Abstract
Microinjection of opioids into the ventrolateral periaqueductal gray (vlPAG) produces antinociception in part by binding to mu-opioid receptors (MOPrs). Although both high and low efficacy agonists produce antinociception, low efficacy agonists such as morphine produce limited MOPr internalization suggesting that MOPr internalization and signaling leading to antinociception are independent. This hypothesis was tested in awake, behaving rats using DERM-A594, a fluorescently labeled dermorphin analog, and internalization blockers. Microinjection of DERM-A594 into the vlPAG produced both antinociception and internalization of DERM-A594. Administration of the irreversible opioid receptor antagonist beta-CNA prior to DERM-A594 microinjection reduced both the antinociceptive effect and the number of DERM-A594 labeled cells demonstrating that both effects are opioid receptor-mediated. Pretreatment with the internalization blockers dynamin dominant-negative inhibitory peptide (dynamin-DN) and concanavalinA (ConA) attenuated both DERM-A594 internalization and antinociception. Microinjection of dynamin-DN and ConA also decreased the antinociceptive potency of the unlabeled opioid agonist dermorphin when microinjected into the vlPAG as demonstrated by rightward shifts in the dose-response curves. In contrast, administration of dynamin-DN had no effect on the antinociceptive effect of microinjecting the GABAA antagonist bicuculline into the vlPAG. The finding that dermorphin-induced antinociception is attenuated by blocking receptor internalization indicates that key parts of opioid receptor-mediated signaling depend on internalization.
Keywords: endocytosis, periaqueductal gray, opiate, analgesia, pain modulation
The antinociceptive effect of opioids is mediated primarily by mu-opioid receptors (MOPr). MOPr activity is regulated by endocytotic trafficking to and from the plasma membrane (Finn and Whistler, 2001; Tanowitz and von Zastrow, 2003; von Zastrow et al., 2003). Recently, distinct signaling from receptors located on the plasma membrane and from endosomes (Murphy et al., 2009; Sorkin and von Zastrow, 2009) suggests that internalization of MOPr could play a role in the signaling that leads to antinociception. Whether receptor trafficking contributes to the signaling that leads to antinociception is unclear. Antinociception and opioid internalization have been shown to correlate following administration of opioid agonists (Trafton and Basbaum, 2000; Pradhan et al., 2009). However, studies in cell culture find that some agonists, such as morphine, do not readily induce internalization (Alvarez et al., 2002; Borgland et al., 2003; Arttamangkul et al., 2008) suggesting that signaling precedes MOPr internalization.
Development of a fluorescent label for the MOPr agonist dermorphin (DERM-A594) has allowed in vitro characterization of MOPr trafficking in locus coeruleus neurons (Arttamangkul et al., 2000; Arttamangkul et al., 2006). In these studies, visualization of internalized DERM-A594 occurs rapidly upon administration and is blocked with prior administration of the internalization blocker Concanavalin A (ConA). Simultaneous electrophysiological experiments demonstrated that in the presence of ConA, DERM-induced desensitization of G protein-coupled inwardly rectifying potassium channels (GIRK) is unaffected, indicating that MOPr internalization and the signaling that leads to desensitization are separate processes.
The ventrolateral periaqueductal gray (vlPAG) is an ideal structure to study the relationship between MOPr internalization and antinociception. Microinjection of opioids into the vlPAG produces antinociception (Jacquet and Lajtha, 1976; Bodnar et al., 1991; Morgan et al., 1998), and blocking opioid action in the vlPAG attenuates antinociception produced by systemic morphine administration (Zambotti et al., 1982; Randich et al., 1992; Lane and Morgan, 2005; Lane et al., 2005). Although in vitro studies indicate that MOPr internalization and signaling are independent, the objective of the present study was to test this hypothesis in awake, behaving animals. The first step was to correlate DERM-A594 internalization and antinociception following microinjection of DERM-A594 into the vlPAG. The second step was to determine whether blocking receptor internalization with ConA and dynamin dominant-negative inhibitory peptide (dynamin-DN) alters dermorphin-induced antinociception.
Experimental procedures
Animals
Experiments were performed in adult male Sprague-Dawley rats (250 – 350 g; Animal Technologies, Livermore, CA). All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the IACUC at Washington State University. Efforts were made to minimize the number of experimental subjects (e.g. using a within subjects design when possible).
Microinjections
Rats were anesthetized with pentobarbital (60 mg/kg, i.p.) and implanted with a guide cannula (23 gauge, 9 mm long) aimed at the vlPAG (AP: +1.7 mm, ML: ±0.6 mm, DV: −5.0 mm from lambda) using stereotaxic techniques. The guide cannula was attached to two screws in the skull by dental cement. At the end of the surgery, a stylet was inserted to plug the guide cannula. The rat was maintained under a heat lamp until awake. Following surgery, rats were housed individually. The animal housing room was maintained on a reverse light/dark schedule (lights off at 7:00 AM) so rats could be tested during the active dark phase. Food and water were available at all times except during testing. Rats were handled daily before and after surgery. Testing began at least 7 days after surgery.
Drugs were administered directly into the vlPAG through a 31-gauge injection cannula (0.25 mm OD and 0.127 mm ID) inserted into and extending 2 mm beyond the tip of the guide cannula. One day before testing, rats received a sham injection in which an injector was inserted into the guide cannula but no drug was administered. This procedure reduces confounds resulting from mechanical stimulation of neurons on the test day and habituates the rat to the microinjection procedure. Testing with drug administration began 1 day later. Drugs were microinjected at a rate of 0.1 µl/10 s while the rat was gently restrained by hand. The injection cannula remained in place an additional 20 s to minimize backflow of the drug up the cannula track. Following the injection, the stylet was replaced and the rat was returned to its home cage.
Behavioral testing
Nociception was assessed using the hot plate test. The hot plate test consisted of measuring the latency to lick the hind paw when placed on a 52.5°C plate. The rat was removed from the hot plate if no response occurred within 50 s.
Experiment 1: Does Microinjection of DERM-A594 Produce Antinociception and Internalization?
DERM-A594 (300 ng/0.5 µl) was microinjected into the vlPAG to determine whether it produced antinociception and MOPr internalization in intact, awake rats. The irreversible opioid receptor antagonist beta-chlornaltrexamine (beta-CNA; 270 ng/0.5 µl) (Sigma-Aldrich, St. Louis, MO) or saline were microinjected into the vlPAG 6 hrs and again 30 min prior to microinjection of DERM-A594 to determine whether DERM-A594 internalization was a MOPr mediated effect. Nociception was assessed using the hot plate test 30 min following microinjection of DERM-A594.
