Abstract
Precision and accuracy of the quantitative magnetic resonance (QMR) system for measuring fat in phantoms and total body fat (TBF) in humans were investigated. Measurements were made using phantoms: oil, beef with water, beef with oil, and humans with oil and water. TBFQMR in humans was compared with TBF by a four-compartment model (TBF4C). The coefficient of variation (CV) for replicate TBFQMR was 0.437%. QMR fat was lower at 23 °C vs. 37 °C. The fat increase in QMR phantom studies was consistent with the oil increase. When oil was added with humans, the increase in TBFQMR was >250 g for the initial 250 g of oil. With additional oil increments, the increase in TBFQMR was consistent with the amount of oil added. When water was added with humans, the TBFQMR increased independent of the amount of water added. TBFQMR was significantly less (mean ± s.e.) than TBF4C (females: −0.68 ± 0.27 kg, males: −4.66 ± 0.62 kg; P = 0.0001), TBFBV (females: −1.90 ± 0.40 kg; males: −5.68 ± 0.75 kg; P = 0.0001), and TBFD2O for males, but greater for females (1.19 ± 0.43 kg vs. −3.69 ± 0.81 kg for males; P = 0.0003). TBFQMR was lower than TBFiDXA with the difference greater in males (P = 0.001) and decreased with age (P = 0.011). The strong linear relationships between TBFQMR and TBF4C, TBFBV, and TBFD2O with slopes consistent with unity suggest that modifications are required to improve the accuracy. Should the latter be accomplished, QMR holds promise as a highly precise, rapid, and safe, noninvasive method for estimating the amount of and changes in TBF in overweight and severely obese persons.
INTRODUCTION
There is high demand for noninvasive and in vivo body composition methods that precisely and accurately measure small changes in total body fat (TBF) and water in humans. Accurate measurement of the amount of, and changes in, adipose tissue mass and closely related fat is essential for quantitative analysis of the mechanisms leading to overweight and obesity as well as the efficacy of weight-loss treatments.
The precision of an instrument to determine fat refers to the instrument’s ability to provide the same fat value each time the same subject’s fat is measured, assuming that the subject’s body composition has not changed between measurements. For a subject with a stable body composition, the standard deviation of fat values obtained from multiple measurements can be used to estimate variability and define an instrument’s precision. The coefficient of variation (CV; s.d./mean), expressed as a percent, is a parameter frequently used to define precision. Two critical first steps when a new instrument becomes available are to determine the precision with which the new instrument can measure a body composition variable and the accuracy of that instrument to measure it. Understanding the inherent precision and accuracy of an instrument is vitally important when conducting longitudinal measurements.
Multiple in vivo body composition measurement methods are available for use in humans, each with varying levels of precision and accuracy (1,2). Dual-energy X-ray absorptiometry (DXA) is currently the most commonly used measurement technique. Depending on the system, fat, lean soft tissue, and bone mineral estimates obtained by the DXA technique are highly reproducible with respective CVs of 1.89, 1.11, and 1.10% (ref. 3). In our own laboratory, five subjects were each scanned three times within a week with CVs of 3.43% for fat, 1.17% for lean soft tissue, and 1.03% for bone mineral estimates (unpublished data).
A recently available quantitative magnetic resonance (QMR) system (EchoMRI-AH; Echo Medical Systems, Houston, TX) relies on proton nuclear magnetic resonance (NMR) to measure human body composition (4). Using various pulse sequences, the QMR system provides estimates of fat mass, lean tissue mass, free water, and total body water (TBW). Napolitano et al. (4) reported a precision (s.d. of repeated measurements) of 0.25 kg for fat mass and 0.51 kg for lean mass in nonobese subjects, and 0.43 and 0.81 kg for fat and lean mass in obese subjects. With respect to accuracy, fat was underestimated by an average of 3.6 kg (14%) by QMR compared to the reference four-compartment (4C) model. The between-method difference between QMR and the 4C model increased with increasing fat mass and was found to be greater in males (4.6 kg) than females (2.1 kg).
Although conventional magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS) provide body composition estimates along with many other measures relevant to the study of excess adiposity including volume of adipose tissue depots, the QMR system offers the potential of a safe, rapid, practical, accurate, precise, and affordable means of quantifying fat, lean tissue mass, and body water.
In the present evaluation, a series of nonhuman studies involving phantoms were conducted to test the accuracy of the QMR system in measuring substances selected to represent physiologically meaningful body components. Thereafter, a two-part human study was conducted with the following aims: (i) part 1 assessed the precision and accuracy of Echo QMR for measuring body composition by taking body composition measurements with subjects in the presence and absence of known quantities of oil and water phantoms; (ii) part 2 compared the body composition for each subject as measured by QMR vs. the 4C model. In addition, Echo QMR fat values also were compared with estimates of fat mass measured by DXA, air displacement plethysmography (BodPod), and deuterium dilution techniques.
METHODS AND PROCEDURES
Seven linked studies were conducted to test the QMR system, five with nonhuman phantoms and two with human subjects.
Nonhuman studies
The nonhuman studies all involved various oil, water, and ground beef phantoms. In study 1, QMR fat was measured using 5–75 kg of oil in increments of 5 kg. Three QMR measurements were acquired for each amount of oil. The procedure was repeated on three different days. In studies 2 and 3, QMR fat was measured using 10 kg of beef at three temperatures, 23 °C, 36 °C, and 37 °C, with 0, 250, 500, and 1,000 g of water placed in the instrument with the beef. In study 4, QMR fat was measured with 0, 250, 500, and 1,000 g of oil placed in the instrument with 10 kg of beef and 1,000 g of water at 37 °C. In study 5, QMR fat was measured with 0, 250, 500, and 1,000 g of oil placed in the instrument with 10 kg of beef at a temperature of 36 °C. Phantom lipid composition was measured using previously reported methods (5,6).
