Abstract
Background and Purpose
High stroke event rates among carotid artery stenting (CAS)-treated patients in the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) lead-in registry generated an a priori hypothesis that age may modify the relative efficacy of CAS versus carotid endarterectomy (CEA). In the primary CREST report, we previously noted significant effect modification by age. Here we extend this investigation by examining the relative efficacy of the components of the primary endpoint, the treatment-specific impact of age, and contributors to the increasing risk in CAS-treated patients at older ages.
Methods
Among 2,502 CREST patients with high-grade carotid stenosis, proportional hazards models were used to examine the impact of age on the CAS-to-CEA relative efficacy, and the impact of age on risk within CAS-treated and CEA-treated patients.
Results
Age acted as a treatment effect modifier for the primary endpoint (Pinteraction=0.02), with the efficacy of CAS and CEA approximately equal at age 70 years. For CAS, risk for the primary endpoint increased with age (P<0.0001) by 1.77-times (95% confidence interval, 1.38 to 2.28) per 10-year increment; however, there was no evidence of increased risk for CEA-treated patients (P=0.27). Stroke events were the primary contributor to the overall effect modification (Pinteraction=0.033), with equal risk at ≈ 64 years. The treatment-by-age interaction for CAS and CEA was not altered by symptomatic status (P=0.96) nor by sex (P=0.45).
Conclusions
Outcomes after CAS versus CEA were related to patient age, attributable to increasing risk for stroke after CAS at older ages. Patient age should be an important consideration when choosing between the two procedures for treating carotid stenosis.
Keywords: carotid artery, carotid endarterectomy, cerebrovascular disease, stents, vascular surgery, prevention
Introduction
Patient age has been shown to influence the outcomes after carotid revascularization.1–6 The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) protocol was developed in 19977 when age and vascular anatomy8, 9 were not yet recognized as predictors of complications of carotid-artery stenting (CAS). To the contrary, it was postulated that CAS might be safer than carotid endarterectomy (CEA) in the elderly. However, during the conduct of the lead-in phase of the study a high risk of stroke events was observed among the CAS-treated patients, and octogenarians were subsequently excluded from this portion of the trial (but were continued in the randomized phase to assess if equivalent risks were present the CEA-treated patients).10 At this time (on the basis of lead-in data only and prior to unblinding of randomized data), the study investigators committed to the preplanned formal assessment of the impact of age on relative efficacy reported herein.
Methods
Study Participants and Measurement
CREST is a randomized clinical trial assessing the relative efficacy of CAS versus CEA. The study enrolled 1,321 symptomatic patients and 1,181 asymptomatic patients. Endpoints were adjudicated by committees blinded to treatment assignment. Details of the study are provided elsewhere.11–13 The protocol was approved by the Institutional/Ethics Review Board of all participating sites. All patients gave written informed consent.
Statistical Analysis
The focus of these analyses is to assess if the age of the patient influences the relative efficacy of CAS and CEA, and if so, what are the contributors of the effect modification. As such, the primary evaluation of efficacy was assessed on an intention-to-treat analysis using proportional hazards analysis to evaluate the potential of an age-by-treatment interaction after adjustment for symptomatic status, and sex. The primary outcome of the trial was stroke, myocardial infarction or death during a periprocedural period (30 days post procedure for those receiving treatment within 30 days, or 36 days post randomization for those not receiving treatment within 30 days), or ipsilateral stroke over a follow-up period extending 4-years from randomization. Potential effect modification by age was analyzed assuming a linear effect of age (after confirming the linear assumption was reasonable). The stroke component was defined as any stroke during the periprocedural period and stroke ipsilateral to the study artery for the subsequent 4-year period. The MI component was defined as elevated enzymes plus either symptoms or electrocardiographic (ECG) evidence of an event during the periprocedural period. Too few deaths occurred during the periprocedural period to permit meaningful analyses of this component on its own.
The modeling approach used the addition of interaction terms to proportional hazard models predicting the composite endpoint, the stroke endpoint and the MI endpoint. A priori, P < 0.10 were considered suggestive of effect modification. We also provide secondary analyses within specific age strata (younger than 65, 65 to 74, and 75 years or older) to provide the reader details including: 1) the number of events in these broad age range, 2) an assessment of the linearity of the primary analysis of age-by-treatment interaction, 3) event rates within age strata for each treatment for comparisons to other studies, and 4) crude estimates of CAS-to-CEA relative efficacy for these age strata.
We also assessed if any potential age-by-treatment effect modification was consistent by symptomatic status or sex, by adding higher order interaction terms to the model. Proportional hazards models were fit separately for those CAS-treated and CEA-treated patients to describe the age-related changes in risk within each treatment contributing to their relative CAS-to-CEA efficacy differences.
Finally, to identify potential causes underlying the age-treatment interaction, we conducted a mediation analysis14 to identify if the increased risk at older ages for CAS-treated patients was attributable to an increased prevalence of risk factors (hypertension, diabetes, or dyslipidemia), differences in the characteristics of the lesion (lesion length, eccentric lesions, ulcerated lesion, or percent stenosis), or differences in the procedure (fluoroscopy time or total procedure time) by entering these factors into the model and observing the change in the estimated hazard ratio associated with age. Characteristics of the lesion were determined by the local clinic. Anatomic characteristics such as aortic arch anatomy, vessel tortuosity and calcification known to be associated with age and CAS complications were not available for analysis. The standard error of the mediation was estimated using bootstrap techniques.