Experiment 2: Do Internalization Blockers Prevent DERM-A594 Internalization?
If DERM-A594 enters neurons through internalization of G-protein coupled receptors (GPCRs), then blocking receptor internalization should block DERM-A594 internalization. This hypothesis was tested by microinjecting dynamin-DN (100 ng/0.4 ml; 200 mM) or the scrambled dynamin peptide (dynamin-scr) (100 ng/0.4 ml; 200 mM) (Tocris Cookson, Inc. Ellisville MO) into the vlPAG 20 min prior to DERM-A594 administration. Dynamin-DN blocks receptor internalization by competing with GTPase dynamin that moves the receptor/beta-arrestin complex from the membrane. Nociception was assessed using the hot plate test 30 min after microinjection of DERM-A594. The brain was removed 5 min after the hot plate test for confocal analysis of DERM-A594 in vlPAG neruons.
A separate group of rats was injected with ConA (800 ng/0.8 ml) (Sigma-Aldrich, St. Louis, MO) or saline 20 min prior to administration of DERM-A594 into the vlPAG. ConA is a lectin that blocks receptor internalization by preventing receptor clustering (Pippig et al., 1995). This experiment was conducted to test whether independent methods of blocking MOPr internalization would have the same effect. Changes in hot plate latency and MOPr internalization were measured as described above.
Experiment 3: Do Internalization Blockers Prevent Dermorphin-Induced Antinociception?
The internalization blockers dynamin-DN and ConA were microinjected into the vlPAG 20 min prior to administration of the unlabeled MOPr agonist dermorphin to test the hypothesis that MOPr internalization contributes to opioid antinociception. Animals received microinjections of dynamin-DN (100 ng/0.4 ml; 200 mM) or the scrambled dynamin peptide (100 ng/0.4 ml; 200 mM) 20 minutes before microinjection of cumulative third log doses of unlabeled dermorphin (0.22, 0.46, 1.0, & 2.2 µg/0.4 µl). Dermorphin was injected every 20 min, and nociception was assessed using the hot plate test 15 min after each injection. This procedure has been shown to produce reliable dose response curves for opioids such as morphine, DAMGO, and fentanyl (Morgan et al., 2006; Bobeck et al., 2009).
A separate group of rats were injected with Con A (800 ng/0.8 µl) or saline (0.8 µl) into the vlPAG 20 min before microinjection of dermorphin (0.22, 0.46, 1.0, & 2.2 µg/0.4 µl). A large volume of ConA was used to keep the concentration low and cover a large region of the vlPAG. Rats were tested as described above. A within-subjects design was used in which half the rats were pretreated ConA or saline on one day and treated with the reverse treatment 2 days later.
The specificity of dynamin-DN for GPCRs was assessed by microinjecting dynamin-DN (100 ng/0.4 ml; 200 mM) or the scrambled dynamin peptide (100 ng/0.4 ml; 200 mM) into the vlPAG 20 minutes before microinjection of cumulative third log doses of the GABAA receptor antagonist bicuculline (4.6, 10, 22, & 46 ng/0.4 µl) (Sigma-Aldrich, St. Louis, MO). Previous research has shown that blocking tonic GABA inhibition by microinjecting bicuculline into the vlPAG produces a rapid and potent antinociception (Morgan and Clayton, 2005; Bobeck et al., 2009). Bicuculline was injected every 7 minutes, and nociception was assessed using the hot plate test 5 min after each injection. A within subjects design was used in which half the rats were pretreated with either scrambled-dynamin or dynamin-DN in the dermorphin dose-response test. On a second test, one week later, rats were pretreated with the reverse treatment, followed by bicuculline.
Histology
Rats were anesthetized with Halothane 5 – 10 min after the hot plate test and perfused with saline and then formalin (10%). The brain was removed and placed in formalin (10%) for subsequent immunohistochemistry and confocal analysis of DERM-A594 internalization (see methods below). The brain was sectioned coronally (60 µm) at least two days after being removed from the rat. The location of the injection site was identified by looking for the end of the cannula track (Paxinos and Watson, 2005). These brain sections were then prepared for confocal microscopy (see below).
Confocal microscopy
Coronal brain slices containing vlPAG were incubated in 5% goat serum (Sigma-Aldrich, St. Louis, MO), 3% BSA (Sigma-Aldrich), and 0.5% Triton X-100 (Sigma-Aldrich) in phosphate buffered saline (PBS) for 1 hr at room temperature. Sections were washed three times with PBS. All slices were then incubated in mouse anti-NeuN (1/500, Chemicon, Temecula, CA) antibody overnight, washed in PBS three times, and then incubated for 1 hr in secondary antibody (goat anti-mouse IgG 488 Alexa Fluor conjugate (1/800, Invitrogen, Carlsbad, CA)). Slices were washed three times in PBS.
Confocal analysis targeted the vlPAG adjacent to the aqueduct (Aq) and within 100–200 uM of the injection site (Figure 1B). During the image collection, the experimenter was blind to treatments. The size of the sampling region (180 µm2) was the same in each animal. The aqueduct was centered under the 10× objective. Using the 20× objective, the aqueduct was positioned in the upper left corner of the 20× field. Without repositioning the sample, the 63× oil objective was lowered and images were collected. NeuN and DERM-A594 labeling in the vlPAG were examined in each case. NeuN labeling was used to verify that the number of cells in the different treatment groups were comparable (96–110 total NeuN cells were counted for each treatment group with the mean ranging from 13.7 to 18.3 neurons for each animal). NeuN and DERM-A594 colocalization was not determined in this study. Images of NeuN and DERM-A594 labeling in vlPAG were collected on a Zeiss LSM 510 META confocal microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY) using a single pass, multi-channel format with band pass filters to spectrally separate the NeuN and DERM-A594 labeling. We used a pinhole of 1.0 airy unit and objectives of 10× (numerical aperture (NA) 0.3), 20× (numerical aperture (NA) 0.75), and 63× oil (NA 1.4), resulting in estimated optical section thicknesses (full width at half-maximum) of 2.53, 1.01, and 0.62 µm, respectively. We determined the vertical extent of NeuN and DERM-A594 labeling through the section and then acquired a single 12-bit image of the optical section in which labeling for both NeuN and DERM-A594 was optimal using a fast scan (z-interval). Optical sections were acquired at a digital size of 1024×1024 pixels and averaged four times to reduce noise.
