Abstract
BACKGROUND AND PURPOSE
The aim of this study was to explore the effects of CB2 receptor agonist and antagonist in the regulation of anxiety-like behaviours.
EXPERIMENTAL APPROACHES
Effects of acute and chronic treatment with the CB2 receptor agonist JWH133 and CB2 receptor antagonist AM630 were evaluated in the light-dark box (LDB) and elevated plus maze (EPM) tests in Swiss ICR mice. CB2 receptor, GABAAα2 and GABAAγ2 gene and protein expression in the cortex and amygdala of mice chronically treated with JWH133 or AM630 were examined by RT-PCR and Western blot. Effects of chronic AM630 treatment were evaluated in spontaneously anxious DBA/2 mice in LDB.
KEY RESULTS
Acute JWH133 treatment failed to produce any effect. Acute AM630 treatment increased anxiety and was blocked by pre-treatment with JWH133. Chronic JWH133 treatment increased anxiety-like behaviour whereas chronic AM630 treatment was anxiolytic in LDB and EPM tests. Chronic AM630 treatment increased gene and reduced protein expression of CB2 receptors, GABAAα2 and GABAAγ2 in cortex and amygdala. Chronic JWH133 treatment resulted in opposite gene and protein alterations. In addition, chronic AM630 administration decreased the anxiety of DBA/2 mice in the LDB test.
CONCLUSIONS AND IMPLICATIONS
The opposing behavioural and molecular changes observed after chronic treatment with AM630 or JWH133 support the key role of CB2 receptors in the regulation of anxiety. Indeed, the efficacy of AM630 in reducing the anxiety of the spontaneously anxious DBA/2 strain of mice strengthens the potential of the CB2 receptor as a new target in the treatment of anxiety-related disorders.
Keywords: CB2 receptor, anxiety disorders, GABAAα2, GABAAγ2, JWH133, AM630, DB/A2 mice
Introduction
The incomplete knowledge of the mechanisms involved in the regulation of emotional states represents the main limiting factor of the efficacy of anxiolytic and antidepressant drugs in the treatment of anxiety and mood disorders. The discovery of new neurochemical elements involved in the regulation of emotional states may help to identify new therapeutic targets and contribute to a better understanding of the differences in the response of anxiolytics in certain clinical situations.
Controversial information is available about the pattern of expression of the cannabinoid CB2 receptors (receptor nomenclature follows Alexander et al., 2011). Initially, CB2 receptors were identified in the rat spleen and leucocyte subpopulation in humans (Munro et al., 1993; Galiègue et al., 1995). In addition, CB2 receptors were also found in the brain only under pathological conditions such as senile plaques in Alzheimer's disease (Benito et al., 2003), activated microglial cells/macrophages in multiple sclerosis (Yiangou et al., 2006), the spinal cord in amyotrophic lateral sclerosis (Yiangou et al., 2006) and in the vicinity of tumours (Sánchez et al., 2001). However, no expression of CB2 receptors was found in the brain under normal conditions (Hohmann and Herkenham, 1999a,b; Griffin et al., 1999; McCoy et al., 1999; Price et al., 2003; Derbenev et al., 2004). In contrast, recent reports have identified the expression of CB2 receptors in the CNS under normal conditions (see Atwood and Mackie, 2010): (i) presence of gene and protein expression of CB2 receptors in the microglia (Carlisle et al., 2002; Klegeris et al., 2003; Walter et al., 2003; Beltramo et al., 2006; Maresz et al., 2007) and in cultured granule cells, granule and Purkinje cell layers of mouse, rat and ferret cerebellum (Van Sickle et al., 2005; Ashton et al., 2006; Gong et al., 2006; Onaivi et al., 2008b); (ii) distribution of gene and protein expression of CB2 receptors in different nuclei of the brainstem of mouse, rat and ferret (Van Sickle et al., 2005; Gong et al., 2006); (iii) identification of CB2 receptor immunoreactivity in several areas of the hippocampal formation, thalamic nuclei, cortex, midbrain and olfactory bulb in rat (Gong et al., 2006; Onaivi, 2006; Onaivi et al., 2006, 2008a,b; Brusco et al., 2008a,b;); and, finally, (iv) CB2 receptor gene expression in the striatum and hypothalamus of rats (Gong et al., 2006; Onaivi, 2006; Onaivi et al., 2008a,b). Additionally, a recent publication (García-Gutiérrez et al., 2010) identified the distribution of CB2 receptor gene expression under normal conditions in several brain regions of mice, such as caudate-putamen nucleus, nucleus accumbens, cingulate cortex, amygdala, hippocampus, ventromedial nucleus and arcuate nucleus of hypothalamus, substantia nigra, dorsal and medial raphe. The wide distribution of CB2 receptors in the CNS of the rodents has increased the number of studies about the potential implication of CB2 receptors in a wide variety of physiological and pathological processes.
Recent findings supporting the role of CB2 receptors in the regulation of anxiety and mood disorders include studies that show CB2 receptor knockout mice (CB2-/-) present an increased vulnerability to stressful stimuli in the light–dark box (LBD), elevated plus maze (EPM) and tail suspension tests (TST) (Ortega-Álvaro et al., 2011). In addition, transgenic mice overexpressing the CB2 receptor in the CNS (CB2xP) exhibited a clear endophenotype resistant to acute (novelty-suppressed feeding and TST) and chronic [chronic mild stress (CMS)] depressive-like stimuli (García-Gutiérrez et al., 2010). Furthermore, CB2xP mice presented an endophenotype resistant to acute anxiogenic stimuli in the LDB and EPM (García-Gutiérrez and Manzanares, 2011). Indeed, the anxiolytic drug alprazolam was without effects in CB2xP mice at either of the doses used (45 and 70 µg·kg−1). These behavioural alterations were accompanied by molecular changes in different key targets involved in the response to stress, anxiety and depression, such as GABAA receptors, brain-derived neurotrophic factor (BDNF), corticotrophin-releasing factor (CRF) and proopiomelanocortin (POMC). CB2xP mice presented significantly higher expression of the neurotrophic factor BDNF in the hippocampus. Moreover, chronic stress failed to produce any modification in BDNF gene and protein expression in the hippocampus of CB2xP mice (García-Gutiérrez et al., 2010). Similarly, restraint stress did not alter CRF gene expression in the paraventricular nucleus and produced a slight increase of POMC gene expression in the arcuate nucleus of CB2xP mice compared with Swiss ICR mice (García-Gutiérrez and Manzanares, 2011). Furthermore, the gene expressions of the main anxiolytic subunits of GABAA receptors (α2 and γ2 subunits) were significantly increased in the amygdala and hippocampus of CB2xP mice (García-Gutiérrez and Manzanares, 2011).
The results obtained by pharmacological manipulation of CB2 receptors suggest the role of this receptor in the regulation of emotional behaviour. Acute or chronic treatment with the cannabinoid CB2 receptor antagonist, 6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl] (4-methoxyphenyl) methanone (AM630), resulted in antidepressant-like effects in both the forced swimming test and CMS test (García-Gutiérrez et al., 2010). Interestingly, chronic treatment with AM630 for 4 weeks blocked the reduction of CB2 receptor gene expression and BDNF gene and protein expression in Swiss ICR mice exposed to CMS (García-Gutiérrez et al., 2010). Conversely, anxiolytic effects were detected after intracerebroventricular administration of antisense oligonucleotide sequence directed against the CB2 receptor gene in mice (Onaivi et al., 2008b).
In the present study, the effects of pharmacological manipulation of CB2 receptors were studied by using the cannabinoid CB2 receptor agonist, (6aR,10aR)-3-(1,1-dimethylbutyl)-6a,7,10,10a-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran (JWH133) and the CB2 receptor antagonist AM630 in Swiss ICR mice. Dose-response effects of acute and chronic administration of JWH133 (0.5, 1 or 2 mg·kg−1, i.p.) and AM630 (1, 2 or 3 mg·kg−1, i.p.) were evaluated in the LDB and EPM tests. CB2 receptor, GABAAα2 and GABAAγ2 gene and protein expressions were studied by real time PCR (RT-PCR) and Western blot in the cortex and amygdala of chronically treated Swiss ICR mice. Furthermore, the anxiolytic effects of chronic AM630 treatment were evaluated in the spontaneously anxious DBA/2 strain of mice.
The results of this study revealed that chronic administration with AM630 produced anxiolytic effects associated with increased and reduced CB2 receptor, GABAAα2 and GABAAγ2 gene and protein expressions, respectively, in the cortex and amygdala. In contrast, chronic administration with JWH133 increased anxiety, reduced gene and increased protein expression of CB2 receptors, GABAAα2 and GABAAγ2 in the cortex and amygdala. Interestingly, the same chronic pattern of administration with AM630 reduced the anxiety of the spontaneously anxious DBA/2 strain of mice. Taken together, these results strongly point to the CB2 receptor as a potential target in the treatment of anxiety-related disorders.