Results
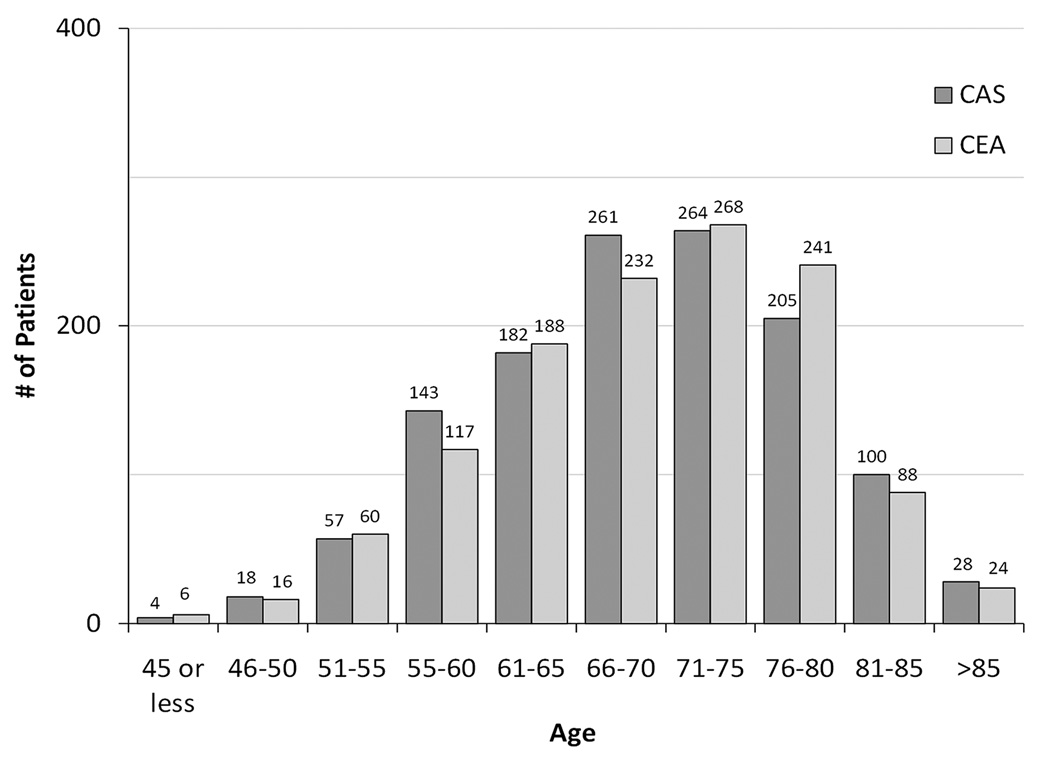
For both treatment groups in CREST, with increasing age participants were more likely to be female, white, and to have higher levels of systolic blood pressure and lower levels of diastolic blood pressure; however, they were less likely to have diabetes, dyslipidemia, or to be a current smoker (Table 1). There were no significant differences between treatment groups for these factors in any age strata. Figure 1 shows the distribution of the ages for CAS and CEA.
Table 1.
Description of study population by treatment and age strata.*
| Younger than 65 | 65–75 | 75 and Older | |||||
|---|---|---|---|---|---|---|---|
| CAS (n = 404) |
CEA (n = 387) |
CAS (n = 525) |
CEA (n = 500) |
CAS (n = 333) |
CEA (n = 353) |
||
| Male - % | 64.6 | 66.7 | 67.4 | 67.4 | 57.7 | 64.6 | |
| White -% | 90.6 | 92.8 | 93.1 | 92.8 | 95.2 | 95.5 | |
| Asymptomatic arteries - % | 43.8 | 41.6 | 53.1 | 53.0 | 41.4 | 45.6 | |
| Risk Factor Status | Hypertension - % | 81.4 | 84.4 | 88.7 | 87.9 | 86.4 | 85.2 |
| Diabetes - % | 32.9 | 30.5 | 31.6 | 34.2 | 26.0 | 25.0 | |
| Dyslipidemia - % | 84.1 | 85.9 | 84.3 | 88.3 | 79.3 | 82.2 | |
| Current smoker - % | 46.3 | 47.9 | 22.3 | 21.0 | 8.6 | 9.0 | |
| Prior cardiovascular disease - % | 36.3 | 40.8 | 46.9 | 50.1 | 42.9 | 42.4 | |
| Prior coronary artery bypass - % | 15.9 | 18.2 | 22.3 | 24.7 | 21.1 | 20.8 | |
| Systolic blood pressure (mean ± SD) mmHg |
137 ± 20 | 138 ± 20 | 142 ± 20 | 141 ± 21 | 147 ± 20 | 145 ± 20 | |
| Diastolic blood pressure (mean ± SD) mmHg |
76 ± 12 | 76 ± 12 | 74 ± 11 | 73 ± 11 | 72 ± 12 | 73 ± 12 | |
| Stenosis Measures |
Moderate (<70%) | 11.1 | 12.4 | 13.3 | 17.6 | 15.0 | 13.9 |
| Severe (≥ 70%) | 88.9 | 87.6 | 86.7 | 82.4 | 85.0 | 86.1 | |
| Left carotid treated - % | 50.7 | 53.2 | 47.8 | 52.6 | 55.0 | 50.7 | |
| Contralateral occlusion - % | 3.4 | 4.7 | 1.9 | 3.3 | 3.3 | 1.5 | |
| Median day from randomization to treatment | 6.0 | 7.0 | 7.0 | 7.0 | 6.0 | 7.0 | |
Sample sizes vary for specific characteristics (rows) because of missing data on specific items for a small number of patients
SD indicates standard deviation; CEA, carotid endarterectomy; CAS, carotid artery stenting
Figure 1.