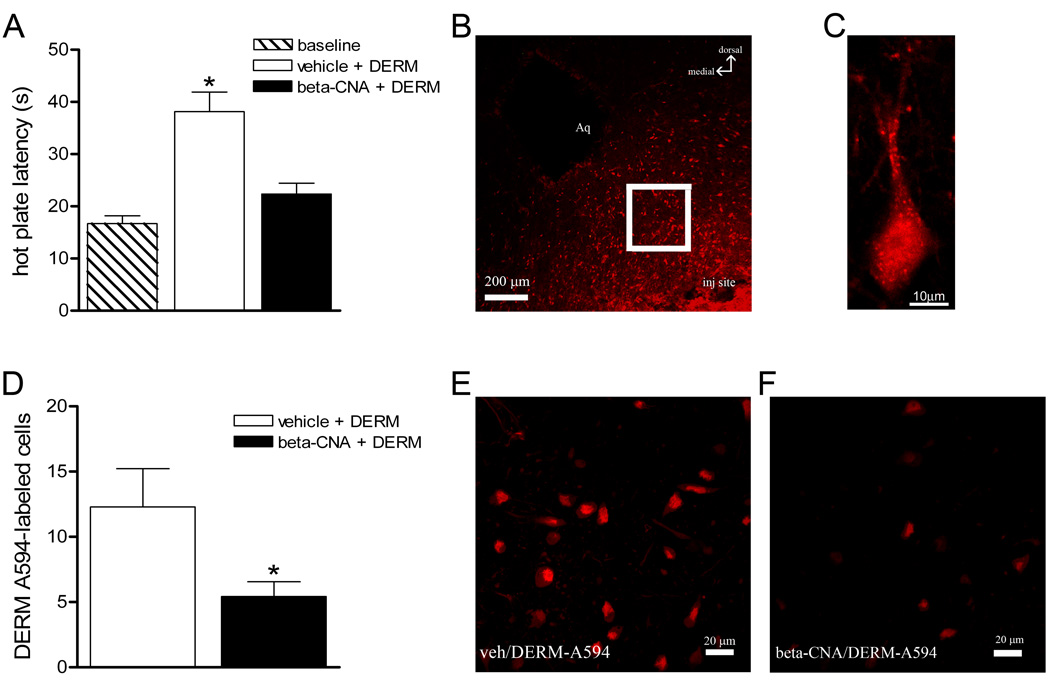
Figure 1. Microinjection of DERM-A594 into the vlPAG produces antinociception and internalization.
Rats were microinjected with vehicle or beta-CNA, followed by DERM-A594 into the vlPAG. A: Microinjection of DERM-A594 produced an increase in hot plate latency compared to baselines and this increase was blocked by pretreating rats with beta-CNA. B: The number of cells labeled with DERM-A594 (red) was assessed using confocal microscopy in the vlPAG at least 100 µm from the microinjection site. C: View of a single vlPAG neuron with DERM-A594 labeling. D: The number of DERM-A594 labeled neurons was decreased in rats pretreated with beta-CNA compared to saline-pretreated animals. E: Labeling for DERM-A594 from a representative animal pretreated with vehicle. F: Labeling for DERM-A594 from a representative animal pretreated with beta-CNA.
Data analysis
Data were analyzed and plotted using Prism 4 (GraphPad Software, San Diego, CA, USA). Differences in hot plate latency were compared using an one-way ANOVA followed by a Tukey’s post hoc test or Student’s t-tests between experimental and control groups. Dermorphin and bicuculline dose-response curves and D50 values (dose for half maximal antinociception) (Tallarida, 2001) were calculated using non-linear regression (Prism 4, GraphPad Software, San Diego, CA, USA). Only rats with injection cannula in or on the border of the vlPAG were included in data analysis (Paxinos and Watson, 2005).
DERM-A594 labeled cells were quantified using Image J (NIH, Bethesda, MD, USA). The experimenter was blind to treatments during analyses. The number of cells with DERM-A594 labeling was counted for each animal. The average number of DERM-A594 positive cells (± SEM) for animals in each condition was calculated and compared to the number of cells counted from other treatment conditions using Student’s t-test.
Results
Experiment 1: Does Microinjection of DERM-A594 Produce Antinociception and Internalization?
The objective of this experiment was to determine whether microinjection of DERM-A594 into the vlPAG produces both MOPr internalization and antinociception. Microinjection of DERM-A594 (300 ng/0.5µl) into the vlPAG produced an increase in hot plate latency compared to baseline (38.1 ± 3.7 s vs. 19.0 ± 2.5 s; N = 9; F (2, 35) = 22.13, p < 0.001). Confocal analysis of vlPAG neurons located immediately adjacent to the microinjection site revealed internalization of DERM-A594. Pretreatment with the irreversible MOPr antagonist beta-CNA (N = 11) reversed both DERM-A594 internalization (t (12) = 2.176, p = 0.05) and antinociception (p > 0.05 compared to baseline; Figure 1). This finding demonstrates that DERM-A594 produces antinociception and internalization by binding to opioid receptors in the vlPAG.
Experiment 2: Do Internalization Blockers Prevent DERM-A594 Internalization?
