Abstract
Objective
To identify markers of ovarian age that best match the pattern of oocyte loss seen in histology specimens.
Design
Cross-sectional study.
Setting
University.
Patient(s)
Caucasian women (n = 252) aged 25–45 years.
Intervention(s)
none.
Main Outcome Measure(s)
The relationship between antral follicle count (AFC), antimüullerian hormone (AMH), inhibin B, FSH, and E2 with age was estimated using the power model, which previously has been shown to most accurately describe oocyte loss in histologic specimens. The power model was fit to each marker and used to compare the rates of change at ages 30 and 40 with the histologic pattern. Among those markers following the pattern, R2 was used to compare the degree of relationship with age.
Result(s)
Both AMH levels and AFC exhibited significant progressive declines with age. The average rates of loss per year for AFC and AMH were, respectively, −0.57 and −1.09 at age 30, and −1.33 and −3.06 at age 40. FSH, inhibin B, and E2 did not exhibit progressive rates of change. The R2 for AFC was 27.3% and for AMH was 22.7%.
Conclusion(s)
Only AFC and AMH follow the pattern of oocyte loss observed histologically. Although AMH may be more cost-effective, AFC is a slightly more accurate noninvasive measure for ovarian aging.
Keywords: AFC, AMH, ovarian reserve, fertility, FSH, oocyte, follicle
Wide variability exists between women both in the age at which menopause occurs and in the onset of diminished reproductive capacity (1). As ovarian function has profound impacts on women’s hormonal milieu and their subsequent risk for the development of disease, as well as reproductive potential, improving our understanding of reproductive aging is critical to improving quality of life for all women. The quantity and quality of oocytes (ovarian reserve) has been linked to ovarian function and so there is significant interest in developing noninvasive testing to characterize the rate and pattern of oocyte loss.
Several studies have directly assessed the rate of oocyte loss. Using cross-sectional data from multiple autopsy studies, Faddy et al. developed a mathematical model for rate of follicle count decline (2–4). Their original analysis suggested a sudden acceleration in the decline of oocytes at approximately 37 years of age (i.e., a biphasic relationship) (3). This pattern had been widely accepted because it mirrored the decline in fertility noted earlier and the increase in spontaneous abortion (largely from increasing aneuploidy). However, in a reanalysis of their data, a gradual acceleration in decline fit the data better than the biphasic relationship (5, 6). Recently, Hansen et al. studied the rate of nongrowing follicles within a single population and confirmed a gradual acceleration in the rate of loss over time and demonstrated the power model to better describe this decline (7). We therefore utilized the power model as a platform to determine whether markers of ovarian reserve mirror this pattern of loss.
During the past decade, research has sought to identify noninvasive (indirect) markers of ovarian age. Several studies have correlated noninvasive markers to follicle count in histologic specimens in both the human and animal models (8–10). We recently characterized the relationship of antral follicle count (AFC) with chronological age and found that the pattern corresponded to a gradual acceleration of loss seen by Hansen et al. (7, 11). No studies have determined whether the serum markers of ovarian age follow a similar pattern.
Most data evaluating surrogate markers were derived from small observational studies, or from infertile populations who may or may not have received infertility medications. The values of these surrogate markers may differ between infertile women and the general population (12). In the current study, we quantify the relationship of each with age and compare it to the documented pattern of histologic decline in ovarian reserve. We propose that good surrogate markers of ovarian reserve would follow a pattern of loss with age similar to that of follicle loss observed histologically. Among those markers following the correct pattern, we hypothesized we could further distinguish them according to the quality of their fit with age. Improving our understanding of reproductive aging will have profound economic and social implications given the complex choices women face regarding the timing of childbearing and the growing burden of infertility.
MATERIALS AND METHODS
The study population includes 252 Caucasian women aged 25–45, enrolled in a community-based cohort consisting of women not seeking treatment for fertility or other medical problems. This population is derived from the Ovarian Aging (OVA) study, which is a population-based multiethnic cohort designed to study the natural process of ovarian aging; it so far consists of measurements at a single time point, and our sample comprises the subset of non-Hispanic Caucasians.
Subjects were recruited from a sampling frame consisting of all age-eligible women members of the Kaiser Permanente Northern California Health Plan in geographical areas within reasonable distance to the research clinic. Institutional review board approval was obtained both from Kaiser Permanente and the University of California, San Francisco. Inclusion required subjects to have intact ovaries and regular menses at 22- to 35-day intervals. Exclusion criteria included estrogen- or progestin-containing medications in the 3 months before enrollment, history of endometriosis, or any uterine or ovarian surgery.