Human studies
The two human studies (denoted here as studies 6 and 7) were conducted in accordance with Good Clinical Research Practice guidelines and the Declaration of Helsinki. The study protocol was approved by institutional review board of St Luke’s-Roosevelt Hospital Center (IRB#: 07-018, New York, NY). Specific details on subject management in studies 6 and 7 are provided in Supplementary Methods and Procedures online.
In study 6, the precision and accuracy of the QMR instrument in measuring fat was evaluated on each of 12 subjects using a series of eight test–retest scenarios, each scenario consisting of two consecutive QMR fat measurements. Specifically, in scenario 1 the subject was placed in the instrument and two readings were taken. The second measurement was taken immediately following the completion of the first measurement. In scenario 2, the subject was removed from the instrument following the first measurement and was repositioned on the stretcher. The subject was then returned to the QMR for the second measurement. Scenarios 3, 4, and 5 investigated the effects of adding water on the QMR fat measurement by placing 250, 500, and 1,000 g of water (at 37 °C), respectively, in the instrument with the subject and again taking two QMR fat readings for each added water weight. Similarly, scenarios 6, 7, and 8 investigated the effects of adding oil on the QMR fat measurement by adding 250, 500, and 1,000 g of oil (at 37 °C), respectively. In all except scenario 2, the subjects were not repositioned between the test–retest measurements and the oil or water was placed in the same location, between the subject’s lower legs, without any change in the positioning. This set of eight test– retest scenarios was repeated for each subject during both the morning and afternoon on three separate study days (days 1, 8, and 15).
In study 7, fat mass was measured for each of 34 subjects using four methods, QMR, DXA (iDXA), BodPod (BV), and deuterium oxide (D2O), and fat was also calculated using a 4C model.
Body composition evaluations
EchoMRI-AH
We provide here a description of the system and the general approach used in estimating the four evaluated compartments. A previous description of the EchoMRI-AH QMR system at this level is lacking and leaves an important gap when interpreting the studies that follow. The earlier Echo Medical Systems’ QMR instruments and DXA were developed and validated against classic carcass chemical analysis for use in small animals (7,8). The reported precision (CV) for fat measurement in mice was 0.34–0.71% for QMR compared with 3.06–12.60% for DXA (8).
The QMR system has a patient capacity of up to 250 kg. The resistive magnet bore size is 29 × 29 inches and external dimensions are L × W × H is 144 × 57 × 60 inches. The field of view is 27.5 × 27.5 × 63 inches (X × Y × Z axis) and the system is self-shielded. Static magnetic field homogeneity in the QMR system over the whole body is about 0.3% as measured by a non-NMR method. Measurement of an ECHO WIDTH for samples weighing ~100 kg (i.e., large adult human) demonstrates a static magnetic field homogeneity of ~0.2%. The QMR operating system is based on Windows XP Professional Edition. Measuring time is typically <3 min, with three or four repeat measurements taking about 10 min; there is a recommended daily system test in the most recent software. The system output includes fat mass, lean tissue mass, free water mass, and total water mass in units of kilograms.
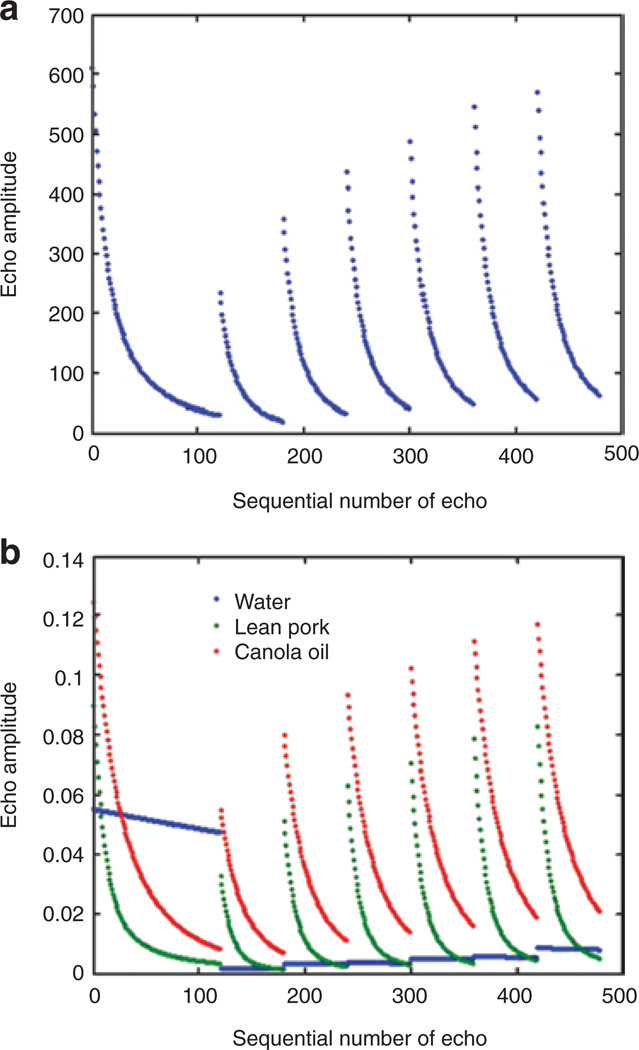
The QMR system produces an optimized sequence of radio pulses consisting of several segments separated by pauses of varying duration that are designed to capture relevant relaxation time scales. The captured signals, as shown in the human example presented in Figure 1a, reflect the combined signals of TBF, lean, and free water. Examples of separate measurements of canola oil, lean pork, and water phantoms normalized to unit mass are shown in Figure 1b.
Figure 1.
Quantitative magnetic resonance (QMR) system echo amplitude vs. sequential echo number (a) for a representative human subject and (b) corresponding representation for canola oil, lean pork, and water (normalized to mass) phantoms. Echo amplitude is in arbitrary (relative) units and not comparable between a and b. Courtesy of Echo Medical Systems, Houston, TX.
