translocate towards and bind to the plasma membrane-bound gp91phox, the catalytic subunit of NOX2. Such multisubunit assembly results in activation of the whole NOX2 system, producing superoxide (O2").
AngII, the brain NOX and psychiatric disorders
In the brain, NOX distributes in somatodendritic and axonal profiles in the NTS, hippocampus and prefrontal cortex. NTS dysregulation may disrupt cardiorespiratory homeostasis that not only leads to hypertension, but also is involved in anxiety disorders. Within the NTS, the ultra-structural analysis revealed an anatomical link between AT1AR and NOX2. AT1ARS were identified in somatodendric, pre- and post-synaptic axon terminal profiles containing NOX2 [4]. These results implicate that NOX2 may be functionally coupled with pre- and postsynaptic signaling. Within the hippocampus, dual labeling of p67phox subunit with the presynaptic marker synaptophysin suggested a close association between NOX and presynaptic sites, which are coupled with glutamate release triggered by NOX-derived ROS [4, 45, 47]. The NMDA receptor-dependent activation of the extracellular signal-regulated kinase (MAPK) was blocked by the NOX inhibitor diphenylene io-donium or absent in p47phox null mice, indicating that NOX-derived ROS are required for MAPK activation [47]. Within the prefrontal cortex, NOX2 also served as a major source of ROS that control glutamate release and were associated with behavioral alterations after acute ketamine exposure [33, 49].
NOX plays important roles in the AngII-linked hypertension [2, 7, 8]. It is accepted that the AngII-linked neurogenic hypertension is attributed, at least in part, to the ROS produced by the brain NOX [7, 33, 35]. The transduction signal pathways underlying the AngII-induced ROS production via NOX in the brain have recently been addressed [4, 8, 33-37]. Moreover, continuous activation of the brain RAS impairs cognitive functions by NOX-derived ROS. In human renin and human angiotensinogen gene chi-meric transgenic mice, AngII expression was significantly increased in both the brain and plasma. More interestingly, an increase in the avoidance rate in these chimeric double transgenic mice was associated with an increase in the brain O2- production derived from the NADPH oxidase. The AT1R antagonist olmesartan or the O2- scavenger tempol improved the cognitive function and oxidative stress in these mice, suggesting that continuous activation of the brain renin-angiotensin system impairs cognitive functions via both AT1Rs and NADPH oxidase (NOX)-derived ROS [34].
Accumulating evidence also supports the hypothesis that NOX-derived ROS are involved in the pathophysiology of anxiety and bipolar disorders [12, 33, 34, 50-53]. Anxiety and mood disorders are closely linked to NOX-mediated oxidative stress [54-58]. Two separate genes encoding glyoxalase 1 and glutathione reductase in correlation with oxidative stress metabolism were involved in anxiety-like behavioral pheno-types. Overexpression of glyoxalase 1 and glutathione reductase in the mouse brain results in increased anxiety-like behaviors. However, Inhibition of glyoxalase 1 expression by siRNA decreases anxiety-like behaviors [56]. Moreover, anxiety disorder was linked to intracellular ROS levels in central neuronal and glial cells [55]. The oxidative stress inducer L-buthionine-(S,R)-sulfoximine produces anxiety-like behaviors, which were antagonized by the NOX inhibitor apocynin and PDE2 inhibitor [57]. NOX2-derived oxidative stress is involved in the development of anxiety disorder after social isolation. The oxidative stress indicator oxidized nucleic acid 8-hydroxy-2'-deoxy-guanosine was increased following social isolation. The oxidative stress could be in part due to NOX2 activation in microglia, because the pretreatment with apocynin prevented both behavioral and pathological alterations induced by social isolation [58]. The roles of oxidative stress in bipolar disorder and schizophrenia are also addressed. The level of tyrosine nitration that reflects the levels of an endogenous ROS ONOO- was significantly higher in bipolar patients than in healthy controls [59]. In addition, a link between an increased NOX activity and resultant superoxide production in interneurons of the ketamine-induced schizophrenic model was reported [60]. NOX expression and activity were upregulated in the temporal region of mild cognitive impairment patients [61]. ROS and NOX also played a critical role in the pathogenesis of Alzheimer's dementia [62, 63].