All subjects underwent transvaginal ultrasound assessment of antral follicle count and ovarian volumes, performed on the second or fourth day of the menstrual cycle. Using a Shimadzu SDU-450XL machine, with a variable 4–8 mHz vaginal transducer, measurements of the transverse, longitudinal, and anteroposterior diameters of each ovary were made using electronic calipers. All echo-free structures in the ovaries with a mean diameter (of two dimensions) between 2 and 10 mm were counted as antral follicles. Antral follicle count for each subject was determined by summing total AFC for both ovaries. All examinations were performed by one of two examiners (M.I.C., M.P.R.). Our internal data have shown excellent correlations between repeated measurements (r2 = 0.92).
Serum hormonal assays were obtained on the second or fourth day of the menstrual cycle and performed in the CLASS Laboratory at University of Michigan. Follicle-stimulating hormone was measured with standardized two-site chemiluminescence immunoassays; intra-assay coefficients of variation (CVs) were 1.9%–2.1% and interassay, 5.2%–6.8%. Inhibin B was assayed using commercially an available ELISA kit from Diagnostic Systems Laboratories (Webster City, TX); intra-assay CV 3.3%–7.2%; interassay CV 7.8%–17%. Estradiol was assayed with an automated chemiluminescent assay using Bayer Diagnostics ACS:180 (Tarrytown, NY). Estradiol intra- and interassay CVs were 6.5%–6.9% and 13.6%–16.1%, respectively. Antimüullerian hormone was assayed using ELISA from Beckman Coulter (Marseille, France), using two-site sandwich-type immunoassay; intra-assay CV was 5.6% and interassay CV was 15.3%.
Descriptive statistics were calculated for demographic variables. The relationship between the serum markers with age was assessed using a power model regression (7): marker = A + B × (Age)C. The shape of the relationship with age was used to help assess accuracy of the markers. We believe that the original scale probably may be related more to biological processes than log-transformed data, and therefore our analysis was performed using original data. We used the power model because previous data have suggested that oocyte loss based on histologic specimens and AFC follows a gradual accelerated loss with age (7, 11). In its original form, the parameters of the power model are not readily interpretable. We therefore rewrote the model using the following three parameters: value at age 30 and yearly rates of change at ages 30 and 40. Confidence intervals were derived for each of these parameters to compare to the trends observed in histologic data. Coefficients of determination (R2) were calculated for each marker of ovarian reserve and were used to compare model fits for each of the markers. A sensitivity analysis was performed by removing outliers (<5th and >95th percentile) and reanalyzing the data. All analyses were performed using SAS, version 9.12 (Cary, NC).
RESULTS
Subject characteristics are given in Table 1. The mean age was 35.4 (range 25–45 years). Notably, the percentage of subjects who reported never smoking was 54.8% and those who had ever given birth was 14.7%.
TABLE 1.
Baseline characteristics of study participants (n = 252).