The key principal of the QMR method is that each scan produces a record of NMR responses (echoes) to a radiofrequency pulse sequence. The sequence is composed of several periodic Carr-Purcell-Meiboom-Gill parts separated by pauses of different duration. The lengths of the periodic parts and the pause durations are designed to capture all relevant characteristic (relaxation) time scales of the NMR responses (transverse, “T2”, and longitudinal, “T1”, relaxation) typical for fat, lean, and free water. The whole-body signal is a linear combination of fat, lean, and free water contributions, and the differences between the relaxation rates of the three basic substances make it possible to use linear regression analysis formulas calibrated to fat (canola oil), lean (chicken breast, small animals; lean pork, larger animals), and free water (tap water) phantoms. The algorithm for optimizing these regression formulas is a variant of multivariate calibration, which is typical of chemometric analyses (9), employing partial least squares optimization combined with principal component analysis for high-dimensional regressions.
Total water is calculated from the same records but in a different way as the contributions of protons associated with proteins and other “solid” materials are negligible at the time scales employed by the QMR system. The “lean” signal therefore comes mainly from water bound within the lean tissues. There is also a substantial contribution from protons in fat molecules. The difference between an estimate of the total amount of protons participating in the record and an estimate of fat found by regression analysis yields an estimate of the amount of total water included in lean together with free water. Therefore, fat mass and TBW are not measured independently of each other.
In sum, the main QMR pulse sequence is used to derive fat, lean mass, and free water using multiple linear regression prediction formulas calibrated against canola oil, lean animal tissues, and tap water, respectively. QMR body mass (BM) is then estimated as the sum of fat mass, lean mass, and free water mass; bone mineral, with high calcium and phosphorus content and gastrointestinal solids are not detected using the QMR system. QMR BM thus reflects the sum of three measured compartments, fat, lean, and free water mass.
4C model
A 4C model was used to estimate body fat (TBF4C) in subjects where the 4C formula incorporates BM measured by a calibrated scale (10), TBW by D2O dilution technique (11), body volume (BV) by air displacement plethysmography (12), and total body bone mineral (Mo) by DXA (13). The propagated error for percent fat estimates by the 4C method (CV) is ~1% (ref. 10) and fat mass by the 4C model is calculated as follows:
iDXA
TBF and bone mineral were measured using the iDXA (software version 10.40; GE Lunar, Madison, WI). The radiation dose for a total body scan set on standard thickness was 0.03 mSv (30% of the radiation dose received in a chest X-ray). Five subjects were each evaluated three times each over 9 days showing CVs of 0.8 and 3.1% for total body bone mineral and percent body fat, respectively.
BodPod
Subjects were clothed in a tight-fitting bathing suit and acrylic bathing cap and were weighed to the nearest 0.01 kg using the air displacement plethysmography system’s electronic scale (Tanita, Tokyo, Japan). BV was computed corrected for thoracic gas volume and a surface area artefact as per manufacturer instructions (12,14). The within-subject within-day CV for percent body fat measurement of 21 adults weighing 44–167 kg was 2.9%.
D2O dilution (D2O)
Each patient was given an oral dose of D2O (ICON, Summit, NJ) calculated as 0.1 g per kg body weight, weighed in a dose cup, accurate to ±0.002 g. With this dose the D2O concentration at equilibrium is <0.03% of body water (15,16). Blood samples (~7 ml each) were collected immediately before and 3 h after intake of the isotope, when equilibration had been reached. The dose concentration in the collected specimen was measured on a single frequency infrared-spectrophotometer (Nicolet 380; Thermo Electron, Madison, WI) after lyophilization. Total body water volume was calculated by dividing the dose by the net D2O concentration in the specimen. The precision for TBW measurement was ±1.0% (ref. 16).
Height was measured to the nearest 0.5 cm with a wall-mounted stadiometer (Holtain, Crosswell, Wales), and weight was measured to the nearest 0.01 kg with a calibrated scale (Tanita).
Statistical methods
For study 1, linear regression methods were used to derive the relationship between QMR fat measurement and the amount of oil. Day was treated as a random effect in the model; therefore, the model parameters were assumed to vary randomly from day to day.
For studies 2 and 3, an ANOVA was used to test the hypotheses that there were no main effects of water or temperature and that there was no interaction between temperature and water on the QMR fat measurement.
For both studies 4 and 5, an ANOVA was used to test the hypothesis that there was no main effect of oil on the Echo QMR fat measurement. Linear regression methods were used to derive the relationship between the Echo QMR–measured fat and the amount of oil.
For study 6, results from the second scenario were used to calculate the precision of the QMR instrument because this scenario required each subject to be repositioned between the pair of test–retest measurements. As 12 subjects were studied in the morning and afternoon on three separate days, there were 72 test–retest pairs. The variance of each pair of test–retest measurements was calculated. The average variance of the 72 pairs was calculated and used to determine the standard deviation. The CV (precision) was calculated using this standard deviation and the average of the QMR fat measurements.
Using results from scenarios 1, 6, 7, and 8, repeated measures ANOVA was used to test for the effects of day, am/pm, and amount of oil and their interactions on the Echo QMR–measured fat. There were no significant interactions between the amount of oil and any of the other factors; therefore, the main effect of oil was analyzed using the mean Echo QMR–measured fat for each subject. A mixed model ANOVA was used to test for the effect of oil on the mean QMR–measured fat. The amount of oil was included as a fixed effect and subject was considered as a random effect. A linear combination of the means was used to estimate the slope of the relationship between the mean Echo QMR– measured fat and the amount of oil. Using results from scenarios 1, 3, 4, and 5, repeated measures ANOVA was used to test for the effects of day, am/pm, and amount of water and their interactions on the QMR–measured fat. There were no significant interactions between the amount of water and any of the other factors; therefore, the main effect of water was analyzed using the mean Echo QMR–measured fat for each subject. A mixed model ANOVA was used to test for the effect of water on the mean Echo QMR–measured fat. The amount of water was included as a fixed effect and subject was considered as a random effect.