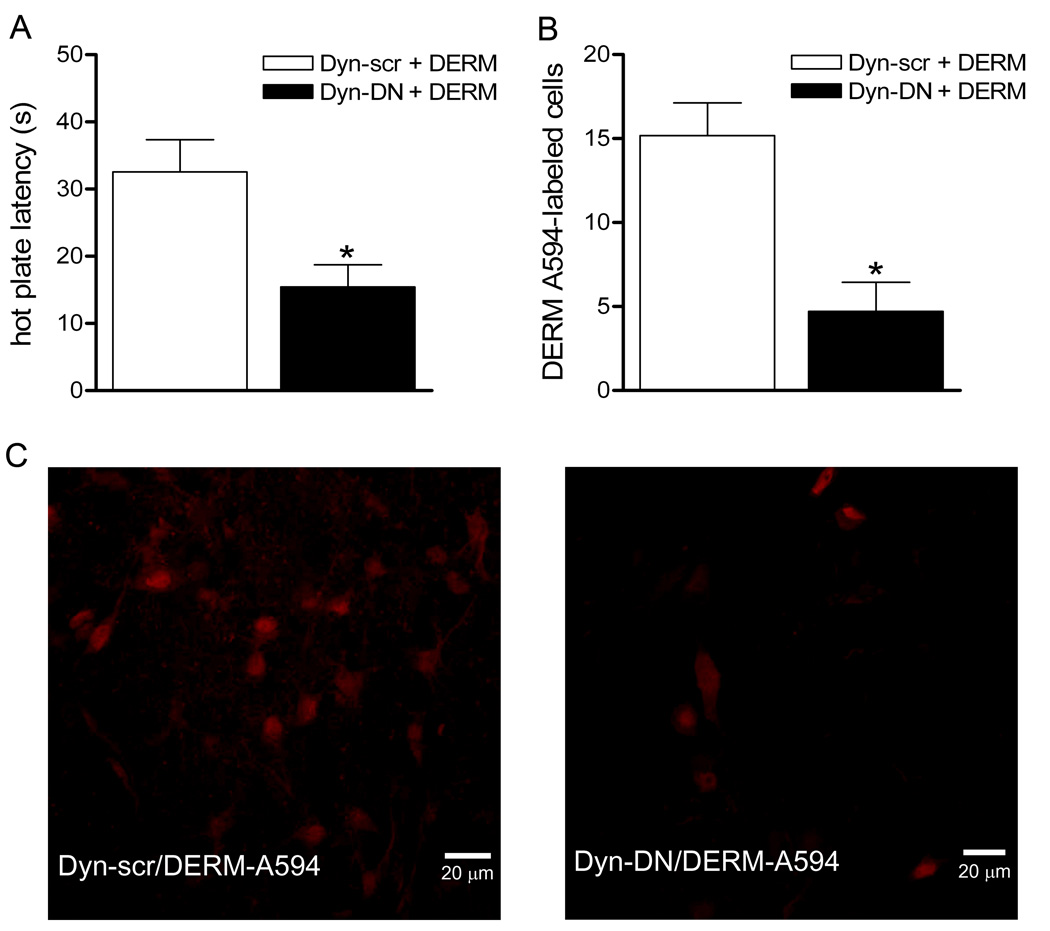
The objective of this experiment was to test the hypothesis that DERM-A594 enters neurons via receptor internalization. Blockade of receptor internalization by microinjection of dynamin-DN into the vlPAG (N = 7) reduced the number of neurons showing DERM-A594 labeling compared to rats injected with the scrambled dynamin control peptide (N = 6) (t (11) = 4.020, p < 0.01, Figure 2). Microinjection of dynamin-DN also prevented the increase in hot plate latency produced by microinjection of DERM-A594 (t (12) = 3.045, p<0.01, Figure 2).
Figure 2. Microinjection of dynamin-DN into the vlPAG reduces DERM-A594 antinociception and internalization.
Rats were microinjected with the dynamin inhibitory peptide (dyn-DN) or the scrambled peptide (dyn-scr) as a control prior to microinjection of DERM-A594 into the vlPAG. A: Pretreatment with dynamin-DN reduced the antinociceptive effect of microinjecting DERM-A594 into the vlPAG compared to pretreatment with the scrambled peptide. B: The number of DERM-A594 labeled cells was significantly decreased in rats pretreated with dynamin-DN compared to scrambled-dynamin pretreated animals. C, Panel 1, Labeling for DERM-A594 from a representative animal pretreated with scrambled-dynamin (left) compared to a representative animal pretreated with dynamin-DN (right).
Blocking receptor internalization by microinjecting ConA into the vlPAG also attenuated both DERM-A594-mediated internalization and antinociception. Confocal analysis of DERM-A594 labeling showed a decrease in fluorescent cells in ConA pretreated rats (mean = 5.4 ± 1.0 cells per animal, N = 7) compared to saline treated animals (12.3 ± 2.9 cells per animal, N = 7; t(12) = 2.224, p< .05). Pretreatment with ConA (N = 12) also caused a significant decrease in DERM-A594 mediated antinociception from 38.1 ± 3.7 s to 24.8 ± 2.9 s (t (19) = 2.882, p < 0.05). These data show that blocking internalization, whether by dynamin-DN or ConA, prevents both DERM-A594 internalization and antinociception.
Experiment 3: Do Internalization Blockers Prevent Dermorphin-Induced Antinociception?
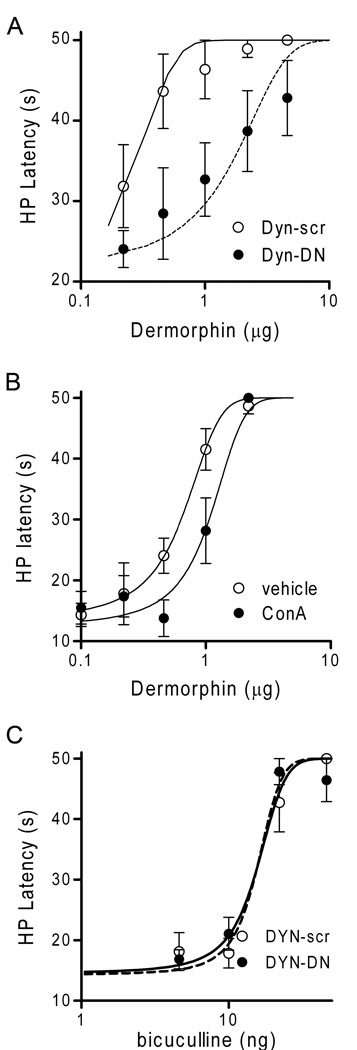
The hypothesis that MOPr internalization is a necessary signaling step for dermorphin-mediated antinociception was tested by measuring the antinociceptive effect of microinjecting unlabeled dermorphin into the vlPAG in rats pretreated with the receptor internalization blockers dynamin-DN and ConA. Microinjection of dermorphin into the vlPAG produced a dose-dependent increase in hot plate latency (Figure 3). This dermorphin-induced antinociception was significantly reduced in rats pretreated with dynamin-DN (D50 = 1.1 ± 0.3 µg, N = 7) compared to scrambled dynamin pretreated animals (D50 = 0.2 ± 0.05 µg, N = 7) (F (1, 79) = 10.04, p < 0.01, Figure 3A). Pretreatment with ConA also significantly decreased dermorphin-induced antinociception (D50 = 1.1 ± 0.15 µg) compared to vehicle-pretreated animals (D50 = 0.6 ± 0.1 µg) (F (1, 51) = 12.52; p < 0.01; N= 4, Figure 3B).
Figure 3. Blocking internalization attenuates dermorphin-induced antinociception.
Rats were administered internalization blockers prior to cumulative microinjections of dermorphin. A: Microinjection of dermorphin produced a dose dependent increase in hot plate latency. Antinociceptive potency was decreased in rats pretreated with dynamin-DN (dyn-DN) compared to scrambled dynamin (dyn-scr) pretreated animals. B: The potency of dermorphin-induced antinociception also was decreased in rats pretreated with ConA compared to saline-pretreated rats. C: The antinociceptive potency of microinjecting the GABA antagonist bicuculline into the vlPAG was not affected by pretreatment with dynamin-DN peptide as would be expected given that blocking GABA does not produce antinociception via a GPCR.