| Variable | Mean ± SD | Minimum | Maximum |
|---|---|---|---|
| Age | 35.38 ± 4.97 | 25 | 45 |
| AFC | 15.70 ± 9.37 | 1 | 58 |
| Height (cm) | 166.35 ± 6.13 | 148.20 | 182.40 |
| Weight (kg) | 67.74 ± 16.36 | 48.70 | 146.60 |
| BMI | 24.46 ± 5.63 | 17.41 | 58.35 |
| Waist hip ratio | 0.75 ± 0.05 | 0.42 | 0.96 |
| Smoker | |||
| Never | 54.76% | ||
| Current | 13.49% | ||
| Past | 31.75% | ||
| Parity | |||
| None | 64.1% | ||
| 1 | 15.4% | ||
| >1 | 20.5% |
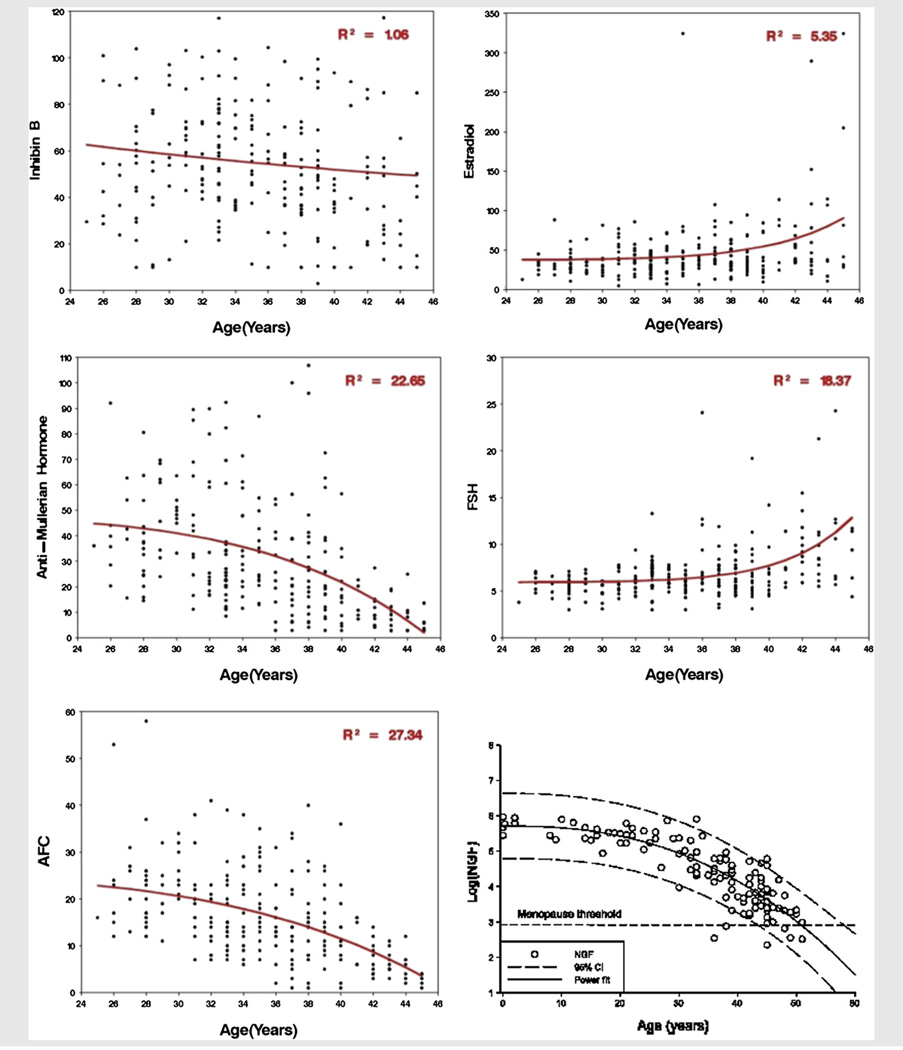
Figure 1 displays the fits of the power model relationships between each ovarian reserve marker and age, with the relationship of follicle counts of histologic ovarian specimens and age. Coefficients of determination are noted. All but one of the markers has a relationship with age that mirrors the histologic relationship (panel F). Antimüullerian hormone levels and AFCs exhibit gradual acceleration of oocyte loss with age, and FSH and E2 levels exhibit an upturn with age. However, the upturn in FSH and E2 levels occurs later than the downturn in follicle number in histologic specimens. Inhibin B does not match the histologic relationship.
FIGURE 1.
Serum markers of ovarian reserve with age.
Table 2 lists the estimates for the average values for the ovarian reserve markers at age 30 and rate of change per year at ages 30 and 40 that were derived from the fitted model. The average rates of loss are significant for AFC and AMH and are, respectively, −0.57 and −1.09 at age 30, and −1.33 and −3.06 at age 40. Estradiol, FSH, and inhibin B do not have a significant change at age 30 but do exhibit a significant change at age 40.
TABLE 2.
Estimates for the average values for the ovarian reserve markers at age 30 and rate of change per year at ages 30 and 40 that were derived from the fitted model.
