Abstract
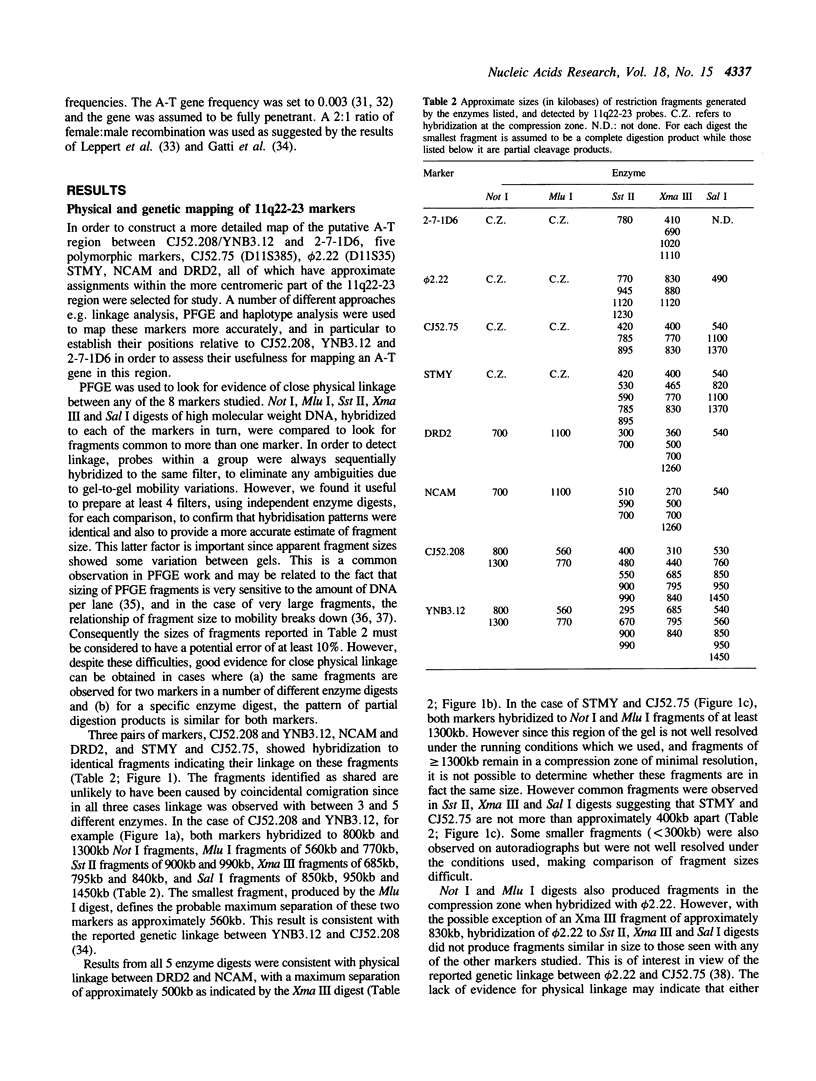
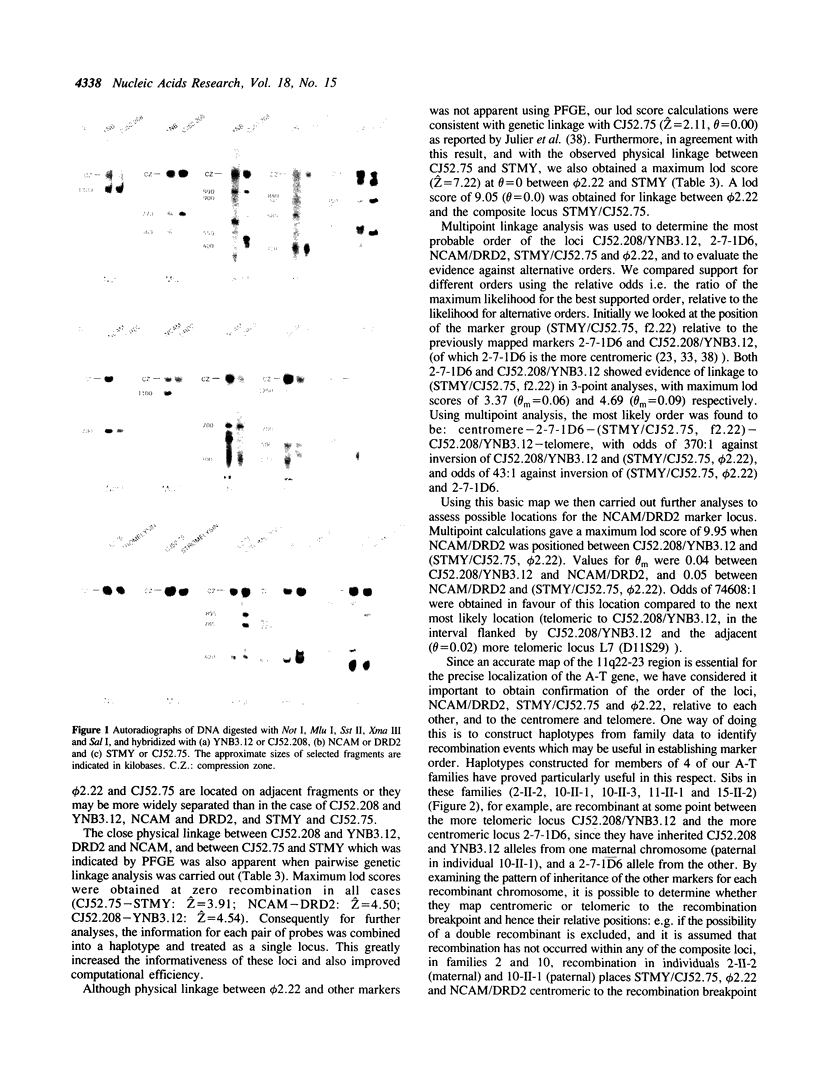
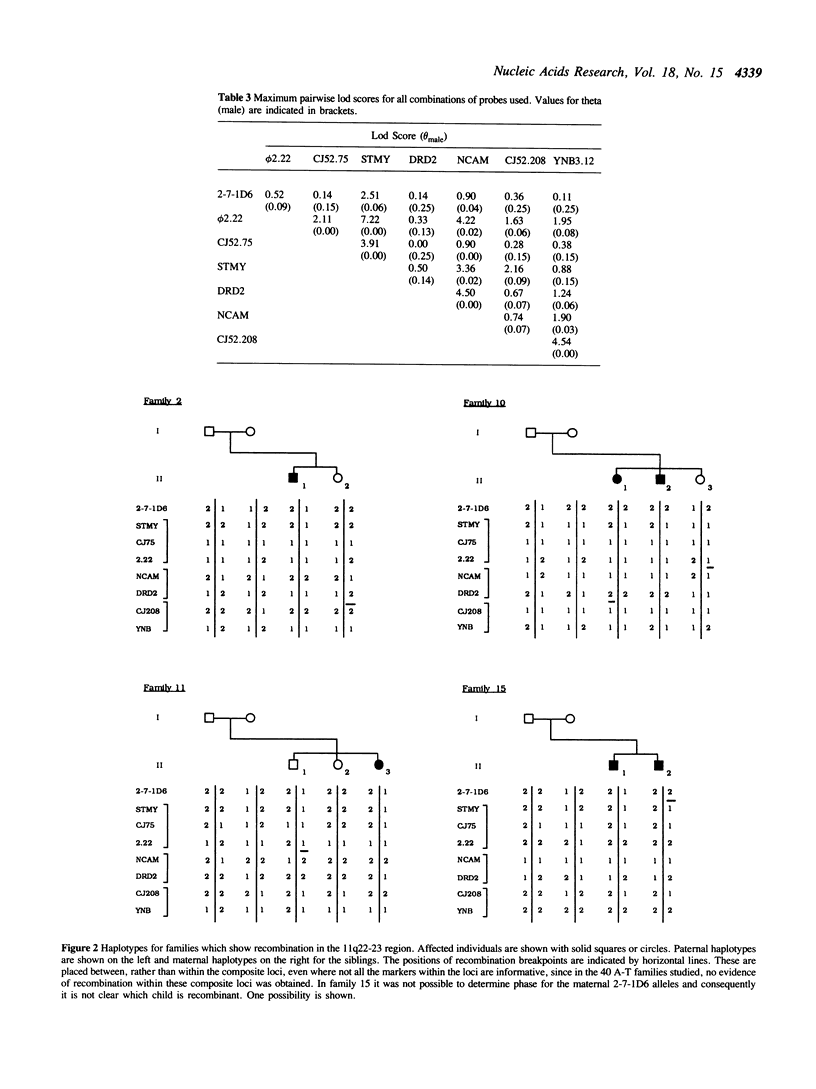
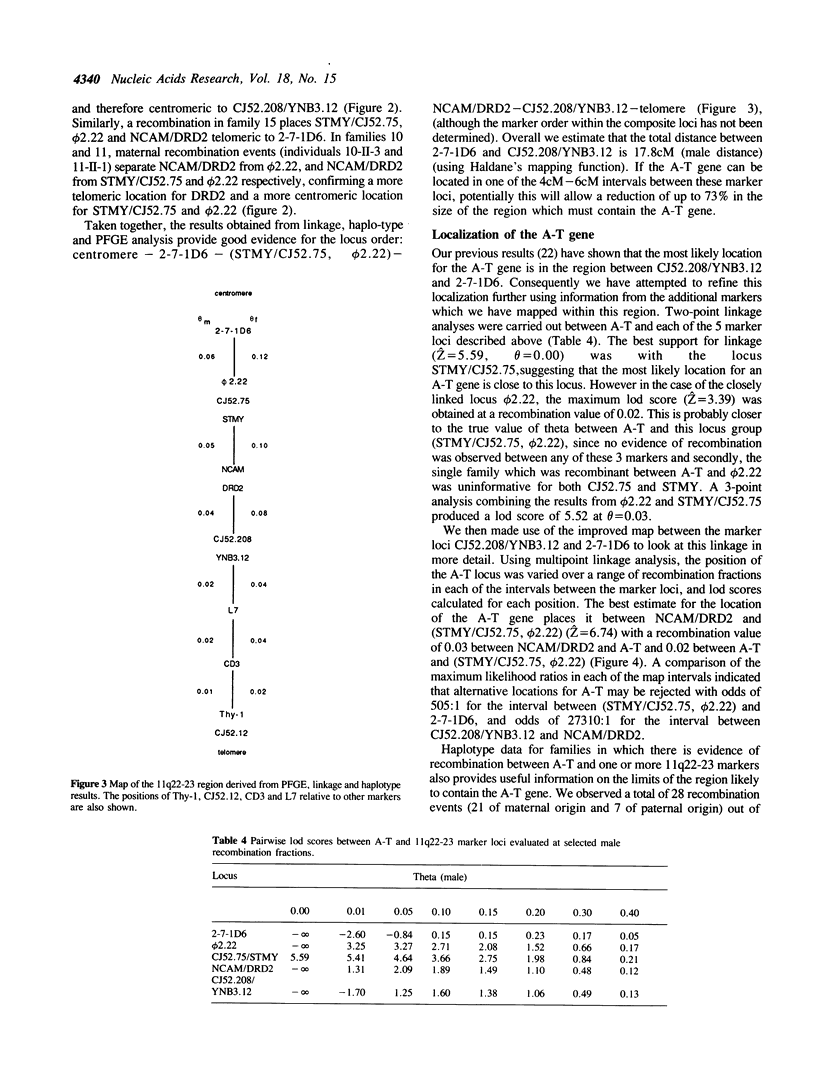
Using pulsed-field gel electrophoresis, and a range of different enzyme digests, we have established that both markers of each of the pairs CJ52.208/YNB3.12, NCAM/DRD2, and STMY/CJ52.75, on chromosome 11q22-23, show physical linkage on a single DNA fragment. We have also shown, using genetic linkage and haplotype analyses, that these markers lie within a region of approximately 18cM, which, it has been shown previously, is likely to contain the A-T gene. The relative positions of these marker loci, and the distance between them was determined in order to construct a detailed map which has allowed a more precise localization of the A-T gene. We have shown that in pairwise linkage analysis the strongest support for linkage to the A-T gene was with the STMY/CJ52.75 locus (Z = 5.59, theta = 0.0). A three-point analysis using the results from STMY/CJ52.75 and the closely linked marker phi 2.22 gave Z = 5.55, theta = 0.03. Despite persisting evidence of some linkage to Thy-1 our results are consistent with the existence of a single A-T locus on chromosome 11q22-23 and our best estimate of the position of this locus places it between NCAM/DRD2 and (STMY/CJ52.75, F2.22) (Z = 6.74), a region of approximately 5cM in males.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer R., Heppell A., Taylor A. M., Rabbitts P. H., Boullier B., Rabbitts T. H. The breakpoint of an inversion of chromosome 14 in a T-cell leukemia: sequences downstream of the immunoglobulin heavy chain locus are implicated in tumorigenesis. