Abstract
The hot plate is a widely used test to assess nociception. The effect of non-nociceptive factors (weight, sex, activity, habituation, and repeated testing) on hot plate latency was examined. Comparison of body weight and hot plate latency revealed a small but significant inverse correlation (light rats had longer latencies). Habituating rats to the test room for 1 hr prior to testing did not decrease hot plate latency except for female rats tested on Days 2 - 4. Hot plate latency decreased with repeated daily testing, but this was not caused by a decrease in locomotor activity or learning to respond. Activity on the hot plate was consistent across all four trials, and prior exposure to a room temperature plate caused a similar decrease in latency as rats tested repeatedly on the hot plate. Despite this decrease in baseline hot plate latency, there was no difference in morphine antinociceptive potency. The present study shows that weight, habituation to the test room, and repeated testing can alter baseline hot plate latency, but these effects are small and have relatively little impact on morphine antinociception.
Keywords: Pain, Nociception, Sex differences, Hyperalgesia, Morphine
Introduction
The hot plate test is one of the oldest 6, 13, 29 and most widely used experimental methods to assess nociception in rats and mice 16. The test consists of placing a rodent on an enclosed hot plate and measuring the latency to lick a hindpaw or jump out of the enclosure 2. The advantages of this test are that it is objective, quantifiable, can be administered repeatedly without causing inflammation, and assesses supraspinally-organized responses to a noxious stimulus.
There appears to be a good correspondence between drugs that produce antinociception on the hot plate test and drugs used clinically to treat pain 26. Low intensity hot plates seem to be especially sensitive to analgesic drugs 1, 21, 25, although antinociceptive potency is difficult to compare when different hot plate temperatures are used because baseline latency and the operational definition of antinociception will vary significantly 19. A number of other factors in addition to stimulus intensity and nociceptive sensitivity may influence hot plate latency. For example, numerous studies have shown that hot plate latency decreases with repeated testing 3, 7-9, 14, 15, 22. This decrease could be caused by learning, a reduction in stress, habituation to the stimuli associated with the test, or other unknown factors 10, 13, 22.
The objective of the present study was to assess whether factors such as weight, activity, habituation time, and prior exposure to the apparatus alter hot plate latency. It is hypothesized that a heavy rat will have a shorter latency because of greater contact with the hot plate and an active rat will have a longer latency because of less contact with the hot plate. These hypotheses were assessed by placing rats on the hot plate once a day for four consecutive days while simultaneously measuring locomotor activity.
Materials and Methods
Subjects
Sprague-Dawley rats (Harlan Laboratories, Livermore, CA) were housed 2 or more to a cage in a room maintained at approximately 22 °C. Lights were set on a reverse cycle (off at 7:00 AM) so rats could be tested during the active dark phase. Food and water were provided ad lib except during testing. Experiments were approved by the Animal Care and Use Committee at Washington State University. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Rats were treated with care and respect before, during, and after testing.
Nociception was assessed using the hot plate test (Columbus Instruments, Columbus, OH). The latency to lick a hindpaw when the rat was placed on a 52.5 °C plate was measured. The plate was enclosed with four Plexiglas walls so the rat could not escape. The rat was removed from the plate immediately upon licking a hindpaw or if no response occurred within 50 s.
Experiment 1: Correlation Between Weight & Hot Plate Latency
The effect of body weight on hot plate latency was assessed by measuring whether these two factors correlate. Baseline hot plate data were collated from 201 male rats used in previous experiments 11 in order to acquire a large sample size and compare a wide range of weights (72 – 376 g). Each rat was weighed and then moved to an adjacent room for the hot plate test. Only drug-naïve rats tested on the hot plate for the first time were included.
Experiment 2: Effect of Sex, Habituation, and Activity on Hot Plate Latency
The objective of this experiment was to determine whether repeated testing, habituation to the test room, and activity influence hot plate latency in female and male rats. Female (152 – 332 g) and male (190 – 420 g) rats were weighed and then moved to an adjacent room for the hot plate test. Half of the rats were habituated to the testing room for 1 hr prior to being placed on the hot plate whereas the other half were tested within 5 min. Each rat was tested in the same manner for four consecutive days. Rats remained in their home cage except when being weighed or tested on the hot plate.