| Estimate values at age 30 |
Estimate rate of change/y at age 30 |
Estimate values at age 40 |
Estimate rate of change/y at age 40 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | R2 | |||||
| AFC | 20.63 | 19.10 | 22.16 | −0.57 | −0.95 | −0.19 | 11.50 | 10.06 | 12.93 | −1.33 | −1.72 | −0.93 | 27.34 |
| AMH (pmol/L) | 41.29 | 37.37 | 45.22 | −1.09 | −1.99 | −0.20 | 21.75 | 18.10 | 25.40 | −3.06 | −4.03 | −2.08 | 22.65 |
| E2 (pg/mL) | 37.25 | 26.91 | 47.59 | 0.80 | −1.05 | 2.65 | 57.22 | 47.09 | 67.35 | 3.81 | 1.46 | 6.16 | 5.35 |
| FSH (IU/L) | 5.99 | 5.41 | 6.57 | 0.02 | −0.02 | 0.06 | 7.49 | 6.80 | 8.17 | 0.47 | 0.33 | 0.60 | 18.37 |
| Inhibin B (pg/mL) | 58.19 | 52.48 | 63.89 | −0.16 | −0.90 | 0.58 | 52.24 | 46.25 | 58.22 | −1.38 | −2.57 | −0.20 | 2.19 |
The quality of fit for the power model for each marker exhibiting the correct relationship is the following: AFC had the highest R2 (27.3%), followed by AMH (22.7%), FSH (18.4%), and E2 (5.4%). The sensitivity analyses showed no alterations in the relationships between any of the noninvasive markers and age.
DISCUSSION
In this study, we evaluated how serum markers of ovarian reserve decline with age in a community-based, regularly cycling Caucasian population and compared these patterns to those identified from histologic data (7). Of the markers considered, only AMH and AFC exhibited significant progressive declines with age, with AFC having a slightly better fit to the basic pattern observed histologically (Table 2); E2 and inhibin B did not and therefore the data do not provide support for them as markers of ovarian reserve.
Antimüullerian hormone levels therefore appear to be the best serum marker of ovarian reserve (13–15). Simple correlation coefficients between age and AMH have been reported previously. A recent study evaluating the relationship between AMH and age at menopause in 144 participants reported an accelerated rate of decline with age (16). Reported correlations between age and AMH are dependent on the population studied (r = −0.30 to −0.66) (17–19). Perhaps the most similar patient group to ours is a subset analysis of 81 participants of the 162 subjects in their overall cohort; for that study, the reported correlation (r) was −0.66, whereas we found an r of −0.46. Unlike our results, they do not report quantitative loss across ages and judge whether that marker fit the histologic pattern of oocyte loss.
Among those markers following the pattern, R2 was used to compare the degree of relationship with age. Antral follicle count had a slightly better fit with the power model compared with AMH (R2 = 27.3% vs 22.7%, respectively). However, the R2 for nongrowing follicles with age using the power model was reported by Hansen et al. to be 0.83 (7), much higher than ours. However, the population studied was from ages <1 month to 51 years. For comparison, we reanalyzed their data and restricted the ages to that of our study sample (25–45 years) and found a reduced R2 of approximately 0.30 using either the raw or log-transformed values of AMH, much more similar to our results. The R2 for our data for the log-transformed AMH levels was 0.38, and for AFC it was 0.37.
Inhibin B is a biologically plausible biomarker of ovarian reserve because its decline leads to a rise in FSH levels, and it is produced by small preantral and antral follicles (20–22). Danforth et al. showed a statistically significant correlation of −0.54 between inhibin B and age (20). Although their study included healthy volunteers and not infertile patients, it was small (n = 25), and only included women between the ages of 39 and 52, not addressing correlation across the full reproductive age span. A larger study by Scheffer et al. composed of 162 participants from the general population, aged 25–46 years, showed that inhibin B was not significantly correlated with age (r = −0.12 and NS) (23). In our study, inhibin B levels did not show the expected gradual accelerated decline with age the other markers exhibited and thus was consistent with the work of Scheffer et al., suggesting poor correlation with age overall (r = 0.11, P<.07).
Day 3 FSH and E2 levels have been utilized as a marker of ovarian reserve since the 1980s and was the first such marker (24–26). Elevated day 3 FSH and E2 levels correlate well with those in late perimenopause and menopause, and milder elevations have been considered the hallmark for ovarian aging (27). Earlier studies showed FSH levels to be significantly higher starting in the fifth decade of life (28, 29). Scheffer et al. showed that FSH (r = 0.25, P<.05) and day 3 E2 (r = 0.29, P<.05) levels were significantly correlated with age (23). We similarly found that FSH (r = 0.37, P<.001) and day 3 E2 (r = 0.22, P<.001) levels were correlated with age, and found that the yearly rate of change was significant only at older ages (Fig. 1, Table 2). Furthermore, the power model fit for day 3 E2 shows a relatively poor association with age (Fig. 1). This suggests that E2 alone is not an accurate marker of ovarian aging.