For study 7, the primary objective was to compare fat measurement by QMR with fat calculated using a 4C model. As fat measurements were also available by iDXA, BodPod, and D2O, they were also compared with the QMR fat measurements. Descriptive statistics were calculated for fat measured by each of the four methods. The paired t-test was used to test the hypothesis that the mean Echo QMR–measured fat was equal to the mean fat measured by each of the other four methods. Multiple linear regression methods were used to model the fat measured by each of the other four methods and Echo QMR. The dependent variable was fat measured by each method and the independent variables were Echo QMR fat, age, gender, and interactions of gender and with Echo QMR fat and age.
The level of significance for statistical tests of hypothesis was 0.05. All statistical calculations were performed using SAS (version 9.1; SAS Institute, Cary, NC) and STRATA STATA statistical software (version 10; College Station, TX) programs for personal computers.
RESULTS
Precision
The estimated standard deviation for the replicate measurements of QMR fat was 0.131 kg (131 g) and the average fat value was 30.057 kg, resulting in a CV of 0.437%. The observed standard deviation of 131 g indicates that the QMR instrument is capable of detecting fat mass changes on the order of 250 g. Assuming a standard deviation of 131 g, taking five premeasurements and five postmeasurements of an individual’s fat has a power of 80% for detecting a change of 250 g in the mean fat using a t-test at a level of significance of 0.05.
Accuracy
QMR fat measurements of beef alone at 37 °C were made in studies 2 and 4. Overall, the beef chemical analysis indicated 1.65 kg of fat are present per 10 kg beef. The average QMR fat measurements were 1.697 kg (95% confidence interval (CI): 1.639–1.755) for study 2; and 1.677 kg (95% CI: 1.620–1.734 kg) for study 4. The differences between the average QMR fat measurements and chemical analysis were 47 g in study 2 and 27 g in study 4. For studies 2 and 4, the average QMR fat measurement was consistent with the amount of fat determined by chemical analysis
The effect of adding an oil phantom on the QMR fat measurement was investigated in studies 1 (oil only), 4 (beef+water+oil), 5 (beef+oil), and 6 (human+oil), scenarios 1, 6, 7, and 8. The null hypothesis for each experiment was that the slope of the linear regression line was equal to unity (i.e., the addition of 1 kg of oil would increase the QMR fat measurement by 1 kg). Table 1 presents the slopes of the linear regression lines estimated from each condition. The estimated slopes were significantly greater than unity.
Table 1.
Descriptive and inferential statistics for the rate of increase in Echo QMR fat measurement per kilogram of oil
| 95% Confidence interval | |||
|---|---|---|---|
| Condition | Slope (kg/kg) | Lower limit (kg/kg) |
Upper limit (kg/kg) |
| Oil | 1.037a | 1.028 | 1.046 |
| Oil with beef | 1.064a | 1.017 | 1.111 |
| Oil with human | 1.092a | 1.046 | 1.138 |
QMR, quantitative magnetic resonance.
Slope is significantly greater than unity.
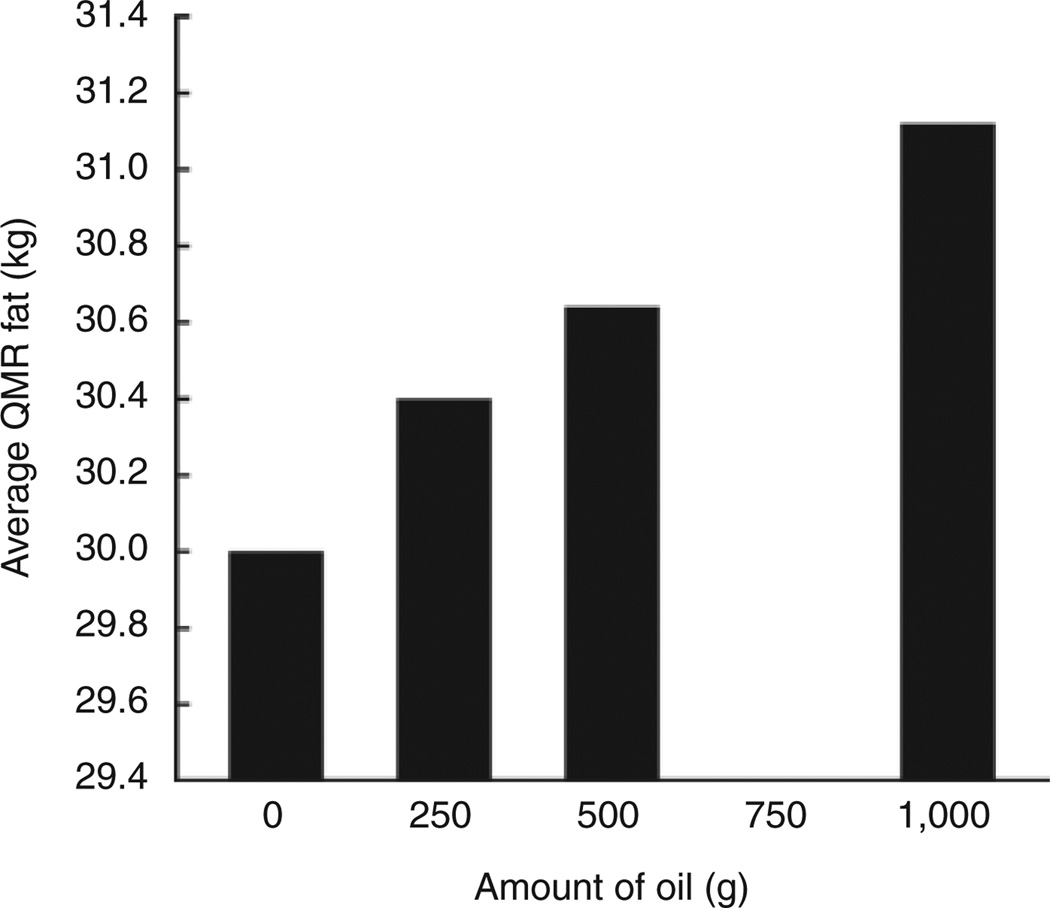
Figure 2 shows the effect of adding a known amount of oil on QMR fat measurement in humans (study 6). The addition of 250 g of oil in the QMR instrument with subjects present increased the average fat mass measurement by 398 g (95% CI: 350–446 g), which significantly exceeded the weight of the added oil. The inclusion of an additional 250 g of oil in the instrument increased the average QMR fat measurement by 240 g (95% CI: 192–288 g), which did not significantly differ from the weight of the added oil. Further addition of 500 g of oil in the instrument increased the average QMR fat estimate by 485 g (95% CI: 437–533 g), which did not significantly differ from the amount added. Although the QMR instrument overestimated the initial 250 g phantom of oil by an average of almost 150 g, it was able to accurately detect subsequent increments in oil of 250 and 500 g with human subjects in the magnet.