Perspective
It is widely accepted that the interplay of genetic, developmental and environmental factors contributes to the pathogenesis of anxiety and mood disorders. AngII generated in the brain by the brain RAS functions as a peptidergic neuro-modulator and has many neuropsychopharmo-cological effects. As of the genetic aspect, it has been determined that the ACE DD gene polymorphism may serve as a susceptibility gene for mood disorder since it reproduces the pheno-type that is associated with the high ACE activity, high AngII concentration and/or high NADPH oxidase activity in the brain of subjects with hypertension and mood disorder.
Acknowledgments
The authors acknowledge the support of National Institute of Health (NIH HL096571 and MH40342).
References
- 1.Phillips MI, de Oliveira EM. Brain renin angiotensin in disease. J Mol Med (Berl) 2008;86:715–722. doi: 10.1007/s00109-008-0331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen Dinh Cat A, Touyz RM. Cell signaling of angiotensin II on vascular tone: novel mechanisms. Curr Hypertens Rep. 2011;13:122–128. doi: 10.1007/s11906-011-0187-x. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson AV, Washburn DL, Latchford KJ. Hormonal and neurotransmitter roles for angiotensin in the regulation of central autonomic function. Exp Biol Med (Maywood) 2001;226:85–96. doi: 10.1177/153537020122600205. [DOI] [PubMed] [Google Scholar]
- 4.Wang G, Anrather J, Huang J, Speth RC, Pickel VM, Iadecola C. NADPH oxidase contributes to angiotensin II signaling in the nucleus tractus solitarius. J Neurosci. 2004;24:5516–5524. doi: 10.1523/JNEUROSCI.1176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karamyan VT, Speth RC. Distribution of the non -AT1, non-AT2 angiotensin-binding site in the rat brapreliminary characterization. Neuroendocrinology. 2008;88:256–265. doi: 10.1159/000140635. [DOI] [PubMed] [Google Scholar]
- 6.Davisson RL, Oliverio MI, Coffman TM, Sigmund CD. Divergent functions of angiotensin II receptor isoforms in the brain. J Clin Invest. 2000;106:103–106. doi: 10.1172/JCI10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chrissobolis S, Faraci FM. The role of oxidative stress and NADPH oxidase in cerebrovascular disease. Trends Mol Med. 2008;14:495–502. doi: 10.1016/j.molmed.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirooka Y, Kishi T, Sakai K, Takeshita A, Sunagawa K. Imbalance of central nitric oxide and reactive oxygen species in the regulation of sympathetic activity and neural mechanisms of hypertension. Am J Physiol Regul Integr Comp Physiol. 2011;300:R818–826. doi: 10.1152/ajpregu.00426.2010. [DOI] [PubMed] [Google Scholar]
- 9.Ohishi M, Dusting GJ, Fennessy PA, Mendelsohn FA, Li XC, Zhuo JL. Increased expression and co-localization of ACE, angiotensin II AT(1) receptors and inducible nitric oxide synthase in atherosclerotic human coronary arteries. Int J Physiol Pathophysiol Pharmacol. 2010;2:111–124. [PMC free article] [PubMed] [Google Scholar]
- 10.Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR Fourth Edition. American Psychiatric Association. 2004.