Histogram of the number of patients within age strata by treatment assignment. CAS indicates carotid artery stenting; CEA, carotid endarterectomy.
Table 2 provides the observed number of MIs, strokes, and primary endpoints within approximate tertiles of age strata for both the periprocedural period and for the 4-year outcome, and Figure 2 provides the associated Kaplan-Meier estimates of the proportion of participants with a primary endpoint for each age-treatment strata, showing the similarity of time-to-event across age strata for CEA-treated patients, but the differences of time-to-event across age strata for CAS-treated patients. As previously reported for the primary endpoint at 4-years, there was evidence of a treatment-by-age interaction (P=0.02). The CAS-to-CEA risk increased with advancing age, from 0.60 (95% confidence interval (CI), 0.31-1.18) for patients younger than 65, to approximate equal risk for those aged 65 to 74 (hazard ratio, 1.08; 95% CI, 0.65-1.78), and to 1.63 (95% CI, 0.99-2.69) for those aged 75 and over. This increasing risk was associated with increasing event rates in the CAS-treated patients (3.9% in the youngest age strata, 6.3% in the middle, and 12.7% in the oldest), while risk was relatively stable in the CEA-treated patients (respective rates 6.1% youngest, 6.8% middle, and 7.4% oldest). This increasing risk was driven by the stroke endpoint, with a higher (P=0.033) CAS-to-CEA risk across age strata with hazard ratios of 0.78 (95% CI, 0.37-1.62), 1.42 (95% CI, 0.78-2.60), and 2.15 (95% CI, 1.19-3.91). The increasing CAS-to-CEA risk at older ages is associated with increasing stroke event rates for those CAS-treated (3.7%, 5.1%, and 10.9%), but not in those CEA-treated (4.5%, 4.6%, and 4.9%). A similar pattern of effects (increasing CAS-to-CEA risk largely driven by increasing risk at older ages in the CAS-treated patients) was observed during the periprocedural period for both the composite and stroke endpoints; however these trends failed to reach a level of statistical significance (p > 0.1). Contralateral strokes occurring during the periprocedural period were a component of the composite outcome and the stroke outcome, but contralateral strokes after the periprocedural period are not part of these outcomes. For the “All Stroke” endpoint (including contralateral strokes occurring after the periprocedural period), the treatment differences across the age spectrums are diluted (P=0.19) by the addition of the stroke events beyond the periprocedural period.
Table 2.
Number of events and event rates by age category for patients treated with carotid artery stenting and carotid endarterectomy
| CAS Younger than 65 65–74 75 or older |
N=404 N=525 N=333 |
CEA Younger than 65 65–74 75 or older |
N=387 N=500 N=353 |
Periprocedural Period*† | Four-year Period | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAS No. of events (rate ± SE‡) |
CEA No. of events (rate ± SE‡) |
Hazard Ratio (95% CI)§ |
P- value‖ |
Treatment by Age Interaction P- value# |
CAS No. of events (rate ± SE‡) |
CEA No. of events (rate ± SE‡) |
Hazard Ratio (95% CI)§ |
P- value‖ |
Treatment by Age Interaction P-value# |
||||
| Primary endpoint (any stroke, MI or death within periprocedural† period ± post-procedural ipsilateral stroke) | Younger than 65 | 10 (2.5±0.8) |
14 (3.6±0.9) |
0.69 (0.31-1.55) |
0.37 | 0.13 | 14 (3.9±1.1) |
22 (6.1±1.3) |
0.60 (0.31-1.18) | 0.14 | 0.02 | ||
| 65–74 | 26 (5.0±0.9) |
21 (4.2±0.9) |
1.22 (0.68-2.16) |
0.51 | 32 (6.3±1.1) |
29 (6.8±1.3) |
1.08 (0.65-1.78) | 0.78 | |||||
| 75 or older | 30 (9.0±1.6) |
21 (5.9±1.3) |
1.49 (0.85-2.60) |
0.16 | 39 (12.7±1.9) |
25 (7.4±1.4) |
1.63 (0.99-2.69) | 0.057 | |||||
| Stroke endpoint (any stroke within periprocedural†period ± post-procedural ipsilateral stroke) | Younger than 65 | 9 (2.2±0.7) |
8 (2.1±0.7) |
1.10 (0.42-2.84) |
0.85 | 0.27 | 13 (3.7±1.0) |
16 (4.5±1.1) |
0.78 (0.37-1.62) | 0.50 | 0.033 | ||
| 65–74 | 20 (3.8±0.8) |
10 (2.0±0.6) |
1.98 (0.93-4.23) |
0.08 | 26 (5.1±1.0) |
18 (4.6±1.1) |
1.42 (0.78-2.60) | 0.25 | |||||
| 75 or older | 23 (6.9±1.4) |
11 (3.1±0.9) |
2.17 (1.06-4.45) |
0.035 | 33 (10.9.0±1.8) |
16 (4.9±1.2) |
2.15 (1.19-3.91) |
0.01 | |||||
| All strokes (any stroke up to 4 year follow-up) | Younger than 65 | 9 (2.2±0.7) |
8 (2.1±0.7) |