Figure 2.
The effect of adding increments of oil with human subject to the Echo QMR measurement of fat (kg). The standard error of the difference between two means is 0.024 kg. There were 12 subjects with 12 measurements for each subject. QMR, quantitative magnetic resonance.
For studies 4 and 5, the increases in the QMR fat measurements of beef plus oil were consistent with the increases in the amount of oil. Adding 250 g of oil increased the average QMR fat measurement by 274 g (95% CI: 217–331 g). Increasing the amount of oil by another 250 g increased the average QMR fat measurements by 283 g (95% CI: 226–340 g). Adding another 500 g of oil increased the average QMR fat measurements by 509 g (95% CI: 454–564 g). The increases in average QMR fat measurements for the phantom studies were consistent with the actual increases in the amounts of oil. The differences between the increases in average QMR fat and the actual amount of oil added were 24, 33, and 9 g.
The effect of adding water on the QMR fat measurement was investigated in studies 2 and 3 (beef+water at 23, 36, and 37 °C), and 6 (human+oil), scenarios 1, 3, 4, and 5. The null hypothesis for each study was that the addition of water would not influence the QMR fat measurement (i.e., QMR fat measurements would not change with the addition of water). The estimates of the effect of water are presented in Table 2 for each condition.
Table 2.
Descriptive and inferential statistics for the effect of adding water on measurement of fat
| 95% Confidence interval | |||
|---|---|---|---|
| Condition | Effect (kg) | Lower limit (kg) |
Upper limit (kg) |
| Water with beef | 0.005a | −0.034 | 0.044 |
| Water with human | 0.116 | 0.087 | 0.144 |
Water effect is not significantly different from zero.
The effect of water on the QMR fat measurement was estimated as the difference between the average of the three mean QMR fat measurements at 250, 500, and 1,000 g of water and the mean QMR fat measurement at 0 g of water. The addition of water to the QMR instrument did not significantly affect the average fat measurement of beef; however, the addition of a water phantom significantly increased the average QMR fat measurement of human subjects by 116 g. There was no significant effect of the amount of water placed in the instrument with the human subject (i.e., the increase in average QMR fat did not depend on the actual amount of water being added 250, 500, or 1,000 g).
The effect of temperature on the nonhuman QMR fat measurements was investigated in studies 2 and 3. There was a significant temperature effect (P < 0.001). The average QMR fat was 0.878 kg (95% CI: 0.848–0.907 kg) at 23 °C, 1.750 kg (95% CI: 1.721–1.779 kg) at 36 °C, and 1.699 kg (95% CI: 1.670– 1.728 kg) at 37 °C. An estimate of the effect of temperature on the QMR fat measurements was calculated as the difference between the average QMR fat measured at 37 °C minus the average QMR fat measured at 23 °C. The average QMR fat measurement was significantly reduced from 1.699 kg at 37 °C to 0.878 kg at 23 °C, resulting in a difference of 0.822 kg (95% CI: 0.780–0.863 kg).
The baseline demographic characteristics of subjects evaluated in studies 6 and 7 are presented in Table 3. Descriptive statistics for body fat measured in study 7 by each method are summarized in Table 4 for females and males. Descriptive statistics for the differences in body-fat measurements as assessed by each method vs. TBFQMR are presented in Table 5 for females and males.
Table 3.
Descriptive statistics for subjects (2 females, 10 males) participating in study 6 and subjects (16 females, 18 males) participating in study 7
| Study | Variable | Mean | s.d. | Minimum | Maximum |
|---|---|---|---|---|---|
| 6 | Age (years) | 38.5 | 13.0 | 23 | 55 |
| Weight (kg) | 96.7 | 10.9 | 81 | 108 | |
| Height (cm) | 174.7 | 5.4 | 166.7 | 182.7 | |
| BMI (kg/m2) | 31.9 | 4.9 | 25.1 | 37.9 | |
| 7 | Age (years) | 45.2 | 8.9 | 23 | 55 |
| Weight (kg) | 91.2 | 16.8 | 62 | 128 | |
| Height (cm) | 168.3 | 9.6 | 151 | 185 | |
| BMI (kg/m2) | 32.0 | 4.0 | 25.5 | 39.2 |
Table 4.
Descriptive statistics for fat for each method by gender
| Gender | Method | N | Mean (kg) | s.d. (kg) | Minimum (kg) | Maximum (kg) |
|---|---|---|---|---|---|---|
| Female | TBFQMR | 16 | 35.56 | 8.79 | 20.90 | 48.70 |
| TBF4C | 16 | 36.23 | 8.41 | 22.50 | 48.99 | |
| TBFiDXA | 16 | 35.53 | 8.21 | 22.78 | 48.41 | |
| TBFBV | 16 | 37.46 | 8.56 | 23.24 | 49.03 | |
| TBFD2O | 16 | 34.37 | 8.67 | 21.64 | 47.99 | |
| Male | TBFQMR | 18 | 29.49 | 9.87 | 15.11 | 49.62 |
| TBF4C | 18 | 34.15 | 10.30 | 22.48 | 52.62 | |
| TBFiDXA | 18 | 34.73 | 9.39 | 22.81 | 52.76 | |
| TBFBV | 18 | 35.17 | 10.09 | 18.63 | 53.75 | |
| TBFD2O | 18 | 33.18 | 10.51 | 20.08 | 52.83 |
BV, body volume by BodPod; D2O, deuterium dilution technique; iDXA, dual-energy X-ray absorptiometry; QMR, quantitative magnetic resonance; TBF, total body fat; 4C, four-compartment model.