- 11.Zubenko GS, Nixon RA. Mood-elevating effect of captopril in depressed patients. Am J Psychiatry. 1984;141:110–111. doi: 10.1176/ajp.141.1.110. [DOI] [PubMed] [Google Scholar]
- 12.Gard PR. Angiotensin as a target for the treatment of Alzheimer's disease, anxiety and depression. Expert Opin Ther Targets. 2004;8:7–14. doi: 10.1517/14728222.8.1.7. [DOI] [PubMed] [Google Scholar]
- 13.Saavedra JM, Benicky J. Brain and peripheral angiotensin II play a major role in stress. Stress. 2007;10:185–193. doi: 10.1080/10253890701350735. [DOI] [PubMed] [Google Scholar]
- 14.Arinami T, Li L, Mitsushio H, Itokawa M, Hamaguchi H, Toru M. An insertion/deletion polymorphism in the angiotensin converting enzyme gene is associated with both brain substance P contents and affective disorders. Biol Psychiatry. 1996;40:1122–1127. doi: 10.1016/s0006-3223(95)00597-8. [DOI] [PubMed] [Google Scholar]
- 15.Castellon R, Hamdi HK. Demystifying the ACE polymorphism: from genetics to biology. Curr Pharm Des. 2007;13:1191–1198. doi: 10.2174/138161207780618902. [DOI] [PubMed] [Google Scholar]
- 16.Saab YB, Gard PR, Yeoman MS, Mfarrej B, El-Moalem H, Ingram MJ. Renin-angiotensin-system gene polymorphisms and depression. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1113–1118. doi: 10.1016/j.pnpbp.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Baghai TC, Binder EB, Schule C, Salyakina D, Eser D, Lucae S, Zwanzger P, Haberger C, Zill P, Ising M, Deiml T, Uhr M, Illig T, Wichmann HE, Modell S, Nothdurfter C, Holsboer F, Muller-Myhsok B, Moller HJ, Rupprecht R, Bondy B. Polymorphisms in the angiotensin-converting enzyme gene are associated with unipolar depression, ACE activity and hypercortisolism. Mol Psychiatry. 2006;11:1003–1015. doi: 10.1038/sj.mp.4001884. [DOI] [PubMed] [Google Scholar]
- 18.Sparks DL, Hunsaker JC 3rd, Amouyel P, Malafosse A, Bellivier F, Leboyer M, Courtet P, Helbecque N. Angiotensin I-converting enzyme I/D polymorphism and suicidal behaviors. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:290–294. doi: 10.1002/ajmg.b.30793. [DOI] [PubMed] [Google Scholar]
- 19.Kucukali CI, Aydin M, Ozkok E, Bilge E, Zengin A, Cakir U, Kara I. Angiotensin-converting enzyme polymorphism in schizophrenia, bipolar disorders, and their first-degree relatives. Psychiatr Genet. 2010;20:14–19. doi: 10.1097/YPG.0b013e3283351194. [DOI] [PubMed] [Google Scholar]
- 20.Zou YF, Wang F, Feng XL, Li WF, Pan FM, Huang F. Meta-analysis of ACE gene I/D polymorphism and bipolar disorder susceptibility. Nord J Psychiatry. 2011;65:276–282. doi: 10.3109/08039488.2011.555564. [DOI] [PubMed] [Google Scholar]
- 21.ErhardtA, Lucae S, Kern N, Unschuld PG, Ising M, Lieb R, Uhr M, Hohoff C, Deckert J, Bandelow B, Maier W, Binder EB, Muller-Myhsok B, Keck ME, Holsboer F. Association of polymorphisms in the angiotensin-converting enzyme gene with syndromal panic attacks. Mol Psychiatry. 2008;13:242–243. doi: 10.1038/sj.mp.4002094. [DOI] [PubMed] [Google Scholar]
- 22.Bandelow B, Saleh K, Pauls J, Domschke K, Wedekind D, Falkai P. Insertion/deletion polymorphism in the gene for angiotensin converting enzyme (ACE) in panic disorder: A gender-specific effect? World J Biol Psychiatry. 2010;11:66–70. doi: 10.3109/15622970701459810. [DOI] [PubMed] [Google Scholar]
- 23.San Jose G, Fortuno A, Moreno MU, Robador PA, Bidegain J, Varo N, Beloqui O, Diez J, Zalba G. The angiotensin-converting enzyme insertion/deletion polymorphism is associated with phagocytic NADPH oxidase-dependent superoxide generation: potential implication in hypertension. Clin Sci (Lond) 2009;116:233–240. doi: 10.1042/CS20080057. [DOI] [PubMed] [Google Scholar]