Methods
Animals
All animal care and experimental studies were in compliance with the Royal Decree 223/1998 of 14 March (BOE. 8 18) and the Ministerial Order of 13 October 1989 (BOE 18), as well as with the European Council Directive of 24 November 1986 (86/609/EEC). Male Swiss ICR and DBA/2 Ola Hs mice were used in all experiments (Harlan, Barcelona, Spain). At the beginning of the experiments, mice were between 2 and 3 months old and weighed 25–35 g. All animals were maintained under controlled temperature (23 ± 2°C) and light (light–dark cycle from 0800 to 2000 h), with free access to food (commercial diet for rodents A04 Panlab, Barcelona, Spain) and water. All the experiments were carried out between 0900 and 1300 h. The same conditions were maintained for all the behavioural tests.
Treatments
AM630 is a cannabinoid CB2 receptor antagonist (Ki = 31.2 nM) that displays 165-fold selectivity over CB1 receptors (Pertwee et al., 1995; Hosohata et al., 1997). JWH133 is a potent cannabinoid CB2 receptor agonist that displays approximately 200-fold selectivity over CB1 receptors (Huffman et al., 1999; Pertwee, 1999). Both drugs were obtained from Tocris (Biogen, Madrid, Spain) and dissolved in DMSO, Tween 80 and distilled water (1:1:8) immediately before use. In acute experiments, AM630 (1, 2 or 3 mg·kg−1; 0.3 ml; i.p.) or JWH133 (0.5, 1 or 2 mg·kg−1; 0.3 ml; i.p.) were administered 30 min before the corresponding experimental test. In chronic experiments, AM630 (1, 2 or 3 mg·kg−1; 0.3 ml; i.p.) or JWH133 (0.5, 1 or 2 mg·kg−1; 0.3 mL; i.p.) were administered twice a day (0900 h and 1800 h) for 7 days. On day 8, behavioural assessment was performed 15 h after the last administration of the corresponding drug or vehicle.
Behavioural assessment
LDB
The LDB test uses the natural aversion of rodents to bright areas compared with darker ones (Crawley and Goodwin, 1980). In a two-compartment box, rodents will prefer dark areas, whereas anxiolytic drugs should increase the time spent in the light compartment. The apparatus consisted of two methacrylate boxes (20 × 20 × 15 cm), one transparent, and one black and opaque, separated by an opaque tunnel (4 cm). Light from a 60 W desk lamp placed 25 cm above the light box provided room illumination. Mice were individually tested in 5 min sessions. During this period, the time spent in the light box and the number of transitions between the two compartments was recorded. A mouse whose four paws were in the new box was considered as having changed boxes. The floor of each box was cleaned between sessions with paper towels moistened with water and fully dried. At the beginning of the session, mice were placed in the tunnel facing the dark box.
EPM
The EPM consisted of two open arms and two enclosed horizontal perpendicular arms 50 cm above the floor (Lister, 1987). The junction of four arms formed a central squared platform (5 × 5 cm). The test began with the animal being placed in the centre of the apparatus facing one of the enclosed arms and allowed to explore freely for 5 min. During this period, the time spent in the open arms (as percentages of total test time) and the number of entries from open-arms to closed-arms (and vice versa) was recorded. Arm entry was considered as entry of four paws into the arm. The floor of each box was cleaned between sessions with paper towels moistened with water and fully dried.
Analysis of gene expression of CB2 receptors, GABAAα2 and GABAAγ2 receptor subunits by RT-PCR
The gene expression of CB2 receptors and the main anxiolytic subunits of GABAA receptor, GABAAα2 and GABAAγ2, were examined by RT-PCR in the cortex and amygdala of Swiss ICR mice, chronically treated with AM630 and JWH133. Briefly, 18 h after the last administration of AM630, JWH133 or its corresponding vehicle, mice were killed, and the brains were removed from the skull and frozen over dry ice. Brain sections (500 µm) were cut at different levels containing the cingulated cortex (figure 20 and 1.34 mm from the bregma) and amygdala (basolateral and central amygdaloid nuclei; figure 40, −1.6 mm from the bregma) according to Paxinos and Franklin (2001), mounted onto slides and stored at −80°C. Sections were dissected following the method described by Palkovits (1983). Total RNA was obtained from brain punches using Biozol® Total RNA extraction reagent (Bioflux, Inilab, Madrid, Spain). After DNAse digestion, the reverse transcription was carried out following the instructions of the manufacturer (Epicentre, Tech. Corp., Madison, WI, USA). CB2 receptor, GABAAα2 and GABAAγ2 gene expression was measured by using Taqman® Gene Expression assays (Mm 00438286_m1, Mm00433435_m1 and Mm00433489_m1 respectively; Applied Biosystems, Madrid, Spain) as a double-stranded DNA-specific fluorescent dye and performed on the AbbiPrism 7700 Real Time Cycler (Applied Biosystems). The reference gene used was 18S rRNA, detected using Taqman ribosomal RNA control reagents. All primer-probe combinations were optimized and validated for relative quantification of gene expression. Briefly, data for each target gene were normalized to the endogenous reference gene, and the fold change in target gene abundance was determined using the 2-ΔΔCt method (Livak and Schmittgen, 2001).
Analysis of expression of CB2 receptors, GABAAα2 and GABAAγ2 receptor subunits protein by Western blot
After determination and adjusting protein levels, homogenates of cortex and amygdala tissues were centrifuged (12 000×g, 20 min at 4°C) and mixed with Laemmli sample buffer (Bio-Rad, Hercules, CA, USA; SDS 10%, distilled H2O, glycerol 50%, Tris HCl 1 M pH 6,8, dithiotreitol and blue bromophenol) containing β-mercapthoethanol (50 µL per mL of Laemmli). Once separated by molecular weight, proteins from the electrophoresis gels were blotted onto a nitrocellulose membrane (Amersham Ibérica, Madrid, Spain) by semidry transfer system (Bio-Rad) and incubated with a specific primary antibodies against CB2 receptors (Cayman Chemical, Michigan, USA; 1:1000 dilution), GABAAα2 (Abcam, Cambridge, UK; 1:1000 dilution) and GABAAγ2 antibody (Abcam; 1:1000 dilution). Proteins recognized by the respective horseradish peroxidase-linked secondary antibodies were revealed by ECL™-kit following the manufacturer's instructions (Amersham) and visualised on X-ray film (Amersham). Autoradiographs were quantified by densitometry (program Image J, National Institutes of Health, Bethesda, MD, USA), and several time exposures were analysed to ensure the linearity of the band intensities. All densitometries are expressed in arbitrary units (AU). In all the Western blot analyses, the housekeeping gene β-actin (Sigma, Madrid, Spain) was used as loading control.
Statistical analyses
Statistical analyses were performed using the Student's t-test when comparing two groups, one-way anova followed by the Student–Newman–Keul's test when comparing four groups and two-way anova followed by Student–Newman–Keul's test when comparing four groups and two variables. Differences were considered significant if the probability of error was less than 5%.
Results
Acute treatment with the cannabinoid CB2 receptor-antagonist AM630 and agonist JWH133
Acute treatment with AM630 at the dose of 1 mg·kg−1 failed to produce any modification (Figure 1A). However, the administration of AM630 at the doses of 2 and 3 mg·kg−1 significantly reduced the time spent in the light box compared with vehicle group (one-way anova followed by Student–Newman–Keul's: F(3,29) = 3.530, P = 0.029) (n = 8–7) (Figure 1A). In addition, no difference was observed in the total number of transitions between groups (one-way anova: F(3,27) = 0.423, P = 0.738) (n = 8–7) (Figure 1B).
Figure 1.
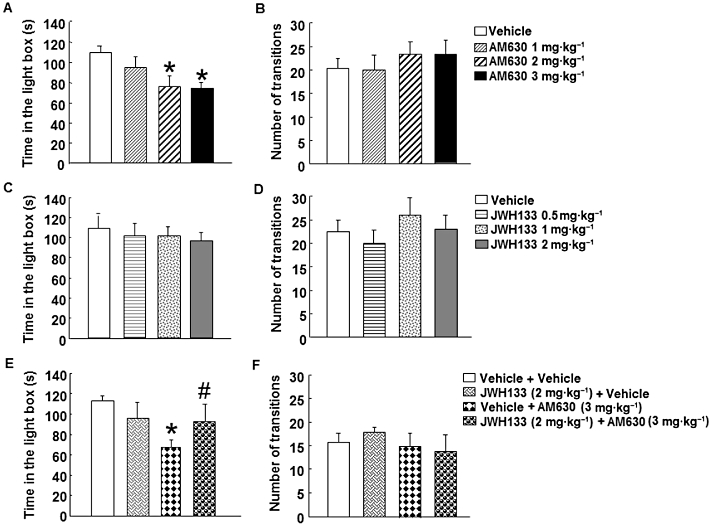
Acute dose-response effects of AM630 and JWH133 in the LDB test in Swiss ICR mice. Panels A–D: mice were injected with AM630 (1, 2 or 3 mg·kg−1, i.p.) (A, B) or JWH133 (0.5, 1 or 2 mg·kg−1, i.p.) (C, D), and 30 min later, were exposed to the LDB test for 5 min. Panels E–F: mice were injected with JWH133 (2 mg·kg−1) or its corresponding saline vehicle (0.3 mL, i.p.), and 30 min later. AM630 (3 mg·kg−1) or its corresponding saline vehicle were administered (0.3 mL). Anxiety-like behaviour was analysed 30 min after the last administration in the LDB test during 5 min. Columns represent the means and vertical lines (SEM of the different parameters. Panel A: *P < 0.05, significantly different from corresponding control group. Panel E: *P < 0.05, significantly different from corresponding control group. #P < 0.05, JWH133 + AM630 significantly different from vehicle + AM630 group.