1.10 (0.42-2.84) |
0.85 | 0.27 | 21 (6.7±1.5) |
20 (6.0±1.4) |
1.0 (0.54-1.84) | 0.99 | 0.19 | ||
| 65–74 | 20 (3.8±0.8) |
10 (2.0±0.6) |
1.98 (0.93-4.23) |
0.08 | 46 (11.4±1.9) |
30 (8.1±1.6) |
1.51 (0.96-2.40) | 0.08 | |||||
| 75 or older | 23 (6.9±1.4) |
11 (3.1±0.9) |
2.17 (1.06-4.45) |
0.035 | 38 (13.1±2.1) |
25 (9.4±2.2) |
1.60 (0.97-2.65) | 0.07 | |||||
| Death | Younger than 65 | 0 (0.0±0.0) |
1 (0.3±0.3) |
NA** | NA** | 0.50 | 19 (6.6±1.6) |
16 (8.2±2.2) |
1.12 (0.58-2.18) | 0.74 | 0.65 | ||
| 65–74 | 4 (0.8±0.4) |
0 (0.0±0.0) |
NA** | NA** | 36 (9.7±1.6) |
30 (12.3±2.6) |
1.15 (0.71-1.87) | 0.57 | |||||
| 75 or older | 4 (1.2±0.6) |
2 (0.6±0.4) |
2.14* (0.39-11.68) |
0.38 | 39 (18.2±3.0) |
37 (18.0±3.0) |
1.10 (0.70-1.72) | 0.69 | |||||
| MI endpoint (periprocedural† MI‡) |
Younger than 65 | 1 (0.2±0.2) |
6 (1.6±0.6) |
0.16* (0.02-1.32) |
0.09 | 0.35 | |||||||
| 65–74 | 6 (1.1±0.5) |
11 (2.2±0.7) |
0.53* (0.20-1.42) |
0.21 | |||||||||
| 75 or older | 7 (2.1±0.8) |
11 (3.1±0.9) |
0.67* (0.26-1.72) |
0.40 | |||||||||
Univariate proportional hazards model used because of the small number of events.
Periprocedural period was defined as the 30-day period after the procedure for all patients receiving treatment within 30 days of randomization, or day 36 for patients not receiving therapy within 30 days of randomization.
Event rates for MI endpoint was calculated as the proportion exposed patients experiencing the endpoint with SE calculated from the binominal distribution, whereas event rates for the stroke endpoint and primary endpoint were calculated using Kaplan-Meier survival function with SE calculated from the Greenwood formula.
Hazard ratios for the primary endpoint and stroke endpoint and death endpoint were adjusted for symptomatic status, and sex, but no adjustments were made in the MI endpoint because of a small number of events.
P-value was calculated from hazard ratio.
Per-protocol, P-value was calculated with age as a continuous variable.
Not available because of unreliable estimates.
Figure 2.
Kaplan-Meier estimates of the proportion of study participants with a primary endpoint. CAS indicates carotid artery stenting; CEA, carotid endarterectomy.
Although we urge caution in interpretation, Supplemental Table 1 (https://stroke.ahajournals.org) provides results similar to Table 2 stratified by symptomatic status. This table requires stratification by both age and symptomatic status, and as such the small sample size in specific stratum could lead to misleading results. We have considered age-by-symptomatic status interactions and found none to be significant (P > 0.1), and differences between symptomatic and asymptomatic patients in the relationships of risk with age could have easily occurred by chance alone. However, these data are provided for comparisons with the results of other studies that do not include both asymptomatic and symptomatic patients.
The primary analysis for this report is shown in Figure 3. Figure 3A shows the CREST primary endpoint as a continuous function of age (identical figure to that shown in primary study results paper11, all other figures and analyses are novel to this paper). The risk of the two procedures is approximately equal at age 70, with CAS showing superiority in younger patients, and increasing benefit for CEA in older patients. The stroke component of the composite endpoint as a function of age is shown in Figure 3B. The steeper slope in this figure implies a larger magnitude of effect modification by age upon the occurrence of stroke (P=0.033) than for occurrence of the primary endpoint. We note that unlike the composite outcome in which CAS-to-CEA risk approaches a significant advantage for CAS at younger ages, the wider CI the stroke endpoint implies the upper limit of the 95% CI bounds remains >1.0; however, the a priori focus of this article was on the trend of risk with differences in age (rather than differences at any specific age). The point of equal risk for CAS and CEA is at age 64 years, 6 years younger than for the primary endpoint. The wider 95% CI bounds imply greater uncertainty for the stroke outcome compared to the primary outcome. There was no evidence (P=0.35) of effect modification by the MI component of the primary endpoint (see Figure 3C).
Figure 3.