Activity was assessed while the rat was on the hot plate (25.4 × 25.4 cm) by measuring the number of times the rat crossed from one quarter of the plate (12.7 × 12.7 cm) to another. Given that the number of crosses increase with the duration on the hot plate, activity was assessed by the average time to cross (hot plate latency/crosses). Activity also was evaluated as the total number of crosses within the first 6 s so rats could be compared independent of hot plate latency.
Experiment 3: Effect of Learning on Hot Plate Latency
The objective of this experiment was to determine whether the decrease in activity with repeated testing is a learned response. This hypothesis was tested by placing male rats (192 – 269 g) on a hot plate (52.5 °C) or room temperature plate twice a day for two consecutive days. Twice daily, instead of once daily tests were used so the results could be generalized to our tolerance paradigm 18. Rats remained on the room temperature plate for 50 s on each trial. A control group was moved into the testing room twice a day for 2 days and handled for 50 s and then returned to their homecage. On Day 3 (Trial 5), baseline hot plate latency was recorded for rats from all three groups. Following baseline assessment, cumulative quarter log doses of morphine sulfate (1.8, 3.2, 5.6, 10, & 18 mg/kg. s.c.) were administered to determine whether differences in baseline latency alter morphine antinociceptive potency. Morphine was injected subcutaneously every 20 min and hot plate latency was assessed 15 min after each injection. Injections and testing were terminated once a rat reached the 50 s cutoff value for antinociception.
Statistical analysis
Data were analyzed using ANOVA. A general linear model with repeated measures was used with weight, habituation, and sex as between-subjects factors, and repeated testing as the within-subjects factor. Hot plate latency and activity were the dependent variables. The dose for half maximal antinociception (D50) following morphine administration was calculated and compared using GraphPad. Statistical significance was defined as an alpha level of 0.05.
Results
Experiment 1: Correlation Between Weight & Hot Plate Latency
The rats in this experiment (N = 201) ranged from 72 – 376 g with a median weight of 195 g and a mean ± standard deviation weight of 194 ± 85 g. Hot plate latency varied from a low of 4.2 s to a high of 33 s with a median of 16.5 s and a mean ± standard deviation of 16.5 ± 5.9 s. Comparison of weight and hot plate latency revealed a small but significant inverse correlation (r = -0.26, p < .05). That is, heavy rats tended to have lower hot plate latencies than light rats (Figure 1). This correlation was particularly strong for rats under 300 g (r = -0.40, p < .05), but disappeared for rats over 300 g (r = 0.12; N = 22).
Figure 1.
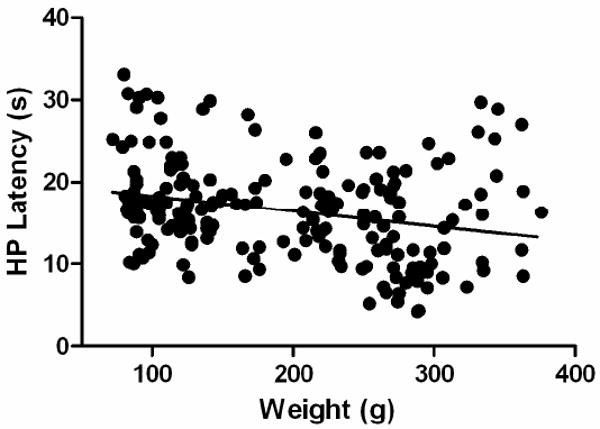
Inverse correlation between body weight and hot plate (HP) latency. There is a slight but significant decrease in hot plate latency as body weight increases (r = -0.26). This correlation breaks down for rats over 300 g.
Experiment 2: Effect of Sex, Habituation, and Activity on Hot Plate Latency
There was a significant decrease in hot plate latency from Trial 1 to 4 (F(3,174) = 15.613, p < .05). Both male (N = 30) and female (N = 31) rats showed this decrease with repeated testing (Figure 2). Female rats showed a decrease in latency of approximately 1 s from one day to the next. The decrease in male rats was evident from the second to the fourth day of testing.
Figure 2.