- 24.Kondo DG, Speer MC, Krishnan KR, McQuoid DR, Slifer SH, Pieper CF, Billups AV, Steffens DC. Association of AGTR1 with 18-month treatment outcome in late-life depression. Am J Geriatr Psychiatry. 2007;15:564–572. doi: 10.1097/JGP.0b013e31805470a4. [DOI] [PubMed] [Google Scholar]
- 25.Golding BJ, Overall A, Gard PR. Strain differences and the role of AT(1) receptor expression in anxiety. Int J Mol Epidemiol Genet. 2011;2:51–55. [PMC free article] [PubMed] [Google Scholar]
- 26.Shelton RC. The molecular neurobiology of depression. Psychiatr Clin North Am. 2007;30:1–11. doi: 10.1016/j.psc.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pavel J, Benicky J, Murakami Y, Sanchez-Lemus E, Saavedra JM. Peripherally administered angiotensin II AT1 receptor antagonists are anti -stress compounds in vivo. Ann N Y Acad Sci. 2008;1148:360–366. doi: 10.1196/annals.1410.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saavedra JM, Sanchez-Lemus E, Benicky J. Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation and ischemia: Therapeutic implications. Psychoneuroendocrinology. 2011;36:1–18. doi: 10.1016/j.psyneuen.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shekhar A, Johnson PL, Sajdyk TJ, Fitz SD, Keim SR, Kelley PE, Gehlert DR, DiMicco JA. Angiotensin-II is a putative neurotransmitter in lactate-induced panic-like responses in rats with disruption of GABAergic inhibition in the dorsomedial hypothalamus. J Neurosci. 2006;26:9205–9215. doi: 10.1523/JNEUROSCI.2491-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson PL, Truitt WA, Fitz SD, Lowry CA, Shekhar A. Neural pathways underlying lactate-induced panic. Neuropsychopharmacology. 2008;33:2093–2107. doi: 10.1038/sj.npp.1301621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shekhar A, Sajdyk TJ, Gehlert DR, Rainnie DG. The amygdala, panic disorder, and cardiovascular responses. Ann N Y Acad Sci. 2003;985:308–325. doi: 10.1111/j.1749-6632.2003.tb07090.x. [DOI] [PubMed] [Google Scholar]
- 32.Garrido AM, Griendling KK. NADPH oxidases and angiotensin II receptor signaling. Mol Cell Endocrinol. 2009;302:148–158. doi: 10.1016/j.mce.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sorce S, Krause KH. NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal. 2009;11:2481–2504. doi: 10.1089/ars.2009.2578. [DOI] [PubMed] [Google Scholar]
- 34.Inaba S, Iwai M, Furuno M, Tomono Y, Kanno H, Senba I, Okayama H, Mogi M, Higaki J, Horiuchi M. Continuous activation of renin-angiotensin system impairs cognitive function in renin/angiotensinogen transgenic mice. Hypertension. 2009;53:356–362. doi: 10.1161/HYPERTENSIONAHA.108.123612. [DOI] [PubMed] [Google Scholar]
- 35.Zimmerman MC. Angiotensin II and angiotensin -1-7 redox signaling in the central nervous system. Curr Opin Pharmacol. 2011;11:138–143. doi: 10.1016/j.coph.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Datla SR, Griendling KK. Reactive oxygen species, NADPH oxidases, and hypertension. Hypertension. 2010;56:325–330. doi: 10.1161/HYPERTENSIONAHA.109.142422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang G, Milner TA, Speth RC, Gore AC, Wu D, Iadecola C, Pierce JP. Sex differences in angiotensin signaling in bulbospinal neurons in the rat rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1149–1157. doi: 10.1152/ajpregu.90485.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tyagi N, Qipshidze N, Sen U, Rodriguez W, Ovechkin A, Tyagi SC. Cystathionine beta synthase gene dose dependent vascular remodeling in murine model of hyperhomocysteinemia. Int J Physiol Pathophysiol Pharmacol. 2011;3:210–222. [PMC free article] [PubMed] [Google Scholar]