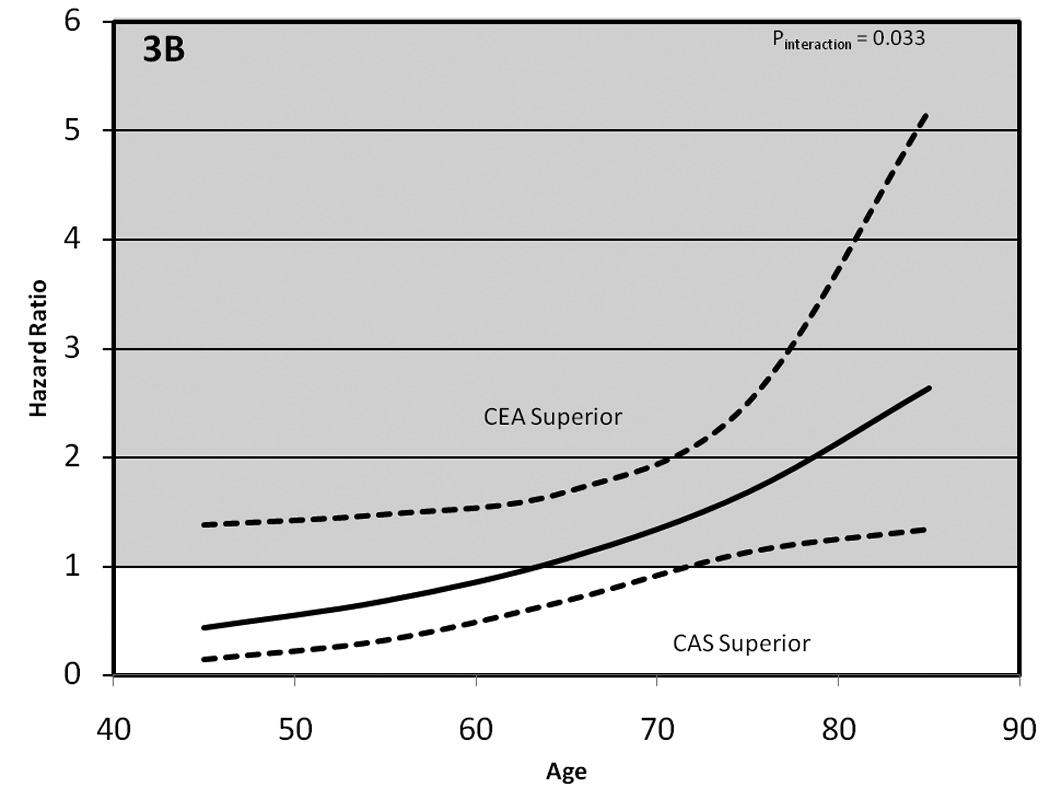

The impact of age on the relative efficacy of carotid artery stenting (CAS) vs carotid endarterectomy (CEA). 3A, hazard for the primary endpoint of any stroke, death, or MI during the periprocedural period, plus ipsilateral strokes over the subsequent 4-year period. Progressively better outcomes were seen with CAS in patients younger than 70 years old and with CEA in those older than 70 years old. 3B, hazard as a function of age for the stroke component of the primary endpoint (any stroke during the periprocedural period plus ipsilateral stroke over the subsequent 4-year period) Progressively better outcomes were seen with CAS in patients younger than 64 years old, and with CEA in those older than 64 years old. 3C, `hazard for the MI component of the primary endpoint (MI during the periprocedural period). The third component, deaths during the periprocedural period, is not provided because of the relatively small number of death events.
For those treated with CAS, there was a 1.77 times increase in risk of primary endpoint event (P<0.0001; 95% CI, 1.38-2.28) and a 1.76 times increase (95% CI, 1.35-2.31) for stroke events with each 10-year difference in age. For those treated with CEA, there was no evidence of a difference in risk across the age spectrum for either the primary endpoint (hazard ratio, 1.16; 95% CI, 0.89-1.50; P=0.27) or for stroke events (hazard ratio, 1.12; 95% CI, 0.82-1.54; P=0.47). Introduction of higher-order interaction terms did not suggest that the age modification of treatment effect was influenced by either symptomatic status (P=0.96) or by sex (P=0.45). The sensitivity analysis using the alternative definition of MI, including 20 biomarker-only MIs, showed a non-significant effect modification of age (P=0.75).
Mediation Analysis
Mediation analysis was performed to assess factors potentially contributing to the age-related risk differences in the CAS treatment group (Table 3), but was not performed for those randomized to CEA because of the lack of evidence for age-related changes for those randomized to CEA. There was no evidence that the effect of age in the CAS group was mediated by differences in the prevalence of hypertension, diabetes, or dyslipidemia, or by differences in observed lesion characteristics or procedure duration (P>0.05). Although total fluoroscopy time was identified as a potential mediator (P=0.046), its effect was modest, only reducing the age hazard ratio from 1.68 to 1.62 for a 10-year difference in age.
Table 3.
Results of mediation analysis showing the hazard ratio for the primary endpoint based upon a 10-year change in age in patients treated with CAS before and after adjustment for a potential mediating factor.