Interestingly, acute treatment of JWH133 failed to produce any modification in the time spent in the light box (one way anova: F(3,23) = 0.202, P = 0.894) (n = 5–7) (Figure 1C) and in the total number of transitions at either of the doses used (one-way anova: F(3,24) = 0.653, P = 0.590) (n = 5–7) (Figure 1D).
To investigate if the anxiogenic effect induced by the blockade of CB2 receptors was receptor specific, mice were pre-treated with the agonist JWH133 (2 mg·kg−1) 30 min before the administration of AM630 (3 mg·kg−1). As expected, the administration of AM630 increased anxiety since the time spent in the light box was reduced compared with its corresponding control group (Figure 1E). Interestingly, pre-treatment with JWH133 blocked the anxiogenic effect of AM630 (Figure 1E). In contrast, the administration of JWH133 alone was without effects (two-way anova followed by Student–Newman–Keul's: JWH133 F(1,28) = 0.134, P = 0.717; AM630 F(1,28) = 4.277, P = 0.049; JWH133 × AM630 F(1,28) = 3.163, P = 0.088) (n = 6–8) (Figure 1E). In addition, no difference was observed in the number of transitions between groups (two-way anova: JWH133 F(1,21) = 0.046, P = 0.833; AM630 F(1,21) = 0.617, P = 0.442; JWH133 × AM630 F(1,21) = 0.413, P = 0.528) (n = 6–8) (Figure 1F).
Chronic treatment with the cannabinoid CB2 receptor antagonist AM630 or agonist JWH133
Assessment of anxiolytic effects in the LDB and EPM tests
Mice treated with AM630 (1, 2 or 3 mg·kg−1, i.p., twice a day) or JWH133 (0.5, 1 or 2 mg·kg−1, i.p., twice a day) for a total of 7 days were exposed to the LDB test, 15 h after the last administration of drug or corresponding vehicle. Chronic AM630 treatment produced a significant anxiolytic effect, increasing the time spent in the light box at all of the doses used (one-way anova followed by Student–Newman–Keul's: F(3, 30) = 7.124, P = 0.001) (n = 6–9) (Figure 2A). In contrast, treatment with JWH133 significantly reduced the time spent in the light box at all doses used. In addition, JWH133 at 2 mg·kg−1 reduced the time spent in the light box compared with JWH133 (0.5 mg·kg−1) group (one-way anova followed by Student–Newman–Keul's: F(3,26) = 9.325, P(0.001) (n = 7–9) (Figure 2C). However, the total number of transitions was not affected by any of the doses of AM630 (one-way anova: F(3,34) = 1.530, P = 0.026) (n = 6–9) (Figure 2B) or JWH133 used (one-way anova: F(3,28) = 0.245, P = 0.864) (n = 7–9) (Figure 2D).
Figure 2.
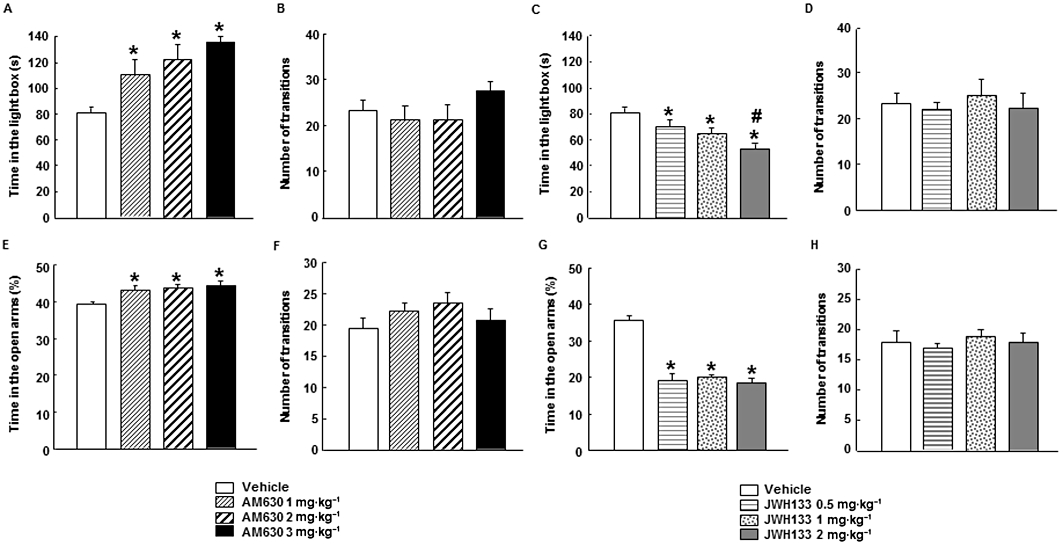
Chronic dose-response effects of AM630 and JWH133 in LDB and EPM tests in Swiss ICR mice. Mice were treated with AM630 (1, 2 or 3 mg·kg−1, i.p., twice a day) (A, B, E, F) or JWH133 (0.5, 1 or 2 mg·kg−1, i.p., twice a day) (C, D, G, H) for 7 days. On day 8, anxiety-like behaviour was evaluated in the LDB or EPM tests during 5 min. Columns represent the means and vertical lines (SEM of the different parameters. *P < 0.05, significantly different from corresponding vehicle-treated groups. #P < 0.05, JWH133 (2 mg·kg−1) significantly different from JWH133 (0.5 mg·kg−1) values.
In the EPM, the percentage of time spent in the open arms significantly increased in mice treated with AM630 at all of the doses used (one-way anova followed by Student–Newman–Keul's: F(3,52) = 3.339, P = 0.027) (n = 11–16) (Figure 2E), without affecting the number of transitions between compartments (one-way anova: F(3,49) = 1.238, P = 0.307) (n = 11–16) (Figure 2F). In contrast, the administration of JWH133 significantly reduced the time spent in the open arms at all the doses used (one-way anova followed by Student–Newman–Keul's: F(3,62) = 38.437, P(0.001) (n = 15–16) (Figure 2G), again without affecting the number of transitions between compartments (one-way anova: F(3,60) = 0.363, P = 0.780) (n = 15–16) (Figure 2H).
Assessment of CB2 receptor, GABAAα2 and GABAAγ2 receptor subunits gene and protein expression
The gene and protein expression of CB2 receptors were examined in cortex and amygdala of mice exposed to chronic JWH133 (0.5, 1 or 2 mg·kg−1, twice a day) or AM630 (1, 2 or 3 mg·kg−1, twice a day) treatments. Moreover, considering the relevance of the GABAergic system in the control of emotional states, the gene and protein expression of the main anxiolytic GABAA subunits, α2 and γ2, were also analysed in the cortex and amygdala. The cortex is a brain region that is thought to be crucially involved in the regulation of mood, aggression and/or impulsivity and decision making (Bechara and Van Der Linden, 2005; Mann, 2003). Similarly, the amygdala is a key element closely involved in the modulation of emotional, cognitive, autonomic and endocrine responses to stress in animals and humans (Swanson and Petrovich, 1998; LeDoux, 2000; Richter-Levin and Akirav, 2000; Kjelstrup et al., 2002; McGaugh et al., 2002; Carrasco and Van de Kar, 2003).
CB2 receptor gene expression
In the cortex, chronic AM630 administration increased CB2 receptor gene expression at all the doses used (one-way anova followed by Student–Newman–Keul's: F(3,31) = 4.266, P = 0.013) (n = 7–9) (Figure 3A). Similarly, in the amygdala, CB2 receptor gene expression was increased by chronic treatment with AM630 (2 and 3 mg·kg−1), compared with vehicle and AM630 (1 mg·kg−1) groups. No effects were observed after treatment with 1 mg·kg−1 AM630(one-way anova followed by Student–Newman–Keul's: F(3,21) = 10.071, P = 0.001) (n = 5–6) (Figure 3C).
Figure 3.