The panels in Figure 1 show that there is a considerable amount of variation with any surrogate marker of ovarian reserve that cannot be explained by age alone. Even with the best surrogate markers, AFC and AMH, more than 70% of the variation in women of reproductive age is left unexplained by age. This finding further illustrates that age is not the sole determinant of ovarian reserve. Several studies have shown that there is considerable variation in the natural age of onset of menopause (30–33). Although the coefficients of variation for the surrogate markers are relatively low, they are similar to what has been observed histologically and therefore we consider them valid markers of ovarian reserve.
A major limitation of this study is that it used cross-sectional data, and so nonlinear longitudinal relationships are not recoverable. Another limitation is that the data set is limited to Caucasians. However, we made this decision to decrease potential ethnic variation. Currently, we are enrolling subjects of different ethnicities to address this shortcoming. Prospective longitudinal studies in the same women over time are planned to more accurately characterize the relationship of these noninvasive markers of ovarian reserve and aging. A strength of this study is the closer approximation to normative as the population was derived from the community and not an infertility clinic. However, determination of which ovarian reserve marker is more reliable is limited because of the absence of direct histologic specimens as an outcome for comparison. More studies are needed to compare these noninvasive tests with histologic assessments.
One must use caution when suggesting these markers are “true markers” of the number of follicles remaining in the ovary. It is noted that the number of growing antral follicles is correlated to the number of primordial follicles (34). Most of these markers of ovarian reserve, other than FSH, are the direct product of growing antral follicles. If there is a disturbance in the number of growing follicles, measures of ovarian reserve may not be reliable (10). For example, there is evidence that AMH levels may be temporally influenced by iatrogenic causes (35). One study showed that AMH levels decrease for a short time after ovarian cystectomy (11). Other studies suggest AMH levels decrease after uterine artery embolization or hysterectomy (36).
This is the first study to characterize the relationship of the noninvasive serum markers of ovarian reserve with age in a large general (noninfertility) population. We found that the only markers that follow the pattern of oocyte loss observed histologically were AFC and AMH. Although AMH may be more cost-effective, AFC was a slightly more accurate noninvasive measure for ovarian aging.
Acknowledgments
The authors wish to express their sincere appreciation to the support staff at the Kaiser Permanente Division of Research for recruiting study participants, and the Center for Reproductive Health at University of California, San Francisco, for coordinating study participation. We also wish to thank the study participants for making this work possible.
Supported by the National Institute of Child Health and Human Development/National Institute of Aging (grant no. R01HD044876) and the National Institutes of Health/Neurobehavioral Core for Rehabilitation Research, University of California at San Francisco–Clinical and Translational Science Institute (grant no. UL1 RR024131).
Footnotes
The contents of the manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.te Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update. 2002;8:141–154. doi: 10.1093/humupd/8.2.141. [DOI] [PubMed] [Google Scholar]
- 2.Richardson SJ, Senikas V, Nelson JF. Follicular depletion during the menopausal transition: evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab. 1987;65:1231–1237. doi: 10.1210/jcem-65-6-1231. [DOI] [PubMed] [Google Scholar]
- 3.Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7:1342–1346. doi: 10.1093/oxfordjournals.humrep.a137570. [DOI] [PubMed] [Google Scholar]