| Covariate potentially mediating impact of age (sample size/number of events) |
Hazard ratio for a 10-year difference in age after adjustment for gender and symptomatic status |
Hazard ratio for a 10- year difference in age after further adjustment for Covariate |
Change in coefficient |
|---|---|---|---|
| Hypertension (1259/85) |
1.77 (1.38 – 2.27) |
1.77 (1.37 – 2.27) |
−0.0021 ± 0.0078 P = 0.79 |
| Diabetes (1257/85) |
1.77 (1.38 – 2.27) |
1.79 (1.39 – 2.30) |
0.0132 ± 0.0153 P = 0.39 |
| Dyslipidemia (1254/85) |
1.78 (1.38 – 2.28) |
1.76 (1.37 – 2.26) |
−0.0077 ± 0.0139 P = 0.58 |
| Lesion Length (mm) (1189 / 83) | 1.73 (1.35 – 2.23) |
1.68 (1.31 – 2.17) |
−0.0286 ± 0.0152 P = 0.060 |
| Eccentric Lesion (1212/84) |
1.75 (1.36 – 2.25) |
1.75 (1.36 – 2.25) |
0.0025 ± 0.0094 P = 0.79 |
| Ulcerated lesion (1207/84) |
1.75 (1.36 – 2.25) |
1.73 (1.34 – 2.22) |
−0.0127 ± 0.0149 P = 0.40 |
| Procedural Angiogram Percent stenosis (1200/83) |
1.73 (1.35 – 2.23) |
1.73 (1.35 – 2.22) |
−0.0001 ± 0.0055 P = 0.98 |
| Fluoroscopy time (minutes) (1156/78) |
1.68 (1.30 – 2.18) |
1.62 (1.26 – 2.09) |
−0.0370± 0.0185 P = 0.046 |
| Total procedure time (minutes) (1210/83) |
1.78 (1.38 – 2.30) |
1.77 (1.37 – 2.27) |
−0.0097 ± 0.0123 P = 0.43 |
Discussion
The current analysis indicates that the age-related differential efficacy observed in CREST11 is primarily attributable to the stroke component of the primary endpoint. In turn, the impact of the stroke component is largely driven by an increasing risk of stroke with increasing age among CAS-treated patients, but little change in the increasing risk of stroke with increasing age among CEA-treated patients.
The point of similarity for the risk of stroke for CAS and CEA is at 64 years, compared to ≈ 70 years for the risk of the primary endpoint. The occurrence of MI following either procedure did not differ with age, suggesting CAS results in fewer MIs across the entire age spectrum. However, since there were fewer MI events (N=42) than stroke events (N=122), there was lesser power to detect effect modification for MI than stroke. There was no evidence that the age-by-treatment relationships differed by symptomatic status or sex.
Our observation of an age effect modification, originally reported in the primary results article,11 was subsequently confirmed by the meta-analysis of the Stent-Protected Angioplasty vs Carotid Endarterectomy (SPACE) trial, the Endarterectomy versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial, and the International Carotid Stenting Study (ICSS).15 For patients 70 years and older, the risk of events in CAS-treated patients was approximately twice that for CEA-treated patients (hazard ratio, 2.04; 95% CI, 1.48 to 2.82). This differential age effect in the meta-analysis was also driven by stroke because MI was not a component of the primary endpoint for this meta-analysis. This meta-analysis showed no differences in risk for patients younger than age 70 (hazard ratio, 1.11; 95% CI, 0.73-1.71), a finding not supported by our analysis where younger CAS-treated patients were shown to be a lower risk. Separately, the SPACE investigators reported a risk of 0.54 times (95% CI, 0.28-1.03) less for CAS relative to CEA for those younger than age 68, and a risk of 1.80 times (95% CI, 0.96-3.40) greater for CAS relative to CEA in those aged 68 and older.16 This age effect is also consistent with reports from the lead-in series of CREST, the Carotid Acculink/Accunet Post-Approval Trial to Uncover Unanticipated or Rare Events (CAPTURE) registry, and in ICSS.10, 17–18 All of these trials used eligibility criteria similar to CREST that did not incorporate anatomical exclusion criteria for CAS now thought to be important in elderly patients.9 None of the analyses included a detailed examination of the separate effect modification by the stroke and MI components of the outcomes.
When CREST was designed, we anticipated that the less invasive CAS would be superior in older age groups compared to the more invasive CEA. Accordingly, the superior performance of CEA in older individuals and the superior performance of CAS in younger individuals were unexpected. This position was challenged by the observation of high risk among the CAS-treated patients in the CREST lead-in registry10 (the observation leading to this pre-planned analysis), a finding confirmed in the analyses reported herein. Observational studies completed before CREST documented age as a predictor of stroke risk8, 19,20 potentially related to the marked increase in aortic arch and carotid artery tortuosity and calcification in the elderly.9 In CREST, characteristics of the carotid lesion such as the degree of stenosis, lesion length, eccentricity, and ulceration had no detectable effect on risk of CAS at older ages. However, the degrees of arterial tortuosity or lesion calcification were not available in the data set, and could contribute to the increased CAS event rates in the elderly.9, 21–24 We hypothesize that the risk of embolization during CAS is increased during navigation of tortuous extracranial arteries, particularly in patients with heavily calcified vessels and “extended” Type II and III aortic arches. Consistent with this hypothesis, we observed that the elderly required longer fluoroscopy time for CAS. Adjustment for this factor partially mediated the magnitude of the increased risk at older ages. Of note, the higher event rates in the elderly were not associated with increase in cardiovascular risk factors, consistent with previous reports.25
The interaction between patient selection, operator experience, and technology may be relevant to the age interaction in this analysis. Recent reports of CAS using updated patient selection criteria suggest that the age differential for CAS may be absent or blunted.26,27 These studies of CAS, also using new proximal protection devices designed to be less affected by arterial tortuosity, were notable for low event rates in the elderly.28,29 Further studies are required to confirm these findings.
Strengths of the CREST analysis include a large cohort of patients with a broad age distribution, inclusion of asymptomatic patients, and age-results consistent with results from the CREST credentialing study and subsequent randomized trials. Limitations include smaller numbers of events than anticipated (because of better than expected safety for both CAS and CEA) and smaller proportions of patients at the tails of the age distribution, 161 (6.4%) aged 55 years and younger and 240 (9.6%) aged 80 years and older (Figure 1). Nonetheless, the finding that the interaction test was significant provides prima facie confirmation that there are sufficient numbers of individuals in the tails of the age distribution to describe the effect of age.