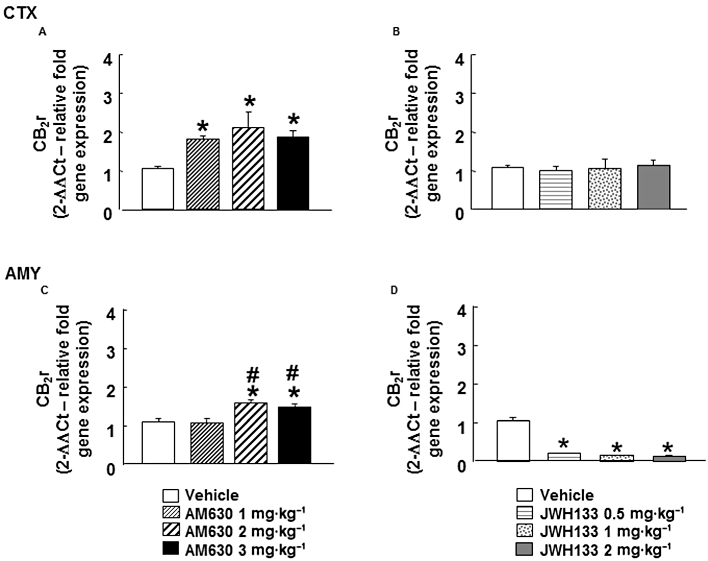
Assessment of CB2 receptor gene expression in the cortex (CTX) and amygdala (AMY) of chronic AM630- or JWH133-treated mice. Relative CB2 receptor (CB2r) gene expression was examined in the cortex and amygdala of Swiss ICR mice treated with AM630 (1, 2 or 3 mg·kg−1, i.p., twice a day) (A, C) or JWH133 (B, D) (0.5, 1 or 2 mg·kg−1, i.p., twice a day) for 7 days. Columns represent the means, and vertical lines represent the (SEM of relative CB2 receptor gene expression. *P < 0.05, significantly different from corresponding vehicle-treated mice. #P < 0.05, AM630 (2 mg·kg−1) and AM630 (3 mg·kg−1) groups significantly different from AM630 (1 mg·kg−1) group.
Interestingly, chronic JWH133 administration produced the opposite effects in CB2 receptor gene expression compared with those obtained with AM630. Mice treated with JWH133 (0.5, 1 or 2 mg·kg−1) had lower CB2 receptor gene expression in the amygdala, compared with its corresponding control group (one-way anova followed by Student–Newman–Keul's: F(3,26) = 177.15, P = 0.001) (n = 5–8) (Figure 3D). In the cortex, however, chronic JWH133 treatment (0.5, 1 or 2 mg·kg−1) did not modify CB2 receptor gene expression (one-way ANOVA: F(3,27) = 0.270, P = 0.847) (n = 6–9) (Figure 3B).
CB2 receptor protein expression
CB2 receptor protein expression was significantly reduced in the cortex of mice chronically treated with AM630 (1, 2 or 3 mg·kg−1) (one-way anova followed by Student–Newman–Keul's: F(3,22) = 5.015, P = 0.010) (n = 6–7) (Figure 4A). However, no modification was observed in the amygdala (one-way anova: F(3,26) = 0.612, P = 0.614) (n = 6–8) (Figure 4C). Interestingly, chronic treatment with JWH133 (2 mg·kg−1) increased CB2 receptor protein expression in the cortex but no changes were observed at 0.5 or 1 mg·kg−1 JWH133 (one-way anova followed by Student–Newman–Keul's: F(3,24) = 3.165, P = 0.046) (n = 6–8) (Figure 4B). However, no modification was observed in the amygdala after chronic treatment with JWH133 (one-way anova: F(3,25) = 0.197, P = 3.165) (n = 5–8) (Figure 4D).
Figure 4.
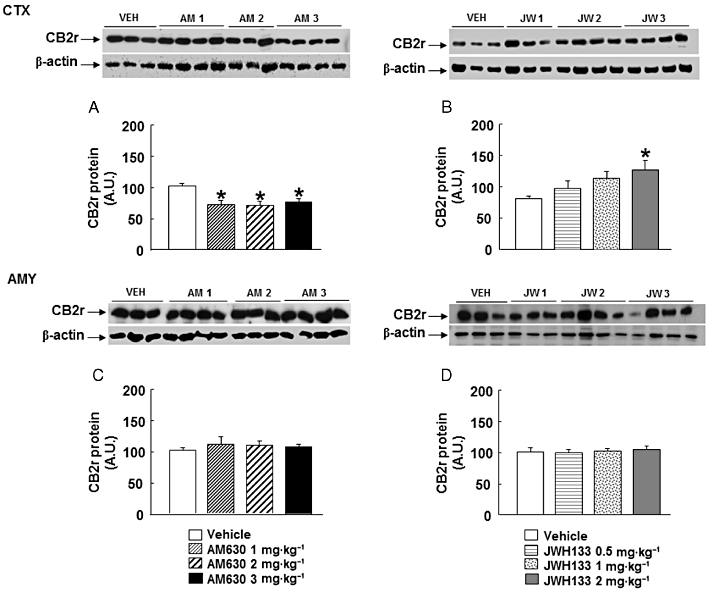
Assessment of CB2 receptor protein expression in the cortex (CTX) and amygdala (AMY) of chronic AM630 or JWH133 treated mice. Western blot and densitometric analysis of CB2 receptor (CB2r) protein were examined in the cortex and amygdala of Swiss ICR mice treated with AM630 (1, 2 or 3 mg·kg−1, twice a day) (A, C) or JWH133 (B, D) (0.5, 1 or 2 mg·kg−1, twice a day) for 7 days. Data are normalized by β-actin (lower band) and are representative of three experiments. Columns represent the means, and vertical lines represent the SEM of CB2 receptor protein expression. *P < 0.05, significantly different from corresponding vehicle (VEH)-treated mice. A.U., arbitrary units.
GABAAα2 gene expression
In the cortex of mice chronically treated with AM630, GABAAα2 gene expression was increased at the highest dose (3 mg·kg−1) but not at the lower doses (1 or 2 mg·kg−1) (one-way anova followed by Student–Newman–Keul's: F(3,25) = 3.548, P = 0.031) (n = 7–8) (Figure 5A). GABAAα2 gene expression in the amygdala of mice chronically treated with AM630 was increased at all doses used (1, 2 or 3 mg·kg−1). Moreover, mice treated with AM630 at highest dose (3 mg·kg−1) presented increased GABAAα2 gene expression compared levels in mice treated with 1 mg·kg−1 AM630 (one-way anova followed by Student–Newman–Keul's: F(3,28) = 7.460, P = 0.001) (n = 6–9) (Figure 5C). In contrast, chronic JWH133 treatment (2 or 3 mg·kg−1, i.p.) significantly reduced GABAAα2 gene expression in the cortex. No modification was observed in mice chronically treated with JWH133 (1 mg·kg−1) (one-way anova followed by Student–Newman–Keul's: F(3,28) = 3.706, P = 0.025) (n = 7–8) (Figure 5A). Similarly, a significant reduction of GABAAα2 gene expression was observed in the amygdala of mice treated with JWH133 (2 mg·kg−1). No modification was observed in mice treated with JWH133 at the doses of 0.5 or 1 mg·kg−1 (one-way anova followed by Student–Newman–Keul's: F(3,26) = 3.815, P = 0.024) (n = 6–7) (Figure 5D).
Figure 5.
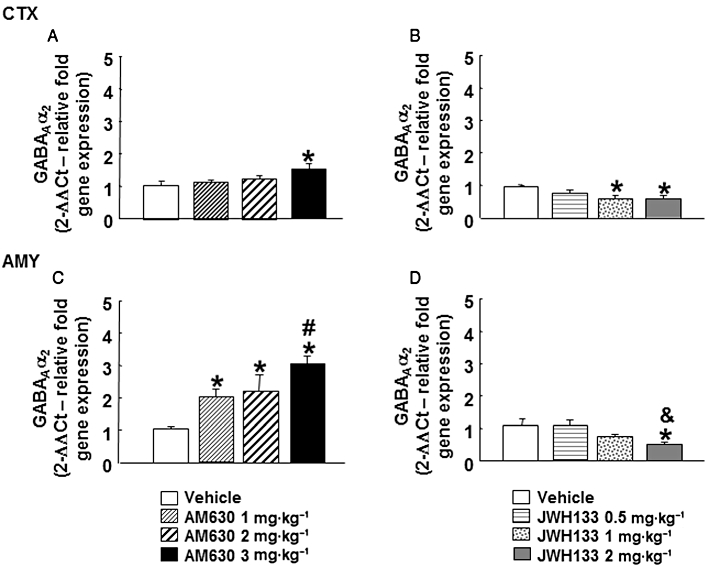
Assessment of GABAAα2 gene expression in the cortex (CTX) and amygdala (AMY) of chronic AM630- or JWH133-treated mice. Relative GABAAα2 gene expression was examined in the cortex and amygdala of Swiss ICR mice chronically treated (7 days) with AM630 (1, 2 or 3 mg·kg−1, twice a day) (A, C) or JWH133 (0.5, 1 or 2 mg·kg−1, twice a day) (B,D). Columns represent the means and vertical lines represent the SEM of relative GABAAα2 gene expression. *P < 0.05, significantly different from corresponding vehicle group. #P < 0.05, AM630 (3 mg·kg−1) significantly different from AM630 (1 mg·kg−1) group. &P < 0.05, JWH133 (2 mg·kg−1) significantly different from JWH133 (0.5 mg·kg−1).