In contrast, there was no difference in the antinociceptive effect of microinjecting the GABAA receptor antagonist bicuculline into the vlPAG in rats pretreated with dynamin-DN (D50 = 16.9 ng, N = 7) or the scrambled peptide (D50 = 14.0 ng, N = 7; F(1, 66) = 2.176; n.s.). Given that bicuculline produces antinociception by blocking the GABAA receptor, this finding demonstrates that the decrease in antinociception following dynamin-DN pretreatment is specific to internalization of the MOPr and not non-specific disruption of cell signaling.
Discussion
The present study shows that microinjection of the fluorescently labeled dermorphin analog DERM-A594 into the vlPAG produces antinociception and internalization of DERM-A594. Internalized DERM-A594 was blocked with prior administration of the irreversible opioid receptor antagonist beta-CNA demonstrating that internalization of DERM-A594 is dependent on opioid receptor binding. Moreover, blocking internalization by administering dynamin-DN or ConA prevented both DERM-A594 internalization and antinociception. Although opioid-induced receptor internalization is a well-known phenomenon, this is the first report showing that blocking receptor internalization can inhibit antinociception.
Internalization of DERM-A594 following microinjection into the vlPAG is consistent with previous studies showing DERM-A594 internalization in cultured locus coeruleus cells (Arttamangkul et al., 2000; Arttamangkul et al., 2006). Although the present manuscript is the first to show DERM-A594 internalization in a whole animal, opioid-induced MOPr internalization has been shown previously in intact animals using immunohistochemistry (Trafton and Basbaum, 2000; He et al., 2002; Trafton and Basbaum, 2004; Chen et al., 2007; Chen et al., 2008; Lao et al., 2008). The advantage of assessing MOPr internalization with a fluorescently labeled opioid agonist in vivo is that internalization is restricted to those receptors bound by agonist at the plasma membrane. This approach allows ligand-induced receptor internalization to be correlated to changes in behavior.
Although dermorphin is approximately 100-fold more selective for MOPr compared to delta-opioid receptors (Amiche et al., 1990), it is possible that delta opioid receptors contribute to DERM-A594 internalization. Mild antinociceptive effects have been reported following microinjection of the delta opioid receptor agonist deltorphin into the PAG (Rossi et al., 1994) (see however Ossipov et al., 1995; Morgan et al., 2009). However, MOPr receptors are primarily responsible for PAG mediated antinociception (Bodnar et al., 1988). Membrane expression of functional delta-opioid receptors primarily occurs in PAG neurons following chronic opioid administration or repeated stress (Commons, 2003; Hack et al., 2005). Kappa-opioid receptors are probably not involved in DERM-A594 internalization because microinjection of kappa receptor agonists into the PAG has no effect on nociception (Fang et al., 1989; Rossi et al., 1994).
DERM-A594 labeling was observed in vlPAG tissue taken from intact rats. DERM-A594 does not cross-link to the tissue during perfusion of the animal therefore limiting both background fluorescence and fluorescence on the plasma membrane. The observations that DERM-A594 only labels a subpopulation of cells in the vlPAG, that there is little background fluorescence, and that internalization of DERM-A594 is significantly inhibited by the irreversible opioid receptor antagonist beta-CNA support evidence that DERM-A594 is internalized following binding to opioid receptors. Much less is known about the fate of DERM-A594 following internalization, but widespread trafficking of fluorescent peptides, including to nuclear compartments, has been reported (Duchardt et al., 2007). Further experiments are needed to provide more information about the endocytotic recycling process.
The regulatory mechanisms underlying MOPr endocytosis have been studied extensively in cell culture systems, but the contribution of this process to antinociception has not been examined. The MOPr is a member of the seven transmembrane GPCR family that undergoes trafficking upon agonist binding. Activation of the receptor induces phosphorylation of the MOPr C-terminus by G-protein receptor kinases (GRKs) and subsequent recruitment of beta-arrestin and other adaptor proteins that promote the formation of clathrin-coated pits and dynamin-dependent endocytosis (for reviews see (Wolfe and Trejo, 2007; Koch and Hollt, 2008). One of the proposed functions of this internalization process is termination of the MOPr signal by uncoupling the receptor from the G-protein and removing the receptor from the membrane (Kim et al., 2008). However, GPCRs have been shown to signal from endosomes (Murphy et al., 2009; Sorkin and von Zastrow, 2009).
The present results showing attenuation of antinociception by blocking receptor internalization suggests that a key part of MOPr signaling occurs following internalization. The effect of blocking receptor internalization on antinociception was assessed using both dynamin-DN and ConA. Microinjection of dynamin-DN peptide into the vlPAG blocked the antinociceptive effect of microinjecting DERM-A594 or unlabeled dermorphin into the vlPAG. Dynamin is a GTPase that interacts with internalization machinery downstream of GRK, beta-arrestin and formation of clathrin-coated pits and functions to release the clathrin-coated pit from the plasma membrane (Wolfe and Trejo, 2007). Dynamin-DN inhibits this process. The lectin ConA binds to cell surface glycoproteins impairing their mobility within the membrane bilayer (Luttrell et al., 1997) and has been used as an effective blocker of GPCR recycling in several cell systems (Xiang et al., 2002; Kim et al., 2004; Arttamangkul et al., 2006). ConA inhibits endocytosis of beta-adrenergic receptors without affecting the initial events in GPCR-signaling, including ligand-binding, G protein-activation, and generation of small molecule second messengers (Wang et al., 1989; Pippig et al., 1995). The effect of ConA on MOPrs appear to be similar to those for the well characterized beta-adrenergic receptors (Keith et al., 1996). Thus, it was not surprising that pretreatment with dynamin-DN or ConA decreased the number of DERM-A594 labeled cells in the vlPAG.
In contrast, the ability of both dynamin-DN and ConA to attenuate the antinociception produced by DERM-A594 and unlabeled dermorphin was surprising because the prevailing hypothesis is that MOPr signaling occurs at the membrane and internalization of GPCRs terminates signaling. According to this hypothesis, manipulations that prevent MOPr internalization should enhance antinociception by promoting additional MOPr signaling from the membrane (Bohn et al., 1999; Bohn et al., 2004). However, decreased antinociception following blockade of MOPr internalization could occur through several potential mechanisms. First, locking MOPrs to the membrane may enhance their ability to desensitize, and internalization of opioid receptors may be required to recover from desensitization (Koch et al., 2005). However, previous research has shown that opioid desensitization is unchanged in locus coeruleus neurons in the presence of ConA (Arttamangkul et al., 2006) and early processes in GPCR activation of beta-adrenergic receptors are not affected by ConA (Wang et al., 1989; Pippig et al., 1995). Second, blockade of internalization may decrease recycling of refreshed MOPrs to the membrane. Differences in opioid-induced activation, desensitization and recycling of the MOPrs indicate that these are distinct processes with varying sensitivities depending on the efficacy of the agonist (Bailey et al., 2003; Dang and Williams, 2004, 2005; Liao et al., 2007; Arttamangkul et al., 2008; Kelly et al., 2008; Virk and Williams, 2008; Ingram, 2009).