- 39.Brown DI, Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lambeth JD, Krause KH, Clark RA. NOX enzymes as novel targets for drug development. Semin Immunopathol. 2008;30:339–363. doi: 10.1007/s00281-008-0123-6. [DOI] [PubMed] [Google Scholar]
- 41.Cheret C, Gervais A, Lelli A, Colin C, Amar L, Ravassard P, Mallet J, Cumano A, Krause KH, Mallat M. Neurotoxic activation of microglia is promoted by a nox1-dependent NADPH oxidase. J Neurosci. 2008;28:12039–12051. doi: 10.1523/JNEUROSCI.3568-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Helmcke I, Heumuller S, Tikkanen R, Schroder K, Brandes RP. Identification of structural elements in Nox1 and Nox4 controlling localization and activity. Antioxid Redox Signal. 2009;11:1279–1287. doi: 10.1089/ars.2008.2383. [DOI] [PubMed] [Google Scholar]
- 43.Cao X, Demel SL, Quinn MT, Galligan JJ, Kreulen D. Localization of NADPH oxidase in sympathetic and sensory ganglion neurons and perivascular nerve fibers. Auton Neurosci. 2009;151:90–97. doi: 10.1016/j.autneu.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peterson JR, Burmeister MA, Tian X, Zhou Y, Guruju MR, Stupinski JA, Sharma RV, Davisson RL. Genetic silencing of Nox2 and Nox4 reveals differential roles of these NADPH oxidase homologues in the vasopressor and dipsogenic effects of brain angiotensin II. Hypertension. 2009;54:1106–1114. doi: 10.1161/HYPERTENSIONAHA.109.140087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serrano F, Chang A, Hernandez C, Pautler RG, Sweatt JD, Klann E. NADPH oxidase mediates beta-amyloid peptide-induced activation of ERK in hippocampal organotypic cultures. Mol Brain. 2009;2:31. doi: 10.1186/1756-6606-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ibi M, Matsuno K, Shiba D, Katsuyama M, Iwata K, Kakehi T, Nakagawa T, Sango K, Shirai Y, Yokoyama T, Kaneko S, Saito N, Yabe-Nishimura C. Reactive oxygen species derived from NOX1/NADPH oxidase enhance inflammatory pain. J Neurosci. 2008;28:9486–9494. doi: 10.1523/JNEUROSCI.1857-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kishida KT, Klann E. Sources and targets of reactive oxygen species in synaptic plasticity and memory. Antioxid Redox Signal. 2007;9:233–244. doi: 10.1089/ars.2007.9.ft-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hidalgo C, Carrasco MA, Munoz P, Nunez MT. A role for reactive oxygen/nitrogen species and iron on neuronal synaptic plasticity. Antioxid Redox Signal. 2007;9:245–255. doi: 10.1089/ars.2007.9.245. [DOI] [PubMed] [Google Scholar]
- 49.Sorce S, Schiavone S, Tucci P, Colaianna M, Jaquet V, Cuomo V, Dubois-Dauphin M, Trabace L, Krause KH. The NADPH oxidase NOX2 controls glutamate release: a novel mechanism involved in psychosis-like ketamine responses. J Neurosci. 2010;30:11317–11325. doi: 10.1523/JNEUROSCI.1491-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andreazza AC, Kapczinski F, Kauer-Sant'Anna M, Walz JC, Bond DJ, Goncalves CA, Young LT, Yatham LN. 3-Nitrotyrosine and glutathione antioxidant system in patients in the early and late stages of bipolar disorder. J Psychiatry Neurosci. 2009;34:263–271. [PMC free article] [PubMed] [Google Scholar]