Table 5.
Descriptive and inferential statistics for the difference, method minus QMR, in fat by gender
| Gender | Method | Mean (kg) | P value | 95% Confidence interval |
Maximum (kg) |
75th Percentile (kg) |
Median (kg) |
25th Percentile (kg) |
Minimum (kg) |
|---|---|---|---|---|---|---|---|---|---|
| Female | TBF4C | 0.68 (1.06) | 0.0222 | 0.11, 1.24 | 2.43 | 1.59 | 0.58 | −0.10 | −1.64 |
| TBFiDXA | −0.02 (1.57) | 0.9557 | −0.86, 0.81 | 2.56 | 1.02 | 0.16 | −1.33 | −3.28 | |
| TBFBV | 1.90 (1.61) | 0.0003 | 1.04, 2.76 | 5.34 | 2.41 | 1.35 | 0.93 | −0.06 | |
| TBFD2O | −1.19 (1.72) | 0.0146 | −2.10, −0.27 | 0.77 | −0.00 | −0.81 | −1.40 | −5.72 | |
| Male | TBF4C | 4.66 (2.65) | 0.0001 | 3.35, 5.98 | 12.19 | 5.90 | 4.36 | 2.83 | 1.63 |
| TBFiDXA | 5.24 (2.60) | 0.0001 | 3.94, 6.53 | 10.53 | 7.46 | 5.27 | 2.94 | 1.14 | |
| TBFBV | 5.68 (3.20) | 0.0001 | 4.09, 7.27 | 11.65 | 8.22 | 4.88 | 3.91 | −1.64 | |
| TBFD2O | 3.69 (3.45) | 0.0003 | 1.98, 5.41 | 12.78 | 5.13 | 3.26 | 1.56 | −1.20 |
BV, body volume by BodPod; D2O, deuterium dilution technique; iDXA, dual-energy X-ray absorptiometry; QMR, quantitative magnetic resonance; 4C, four-compartment model.
The TBF4C and TBFBV values were significantly higher compared with TBFQMR for both male and female subjects. TBFD2O was significantly lower than TBFQMR in female subjects, whereas TBFD2O was significantly higher than TBFQMR in male subjects. There was no statistically significant difference between TBFiDXA and TBFQMR in female subjects; however, the TBFQMR value was significantly less than the TBFiDXA in male subjects.
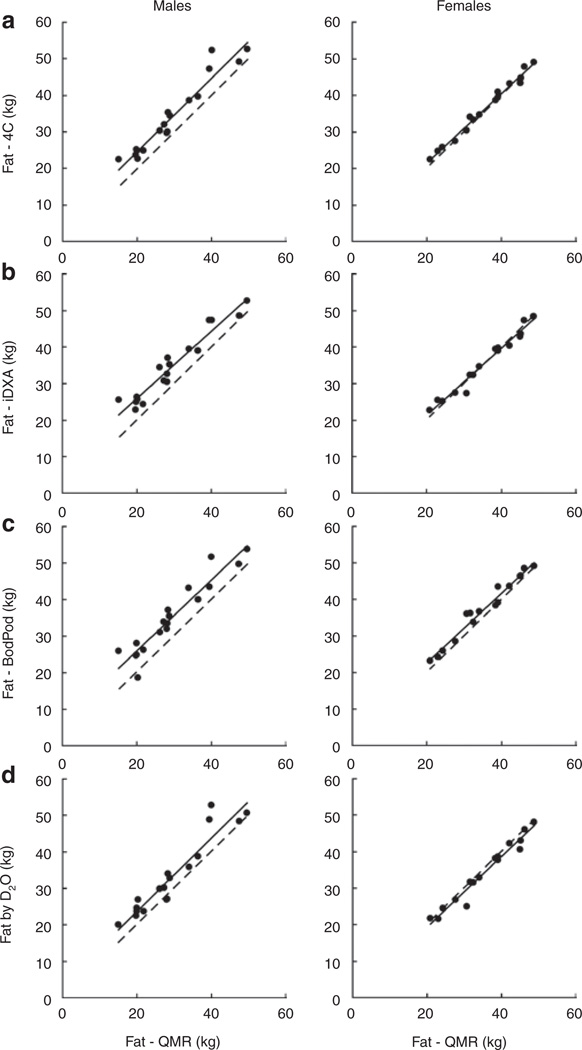
The scatter plots, Figure 3a–d, show fat measurement by each method vs. TBFQMR separately by gender. Within each figure is a linear regression line (solid) relating the two measurements of fat and the line (broken) of identity. TBF4C was highly correlated with TBFQMR in the males (r = 0.967) and females (r = 0.993), r = 0.956 for all subjects (all P values <0.001) (Figure 3a). TBFiDXA was also highly correlated with TBFQMR in the males (r = 0.965) and females (r = 0.985), and r = 0.937 for all subjects (all P values <0.001) (Figure 3b). The fat mass measurements by QMR vs. BodPod (QMR underestimation by −1.90 kg in females, −5.68 kg in males; Figure 3c) and QMR vs. D2O (QMR overestimation by +1.19 kg in females and underestimation by −3.69 kg in males; Figure 3d) were consistent with the QMR vs. the 4C model findings, with the difference between methods being constant.
Figure 3.
Comparisons of relationships between fat measured by different methods by gender. In each graph, the solid line is regression line and the broken line is line of identity. (a) Four-compartment model (4C) vs. Echo QMR fat (r = 0.956; P < 0.0001). (b) Dual-energy X-ray absorptiometry (iDXA) vs. Echo QMR fat (r = 0.937; P < 0.0001). (c) BodPod vs. Echo QMR fat (r = 0.945; P = 0.0001). (d) Deuterium oxide dilution (D2O) vs. Echo QMR fat (r = 0.927; P = 0.0001). QMR, quantitative magnetic resonance.