A more likely mechanism is that signaling by some opioid agonists is dependent on recruitment of internalization machinery. That is, MOPrs may signal from endosomes following internalization of the receptor (Murphy et al., 2009; Sorkin and von Zastrow, 2009). Of course, signaling may be different with high efficacy agonists like dermorphin that produce rapid receptor internalization and low efficacy agonists like morphine that produce limited internalization. Both internalization-dependent (Ignatova et al., 1999) and independent (Kramer and Simon, 2000; Zheng et al., 2008) MAPK signaling pathways have been described. For example, our previous research shows that phosphorylation of ERK1/2 occurs preferentially with high efficacy agonists both in the striatum (Macey et al., 2006) and in vlPAG after chronic morphine (Macey et al., 2009). The present results show for the first time that endosomal signaling may be necessary for opioid-induced antinociception from the vlPAG. Additional studies are needed to distinguish whether dynamin-DN and ConA block a specific internalization-dependent signaling pathway or attenuate antinociception by preventing receptor recycling back to the plasma membrane and/or resensitization.
Acknowledgements
This study was supported in part by the National Institute of Drug Abuse [DA023318, T.A.M, DA016627, S.A.A, and DA015498, M.M.M.], the National Institute on Dental and Craniofacial Research [DE012640, S.A.A.], the National Institute of Neurological Disorders and Stroke [T32NS045553, D.M.H.], and the National Center for Research Resources [RR016858, OHSU]. Charles Jimenez helped synthesize the DERM-A594.
Abbreviations
- Beta-CNA
beta-chlornaltrexamine
- ConA
concanavalinA
- DAMGO
D-Ala2,N-Me4,Gly5-ol]-enkephalin
- DERM-A594
dermorphin conjugated to Alexa Fluor 594
- dynamin-DN
dynamin dominant-negative inhibitory peptide
- dynamin-scr
scrambled dynamin control peptide
- GIRK
G-protein-coupled inwardly rectifying potassium channels
- GPCR
G-protein coupled receptors
- GRK
G-protein receptor kinases
- MOPr
mu-opioid receptor
- PBS
Phosphate buffered saline
- vlPAG
ventrolateral periaqueductal gray
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Tara A. Macey, Department of Psychology, Washington State University Vancouver, Vancouver, WA
Susan L. Ingram, Department of Psychology, Washington State University Vancouver, Vancouver, WA
Erin N. Bobeck, Department of Psychology, Washington State University Vancouver, Vancouver, WA
Deborah M. Hegarty, Department of Physiology and Pharmacology, Oregon Health & Science University, Portland, OR
Sue A. Aicher, Department of Physiology and Pharmacology, Oregon Health & Science University, Portland, OR
Seksiri Arttamangkul, Vollum Institute, Oregon Health & Science University, Portland, OR.
Michael M. Morgan, Department of Psychology, Washington State University Vancouver, Vancouver, WA
References
- Alvarez VA, Arttamangkul S, Dang V, Salem A, Whistler JL, Von Zastrow M, Grandy DK, Williams JT. mu-Opioid receptors: Ligand-dependent activation of potassium conductance, desensitization, and internalization. J Neurosci. 2002;22:5769–5776. doi: 10.1523/JNEUROSCI.22-13-05769.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiche M, Sagan S, Mor A, Pelaprat D, Rostene W, Delfour A, Nicolas P. Characterisation and visualisation of [3H]dermorphin binding to mu opioid receptors in the rat brain. Combined high selectivity and affinity in a natural peptide agonist for the morphine (mu) receptor. Eur J Biochem. 1990;189:625–635. doi: 10.1111/j.1432-1033.1990.tb15531.x. [DOI] [PubMed] [Google Scholar]
- Arttamangkul S, Alvarez-Maubecin V, Thomas G, Williams JT, Grandy DK. Binding and internalization of fluorescent opioid peptide conjugates in living cells. Mol Pharmacol. 2000;58:1570–1580. doi: 10.1124/mol.58.6.1570. [DOI] [PubMed] [Google Scholar]
- Arttamangkul S, Quillinan N, Low MJ, von Zastrow M, Pintar J, Williams JT. Differential activation and trafficking of micro-opioid receptors in brain slices. Mol Pharmacol. 2008;74:972–979. doi: 10.1124/mol.108.048512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arttamangkul S, Torrecilla M, Kobayashi K, Okano H, Williams JT. Separation of mu-opioid receptor desensitization and internalization: endogenous receptors in primary neuronal cultures. J Neurosci. 2006;26:4118–4125. doi: 10.1523/JNEUROSCI.0303-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CP, Couch D, Johnson E, Griffiths K, Kelly E, Henderson G. Mu-opioid receptor desensitization in mature rat neurons: lack of interaction between DAMGO and morphine. J Neurosci. 2003;23:10515–10520. doi: 10.1523/JNEUROSCI.23-33-10515.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobeck EN, McNeal AL, Morgan MM. Drug dependent sex-differences in periaqueducatal gray mediated antinociception in the rat. Pain. 2009;147:210–216. doi: 10.1016/j.pain.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar R, Paul D, Pasternak GW. Synergistic analgesic interactions between the periaqueductal gray and the locus coeruleus. Brain Res. 1991;558:224–230. doi: 10.1016/0006-8993(91)90772-n. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Williams CL, Lee SJ, Pasternak GW. Role of mu 1-opiate receptors in supraspinal opiate analgesia: a microinjection study. Brain Res. 1988;447:25–34. doi: 10.1016/0006-8993(88)90962-6. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Dykstra LA, Lefkowitz RJ, Caron MG, Barak LS. Relative opioid efficacy is determined by the complements of the G protein-coupled receptor desensitization machinery. Mol Pharmacol. 2004;66:106–112. doi: 10.1124/mol.66.1.106. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Connor M, Osborne PB, Furness JB, Christie MJ. Opioid agonists have different efficacy profiles for G protein activation, rapid desensitization, and endocytosis of mu-opioid receptors. J Biol Chem. 2003;278:18776–18784. doi: 10.1074/jbc.M300525200. [DOI] [PubMed] [Google Scholar]