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9069–9073. doi: 10.1073/pnas.84.24.9069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello M. J., Salagnon N., Rey J. A., Guichaoua M. R., Bergé-Lefranc J. L., Jordan B. R., Luciani J. M. Precise in situ localization of NCAM, ETS1, and D11S29 on human meiotic chromosomes. Cytogenet Cell Genet. 1989;52(1-2):7–10. doi: 10.1159/000132828. [DOI] [PubMed] [Google Scholar]
- Charmley P., Foroud T., Wei S., Concannon P., Weeks D. E., Lange K., Gatti R. A. A primary linkage map of the human chromosome 11q22-23 region. Genomics. 1990 Feb;6(2):316–323. doi: 10.1016/0888-7543(90)90572-c. [DOI] [PubMed] [Google Scholar]
- Collins F. S., Cole J. L., Lockwood W. K., Iannuzzi M. C. The deletion in both common types of hereditary persistence of fetal hemoglobin is approximately 105 kilobases. Blood. 1987 Dec;70(6):1797–1803. [PubMed] [Google Scholar]
- Cornforth M. N., Bedford J. S. On the nature of a defect in cells from individuals with ataxia-telangiectasia. Science. 1985 Mar 29;227(4694):1589–1591. doi: 10.1126/science.3975628. [DOI] [PubMed] [Google Scholar]
- Cox R., Debenham P. G., Masson W. K., Webb M. B. Ataxia-telangiectasia: a human mutation giving high-frequency misrepair of DNA double-stranded scissions. Mol Biol Med. 1986 Jun;3(3):229–244. [PubMed] [Google Scholar]
- Drumm M. L., Smith C. L., Dean M., Cole J. L., Iannuzzi M. C., Collins F. S. Physical mapping of the cystic fibrosis region by pulsed-field gel electrophoresis. Genomics. 1988 May;2(4):346–354. doi: 10.1016/0888-7543(88)90024-9. [DOI] [PubMed] [Google Scholar]