- 4.Block E. Quantitative morphological investigations of the follicular system in women; variations at different ages. Acta Anat (Basel) 1952;14:108–123. doi: 10.1159/000140595. [DOI] [PubMed] [Google Scholar]
- 5.Faddy MJ, Gosden RG. A model conforming the decline in follicle numbers to the age of menopause in women. Hum Reprod. 1996;11:1484–1486. doi: 10.1093/oxfordjournals.humrep.a019422. [DOI] [PubMed] [Google Scholar]
- 6.Leidy LE, Godfrey LR, Sutherland MR. Is follicular atresia biphasic? Fertil Steril. 1998;70:851–859. doi: 10.1016/s0015-0282(98)00316-1. [DOI] [PubMed] [Google Scholar]
- 7.Hansen KR, Knowlton NS, Thyer AC, Charleston JS, Soules MR, Klein NA. A new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopause. Hum Reprod. 2008;23:699–708. doi: 10.1093/humrep/dem408. [DOI] [PubMed] [Google Scholar]
- 8.Kevenaar ME, Meerasahib MF, Kramer P, van de Lang-Born BM, de Jong FH, Groome NP, et al. Serum anti-mullerian hormone levels reflect the size of the primordial follicle pool in mice. Endocrinology. 2006;147:3228–3234. doi: 10.1210/en.2005-1588. [DOI] [PubMed] [Google Scholar]
- 9.Morris JL, Thyer AC, Soules MR, Klein NA. Antral follicle count by transvaginal ultrasound is reflective of the actual primordial follicle pool. Fertil Steril. 2002;78:S3. [Google Scholar]
- 10.Sahambi SK, Visser JA, Themmen AP, Mayer LP, Devine PJ. Correlation of serum anti-Mullerian hormone with accelerated follicle loss following 4-vinylcyclohexene diepoxide-induced follicle loss in mice. Reprod Toxicol. 2008;26:116–122. doi: 10.1016/j.reprotox.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Rosen MP, Sternfeld B, Schuh-Huerta SM, Reijo Pera RA, McCulloch CE, Cedars MI. Antral follicle count: absence of significant midlife decline. Fertil Steril. 2010;94:2182–2185. doi: 10.1016/j.fertnstert.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosen MP, Johnstone E, Addauan-Andersen C, Cedars MI. A lower antral follicle count is associated with infertility. Fertil Steril. 2011;95:1950–1954. doi: 10.1016/j.fertnstert.2011.01.151. 1954.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hazout A, Bouchard P, Seifer DB, Aussage P, Junca AM, Cohen-Bacrie P. Serum antimullerian hormone/mullerian-inhibiting substance appears to be a more discriminatory marker of assisted reproductive technology outcome than follicle-stimulating hormone, inhibin B, or estradiol. Fertil Steril. 2004;82:1323–1329. doi: 10.1016/j.fertnstert.2004.03.061. [DOI] [PubMed] [Google Scholar]
- 14.Broer SL, Mol BW, Hendriks D, Broekmans FJ. The role of antimullerian hormone in prediction of outcome after IVF: comparison with the antral follicle count. Fertil Steril. 2009;91:705–714. doi: 10.1016/j.fertnstert.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Elgindy EA, El-Haieg DO, El-Sebaey A. Anti-Mullerian hormone: correlation of early follicular, ovulatory and midluteal levels with ovarian response and cycle outcome in intracytoplasmic sperm injection patients. Fertil Steril. 2008;89:1670–1676. doi: 10.1016/j.fertnstert.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 16.van Disseldorp J, Faddy MJ, Themmen AP, de Jong FH, Peeters PH, van der Schouw YT, et al. Relationship of serum antimullerian hormone concentration to age at menopause. J Clin Endocrinol Metab. 2008;93:2129–2134. doi: 10.1210/jc.2007-2093. [DOI] [PubMed] [Google Scholar]
- 17.van Rooij IA, Broekmans FJ, te Velde ER, Fauser BC, Bancsi LF, de Jong FH, et al. Serum anti-Mullerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002;17:3065–3071. doi: 10.1093/humrep/17.12.3065. [DOI] [PubMed] [Google Scholar]
- 18.van Rooij IA, Broekmans FJ, Scheffer GJ, Looman CW, Habbema JD, de Jong FH, et al. Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83:979–987. doi: 10.1016/j.fertnstert.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 19.van Rooij IA, Tonkelaar I, Broekmans FJ, Looman CW, Scheffer GJ, de Jong FH, et al. Anti-mullerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11:601–606. doi: 10.1097/01.gme.0000123642.76105.6e. [DOI] [PubMed] [Google Scholar]