- Chen W, Song B, Lao L, Perez OA, Kim W, Marvizon JC. Comparing analgesia and mu-opioid receptor internalization produced by intrathecal enkephalin: requirement for peptidase inhibition. Neuropharmacology. 2007;53:664–676. doi: 10.1016/j.neuropharm.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Song B, Zhang G, Marvizon JC. Effects of veratridine and high potassium on micro-opioid receptor internalization in the rat spinal cord: stimulation of opioid release versus inhibition of internalization. J Neurosci Methods. 2008;170:285–293. doi: 10.1016/j.jneumeth.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG. Translocation of presynaptic delta opioid receptors in the ventrolateral periaqueductal gray after swim stress. J Comp Neurol. 2003;464:197–207. doi: 10.1002/cne.10788. [DOI] [PubMed] [Google Scholar]
- Dang VC, Williams JT. Chronic morphine treatment reduces recovery from opioid desensitization. J Neurosci. 2004;24:7699–7706. doi: 10.1523/JNEUROSCI.2499-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang VC, Williams JT. Morphine-Induced mu-opioid receptor desensitization. Mol Pharmacol. 2005;68:1127–1132. doi: 10.1124/mol.105.013185. [DOI] [PubMed] [Google Scholar]
- Duchardt F, Fotin-Mleczek M, Schwarz H, Fischer R, Brock R. A comprehensive model for the cellular uptake of cationic cell-penetrating peptides. Traffic. 2007;8:848–866. doi: 10.1111/j.1600-0854.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- Fang FG, Haws CM, Drasner K, Williamson A, Fields HL. Opioid peptides (DAGO-enkephalin, dynorphin A(1-13), BAM 22P) microinjected into the rat brainstem: comparison of their antinociceptive effect and their effect on neuronal firing in the rostral ventromedial medulla. Brain Res. 1989;501:116–128. doi: 10.1016/0006-8993(89)91033-0. [DOI] [PubMed] [Google Scholar]
- Finn AK, Whistler JL. Endocytosis of the mu opioid receptor reduces tolerance and a cellular hallmark of opiate withdrawal. Neuron. 2001;32:829–839. doi: 10.1016/s0896-6273(01)00517-7. [DOI] [PubMed] [Google Scholar]
- Hack SP, Bagley EE, Chieng BC, Christie MJ. Induction of delta-opioid receptor function in the midbrain after chronic morphine treatment. J Neurosci. 2005;25:3192–3198. doi: 10.1523/JNEUROSCI.4585-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Fong J, von Zastrow M, Whistler JL. Regulation of opioid receptor trafficking and morphine tolerance by receptor oligomerization. Cell. 2002;108:271–282. doi: 10.1016/s0092-8674(02)00613-x. [DOI] [PubMed] [Google Scholar]
- Ignatova EG, Belcheva MM, Bohn LM, Neuman MC, Coscia CJ. Requirement of receptor internalization for opioid stimulation of mitogen-activated protein kinase: biochemical and immunofluorescence confocal microscopic evidence. J Neurosci. 1999;19:56–63. doi: 10.1523/JNEUROSCI.19-01-00056.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram SLaT JR. Role of PKC in functional selectivity at the mu-opioid receptor: From pharmacological curiosity to therapeutic potential. Molecular Interventions epub. 2009 doi: 10.1111/j.1476-5381.2009.00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquet YF, Lajtha A. The periaqueductal gray: site of morphine analgesia and tolerance as shown by 2-way cross tolerance between systemic and intracerebral injections. Brain Res. 1976;103:501–513. doi: 10.1016/0006-8993(76)90448-0. [DOI] [PubMed] [Google Scholar]
- Keith DE, Murray SR, Zaki PA, Chu PC, Lissin DV, Kang L, Evans CJ, von Zastrow M. Morphine activates opioid receptors without causing their rapid internalization. J Biol Chem. 1996;271:19021–19024. doi: 10.1074/jbc.271.32.19021. [DOI] [PubMed] [Google Scholar]
- Kelly E, Bailey CP, Henderson G. Agonist-selective mechanisms of GPCR desensitization. Br J Pharmacol. 2008;153 Suppl 1:S379–S388. doi: 10.1038/sj.bjp.0707604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JA, Bartlett S, He L, Nielsen CK, Chang AM, Kharazia V, Waldhoer M, Ou CJ, Taylor S, Ferwerda M, Cado D, Whistler JL. Morphine-induced receptor endocytosis in a novel knockin mouse reduces tolerance and dependence. Curr Biol. 2008;18:129–135. doi: 10.1016/j.cub.2007.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Kim MY, Lee EJ, Ahn YS, Baik JH. Distinct regulation of internalization and mitogen-activated protein kinase activation by two isoforms of the dopamine D2 receptor. Mol Endocrinol. 2004;18:640–652. doi: 10.1210/me.2003-0066. [DOI] [PubMed] [Google Scholar]
- Koch T, Hollt V. Role of receptor internalization in opioid tolerance and dependence. Pharmacol Ther. 2008;117:199–206. doi: 10.1016/j.pharmthera.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Koch T, Widera A, Bartzsch K, Schulz S, Brandenburg LO, Wundrack N, Beyer A, Grecksch G, Hollt V. Receptor endocytosis counteracts the development of opioid tolerance. Mol Pharmacol. 2005;67:280–287. doi: 10.1124/mol.104.004994. [DOI] [PubMed] [Google Scholar]
- Kramer HK, Simon EJ. mu and delta-opioid receptor agonists induce mitogen-activated protein kinase (MAPK) activation in the absence of receptor internalization. Neuropharmacology. 2000;39:1707–1719. doi: 10.1016/s0028-3908(99)00243-9. [DOI] [PubMed] [Google Scholar]
- Lane DA, Morgan MM. Antinociceptive tolerance to morphine from repeated nociceptive testing in the rat. Brain Res. 2005;1047:65–71. doi: 10.1016/j.brainres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Lane DA, Patel PA, Morgan MM. Evidence for an intrinsic mechanism of antinociceptive tolerance within the ventrolateral periaqueductal gray of rats. Neuroscience. 2005;135:227–234. doi: 10.1016/j.neuroscience.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Lao L, Song B, Chen W, Marvizon JC. Noxious mechanical stimulation evokes the segmental release of opioid peptides that induce mu-opioid receptor internalization in the presence of peptidase inhibitors. Brain Res. 2008;1197:85–93. doi: 10.1016/j.brainres.2007.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Grigoriants OO, Wang W, Wiens K, Loh HH, Law PY. Distinct effects of individual opioids on the morphology of spines depend upon the internalization of mu opioid receptors. Mol Cell Neurosci. 2007;35:456–469. doi: 10.1016/j.mcn.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell LM, Daaka Y, Della Rocca GJ, Lefkowitz RJ. G protein-coupled receptors mediate two functionally distinct pathways of tyrosine phosphorylation in rat 1a fibroblasts. Shc phosphorylation and receptor endocytosis correlate with activation of Erk kinases. J Biol Chem. 1997;272:31648–31656. doi: 10.1074/jbc.272.50.31648. [DOI] [PubMed] [Google Scholar]