Conclusion
This pre-specified analysis of the CREST trial demonstrates that the differential efficacy of CAS compared to CEA across the age spectrum is primarily attributable to stroke events. The pattern of lower relative risk in the CAS group at younger ages and higher relative risk at older ages is driven by increased risk for stroke at older ages for CAS. For CEA, the risk for stroke is relatively constant across the entire age spectrum. We conclude that patient age should be an important factor in selecting the treatment option for carotid stenosis. The anatomical factors that may contribute to these observations require further study.
Acknowledgments
Sources of Funding
This work was supported by the National Institute of Neurological Disorders and Stroke (NINDS) and the NIH (R01 NS 038384) and by supplemental funding from Abbott Vascular Solutions (formerly Guidant), including donations of Accunet and Acculink systems, equivalent to approximately 15% of the total study cost, to CREST centers in Canada and to CREST centers in the United States that were at Veterans Affairs sites.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: J.H. Voeks: None. G. Howard: Consultant/Advisory Board for Bayer Healthcare, member of the ARRIVE Executive Committee, research support from Amgen and Bayer Healthcare, consultant to Abbott Vascular for preparation of FDA materials. G.S. Roubin: Abbott Vascular, Inc., Royalties, Cook Inc., Royalties. M.B. Malas: None. D.J. Cohen: Research support from Boston Scientific, Abbott Vascular, Edwards Lifesciences, MedRad, Merck/Schering-Plough, Medtronic and Eli Lilly-Daiichi Sankyo; consultant to Schering-Plough, Eli Lilly, Medtronic, and Cordis; and Speakers’ Bureau for Eli Lilly and The Medicines Company. W.C. Sternbergh, III: None. H.D. Aronow: Speakers Bureau/Advisory Board for Medtronic. M.K. Eskandari: None. A.J. Sheffet: None. B.K. Lal: None. J.F. Meschia: None. T.G. Brott, for the CREST Investigators: None
References
- 1.McCrory D, Goldstein L, Samsa G, Oddone E, Landsman P, Moore W, et al. Predicting complications of carotid endarterectomy. Stroke. 1993;24:1285–1291. doi: 10.1161/01.str.24.9.1285. [DOI] [PubMed] [Google Scholar]
- 2.Fisher ES, Malenka DJ, Solomon NA, Bubolz TA, Whaley FS, Wennberg JE. Risk of carotid endarterectomy in the elderly. Am J Public Health. 1989;79:1617–1620. doi: 10.2105/ajph.79.12.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothwell PM, Slattery J, Warlow CP. Clinical and angiographic predictors of stroke and death from carotid endarterectomy: systematic review. BMJ. 1997;315:1571–1577. doi: 10.1136/bmj.315.7122.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durward QJ, Ragnarsson TS, Reeder RF, Case JL, Hughes CA. Carotid endarterectomy in nonagenarians. Arch Surg. 2005;140:625–628. doi: 10.1001/archsurg.140.7.625. [DOI] [PubMed] [Google Scholar]
- 5.Salameh JR, Myers JL, Mukherjee D. Carotid Endarterectomy in Elderly Patients: Low Complication Rate With Overnight Stay. Arch Surg. 2002;137:1284–1287. doi: 10.1001/archsurg.137.11.1284. [DOI] [PubMed] [Google Scholar]
- 6.Lau D, Granke K, Olabisi R, Basson MD, Vouyouka A. Carotid endarterectomy in octogenarian veterans: does age affect outcome? A single-center experience. The American Journal of Surgery. 2005;190:795–799. doi: 10.1016/j.amjsurg.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 7.Hobson RW, 2nd, Brott T, Ferguson R, Roubin G, Moore W, Kuntz R, et al. CREST: carotid revascularization endarterectomy versus stent trial. Cardiovasc Surg. 1997;5:457–458. doi: 10.1016/s0967-2109(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 8.Chastain HD, Gomez CR, Iyer S, Roubin GS, Vitek JJ, Terry JB, et al. Influence of age upon complications of carotid artery stenting. JEndovasc Surg. 1999;6:217–222. doi: 10.1583/1074-6218(1999)006<0217:IOAUCO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Lam RC, Lin SC, DeRubertis B, Hynecek R, Kent KC, Faries PL. The impact of increasing age on anatomic factors affecting carotid angioplasty and stenting. Journal of Vascular Surgery. 2007;45:875–880. doi: 10.1016/j.jvs.2006.12.059. [DOI] [PubMed] [Google Scholar]