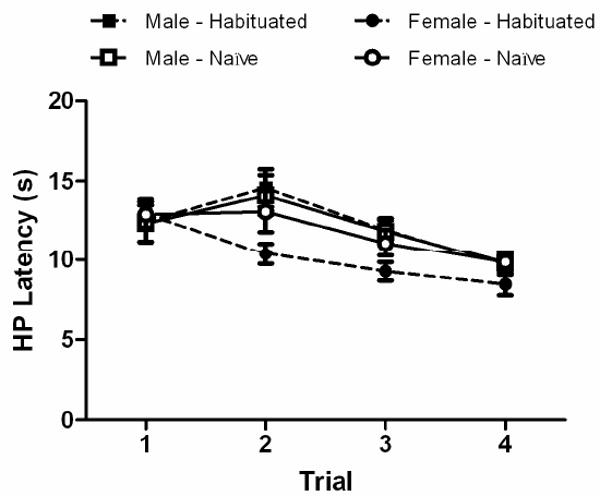
Effect of sex and habituation to the test room on hot plate (HP) latency. There was a significant decrease in hot plate latency from Trial 1 to 4 (F(3,174) = 15.613, p < .05). There was no difference in hot plate latency between male and female rats on Trial 1. Habituation to the test room for 1 hr had no effect on hot plate latency on Trial 1 in female rats, but caused a shorter hot plate latency on Trials 2 – 4 compared to male rats or non-habituated (Naïve) female rats. Habituation to the test room had no effect on hot plate latency for male rats. Sample size varied from 14 – 16 rats in each group.
The significant inverse correlation between weight and hot plate latency reported in Experiment 1, also was evident in the male rats tested in this experiment. This inverse correlation (r = -0.29) on the first trial was nearly identical to that reported in Experiment 1, but it did not reach statistical significance because of the small sample size (N = 29). A non-significant positive correlation occurred on Trials 2 – 4. The relationship between weight and hot plate latency was not significant in female rats on Trial 1 (r = 0.10) or subsequent trials (r = 0.03, 0.29, & 0.0 for Trials 2 – 4, respectively).
There was no difference in hot plate latency between female and male rats on Trial 1 (Figure 2). However, on Trials 2 – 4 female rats habituated to the test room for 1 hr had a lower hot plate latency than female rats habituated for less than 5 min or male rats regardless of habituation. An overall ANOVA comparing habituated and non-habituated female rats across trials did not reach statistical significance (F(1, 29) = 3.401, p = .075) because of the lack of a difference on Trial 1. However, comparison of 95% confidence intervals revealed a significant difference in hot plate latency between habituated and non-habituated female rats on Trials 2 – 4. In contrast, habituation to the test room for 1 hour prior to testing (vs. 5 min) had no effect on hot plate latency on the first or subsequent days in male rats (F(1, 27) = 0.034, n.s.; Figure 2).
The decrease in hot plate latency with repeated testing was not caused by a decrease in activity with repeated exposure to the hot plate. There was no significant difference in the average time to cross from one quadrant to another across trials (F(3,168) = 1.239, n.s.; Figure 3A). Likewise, the mean number of crosses during the first 6 s of being placed on the hot plate did not change across trials (F(3,168) = 0.960 n.s.; Figure 3B). Although females tended to be more active than males, this difference did not reach statistical significance whether measured as time to cross (F(1,56) = 2.091, n.s.) or mean activity during the first 6 s (F(1,56) = 1.943, n.s.). Finally, habituating rats to the test room for 1 hr or 5 min had no effect on activity whether measured as the average time to cross from one quadrant to another F(1,56) = 2.112, n.s.) or the mean number of crosses in the first 6 s (F(1,56) = 0.857, n.s.).
Figure 3.