GABAAα2 protein expression
Chronic AM630 administration reduced GABAAα2 protein expression in the cortex at any of the doses used (one-way anova followed by Student–Newman–Keul's: F(3,26) = 12.267, P = 0.001) (n = 6–8) (Figure 6A). In the amygdala, AM630 at 1 or 2 mg·kg−1 failed to produce any modification in GABAAα2 protein expression. In contrast, AM630 at 3 mg·kg−1 did reduce GABAAα2 protein expression (one-way anova followed by Student–Newman–Keul's: F(3,25) = 17.349, P = 0.001) (n = 6–8) (Figure 6C).
Figure 6.
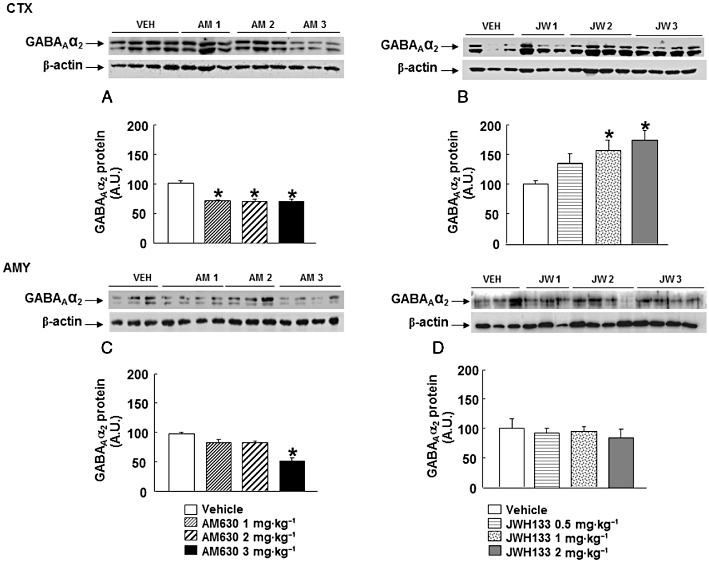
Assessment of GABAAα2 protein expression in the cortex (CTX) and amygdala (AMY) of chronic AM630- or JWH133-treated mice. Western blot and densitometric analysis of GABAAα2 protein were examined in the cortex and amygdala of Swiss ICR mice treated with AM630 (1, 2 or 3 mg·kg−1, twice a day) (A, C) or JWH133 (B, D) (0.5, 1 or 2 mg·kg−1, twice a day) for 7 days. Data are normalized by β-actin (lower band) and are representative of three experiments. Columns represent the means and vertical lines represent the SEM of GABAAα2 protein expression. *P < 0.05, significantly different from corresponding vehicle (VEH)-treated mice. A.U., arbitrary units.
Interestingly, chronic treatment with JWH133 (1 or 2 mg·kg−1) increased GABAAα2 protein expression in the cortex. No modification was observed at 0.5 mg·kg−1 (one-way anova followed by Student–Newman–Keul's: F(3,23) = 4.177, P = 0.019) (n = 5–7) (Figure 6B). In contrast, no modification was observed in the amygdala (one-way anova: F(3,28) = 4.290, P = 0.001) (n = 6–8) (Figure 6D).
GABAAγ2 gene expression
GABAAγ2 gene expression was increased in the cortex of mice chronically treated with AM630 at 3 mg·kg−1 but not at lower doses (1 or 2 mg·kg−1) (one-way anova followed by Student–Newman–Keul's: F(3,29) = 4.441, P = 0.012) (n = 6–8) (Figure 7A). In the amygdala, chronic treatment with AM630 increased GABAAγ2 gene expression at all doses used (1, 2 or 3 mg·kg−1) (one-way anova followed by Student–Newman–Keul's: F(3,24) = 19.583, P = 0.001) (n = 4–7) (Figure 7C).
Figure 7.
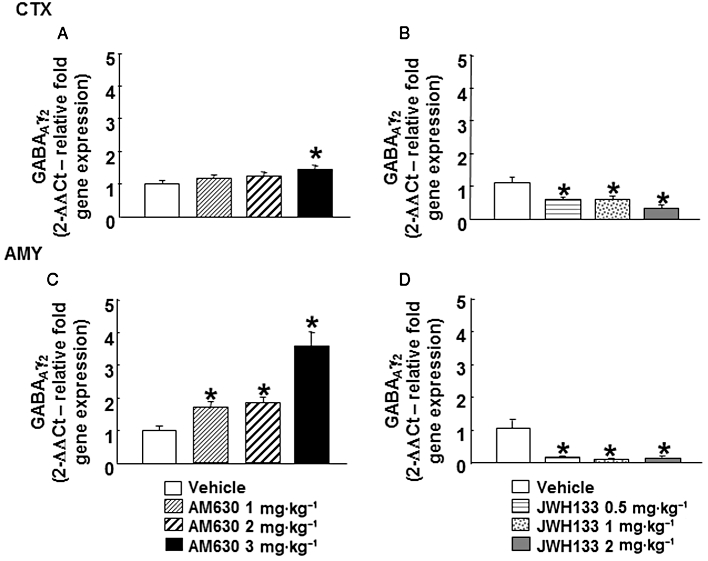
Assessment of GABAAγ2 gene expression in the cortex (CTX) and amygdala (AMY) of chronic AM630- or JWH133-treated mice. Relative GABAAγ2 gene expression was examined in the cortex and amygdala of mice treated with AM630 (1, 2 or 3 mg·kg−1, twice a day) (A, C) or JWH133 (0.5, 1or 2 mg·kg−1, twice a day) (B, D) for 7 days. Columns represent the means and vertical lines represent the SEM of relative GABAAγ2 gene expression. *P < 0.05, significantly different from corresponding vehicle group.
Interestingly, opposite alterations were observed in GABAAγ2 gene of mice chronically treated with JWH133. A reduction of GABAAγ2 gene expression was observed in the cortex (one-way anova followed by Student–Newman–Keul's: F(3,26) = 7.334, P = 0.001) (n = 6–8) (Figure 7B) and amygdala (one way anova followed by Student–Newman–Keul's: F(3,26) = 17.455, P = 0.001) (n = 6–7) (Figure 7D) at all of the doses used.
GABAAγ2 protein expression
Chronic treatment with AM630 at 2 or 3 mg·kg−1, but not 1 mg·kg−1, reduced GABAAγ2 protein expression in the cortex (one-way anova followed by Student–Newman–Keul's: F(3,26) = 6.414, P = 0.003) (n = 7) (Figure 8A). In the amygdala, chronic AM630 administration reduced GABAAγ2 protein expression at all of the doses used (one way anova followed by Student–Newman–Keul's: F(3,21) = 10.658, P = 0.001) (n = 4–6) (Figure 8C).
Figure 8.
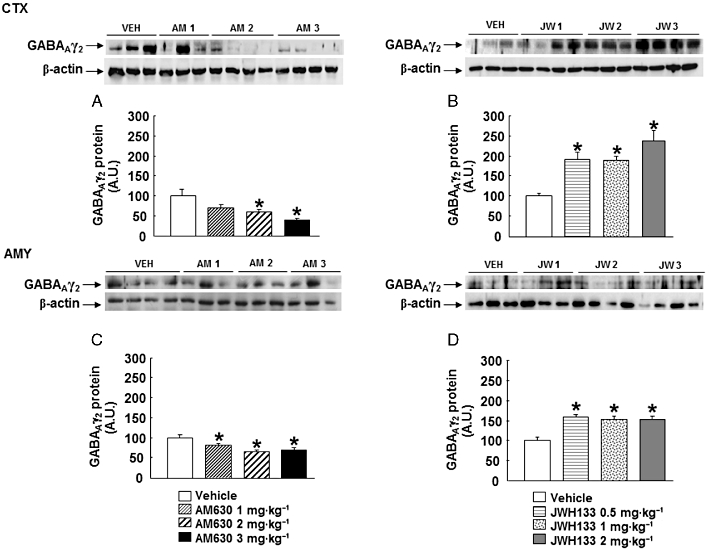
Assessment of GABAAγ2 protein expression in the cortex (CTX) and amygdala (AMY) of chronic AM630- or JWH133-treated mice. Western blot and densitometric analysis of GABAAγ2 protein were examined in the cortex and amygdala of Swiss ICR mice treated with AM630 (1, 2 or 3 mg·kg−1, twice a day) (A, C) or JWH133 (B, D) (0.5, 1 or 2 mg·kg−1, twice a day) for 7 days. Data are normalized by β-actin (lower band) and are representative of three experiments. Columns represent the means and vertical lines represent the SEM of GABAAγ2 protein expression. *P < 0.05, significantly different from corresponding vehicle (VEH)-treated mice. A.U., arbitrary units.
GABAAγ2 protein expression was increased in the cortex (one-way anova followed by Student–Newman–Keul's: F(3,27) = 11.445, P = 0.001) (n = 6–8) (Figure 8B) and in the amygdala (one-way anova followed by Student–Newman–Keul's: F(3,26) = 15.142, P(0.001) (n = 5–8) (Figure 8D) of mice chronically treated with JWH133 at all of the doses used.