- 10.Hobson RW, 2nd, Howard VJ, Roubin GS, Brott TG, Ferguson RD, Popma JJ, et al. Carotid artery stenting is associated with increased complications in octogenarians: 30-day stroke and death rates in the CREST lead-in phase. J Vasc Surg. 2004;40:1106–1111. doi: 10.1016/j.jvs.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 11.Brott TG, Hobson RW, II, Howard G, Roubin GS, Clark WM, Brooks W, et al. Stenting versus Endarterectomy for Treatment of Carotid-Artery Stenosis. N Engl J Med. 2010;363:11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheffet AJ, Roubin G, Howard G, Howard V, Moore W, Meschia J, et al. Design of the Carotid Revascularization Endarterectomy vs. Stenting Trial (CREST) International Journal of Stroke. 2010;5:40–46. doi: 10.1111/j.1747-4949.2009.00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hopkins LN, Roubin GS, Chakhtoura EY, Gray WA, Ferguson RD, Katzen BT, et al. The Carotid Revascularization Endarterectomy vs Stenting Trial: Credentialing of Interventionalists and Final Results of Lead-in Phase. Journal of Stroke and Cerebrovascular Diseases. 2010;19:153–162. doi: 10.1016/j.jstrokecerebrovasdis.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacKinnon DP, Warsi G. A Simulation Study of Mediated Effect Measures. Multivariate Behavioral Research. 1995;30:41. doi: 10.1207/s15327906mbr3001_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Short-term outcome after stenting versus endarterectomy for symptomatic carotid stenosis: a preplanned meta-analysis of individual patient data. The Lancet. 2010;376:1062–1073. doi: 10.1016/S0140-6736(10)61009-4. [DOI] [PubMed] [Google Scholar]
- 16.Eckstein HH, Ringleb P, Allenberg JR, Berger J, Fraedrich G, Hacke W, et al. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol. 2008;7:893–902. doi: 10.1016/S1474-4422(08)70196-0. [DOI] [PubMed] [Google Scholar]
- 17.Gray WA, Yadav JS, Verta P, Scicli A, Fairman R, Wholey M, et al. The CAPTURE registry: results of carotid stenting with embolic protection in the post approval setting. Catheter Cardiovasc Interv. 2007;69:341–348. doi: 10.1002/ccd.21050. [DOI] [PubMed] [Google Scholar]
- 18.Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. The Lancet. 2010;375:985–997. doi: 10.1016/S0140-6736(10)60239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathur A, Roubin GS, Iyer SS, Piamsonboon C, Liu MW, Gomez CR, et al. Predictors of stroke complicating carotid artery stenting. Circulation. 1998;97:1239–1245. doi: 10.1161/01.cir.97.13.1239. [DOI] [PubMed] [Google Scholar]
- 20.Zahn R, Ischinger T, Hochadel M, Zeymer U, Schmalz W, Treese N, et al. Carotid artery stenting in octogenarians: results from the ALKK Carotid Artery Stent (CAS) Registry. European Heart Journal. 2007;28:370–375. doi: 10.1093/eurheartj/ehl421. [DOI] [PubMed] [Google Scholar]
- 21.Bazan HA, Pradhan S, Mojibian H, Kyriakides T, Dardik A. Increased aortic arch calcification in patients older than 75 years: Implications for carotid artery stenting in elderly patients. Journal of Vascular Surgery. 2007;46:841–845. doi: 10.1016/j.jvs.2007.06.048. [DOI] [PubMed] [Google Scholar]
- 22.Chiam PT, Roubin GS, Iyer SS, Green RM, Soffer DE, Brennan C, et al. Carotid artery stenting in elderly patients: importance of case selection. Catheter Cardiovasc Interv. 2008;72:318–324. doi: 10.1002/ccd.21620. [DOI] [PubMed] [Google Scholar]
- 23.White CJ. Carotid Artery Stent Placement. JACC: Cardiovascular Interventions. 2010;3:467–474. doi: 10.1016/j.jcin.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Chaturvedi S, Matsumura JS, Gray W, Xu C, Verta P. CAPTURE 2 Investigators and Executive Committee. Carotid Artery Stenting in Octogenarians: Periprocedural Stroke Risk Predictor Analysis From the Multicenter Carotid ACCULINK/ACCUNET Post Approval Trial to Uncover Rare Events (CAPTURE 2) Clinical Trial. Stroke. 2010;41:757–764. doi: 10.1161/STROKEAHA.109.569426. [DOI] [PubMed] [Google Scholar]
- 25.Stanziale SF, Marone LK, Boules TN, Brimmeier JA, Hill K, Makaroun MS, et al. Carotid artery stenting in octogenarians is associated with increased adverse outcomes. Journal of Vascular Surgery. 2006;43:297–304. doi: 10.1016/j.jvs.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 26.Velez CA, White CJ, Reilly JP, Jenkins JS, Collins TJ, Grise MA, et al. Carotid artery stent placement is safe in the very elderly (> or =80 years) Catheter Cardiovasc Interv. 2008;72:303–308. doi: 10.1002/ccd.21635. [DOI] [PubMed] [Google Scholar]
- 27.Chiam PTL, Roubin GS, Panagopoulos G, Iyer SS, Green RM, Brennan C, et al. One-Year Clinical Outcomes, Midterm Survival, and Predictors of Mortality After Carotid Stenting in Elderly Patients. Circulation. 2009;119:2343–2348. doi: 10.1161/CIRCULATIONAHA.108.805465. [DOI] [PubMed] [Google Scholar]
- 28.Micari A, Stabile E, Cremonesi A, Vadalà G, Castriota F, Pernice V, et al. Carotid artery stenting in octogenarians using a proximal endovascular occlusion cerebral protection device: A multicenter registry. Catheterization and Cardiovascular Interventions. 2010;76:9–15. doi: 10.1002/ccd.22503. [DOI] [PubMed] [Google Scholar]
- 29.Myla S, Bacharach JM, Ansel GM, Dippel EJ, McCormick DJ, Popma JJ. Carotid artery stenting in high surgical risk patients using the FiberNet® embolic protection system: The EPIC trial results. Catheterization and Cardiovascular Interventions. 2010;75:817–822. doi: 10.1002/ccd.22386. [DOI] [PubMed] [Google Scholar]