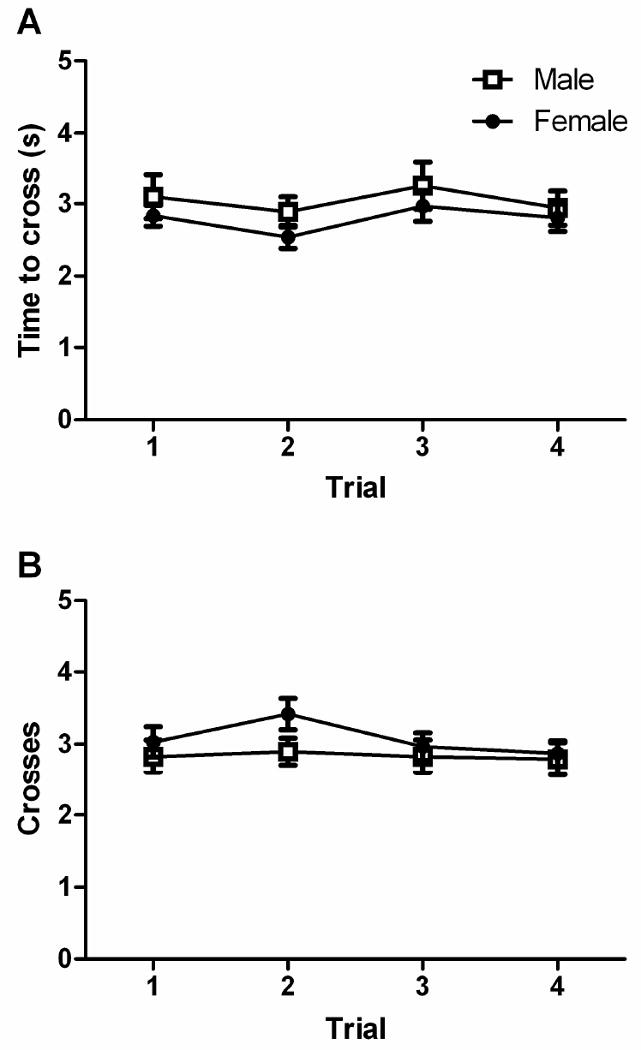
No change in locomotor activity on the hot plate in male or female rats across trials. Locomotion on the hot plate was consistent across trials whether measured as the average time to cross to a new quadrant (Figure A; hot plate latency/crosses) or the number of crosses during the first 6 s of the test period (Figure B). These data indicate that changes in activity are not responsible for the decrease in hot plate latency with repeated testing.
Experiment 3: Effect of Learning on Hot Plate Latency
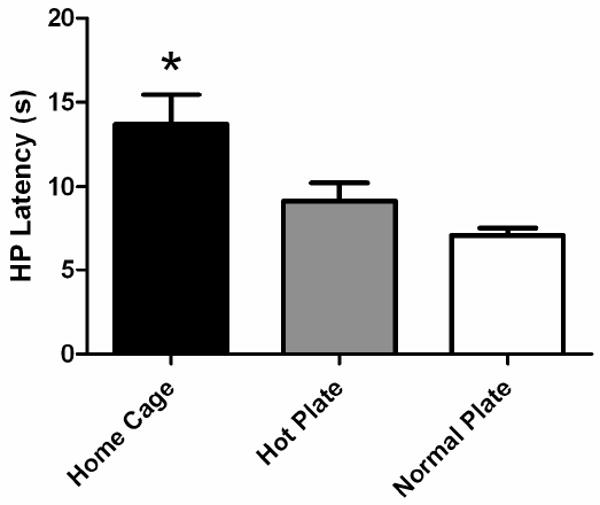
The objective of this experiment was to determine whether the decrease in hot plate latency across trials is caused by exposure to the hot plate apparatus. Analysis of baseline hot plate latency on Trial 5 revealed a significant effect of pretreatment (F(2,22) = 8.400, p < .05). Rats previously exposed to the hot plate, whether at room temperature (22 °C) or 52 °C had hot plate latencies lower than rats placed on the hot plate for the first time (Tukey test, p < .05). The hot plate latency for rats previously exposed to the room temperature plate did not differ from rats tested repeatedly on the hot plate (Figure 4).
Figure 4.
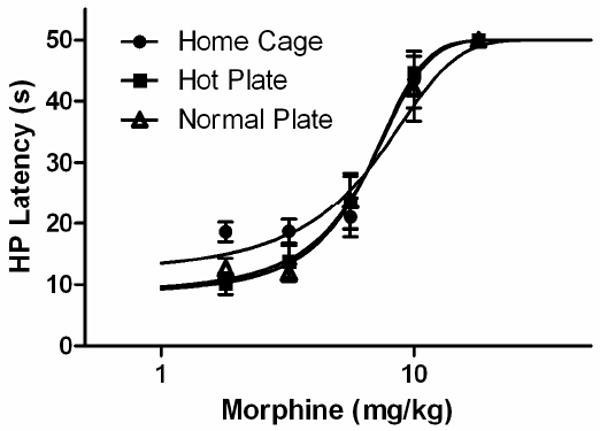
Decrease in hot plate latency following pre-exposure to a room temperature plate. The decrease in hot plate (HP) latency with repeated testing occurs whether the hot plate is heated (52.5 °C) or not (Normal Plate) compared to rats that are naïve to the hot plate apparatus (Home Cage; *p < .05). Data are compared from Trial 5. N = 8 or 9/group.