- Ellis T. H., Cleary W. G., Burcham K. W., Bowen B. A. Ramped field inversion gel electrophoresis: a cautionary note. Nucleic Acids Res. 1987 Jul 10;15(13):5489–5489. doi: 10.1093/nar/15.13.5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber J. P., Weidinger S., Goedde H. W., Ole K. The deficient alpha-I-antitrypsin phenotype PI P is associated with an A-to-T transversion in exon III of the gene. Am J Hum Genet. 1989 Jul;45(1):161–163. [PMC free article] [PubMed] [Google Scholar]
- Gatti R. A., Berkel I., Boder E., Braedt G., Charmley P., Concannon P., Ersoy F., Foroud T., Jaspers N. G., Lange K. Localization of an ataxia-telangiectasia gene to chromosome 11q22-23. Nature. 1988 Dec 8;336(6199):577–580. doi: 10.1038/336577a0. [DOI] [PubMed] [Google Scholar]
- Grandy D. K., Litt M., Allen L., Bunzow J. R., Marchionni M., Makam H., Reed L., Magenis R. E., Civelli O. The human dopamine D2 receptor gene is located on chromosome 11 at q22-q23 and identifies a TaqI RFLP. Am J Hum Genet. 1989 Nov;45(5):778–785. [PMC free article] [PubMed] [Google Scholar]
- Hayashi K., Schmid W. Tandem duplication q14 and dicentric formation by end-to-end chromosome fusions in ataxia telandiectasia (AT). Clinical and cytogenetic findings in 5 patients. Humangenetik. 1975 Nov 6;30(2):135–141. doi: 10.1007/BF00291946. [DOI] [PubMed] [Google Scholar]
- Heppell A., Butterworth S. V., Hollis R. J., Kennaugh A. A., Beatty D. W., Taylor A. M. Breakage of the T cell receptor alpha chain locus in non malignant clones from patients with ataxia telangiectasia. Hum Genet. 1988 Aug;79(4):360–364. doi: 10.1007/BF00282177. [DOI] [PubMed] [Google Scholar]
- Jaspers N. G., Gatti R. A., Baan C., Linssen P. C., Bootsma D. Genetic complementation analysis of ataxia telangiectasia and Nijmegen breakage syndrome: a survey of 50 patients. Cytogenet Cell Genet. 1988;49(4):259–263. doi: 10.1159/000132673. [DOI] [PubMed] [Google Scholar]