- 20.Hofmann GE, Danforth DR, Seifer DB. Inhibin-B: the physiologic basis of the clomiphene citrate challenge test for ovarian reserve screening. Fertil Steril. 1998;69:474–477. doi: 10.1016/s0015-0282(97)00531-1. [DOI] [PubMed] [Google Scholar]
- 21.Welt CK, McNicholl DJ, Taylor AE, Hall JE. Female reproductive aging is marked by decreased secretion of dimeric inhibin. J Clin Endocrinol Metab. 1999;84:105–111. doi: 10.1210/jcem.84.1.5381. [DOI] [PubMed] [Google Scholar]
- 22.Danforth DR, Arbogast LK, Mroueh J, Kim MH, Kennard EA, Seifer DB, et al. Dimeric inhibin: a direct marker of ovarian aging. Fertil Steril. 1998;70:119–123. doi: 10.1016/s0015-0282(98)00127-7. [DOI] [PubMed] [Google Scholar]
- 23.Scheffer GJ. The number of antral follicles in normal women with proven fertility is the best reflection of reproductive age. Hum Reprod. 2003;18:700–776. doi: 10.1093/humrep/deg135. [DOI] [PubMed] [Google Scholar]
- 24.Labhsetwar AP. Age-dependent changes in the pituitary-gonadal relationship. II. A study of pituitary FSH and LH content in the female rat. J Reprod Fertil. 1969;20:21–28. doi: 10.1530/jrf.0.0200021. [DOI] [PubMed] [Google Scholar]
- 25.Parkening TA, Collins TJ, Smith ER. Plasma and pituitary concentrations of LH, FSH and prolactin in aged female C57BL/6 mice. J Reprod Fertil. 1980;58:377–386. doi: 10.1530/jrf.0.0580377. [DOI] [PubMed] [Google Scholar]
- 26.Sherman BM, West JH, Korenman SG. The menopausal transition: analysis of LH, FSH, estradiol, and progesterone concentrations during menstrual cycles of older women. J Clin Endocrinol Metab. 1976;42:629–636. doi: 10.1210/jcem-42-4-629. [DOI] [PubMed] [Google Scholar]
- 27.Metcalf MG, Livesey JH. Gonadotrophin excretion in fertile women: effect of age and the onset of the menopausal transition. J Endocrinol. 1985;105:357–362. doi: 10.1677/joe.0.1050357. [DOI] [PubMed] [Google Scholar]
- 28.Reyes FI, Winter JS, Faiman C. Pituitary-ovarian relationships preceding the menopause. I. A cross-sectional study of serum follice-stimulating hormone, luteinizing hormone, prolactin, estradiol, and progesterone levels. Am J Obstet Gynecol. 1977;129:557–564. [PubMed] [Google Scholar]
- 29.MacNaughton J, Banah M, McCloud P, Hee J, Burger H. Age related changes in follicle stimulating hormone, luteinizing hormone, oestradiol and immunoreactive inhibin in women of reproductive age. Clin Endocrinol (Oxf) 1992;36:339–345. doi: 10.1111/j.1365-2265.1992.tb01457.x. [DOI] [PubMed] [Google Scholar]
- 30.Gold EB, Bromberger J, Crawford S, Samuels S, Greendale GA, Harlow SD, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153:865–874. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- 31.Thomas F, Renaud F, Benefice E, De Meeüs T, Guegan J-F. International variability of ages at menarche and menopause: patterns and main determinants. Human Biology. 2001;73:271–290. doi: 10.1353/hub.2001.0029. [DOI] [PubMed] [Google Scholar]
- 32.Murabito JM, Yang Q, Fox C, Wilson PWF, Adrienne Cupples L. Heritability of age at natural menopause in the Framingham Heart Study. J Clin Endocrinol Metab. 2005;90:3427–3430. doi: 10.1210/jc.2005-0181. [DOI] [PubMed] [Google Scholar]
- 33.Treloar SA, Do KA, Martin NG. Genetic influences on the age at menopause. Lancet. 1998;352:1084–1085. doi: 10.1016/S0140-6736(05)79753-1. [DOI] [PubMed] [Google Scholar]
- 34.Gougeon A, Ecochard R, Thalabard JC. Age-related changes of the population of human ovarian follicles: increase in the disappearance rate of non-growing and early-growing follicles in aging women. Biol Reprod. 1994;50:653–663. doi: 10.1095/biolreprod50.3.653. [DOI] [PubMed] [Google Scholar]
- 35.Jayaprakasan K, Campbell B, Hopkisson J, Clewes J, Johnson I, Raine-Fenning N. Establishing the intercycle variability of three-dimensional ultrasonographic predictors of ovarian reserve. Fertil Steril. 2008;90:2126–2132. doi: 10.1016/j.fertnstert.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 36.Hehenkamp WJ, Volkers NA, Broekmans FJ, de Jong FH, Themmen AP, Birnie E, et al. Loss of ovarian reserve after uterine artery embolization: a randomized comparison with hysterectomy. Hum Reprod. 2007;22:1996–2005. doi: 10.1093/humrep/dem105. [DOI] [PubMed] [Google Scholar]