Despite this difference in baseline hot plate latency, there was no difference in the potency for morphine antinociception (F(2,138) = 0.264, n.s.). D50s ranged from 6.3 – 6.7 mg/kg for the three groups (Figure 5). The difference in hot plate latency following administration of the lowest dose of morphine was consistent with the difference in baseline hot plate latency, but this difference disappeared as the dose of morphine increased.
Figure 5.
No effect of pre-exposure treatment on morphine potency. Dose response curves for morphine antinociception were the same despite differences in baseline hot plate latencies caused by pre-exposure to the hot plate apparatus. Data were collected on Trial 5 after twice daily exposure to the hot plate (52.5 °C) or a room temperature plate (Normal Plate) for 2 days. Control rats (Home Cage) were handled for 50 s on each trial and then returned to their home cage. N = 8 or 9/group.
Discussion
The present data show that hot plate latency is influenced by weight, prior exposure to the hot plate, and duration of habituation to the test room, but not locomotor activity. The lower hot plate latencies with prior exposure to the hot plate do not appear to be the result of a learned response because it happens whether rats are exposed to a hot or room temperature plate.
The inverse correlation between weight and hot plate latency has not been reported previously, but is likely related to greater contact with the plate in heavier rats. This difference also is evident when comparing mice (20 - 30 g) and rats (200 - 300 g). Mice are typically tested on a significantly warmer hot plate (55 °C) compared to rats (52 °C) because testing mice on a 52 °C hot plate results in long hot plate latencies 23. However, the relationship between weight and hot plate latency is fragile. Our finding that the correlation disappears for rats over 300 g and does not occur in female rats indicates that other unknown factors (e.g., changes in skin sensitivity, hormone levels) mitigate this relationship.
The decrease in hot plate latency with repeated testing has been reported numerous times before 3, 7-9, 14, 15, 22. This decrease occurs whether animals are tested once a day or once a week, but is less likely to occur with repeated tests during a single session 10. Learning to respond appears to contribute when jumping out of the box is the endpoint 12, 13, but not when the endpoint is licking the hindpaw. Our data are consistent with others showing that exposure to a room temperature plate in which no hindpaw licking occurs causes the same decrease in hot plate latency as in animals tested repeatedly on the hot plate 3, 10. This decrease is not caused by learning, but is probably the result of habituation to stimuli associated with the test apparatus that inhibit nociception 3, 10.
It was hypothesized that shorter hot plate latencies would occur in inactive rats because of constant exposure to the plate compared to walking rats. This hypothesis was not supported. There was no decrease in locomotor activity with repeated testing despite the decrease in hot plate latency. Activity in a novel environment is known to decrease with repeated exposure 28. The present data showing no change in activity across trials could be caused by the short duration of testing (6 s) or stress associated with the hot plate test.
Despite the decrease in baseline hot plate latency following repeated testing, administration of morphine produced comparable antinociception in rats subjected to repeated testing or tested for the first time. A higher hot plate latency was evident in rats tested for the first time following administration of the lowest morphine dose as would be expected given the higher baseline latency in these rats. However, as the dose of morphine increased, the antinociceptive potency of morphine was the same regardless of the prior baseline latency. Similar results have been reported by others when nociception is assessed using the latency to lick a hind paw 17, 22, 27. These findings demonstrate that the hot plate is a good way to assess the antinociceptive effects of opioids even when baseline sensitivity differs.
Habituating animals to the test room prior to the hot plate test is a common and recommended practice 2. The present data show that habituation has a minimal effect on hot plate latency. There was no difference in hot plate latency on Trial 1 between rats habituated to the room for 5 min or 1 hr. However, 1 hr of habituation resulted in lower hot plate latencies for female rats on Trials 2 – 4 than in male rats or female rats habituated for less than 5 min. Thus, habituation to the room has a much smaller effect on nociception than habituation to the hot plate itself as described above.
Although the present study did not assess the effects of estrus cycle or light phase on nociception, previous studies have shown that hot plate latency tends to be lower when rats are tested during estrus 24 and during the dark phase of the diurnal cycle 5. Other factors that have been shown to influence nociception in mice include strain, experimenter, season, humidity, and cage density 4. These factors would probably have similar effects in rats. However, the present data indicate that most factors have relatively small effects on hot plate latency.