- Julier C., Nakamura Y., Lathrop G. M., Lalouel J. M., White R. Isolation and mapping of a polymorphic DNA sequence (pCJ52.75M1) on chromosome 11 [D11S385]. Nucleic Acids Res. 1989 Nov 25;17(22):9505–9505. doi: 10.1093/nar/17.22.9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keats B., Ott J., Conneally M. Report of the committee on linkage and gene order. Cytogenet Cell Genet. 1989;51(1-4):459–502. doi: 10.1159/000132805. [DOI] [PubMed] [Google Scholar]
- Kennaugh A. A., Butterworth S. V., Hollis R., Baer R., Rabbitts T. H., Taylor A. M. The chromosome breakpoint at 14q32 in an ataxia telangiectasia t(14;14) T cell clone is different from the 14q32 breakpoint in Burkitts and an inv(14) T cell lymphoma. Hum Genet. 1986 Jul;73(3):254–259. doi: 10.1007/BF00401239. [DOI] [PubMed] [Google Scholar]
- Kidd K. K., Bowcock A. M., Schmidtke J., Track R. K., Ricciuti F., Hutchings G., Bale A., Pearson P., Willard H. F., Gelernter J. Report of the DNA committee and catalogs of cloned and mapped genes and DNA polymorphisms. Cytogenet Cell Genet. 1989;51(1-4):622–947. doi: 10.1159/000132810. [DOI] [PubMed] [Google Scholar]
- Kojis T. L., Schreck R. R., Gatti R. A., Sparkes R. S. Tissue specificity of chromosomal rearrangements in ataxia-telangiectasia. Hum Genet. 1989 Nov;83(4):347–352. doi: 10.1007/BF00291379. [DOI] [PubMed] [Google Scholar]
- Komatsu K., Kodama S., Okumura Y., Koi M., Oshimura M. Restoration of radiation resistance in ataxia telangiectasia cells by the introduction of normal human chromosome 11. Mutat Res. 1990 Mar;235(2):59–63. doi: 10.1016/0921-8777(90)90058-d. [DOI] [PubMed] [Google Scholar]