Assessment of potential anxiolytic-like effect in spontaneously anxious DBA/2 mice
To test the validity of the potential therapeutic actions of AM630 in the treatment of disorders associated with high level of anxiety, the effects of chronic AM630 were evaluated in the spontaneously anxious DBA/2 strain of mice (Griebel et al., 2000; Ohl et al., 2003; Yilmazer-Hanke et al., 2003). Firstly, the response of DBA/2 strain mice to anxiogenic stimuli was evaluated using the LDB and showed a reduction in the time spent in the lighted box compared with Swiss ICR mice (Student's t-test: t = 3.689, P = 0.004, 10 d.f.) (n = 5–7) (Figure 9A). In addition, there were a fewer transitions observed with DBA/2 mice, than with Swiss ICR mice (Student's t-test: t = 6.817, P = 0.001, 10 d.f.) (n = 5–7) (Figure 9B). Secondly, chronic administration of AM630 (1, 2 or 3 mg·kg−1; 7 days) significantly increased the time spent in the light box by DBA/2 mice (one-way anova followed by Student–Newman–Keul's, F(3,22) = 9.280, P = 0.001) (n = 5–9) (Figure 9C). No difference was observed between the different doses used. DBA/2 mice treated with AM630 (1 mg·kg−1) increased the number of transitions compared with vehicle group. In addition, no difference was observed in the total number of transitions between DBA/2 mice treated with AM630 (2 or 3 mg·kg−1) compared with the vehicle group (one-way anova followed by Student–Newman–Keul's, F(3,31) = 5.847, P = 0.003) (n = 5–9) (Figure 9D).
Figure 9.
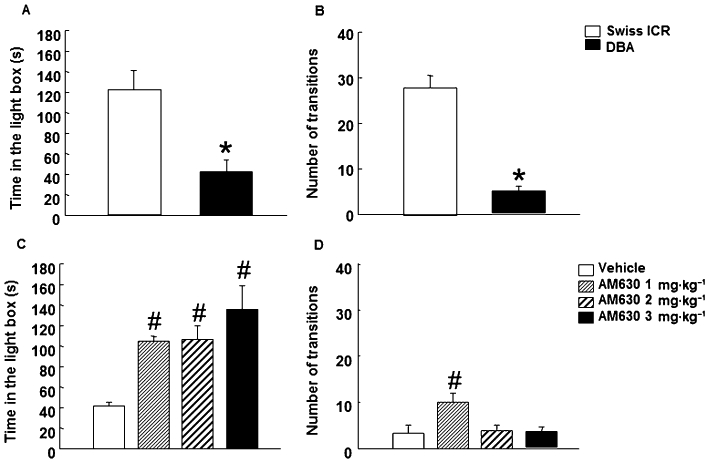
Assessment of anxiety-like behaviors of DBA/2 mice in the LDB paradigm. Panels A–B: assessment of DBA/2 mice response to anxiety-like stimuli in the LDB test. The time spent in the light box was recorded for 5 min. Panels C–D: chronic dose-response effects of AM630 in DBA/2 mice in the LDB test. Mice were injected with AM630 (1, 2 or 3 mg·kg−1, i.p., twice a day) or its saline vehicle for 7 days. On day 8, treated mice were exposed to LDB test for 5 min. Columns represent the mean and vertical lines represent the SEM of the different parameters. * DBA/2 mice significantly different from Swiss ICR mice. #P < 0.05, significantly different from vehicle group.
Discussion and conclusions
The results of the present study support the involvement of the CB2 receptor in the regulation of emotional behaviour and suggest that this receptor may result a relevant target for the treatment of anxiety-like disorders. This assumption was based on the following findings: (i) pretreatment with the cannabinoid CB2 receptor agonist JWH133 blocked the anxiogenic action of acute administration of AM630; (ii) chronic AM630 administration (1, 2 and 3 mg·kg−1) induced anxiolytic effects associated with increased and decreased CB2 receptors, GABAAα2 and GABAAγ2 gene and protein expression, respectively, in the cortex and amygdala; (iii) interestingly, chronic administration of JWH133 (0.5, 1 and 2 mg·kg−1) resulted in behavioural and molecular changes, opposite to those found with chronic treatment with AM630; and (iv) chronic treatment with AM630 (1, 2 and 3 mg·kg−1) reduced anxiety-like behaviour in the spontaneously anxious DBA/2 strain of mice.
The identification of CB2 receptors in the different brain areas related to the response to stress and anxiety suggests the involvement of this receptor in the regulation of emotional behaviours. In this respect, few studies have examined the effects of cannabinoid CB2 receptor agonists and antagonists in the regulation of emotional behaviours. Previous studies reported that acute administration of the cannabinoid CB2 receptor agonist JWH015 (20 mg·kg−1) produced anxiogenic effects in the LDB test (Onaivi et al., 2006). In contrast, the same group demonstrated that JWH015 induced anxiolytic effects in the EPM test (Onaivi, 2006). Additional studies revealed anxiolytic actions induced by the cannabinoid CB2 receptor agonist GW405833 (100 mg·kg−1) in the marble burying test (Valenzano et al., 2005). The fact that the doses of both CB2 receptor agonists evaluated in these studies resulted in alterations in motor activity makes the interpretation of these behavioural effects difficult.
In the present study, the doses of JWH133 and AM630 used were selected based on our previous studies obtained in the open field test. Higher doses of JWH133 (2 mg·kg−1) reduced motor activity in Swiss ICR mice (data not shown). In order to avoid potential motor alterations that may mask the interpretation of the behavioural results, the JWH133 doses used in the present study were below 2 mg·kg−1. Previous pharmacological MRI studies revealed that AM630 at higher doses (3 mg·kg−1) may act as a weak partial/inverse agonist at CB2 receptors (Chin et al., 2008). To avoid this potential loss of CB2 receptor selectivity by AM630, the doses used were below 3 mg·kg−1. Indeed, the doses of AM630 used (1, 2 or 3 mg·kg−1, i.p.) did not alter motor activity in the open field test (data not shown).
Firstly, the effects of acute administration of JWH133 and AM630 were evaluated in the LDB tests. Acute JWH133 administration was without effects at any of the doses used (0.5, 1 and 2 mg·kg−1.). It is possible that the lack of effects may be due to the fact that the doses used were not able to activate the CB2 receptors to produce specific behavioural changes. The fact that higher doses of JWH133 (2 mg·kg−1) modified motor behaviour ruled out the evaluation of additional doses. In contrast, the acute blockade of CB2 receptors by AM630 (2 and 3 mg·kg−1) increased the anxiety-like behaviour of Swiss ICR mice in the LDB test. Furthermore, no modification in the total number of transitions was observed. Taking into account that the number of transitions is considered as an indirect measure of the motor activity, the lack of alterations in this parameter supports the absence of potential motor effects.
In order to further investigate the relative specificity of the CB2 receptor in the mechanism underlying the anxiogenic effects of AM630, mice were pre-treated with the CB2 receptor agonist JWH133. Pre-treatment with JWH133 blocked the anxiogenic effects of AM630. These results supported the involvement of CB2 receptors as the target involved in the acute effects of AM630 on emotional behaviour.
Based on the fact that chronic administration often induces different results from those induced by acute administration, AM630 and JWH133 were administered chronically for 7 days. Interestingly, chronic blockade of CB2 receptors by AM630 (1, 2 and 3 mg·kg−1) resulted in anxiolytic effects in the LDB and EPM tests. In contrast, chronic activation of CB2 receptors induced by chronic treatment with JWH133 (0.5, 1 and 2 mg·kg−1) increased anxiety-like behaviours of Swiss ICR mice in both tests.
Additional gene and protein analyses were carried out in cortex and amygdala of mice chronically treated with AM630 or JWH133 with the aim to identify the molecular mechanisms underlying these behavioural alterations. The anxiolytic effects induced by chronic administration with AM630 were associated with increased CB2 receptor gene expression in the cortex and amygdala and reduced CB2 receptor protein expression in the cortex. Interestingly, the anxiogenic effects of JWH133 were accompanied by opposing changes in CB2 receptors. Chronic administration with JWH133 reduced CB2 receptor gene expression in the amygdala and increased CB2 receptor protein expression in the cortex.
The GABAergic system has been considered a key element in the regulation of emotional states and, therefore, an important therapeutic target controlling anxiety-related disorders. The pentameric GABAA receptors are formed by the assembly of different subunits containing α1, α2, α3 or α5 in combination with β and γ2 subunits. Previous studies suggest that GABAA receptors containing the α2 and γ2 subunits mediate the anxiolytic effect of benzodiazepines (Low et al., 2000). Both subunits are expressed in the limbic system and cortex (Pirker et al., 2000; Mohler et al., 2001; Nyiri et al., 2001). Genetic manipulation of CB2 receptors resulted in changes of GABAA receptors. Transgenic mice that overexpressed CB2 receptors (CB2xP mice) presented increased GABAAα2 and GABAAγ2 gene expression in the hippocampus and amygdala (García-Gutiérrez and Manzanares, 2011). Additionally, recent studies revealed a suppression of GABAergic inhibitory signalling in the entorhinal cortex-hippocampal slices following the administration of the CB2 receptor agonist JWH133 (50 nM). Interestingly, these effects could be blocked by prior administration of AM630 (50 nM) (Morgan et al., 2009) supporting the involvement of CB2 receptors in the effects of JWH133 on GABAergic signalling. These results further strengthen the involvement of CB2 receptors in the regulation of GABAergic release from neuronal terminals.