In sum, the present data show that hot plate latency is affected by body weight, repeated testing, and habituation to the test room, but these changes are small and do not impact subsequent assessment of morphine antinociception. Other factors such as locomotor activity have little or no effect on hot plate latency. The fact that these effects are small, when they do occur, indicates that the hot plate is a good method to assess nociception. Other advantages of using the hot plate test to assess nociception is that it is objective, easy to use, can be used repeatedly, and measures supraspinal responses to nociception. This is an important distinction from reflexive nociceptive tests (e.g., tail flick) because human pain is supraspinally mediated and the modulation of supraspinal responses has been shown to differ from spinal nociceptive reflexes 20. On the other hand, manipulations that affect motivation or motor function could increase hot plate latency in the absence of a direct effect on nociception. Of course, the advantages and disadvantages of any test must be evaluated in relation to the goals of a particular study.
Acknowledgments
The assistance of Katie Suchland is greatly appreciated. Supported by NIH grant DA015498 and by funds provided for medical and biological research by the State of Washington Initiative Measure No. 171. Morphine sulfate was a gift from NIDA.
Footnotes
Perspective: This manuscript shows that non-nociceptive factors such as body weight, habituation, and repeated testing can alter hot plate latency, but these factors do not alter morphine potency. In sum, the hot plate test is an easy to use and reliable method to assess supraspinally organized nociceptive responses.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ankier SI. New hot plate tests to quantify antinociceptive and narcotic antagonist activities. Eur J Pharmacol. 1974;27:1–4. doi: 10.1016/0014-2999(74)90195-2. [DOI] [PubMed] [Google Scholar]
- 2.Bannon AW, Malmberg AB. Models of nociception: hot-plate, tail-flick, and formalin tests in rodents. Curr Protoc Neurosci. 2007;Chapter 8: Unit 8 9 doi: 10.1002/0471142301.ns0809s41. [DOI] [PubMed] [Google Scholar]
- 3.Bardo MT, Hughes RA. Exposure to a nonfunctional hot plate as a factor in the assessment of morphine-induced analgesia and analgesic tolerance in rats. Pharmacol Biochem Behav. 1979;10:481–5. doi: 10.1016/0091-3057(79)90221-1. [DOI] [PubMed] [Google Scholar]
- 4.Chesler EJ, Wilson SG, Lariviere WR, Rodriguez-Zas SL, Mogil JS. Identification and ranking of genetic and laboratory environment factors influencing a behavioral trait, thermal nociception, via computational analysis of a large data archive. Neurosci Biobehav Rev. 2002;26:907–23. doi: 10.1016/s0149-7634(02)00103-3. [DOI] [PubMed] [Google Scholar]
- 5.Christina A, Merlin N, Vijaya C, Jayaprakash S, Murugesh N. Daily rhythm of nociception in rats. J Circadian Rhythms. 2004;2:2. doi: 10.1186/1740-3391-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eddy NB, Leimbach D. Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. J Pharmacol Exp Ther. 1953;107:385–93. [PubMed] [Google Scholar]
- 7.Gamble GD, Milne RJ. Repeated exposure to sham testing procedures reduces reflex withdrawal and hot-plate latencies: attenuation of tonic descending inhibition? Neurosci Lett. 1989;96:312–7. doi: 10.1016/0304-3940(89)90397-2. [DOI] [PubMed] [Google Scholar]
- 8.Gebhart GF, Mitchell CL. Further studies on the development of tolerance to the analgesic effect of morphine: the role played by the cylinder in the hot plate testing procedure. Arch Int Pharmacodyn Ther. 1971;191:96–103. [PubMed] [Google Scholar]
- 9.Gebhart GF, Mitchell CL. The relative contributions of the testing cylinder and the heated plate in the hot plate procedure to the development of tolerance to morphine in rats. Eur J Pharmacol. 1972;18:56–62. doi: 10.1016/0014-2999(72)90131-8. [DOI] [PubMed] [Google Scholar]
- 10.Hunskaar S, Berge OG, Hole K. A modified hot-plate test sensitive to mild analgesics. Behav Brain Res. 1986;21:101–8. doi: 10.1016/0166-4328(86)90088-4. [DOI] [PubMed] [Google Scholar]