- Kunkel L. M., Smith K. D., Boyer S. H., Borgaonkar D. S., Wachtel S. S., Miller O. J., Breg W. R., Jones H. W., Jr, Rary J. M. Analysis of human Y-chromosome-specific reiterated DNA in chromosome variants. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1245–1249. doi: 10.1073/pnas.74.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3443–3446. doi: 10.1073/pnas.81.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan A. T., Meyers M. Chromosomal radiosensitivity of ataxia telangiectasia cells at different cell cycle stages. Hum Genet. 1979 Nov 1;52(1):127–132. doi: 10.1007/BF00284606. [DOI] [PubMed] [Google Scholar]
- Painter R. B. Inhibition of mammalian cell DNA synthesis by ionizing radiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1986 May;49(5):771–781. doi: 10.1080/09553008514552981. [DOI] [PubMed] [Google Scholar]
- Shiloh Y., Tabor E., Becker Y. Abnormal response of ataxia-telangiectasia cells to agents that break the deoxyribose moiety of DNA via a targeted free radical mechanism. Carcinogenesis. 1983 Oct;4(10):1317–1322. doi: 10.1093/carcin/4.10.1317. [DOI] [PubMed] [Google Scholar]
- Swift M., Morrell D., Cromartie E., Chamberlin A. R., Skolnick M. H., Bishop D. T. The incidence and gene frequency of ataxia-telangiectasia in the United States. Am J Hum Genet. 1986 Nov;39(5):573–583. [PMC free article] [PubMed] [Google Scholar]
- Taylor A. M., Harnden D. G., Arlett C. F., Harcourt S. A., Lehmann A. R., Stevens S., Bridges B. A. Ataxia telangiectasia: a human mutation with abnormal radiation sensitivity. Nature. 1975 Dec 4;258(5534):427–429. doi: 10.1038/258427a0. [DOI] [PubMed] [Google Scholar]