- 51.Tsaluchidu S, Cocchi M, Tonello L, Puri BK. Fatty acids and oxidative stress in psychiatric disorders. BMC Psychiatry. 2008;8(Suppl 1):S5. doi: 10.1186/1471-244X-8-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yumru M, Savas HA, Kalenderoglu A, Bulut M, Celik H, Erel O. Oxidative imbalance in bipolar disorder subtypes: a comparative study. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1070–1074. doi: 10.1016/j.pnpbp.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 53.Steckert AV, Valvassori SS, Moretti M, Dal-Pizzol F, Quevedo J. Role of oxidative stress in the pathophysiology of bipolar disorder. Neurochem Res. 2010;35:1295–1301. doi: 10.1007/s11064-010-0195-2. [DOI] [PubMed] [Google Scholar]
- 54.Nair D, Dayyat EA, Zhang SX, Wang Y, Gozal D. Intermittent hypoxia-induced cognitive deficits are mediated by NADPH oxidase activity in a murine model of sleep apnea. PLoS One. 2011;6:e19847. doi: 10.1371/journal.pone.0019847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rammal H, Bouayed J, Younos C, Soulimani R. Evidence that oxidative stress is linked to anxiety-related behaviour in mice. Brain Behav Immun. 2008;22:1156–1159. doi: 10.1016/j.bbi.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 56.Hovatta I, Tennant RS, Helton R, Marr RA, Singer O, Redwine JM, Ellison JA, Schadt EE, Verma IM, Lockhart DJ, Barlow C. Glyoxalase 1 and glutathione reductase 1 regulate anxiety in mice. Nature. 2005;438:662–666. doi: 10.1038/nature04250. [DOI] [PubMed] [Google Scholar]
- 57.Masood A, Nadeem A, Mustafa SJ, O'Donnell JM. Reversal of oxidative stress-induced anxiety by inhibition of phosphodiesterase-2 in mice. J Pharmacol Exp Ther. 2008;326:369–379. doi: 10.1124/jpet.108.137208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schiavone S, Sorce S, Dubois-Dauphin M, Jaquet V, Colaianna M, Zotti M, Cuomo V, Trabace L, Krause KH. Involvement of NOX2 in the development of behavioral and pathologic alterations in isolated rats. Biol Psychiatry. 2009;66:384–392. doi: 10.1016/j.biopsych.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 59.Kunz M, Gama CS, Andreazza AC, Salvador M, Cereser KM, Gomes FA, Belmonte-de-Abreu PS, Berk M, Kapczinski F. Elevated serum superoxide dismutase and thiobarbituric acid reactive substances in different phases of bipolar disorder and in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1677–1681. doi: 10.1016/j.pnpbp.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 60.Behrens MM, Ali SS, Dugan LL. Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J Neurosci. 2008;28:13957–13966. doi: 10.1523/JNEUROSCI.4457-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bruce-Keller AJ, Gupta S, Parrino TE, Knight AG, Ebenezer PJ, Weidner AM, LeVine H 3rd, Keller JN, Markesbery WR. NOX activity is increased in mild cognitive impairment. Antioxid Redox Signal. 2010;12:1371–1382. doi: 10.1089/ars.2009.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saxena U. Bioenergetics breakdown in Alzheimer's disease: targets for new therapies. Int J Physiol Pathophysiol Pharmacol. 2011;3:133–139. [PMC free article] [PubMed] [Google Scholar]
- 63.Park L, Wang G, Zhou P, Zhou J, Pitstick R, Previti ML, Younkin L, Younkin SG, Van Nostrand WE, Cho S, Anrather J, Carlson GA, Iadecola C. Scavenger receptor CD36 is essential for the cerebrovascular oxidative stress and neurovascular dysfunction induced by amyloid-beta. Proc Natl Acad Sci USA. 2011;108:5063–5068. doi: 10.1073/pnas.1015413108. [DOI] [PMC free article] [PubMed] [Google Scholar]