- 11.Ingram SL, Fossum EN, Morgan MM. Behavioral and electrophysiological evidence for opioid tolerance in adolescent rats. Neuropsychopharmacology. 2007;32:600–6. doi: 10.1038/sj.npp.1301139. [DOI] [PubMed] [Google Scholar]
- 12.Jacob J. Psychopharmacological Methods. Macmillan; Ne York: 1963. Some effects of morphine adaptive and learning behavior; pp. 70–79. Editor⌃Editors. [Google Scholar]
- 13.Knoll J, Kelemen K, Knoll B. Experimental studies on the higher nervous activity of animals: I. A method for the eleboration of a non-extinguishable conditioned reflex in the rat. Acta Physiol Hung. 1955;8:327–44. [Google Scholar]
- 14.Lai YY, Chan SH. Shortened pain response time following repeated algesiometric tests in rats. Physiol Behav. 1982;28:1111–3. doi: 10.1016/0031-9384(82)90184-6. [DOI] [PubMed] [Google Scholar]
- 15.Lane DA, Morgan MM. Antinociceptive tolerance to morphine from repeated nociceptive testing in the rat. Brain Res. 2005;1047:65–71. doi: 10.1016/j.brainres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- 17.Milne RJ, Gamble GD, Holford NH. Behavioural tolerance to morphine analgesia is supraspinally mediated: a quantitative analysis of dose-response relationships. Brain Res. 1989;491:316–27. doi: 10.1016/0006-8993(89)90066-8. [DOI] [PubMed] [Google Scholar]
- 18.Morgan MM, Fossum EN, Levine CS, Ingram SL. Antinociceptive tolerance revealed by cumulative intracranial microinjections of morphine into the periaqueductal gray in the rat. Pharmacol Biochem Behav. 2006;85:214–9. doi: 10.1016/j.pbb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Morgan MM, Fossum EN, Stalding BM, King MM. Morphine antinociceptive potency on chemical, mechanical, and thermal nociceptive tests in the rat. J Pain. 2006;7:358–66. doi: 10.1016/j.jpain.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Morgan MM, Sohn JH, Liebeskind JC. Stimulation of the periaqueductal gray matter inhibits nociception at the supraspinal as well as spinal level. Brain Res. 1989;502:61–6. doi: 10.1016/0006-8993(89)90461-7. [DOI] [PubMed] [Google Scholar]
- 21.O'Callaghan JP, Holtzman SG. Quantification of the analgesic activity of narcotic antagonists by a modified hot-plate procedure. J Pharmacol Exp Ther. 1975;192:497–505. [PubMed] [Google Scholar]
- 22.Plone MA, Emerich DF, Lindner MD. Individual differences in the hotplate test and effects of habituation on sensitivity to morphine. Pain. 1996;66:265–70. doi: 10.1016/0304-3959(96)03048-5. [DOI] [PubMed] [Google Scholar]
- 23.Romero A, Miranda HF, Puig MM. Analysis of the Opioid-Opioid Combinations According to the Nociceptive Stimulus in Mice. Pharmacol Res. 2010 doi: 10.1016/j.phrs.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Stoffel EC, Ulibarri CM, Craft RM. Gonadal steroid hormone modulation of nociception, morphine antinociception and reproductive indices in male and female rats. Pain. 2003;103:285–302. doi: 10.1016/s0304-3959(02)00457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suh HH, Fujimoto JM, Tseng LF. Different radiant heat intensities differentiate intracerebroventricular morphine- from beta-endorphin-induced inhibition of the tail-flick response in the mouse. Eur J Pharmacol. 1992;213:337–41. doi: 10.1016/0014-2999(92)90622-b. [DOI] [PubMed] [Google Scholar]
- 26.Taber RI. Narcotic Antagonists. Vol. 8. Raven Press; New York: 1974. Predictive value of analgesic assays in mice and rats; pp. 191–211. Editor⌃Editors. [PubMed] [Google Scholar]
- 27.Van Ree JM, Leys A. Behavioral effects of morphine and phencyclidine in rats: the influence of repeated testing before and after single treatment. Eur J Pharmacol. 1985;113:353–62. doi: 10.1016/0014-2999(85)90083-4. [DOI] [PubMed] [Google Scholar]
- 28.Van Waas M, Soffie M. Differential environmental modulations on locomotor activity, exploration and spatial behaviour in young and old rats. Physiol Behav. 1996;59:265–71. doi: 10.1016/0031-9384(95)02151-5. [DOI] [PubMed] [Google Scholar]
- 29.Woolf G, MacDonald AL. The evaluation of the analgesic action of pethidine hydrochloride (Demerol) J Pharmacol Exp Ther. 1944;80:300–7. [Google Scholar]