Regression models were derived for fat measured by each specific method using independent variables TBFQMR, age, and gender. For TBF4C, TBFBV, and TBFD2O, there was no significant effect of age and no significant gender interaction with age or TBFQMR. Gender was significant in these three models. The male coefficient was positive; therefore, for a given measured TBFQMR, males have higher fat measurements by each of the other three methods than females.
For TBFiDXA, there was a significant effect of age, but no significant gender interaction with age or TBFQMR. Gender was also significant in the model. The male coefficient was positive; therefore, for a given measured TBFQMR, males have higher fat values than females. Although the slope (TBFQMR coefficient) was not significantly different from unity, there was a suggestion (P = 0.086) that the slope was less than unity which implies that the difference between fat measured by iDXA and QMR depends on the magnitude of the QMR fat measurement, age, and subject’s gender.
DISCUSSION
The purpose of this study was to assess the precision and accuracy of the Echo QMR instrument in measuring body composition, more specifically fat mass in phantoms and adult humans. The results of this study extend those previously reported by Taicher et al. (7) and Napolitano et al. (4), who used a similar technology in small animals and adult human subjects, respectively. In the current report, the CV for replicate fat measurements in human subjects was <0.5%, thus demonstrating excellent precision of the Echo QMR instrument. By contrast, the CV for replicate fat measurements in human subjects was 3.1% for DXA and 2.9% for BodPod. The high precision of the QMR methodology surpasses that of all other human in vivo body-fat mea surement techniques (1,2). The QMR fat measurements for humans were also highly correlated with those estimated by the 4C method, although systematic differences between the sexes these two methods were present.
Although highly correlated with fat estimates generated by the 4C model, the QMR measurement significantly underestimated fat mass in all subjects and to a greater degree in male (−4.66 kg, 13.7%) compared with that for female subjects (−0.68 kg, 1.9%). The latter is consistent with the only published human QMR validation study in healthy adults, which reported an underestimation of fat by QMR vs. the 4C model which differed by sex (4). Results provided in their article indicate that QMR underestimated fat by −4.56 kg (18.3%) for males and −2.08 kg (8%) for females. Napolitano et al. (4) reported that the difference between QMR and 4C model increased with BM. However, in this study, the difference between the QMR and 4C model was constant such that the difference was not dependent on the subject’s fatness level. The latter is a positive finding as it suggests that the QMR may be capable of accurately assessing changes in fat under conditions of both weight loss and weight gain. The likelihood of a noninvasive instrument being able to detect such small changes in TBF is most promising because none of the currently available methods exhibit this degree of precision (1,2,10).
The fat mass measurements by QMR vs. BodPod (QMR underestimated in both males and females) and QMR vs. D2O (QMR overestimated in females and underestimated in males) were consistent with the QMR vs. the 4C model findings, with the difference between methods being constant. Specific to iDXA, the difference between QMR and iDXA fat values was found to vary as a function of fat, age, and sex. The differences between methods were greater in male subjects vs. female subjects and decreased as fat increased. It is important to note that the differences in the mean fat values (reference method minus QMR) for all subjects were smaller than many of the individual differences. When using an instrument to measure body fat at the individual level (within subject over time), we found that the individual differences were as large as 2.43 kg in females and 12.19 kg in males.
Overall, the difference between QMR and all four methods for measuring fat mass was larger in males than in females, which is consistent with the previous report (4). Although we cannot explain these results, it is possible that body shape or size influences fat estimates by QMR. This hypothesis can be tested in future studies with appropriate phantoms and human study protocols.
The effect of temperature on the QMR fat measurement of beef showed that at 23 °C, the average QMR fat measurement was significantly lower by 0.822 kg (48%) than at 37 °C. The latter indicates that the QMR results are sensitive to the temperature of phantom or subject under measurement. It should be noted that although core human body temperature is generally maintained at 37 °C, limb temperature can vary.
The results of this study indicate that placement of a water phantom in the Echo QMR instrument with a human subject increases the fat mass measurement; however, the quantity of water added to the instrument does not affect the magnitude of the increase in the fat mass measurement. Specifically, the human QMR average fat measurement was significantly increased by 104 g (95% CI: 69–139 g) in the presence of 250 g of water. When an additional 250 g of water was added to the instrument, no significant increase in the human QMR average fat measurement was observed (95% CI: −20 to 49 g). Further adding another 500 g of water also did not significantly increase the human QMR average fat measurement (95% CI: −28 to 41 g). For this study, the effect of placing water in the instrument with the human subject increased the average fat mass measurement by 116 g (95% CI: 87–144 g) or ~0.5%. The phantom water is technically not any of the three measured QMR components (i.e., fat, lean, or free water) as it generates an NMR signal that differs from the protein-bound water signal. However, the phantom water approaches what the NMR visualizes as “free water”. In communications with the company, we have learned that when tap water is placed in a plastic bottle in the magnet, it generates a small fat or lean signal (combined error may be up to ~10%). Therefore, if humans have about 500 g free water, a 10% artefact will add ~50 g to the measured fat mass. This phenomenon should not affect the precision of measuring fat mass and as a consequence should not influence the accuracy of measuring changes in fat mass.
A challenge that we faced when trying to interpret the results was our lack of understanding of how the QMR measures fat and whether the measurements of fat and water are truly independent of each other. The latter has implications for identifying the sources of error inherent in the QMR measurement of body composition components.
Study limitations
A critical assumption in the current study is that the 4C method provides a “true” estimate of TBF. Although the 4C method is generally considered the gold standard technique for measuring TBF (10), any measurement error introduced in any of the measured body components (i.e., body density, total body water, and bone mineral mass) will influence the final estimate. However, iDXA, air displacement, and deuterium dilution fat estimates provided approximately similar comparative outcomes as the 4C method, so our general assumption appears sound. A related question is whether the QMR method is calibrated against a different measure of fat mass than the other methods against which it was evaluated. The QMR is calibrated against nonliving phantoms and fat estimates obtained from animals by carcass chemical analysis. The “size” of the QMR compartment may thus not be identical to that provided by the other methods, all of which are also based on a series of assumptions and calibrations. Of importance, however, is that QMR fat estimates are all highly correlated with those of the other methods and so it likely that resolution of the calibration question is possible with further analysis or by cross- instrument calibration.