- Macey TA, Bobeck EN, Hegarty DM, Aicher SA, Ingram SL, Morgan MM. ERK1/2 activation counteracts morphine tolerance in the periaqueductal gray of the rat. J Pharmacol Exp Ther. 2009 doi: 10.1124/jpet.109.152157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey TA, Lowe JD, Chavkin C. Mu opioid receptor activation of ERK1/2 is GRK3 and arrestin dependent in striatal neurons. J Biol Chem. 2006;281:34515–34524. doi: 10.1074/jbc.M604278200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MM, Ashley MD, Ingram SL, Christie MJ. Behavioral consequences of delta-opioid receptor activation in the periaqueductal gray of morphine tolerant rats. Neural Plast. 2009;2009:516328. doi: 10.1155/2009/516328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MM, Clayton CC. Defensive behaviors evoked from the ventrolateral periaqueductal gray of the rat: comparison of opioid and GABA disinhibition. Behav Brain Res. 2005;164:61–66. doi: 10.1016/j.bbr.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Fossum EN, Levine CS, Ingram SL. Antinociceptive tolerance revealed by cumulative intracranial microinjections of morphine into the periaqueductal gray in the rat. Pharmacol Biochem Behav. 2006;85:214–219. doi: 10.1016/j.pbb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Whitney PK, Gold MS. Immobility and flight associated with antinociception produced by activation of the ventral and lateral/dorsal regions of the rat periaqueductal gray. Brain Res. 1998;804:159–166. doi: 10.1016/s0006-8993(98)00669-6. [DOI] [PubMed] [Google Scholar]
- Murphy JE, Padilla BE, Hasdemir B, Cottrell GS, Bunnett NW. Endosomes: a legitimate platform for the signaling train. Proc Natl Acad Sci U S A. 2009;106:17615–17622. doi: 10.1073/pnas.0906541106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipov MH, Kovelowski CJ, Nichols ML, Hruby VJ, Porreca F. Characterization of supraspinal antinociceptive actions of opioid delta agonists in the rat. Pain. 1995;62:287–293. doi: 10.1016/0304-3959(94)00231-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson SJ. The rat brain, in stereotaxic coordinates. Sydney: Academic Press; 2005. [Google Scholar]
- Pippig S, Andexinger S, Lohse MJ. Sequestration and recycling of beta 2-adrenergic receptors permit receptor resensitization. Mol Pharmacol. 1995;47:666–676. [PubMed] [Google Scholar]
- Pradhan AA, Becker JA, Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Massotte D, Gaveriaux-Ruff C, Kieffer BL. In vivo delta opioid receptor internalization controls behavioral effects of agonists. PLoS One. 2009;4:e5425. doi: 10.1371/journal.pone.0005425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randich A, Thurston CL, Ludwig PS, Robertson JD, Rasmussen C. Intravenous morphine-induced activation of vagal afferents: peripheral, spinal, and CNS substrates mediating inhibition of spinal nociception and cardiovascular responses. J Neurophysiol. 1992;68:1027–1045. doi: 10.1152/jn.1992.68.4.1027. [DOI] [PubMed] [Google Scholar]
- Rossi GC, Pasternak GW, Bodnar RJ. Mu and delta opioid synergy between the periaqueductal gray and the rostro-ventral medulla. Brain Res. 1994;665:85–93. doi: 10.1016/0006-8993(94)91155-x. [DOI] [PubMed] [Google Scholar]
- Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10:609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ. Drug synergism: its detection and applications. J Pharmacol Exp Ther. 2001;298:865–872. [PubMed] [Google Scholar]
- Tanowitz M, von Zastrow M. A novel endocytic recycling signal that distinguishes the membrane trafficking of naturally occurring opioid receptors. J Biol Chem. 2003;278:45978–45986. doi: 10.1074/jbc.M304504200. [DOI] [PubMed] [Google Scholar]
- Trafton JA, Basbaum AI. The contribution of spinal cord neurokinin-1 receptor signaling to pain. J Pain. 2000;1:57–65. doi: 10.1054/jpai.2000.9806. [DOI] [PubMed] [Google Scholar]
- Trafton JA, Basbaum AI. [d-Ala2,N-MePhe4,Gly-ol5]enkephalin-induced internalization of the micro opioid receptor in the spinal cord of morphine tolerant rats. Neuroscience. 2004;125:541–543. doi: 10.1016/j.neuroscience.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Virk MS, Williams JT. Agonist-specific regulation of mu-opioid receptor desensitization and recovery from desensitization. Mol Pharmacol. 2008;73:1301–1308. doi: 10.1124/mol.107.042952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zastrow M, Svingos A, Haberstock-Debic H, Evans C. Regulated endocytosis of opioid receptors: cellular mechanisms and proposed roles in physiological adaptation to opiate drugs. Curr Opin Neurobiol. 2003;13:348–353. doi: 10.1016/s0959-4388(03)00069-2. [DOI] [PubMed] [Google Scholar]
- Wang HY, Berrios M, Malbon CC. Localization of beta-adrenergic receptors in A431 cells in situ. Effect of chronic exposure to agonist. Biochem J. 1989;263:533–538. doi: 10.1042/bj2630533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe BL, Trejo J. Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic. 2007;8:462–470. doi: 10.1111/j.1600-0854.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Devic E, Kobilka B. The PDZ binding motif of the beta 1 adrenergic receptor modulates receptor trafficking and signaling in cardiac myocytes. J Biol Chem. 2002;277:33783–33790. doi: 10.1074/jbc.M204136200. [DOI] [PubMed] [Google Scholar]
- Zambotti F, Zonta N, Parenti M, Tommasi R, Vicentini L, Conci F, Mantegazza P. Periaqueductal gray matter involvement in the muscimol-induced decrease of morphine antinociception. Naunyn Schmiedebergs Arch Pharmacol. 1982;318:368–369. doi: 10.1007/BF00501180. [DOI] [PubMed] [Google Scholar]
- Zheng H, Loh HH, Law PY. Beta-arrestin-dependent mu-opioid receptor-activated extracellular signal-regulated kinases (ERKs) Translocate to Nucleus in Contrast to G protein-dependent ERK activation. Mol Pharmacol. 2008;73:178–190. doi: 10.1124/mol.107.039842. [DOI] [PMC free article] [PubMed] [Google Scholar]