- Taylor A. M., Metcalfe J. A., McConville C. Increased radiosensitivity and the basic defect in ataxia telangiectasia. Int J Radiat Biol. 1989 Nov;56(5):677–684. doi: 10.1080/09553008914551901. [DOI] [PubMed] [Google Scholar]
- Taylor A. M., Metcalfe J. A., Oxford J. M., Harnden D. G. Is chromatid-type damage in ataxia telangiectasia after irradiation at G0 a consequence of defective repair? Nature. 1976 Apr 1;260(5550):441–443. doi: 10.1038/260441a0. [DOI] [PubMed] [Google Scholar]
- Taylor A. M., Oxford J. M., Metcalfe J. A. Spontaneous cytogenetic abnormalities in lymphocytes from thirteen patients with ataxia telangiectasia. Int J Cancer. 1981 Mar 15;27(3):311–319. doi: 10.1002/ijc.2910270309. [DOI] [PubMed] [Google Scholar]
- Thacker J. The use of integrating DNA vectors to analyse the molecular defects in ionising radiation-sensitive mutants of mammalian cells including ataxia telangiectasia. Mutat Res. 1989 Mar-May;220(2-3):187–204. doi: 10.1016/0165-1110(89)90024-9. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A., Broder S., Goldman C. K., Frost K., Korsmeyer S. J., Medici M. A. Disorders of B cells and helper T cells in the pathogenesis of the immunoglobulin deficiency of patients with ataxia telangiectasia. J Clin Invest. 1983 Feb;71(2):282–295. doi: 10.1172/JCI110768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S., Rocchi M., Archidiacono N., Sacchi N., Romeo G., Gatti R. A. Physical mapping of the human chromosome 11q23 region containing the ataxia-telangiectasia locus. Cancer Genet Cytogenet. 1990 May;46(1):1–8. doi: 10.1016/0165-4608(90)90002-r. [DOI] [PubMed] [Google Scholar]
- Woods C. G., Bundey S. E., Taylor A. M. Unusual features in the inheritance of ataxia telangiectasia. Hum Genet. 1990 May;84(6):555–562. doi: 10.1007/BF00210809. [DOI] [PubMed] [Google Scholar]