Conclusions
In this study, we conducted a series of extensive nonhuman and human experiments to critically evaluate the newly introduced QMR system for adult human body composition. The QMR approach has important advantages over currently available methods as it provides body composition estimates with high precision and without the use of ionizing radiation. The system, moreover, can accommodate subjects up to 250 kg, almost double that of the widely used DXA approach. Our analysis revealed systematic differences in body-fat estimates between the QMR and other available research methods and further exploration is needed to identify these sources of and potential correction approaches for these differences. The QMR approach, with these provisos, thus represents an important addition to research-based methods for adult human body composition analysis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Amy O. Johnson-Levonas, Merck & co., Inc. for her assistance with preparing the manuscript for publication. We also acknowledge the contributions of Alexander Ramirez, St. Luke’s-Roosevelt Hospital, New York, NY for his assistance with study coordination. Study concept: S.B.H. and R.K.; study design and protocol development: S.B.H., R.K., J.B., T.E.B., J.W., D.G.; data collection: Q.H., W.Y., D.G.; study oversight by sponsor: J.B. and V.M.R.; data analysis: J.C.T.; interpretation of data: J.W., S.B.H., J.C.T., R.K., D.G.; manuscript writing: D.G., J.C.T., S.B.H., R.K.; critical review of manuscript for intellectual content: D.G., J.C.T., T.E.B., S.B.H.
The research funding for this study was provided by Merck & co., Inc. the research was also supported in part by National Institutes of Health Grants P30-DK 26687 and RR 024156. Authors T.E.B., J.B., S.B.H., V.M.R., and R.K. are or were employees of Merck & co., Inc. and may own stock or hold stock options in the company.
Footnotes
DISCLOSURE
Authors D.G., J.C.T., Q.H., J.W., and W.Y. declare no conflict of interest.
REFERENCES
- 1.Gallagher D, Javed F. Assessment of human body composition. In: Allison DB, Baskin ML, editors. Handbook of Assessment Methods for Eating Behaviors and Weight-related Problems. 2nd edn. Thousand Oaks, CA: Sage Publications; 2009. pp. 481–528. [Google Scholar]
- 2.Wang ZM, Shen W, Withers R, Heymsfield SB. Multicomponent molecular-level models of body composition analysis. In: Heymsfield SB, Lohman TG, Wang ZM, Going SB, editors. Human Body Composition. 2nd edn. Champaign, IL: Human Kinetics Publishers; 2005. [Google Scholar]
- 3.Aasen G, Fagertun H, Halse J. Body composition analysis by dual X-ray absorptiometry: in vivo and in vitro comparison of three different fan-beam instruments. Scand J Clin Lab Invest. 2006;66:659–666. doi: 10.1080/00365510600898214. [DOI] [PubMed] [Google Scholar]
- 4.Napolitano A, Miller SR, Murgatroyd PR, et al. Validation of a quantitative magnetic resonance method for measuring human body composition. Obesity (Silver Spring) 2008;16:191–198. doi: 10.1038/oby.2007.29. [DOI] [PubMed] [Google Scholar]
- 5.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 6.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 7.Taicher GZ, Tinsley FC, Reiderman A, Heiman ML. Quantitative magnetic resonance (QMR) method for bone and whole-body-composition analysis. Anal Bioanal Chem. 2003;377:990–1002. doi: 10.1007/s00216-003-2224-3. [DOI] [PubMed] [Google Scholar]
- 8.Tinsley FC, Taicher GZ, Heiman ML. Evaluation of a quantitative magnetic resonance method for mouse whole body composition analysis. Obes Res. 2004;12:150–160. doi: 10.1038/oby.2004.20. [DOI] [PubMed] [Google Scholar]
- 9.Bruns RE, Scarminio IS, de Barros Neto B. Statistical Design—Chemometrics. Amsterdam, The Netherlands: Elsevier; 2006. [Google Scholar]
- 10.Withers RT, Laforgia J, Heymsfield SB. Critical appraisal of the estimation of body composition via two-, three-, and four-compartment models. Am J Hum Biol. 1999;11:175–185. doi: 10.1002/(SICI)1520-6300(1999)11:2<175::AID-AJHB5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 11.Schloerb PR, Friis-Hansen BJ, Edelman IS, Solomon AK, Moore FD. Critical appraisal of the estimation of body composition via two-, three-, and four-compartment models . J Clin Invest. 1950;29:1296–1310. doi: 10.1172/JCI102366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dempster P, Aitkens S. A new air displacement method for the determination of human body composition. Med Sci Sports Exerc. 1995;27:1692–1697. [PubMed] [Google Scholar]
- 13.Mazess RB, Peppler WW, Chesnut CH, 3rd, et al. Total body bone mineral and lean body mass by dual-photon absorptiometry. II. Comparison with total body calcium by neutron activation analysis . Calcif Tissue Int. 1981;33:361–363. doi: 10.1007/BF02409456. [DOI] [PubMed] [Google Scholar]
- 14.Ginde SR, Geliebter A, Rubiano F, et al. Air displacement plethysmography: validation in overweight and obese subjects. Obes Res. 2005;13:1232–1237. doi: 10.1038/oby.2005.146. [DOI] [PubMed] [Google Scholar]
- 15.Schoeller DA. Hydrometry. In: Roche AF, Heymsfield SB, Lohman TG, editors. Human Body Composition. Champaign, IL: Human Kinetics Publishers; 1996. pp. 25–44. [Google Scholar]
- 16.Yu W, Faruque O, Gallagher D, Heymsfield SB, Horlick M, Thornton JC, Wang J. An easy and inexpensive phantom for calibrating longitudinal water measurements by tracer dilution. FASEB J. 2005;19:A66. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.