Chronic blockade of CB2 receptors by AM630 increased GABAAα2 and GABAAγ2 gene expressions in the cortex and amygdala. In contrast, the protein expression of these genes was reduced by chronic treatment with AM630 in the brain regions analysed (cortex and amygdala). Interestingly, activation of CB2 receptors by JWH133 reduced the GABAAα2 and GABAAγ2 gene expression and increased its protein expression in the cortex and amygdala. Additional neurobiological systems (hypothalamic–pituitary–adrenal axis, 5-hydroxytryptaminergic system, neuropeptide Y) and brain regions may be involved in the behavioural effects of chronic AM630 or JWH133 administration. However, the opposing behavioural and molecular changes observed between chronic blockade (AM630) or activation (JWH133) of CB2 receptors supported the key role of these targets (CB2 receptors, GABAAα2 and GABAAγ2) in the behavioural effects of AM630 or JWH133. These results provide new insights into the different molecular events related to GABAA receptor gene and protein expression produced by chronic manipulation of CB2 receptors with CB2 receptor agonists or antagonists.
It is important to note that changes found in gene expression are not often coincident with alterations in protein expression (Impagnatiello et al., 1996; Pesold et al., 1997). The gene expression measurement is a dynamic factor considered as an index of synthesis that may be altered by several factors such as the action of antagonist or agonist. Protein expression is a factor representing an index of end product resulting from release and metabolic processes. Therefore, kinetic and dynamic mechanisms regulating gene and protein expression may have very different outcomes. Several factors, such as duration and dose of the drug effect, pattern of drug administration, abundance of gene and protein in different tissues, time after drug administration and sensitivity of the technique used to measure either protein or gene expression may differentially alter the changes found between gene and protein expressions.
In the present study, most of the gene expression alterations analysed were accompanied by opposing changes at the protein expression level, whereas in a few occasions, no modification was observed in the gene and protein expression (e.g. in the amygdala of mice chronically treated with JWH133 or AM630). These results are in agreement with previous reports. In rat cerebellar granule cells exposed to flumazenil, the changes in the expression of subunit proteins were sometimes either dissociated from a change in their respective gene expression or associated with gene changes in the opposite direction (Zheng et al., 1996). The frequent discrepancies between activity-dependent alterations in gene and protein expression are not surprising because of the temporal delay between the onset of changes in gene expression and in their translation products. It is also possible that not all proteins detected by immunolabelling techniques are functionally operative, and that protein expression depends on translation rate and mRNA stability, rather than DNA transcription rates, exclusively. The reduction of CB2 receptor, GABAAα2 and GABAAγ2 protein expression may induce the expression of the corresponding gene expression. The switch in gene expression of CB2 receptors and GABAAα2 and GABAAγ2 subunits may be necessary to keep unchanged the number of receptors and maintain an adequate homeostatic regulation of the receptors.
The anxiolytic effects of chronic AM630 administration in Swiss ICR mice exposed to LDB test do not necessarily predict anxiolytic action in mice displaying spontaneously a high level of anxiety. To further explore the potential therapeutic action of this cannabinoid receptor antagonist in the treatment of anxiety-related disorders, the effects of chronic AM630 administration were evaluated in the highly anxious DBA/2 strain of mice. The evaluation of the basal response to anxiogenic stimuli of DBA/2 mice revealed high level of anxiety compared with Swiss ICR mice. These results are in agreement with previous results obtained in the EPM (Yilmazer-Hanke et al., 2003) and hole-board tests (Ohl et al., 2003). The results revealed that chronic AM630 clearly decreased the anxious state of DBA/2 mice at all the doses used.
In summary, these findings strongly support the role of CB2 receptors in the regulation of emotional states. Pharmacological manipulation of CB2 receptors resulted in behavioural changes that are associated with molecular changes in CB2 receptor, GABAAα2 and GABAAγ2 protein and gene expressions. Furthermore, chronic AM630 treatment reduced the anxiogenic-like behaviour of DBA/2 mice. Taken together, these results point out this receptor as a new target for the treatment of anxiety-related disorders. Further pharmacological studies are necessary to explore the potential therapeutic uses of cannabinoid CB2 receptors in humans and the precise mechanisms underlying these effects.
Acknowledgments
This research was supported by grants from Ministry of Science and Innovation (SAF 2008-01106) and Ministry of Health (RETICS RD06/0001/1004, Red de Trastornos Adictivos, Instituto de Salud Carlos III, MICINN and FEDER and PNSD 2007/061) to JM. MSGG is a predoctoral fellow of the Ministry of Science and Innovation. We thank Patricia Rodríguez and Analía Rico for their excellent technical assistance. AR is a technician supported by RETICS.
Glossary
- AM630
(6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl](4-methoxyphenyl)methanone)
- BDNF
brain-derived neurotrophic factor
- CB2xP
transgenic mice overexpressing the CB2 receptor in the CNS
- CMS
chronic mild stress
- CRF
corticotrophin-releasing factor
- EPM
elevated plus maze test
- GABAAα2
GABAA receptor subunit α 2
- GABAAγ2
GABAA receptor subunit γ 2
- JWH133
((6aR,10aR)-3-(1,1-dimethylbutyl)-6a,7,10,10a-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran)
- LDB
light–dark box test
- POMC
proopiomelanocortin
- RT-PCR
reverse transcription PCR
- TST
tail suspension test
Conflicts of interest
All authors state that they have no biomedical financial interests or potential conflicts of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edn. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton JC, Friberg D, Darlington CL, Smith PF. Expression of the cannabinoid CB2 receptor in the rat cerebellum: an immunohistochemical study. Neurosci Lett. 2006;396:113–116. doi: 10.1016/j.neulet.2005.11.038. [DOI] [PubMed] [Google Scholar]
- Atwood BK, Mackie K. CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol. 2010;160:467–479. doi: 10.1111/j.1476-5381.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Van Der Linden M. Decision-making and impulse control after frontal lobe injuries. Curr Opin Neurol. 2005;18:734–739. doi: 10.1097/01.wco.0000194141.56429.3c. [DOI] [PubMed] [Google Scholar]
- Beltramo M, Bernardini N, Bertorelli R, Campanella M, Nicolussi E, Fredduzzi S, et al. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. Eur J Neurosci. 2006;23:1530–1538. doi: 10.1111/j.1460-9568.2006.04684.x. [DOI] [PubMed] [Google Scholar]
- Benito C, Nunez E, Tolon RM, Carrier EJ, Rabano A, Hillard CJ, et al. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer's disease brains. J Neurosci. 2003;23:11136–11141. doi: 10.1523/JNEUROSCI.23-35-11136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusco A, Tagliaferro P, Saez T, Onaivi ES. Postsynaptic localization of CB2 cannabinoid receptors in the rat hippocampus. Synapse. 2008a;62:944–949. doi: 10.1002/syn.20569. [DOI] [PubMed] [Google Scholar]
- Brusco A, Tagliaferro PA, Saez T, Onaivi ES. Ultrastructural localization of neuronal brain CB2 cannabinoid receptors. Ann N Y Acad Sci. 2008b;1139:450–457. doi: 10.1196/annals.1432.037. [DOI] [PubMed] [Google Scholar]
- Carlisle SJ, Marciano-Cabral F, Staab A, Ludwick C, Cabral GA. Differential expression of the CB2 cannabinoid receptor by rodent macrophages and macrophage-like cells in relation to cell activation. Int Immunopharmacol. 2002;2:69–82. doi: 10.1016/s1567-5769(01)00147-3. [DOI] [PubMed] [Google Scholar]
- Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463:235–272. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- Chin CL, Tovcimak AE, Hradil VP, Seifert TR, Hollingsworth PR, Chandran P, et al. Differential effects of cannabinoid receptor agonists on regional brain activity using pharmacological MRI. J Pharmacol. 2008;153:367–379. doi: 10.1038/sj.bjp.0707506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- Derbenev AV, Stuart TC, Smith BN. Cannabinoids suppress synaptic input to neurones of the rat dorsal motor nucleus of the vagus nerve. J Physiol. 2004;559(Pt 3):923–938. doi: 10.1113/jphysiol.2004.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiègue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- García-Gutiérrez MS, Manzanares J. Overexpression of CB2 cannabinoid receptor gene expression decreased vulnerability to anxiety and impaired anxiolytic action of alprazolam in mice. J Psychopharmacol. 2011;25:111–120. doi: 10.1177/0269881110379507. [DOI] [PubMed] [Google Scholar]
- García-Gutiérrez MS, Pérez-Ortiz JM, Gutiérrez-Adán A, Manzanares J. Depression-resistant endophenotype in mice overexpressing cannabinoid CB(2) receptors. Br J Pharmacol. 2010;160:1773–1784. doi: 10.1111/j.1476-5381.2010.00819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, et al. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Griebel G, Belzung C, Perrault G, Sanger DJ. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology (Berl) 2000;148:164–170. doi: 10.1007/s002130050038. [DOI] [PubMed] [Google Scholar]
- Griffin G, Wray EJ, Tao Q, McAllister SD, Rorrer WK, Aung MM, et al. Evaluation of the cannabinoid CB2 receptor-selective antagonist, SR144528: further evidence for cannabinoid CB2 receptor absence in the rat central nervous system. Eur J Pharmacol. 1999;377:117–125. doi: 10.1016/s0014-2999(99)00402-1. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Herkenham M. Cannabinoid receptors undergo axonal flow in sensory nerves. Neuroscience. 1999a;92:1171–1175. doi: 10.1016/s0306-4522(99)00220-1. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Herkenham M. Localization of central cannabinoid CB1 receptor messenger RNA in neuronal subpopulations of rat dorsal root ganglia: a double-label in situ hybridization study. Neuroscience. 1999b;90:923–931. doi: 10.1016/s0306-4522(98)00524-7. [DOI] [PubMed] [Google Scholar]
- Hosohata Y, Quock RM, Hosohata K, Makriyannis A, Consroe P, Roeske WR, et al. AM630 antagonism of cannabinoid-stimulated [35S]GTP gamma S binding in the mouse brain. Eur J Pharmacol. 1997;321:R1–R3. doi: 10.1016/s0014-2999(97)00047-2. [DOI] [PubMed] [Google Scholar]
- Huffman JW, Liddle J, Yu S, Aung MM, Abood ME, Wiley JL, et al. 3-(1′,1′-Dimethylbutyl)-1-deoxy-delta8-THC and related compounds: synthesis of selective ligands for the CB2 receptor. Bioorg Med Chem. 1999;7:2905–2914. doi: 10.1016/s0968-0896(99)00219-9. [DOI] [PubMed] [Google Scholar]
- Impagnatiello F, Pesold C, Longone P, Caruncho H, Fritschy JM, Costa E, et al. Modifications of gamma-aminobutyric acidA receptor subunit expression in rat neocortex during tolerance to diazepam. Mol Pharmacol. 1996;49:822–831. [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci U S A. 2002;99:10825–10830. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klegeris A, Bissonnette CJ, McGeer PL. Reduction of human monocytic cell neurotoxicity and cytokine secretion by ligands of the cannabinoid-type CB2 receptor. Br J Pharmacol. 2003;139:775–786. doi: 10.1038/sj.bjp.0705304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Low K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, et al. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- Mann JJ. Neurobiology of suicidal behavior. Nat Rev Neurosci. 2003;4:819–828. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- Maresz K, Pryce G, Ponomarev ED, Marsicano G, Croxford JL, Shriver LP, et al. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nat Med. 2007;13:492–497. doi: 10.1038/nm1561. [DOI] [PubMed] [Google Scholar]
- McCoy KL, Matveyeva M, Carlisle SJ, Cabral GA. Cannabinoid inhibition of the processing of intact lysozyme by macrophages: evidence for CB2 receptor participation. J Pharmacol Exp Ther. 1999;289:1620–1625. [PubMed] [Google Scholar]
- McGaugh JL, McIntyre CK, Power AE. Amygdala modulation of memory consolidation: interaction with other brain systems. Neurobiol Learn Mem. 2002;78:539–552. doi: 10.1006/nlme.2002.4082. [DOI] [PubMed] [Google Scholar]
- Mohler H, Crestani F, Rudolph U. GABA(A)-receptor subtypes: a new pharmacology. Curr Opin Pharmacol. 2001;1:22–25. doi: 10.1016/s1471-4892(01)00008-x. [DOI] [PubMed] [Google Scholar]
- Morgan NH, Stanford IM, Woodhall GL. Functional CB2 type cannabinoid receptors at CNS synapses. Neuropharmacology. 2009;57:356–368. doi: 10.1016/j.neuropharm.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Nyiri G, Freund TF, Somogyi P. Input-dependent synaptic targeting of alpha(2)-subunit-containing GABA(A) receptors in synapses of hippocampal pyramidal cells of the rat. Eur J Neurosci. 2001;13:428–442. doi: 10.1046/j.1460-9568.2001.01407.x. [DOI] [PubMed] [Google Scholar]
- Ohl F, Roedel A, Binder E, Holsboer F. Impact of high and low anxiety on cognitive performance in a modified hole board test in C57BL/6 and DBA/2 mice. Eur J Neurosci. 2003;17:128–136. doi: 10.1046/j.1460-9568.2003.02436.x. [DOI] [PubMed] [Google Scholar]
- Onaivi ES. Neuropsychobiological evidence for the functional presence and expression of cannabinoid CB2 receptors in the brain. Neuropsychobiology. 2006;54:231–246. doi: 10.1159/000100778. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Perchuk A, Meozzi PA, et al. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann N Y Acad Sci. 2006;1074:514–536. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Carpio O, Ishiguro H, Schanz N, Uhl GR, Benno R. Behavioral effects of CB2 cannabinoid receptor activation and its influence on food and alcohol consumption. Ann N Y Acad Sci. 2008a;1139:426–433. doi: 10.1196/annals.1432.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Meozzi PA, Myers L, et al. Brain neuronal CB2 cannabinoid receptors in drug abuse and depression: from mice to human subjects. Plos ONE. 2008b;3:e1640. doi: 10.1371/journal.pone.0001640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Álvaro A, Aracil-Fernández A, García-Gutiérrez MS, Navarrete F, Manzanares J. Deletion of CB(2) cannabinoid receptor induces schizophrenia-related behaviors in mice. Neuropsychopharmacology. 2011;36:1489–1504. doi: 10.1038/npp.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palkovits M. Punch sampling biopsy technique. Methods Enzymology. 1983;103:368–376. doi: 10.1016/s0076-6879(83)03025-6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. New York, NY: Academic Press. Harcourt Science and Technology Company; 2001. [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid receptor ligands. Curr Med Chem. 1999;6:635–664. [PubMed] [Google Scholar]
- Pertwee R, Griffin G, Fernando S, Li X, Hill A, Makriyannis A. AM630, a competitive cannabinoid receptor antagonist. Life Sci. 1995;56:1949–1955. doi: 10.1016/0024-3205(95)00175-6. [DOI] [PubMed] [Google Scholar]
- Pesold C, Caruncho HJ, Impagnatiello F, Berg MJ, Fritschy JM, Guidotti A, et al. Tolerance to diazepam and changes in GABA(A) receptor subunit expression in rat neocortical areas. Neuroscience. 1997;79:477–487. doi: 10.1016/s0306-4522(96)00609-4. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Price TJ, Helesic G, Parghi D, Hargreaves KM, Flores CM. The neuronal distribution of cannabinoid receptor type 1 in the trigeminal ganglion of the rat. Neuroscience. 2003;120:155–162. doi: 10.1016/S0306-4522(03)00333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter-Levin G, Akirav I. Amygdala-hippocampus dynamic interaction in relation to memory. Mol Neurobiol. 2000;22:11–20. doi: 10.1385/MN:22:1-3:011. [DOI] [PubMed] [Google Scholar]
- Sánchez C, de Ceballos ML, del Pulgar TG, Rueda D, Corbacho C, Velasco G, et al. Inhibition of glioma growth in vivo by selective activation of the CB(2) cannabinoid receptor. Cancer Res. 2001;61:5784–5789. [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Valenzano KJ, Tafesse L, Lee G, Harrison JE, Boulet JM, Gottshall SL, et al. Pharmacological and pharmacokinetic characterization of the cannabinoid receptor 2 agonist, GW405833, utilizing rodent models of acute and chronic pain, anxiety, ataxia and catalepsy. Neuropharmacology. 2005;48:658–672. doi: 10.1016/j.neuropharm.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Walter L, Franklin A, Witting A, Wade C, Xie Y, Kunos G, et al. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci. 2003;23:1398–1405. doi: 10.1523/JNEUROSCI.23-04-01398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Durrenberger P, Chessell IP, Naylor A, Bountra C, et al. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol. 2006;6:12. doi: 10.1186/1471-2377-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmazer-Hanke DM, Roskoden T, Zilles K, Schwegler H. Anxiety-related behavior and densities of glutamate, GABAA, acetylcholine and serotonin receptors in the amygdala of seven inbred mouse strains. Behav Brain Res. 2003;145:145–159. doi: 10.1016/s0166-4328(03)00107-4. [DOI] [PubMed] [Google Scholar]
- Zheng TM, Caruncho HJ, Zhu WJ, Vicini S, Ikonomovic S, Grayson DR, et al. Chronic flumazenil alters GABAA receptor subunit mRNA expression, translation product assembly and channel function in neuronal cultures. J Pharmac Exp Ther. 1996;277:525–533. [PubMed] [Google Scholar]


