Abstract
Parietal epithelial cells (PECs) of the renal glomerulus contribute to the formation of both cellular crescents in rapidly progressive GN and sclerotic lesions in FSGS. Subtotal transgenic ablation of podocytes induces FSGS but the effect of specific ablation of PECs is unknown. Here, we established an inducible transgenic mouse to allow subtotal ablation of PECs. Proteinuria developed during doxycycline-induced cellular ablation but fully reversed 26 days after termination of doxycycline administration. The ablation of PECs was focal, with only 30% of glomeruli exhibiting histologic changes; however, the number of PECs was reduced up to 90% within affected glomeruli. Ultrastructural analysis revealed disruption of PEC plasma membranes with cytoplasm shedding into Bowman’s space. Podocytes showed focal foot process effacement, which was the most likely cause for transient proteinuria. After >9 days of cellular ablation, the remaining PECs formed cellular extensions to cover the denuded Bowman’s capsule and expressed the activation marker CD44 de novo. The induced proliferation of PECs persisted throughout the observation period, resulting in the formation of typical cellular crescents with periglomerular infiltrate, albeit without accompanying proteinuria. In summary, subtotal ablation of PECs leads the remaining PECs to react with cellular activation and proliferation, which ultimately forms cellular crescents.
Parietal epithelial cells (PECs) of the renal glomerulus have only recently received an increasing amount of attention. This has been predominantly due to several major insights. The most significant advance has been made by studies that established a central role of PECs in two very different diseases: FSGS and rapidly progressive glomerulonephritis (RPGN) with extracapillary proliferations. In RPGN, it is now established that PECs as well as podocytes are the first cells to proliferate along the inner aspect of Bowman’s capsule and to form the characteristic extracapillary proliferations (cellular crescents).1–3 In FSGS, PECs become focally activated by multiple different primary injuries and subsequently invade the glomerular tuft via an adhesion. Once there, they displace podocytes, deposit matrix, and induce mesangial (endocapillary) scarring of the affected segment.4 In addition to their role in glomerular disease, it has been shown that in development approximately 10% of the podocytes are recruited from PECs and/or transitional cells.5 In adults, studies have raised the prospect that PECs might function to regenerate other cell types of the kidney, such as proximal tubular cells or podocytes.5–7
Acute and specific ablation of a defined cell population of interest has been instrumental in determining the functional relevance of a specific cell type. This can be achieved by injection of specific antibodies, such as Thy1.1 antibody into rats, which express the antigen specifically on mesangial cells.8 This model recapitulated mesangioproliferative glomerular disease, enabling multiple studies on this entity. More recently, tissue-specific promoters were used to express the human diphtheria toxin (DTA) receptor on target cells in transgenic mice. Because rodents are not susceptible to DTA, injection of DTA resulted in specific depletion of the target cell population. This system was first used to ablate tissues outside of the kidney such as vascular smooth muscle cells,9 macrophages,10 or Foxp3 regulatory T cells.11
Within the kidney, the human DTA receptor has been used to investigate the consequences of subtotal ablation of podocytes in transgenic rats.12 In an alternative experimental approach, a receptor for a Pseudomonas toxin has been expressed specifically on podocytes.13 More recently, transgenic expression of a prokaryotic enzyme to toxify a pharmacological agent in a cell-specific fashion was used to create an alternative model for specific ablation of podocytes.14 These seminal studies consistently established that FSGS can be induced by specific ablation of podocytes.
In this study, we used a transgenic system to ablate PECs in an inducible fashion. For this purpose, a novel transgenic system was used in which expression of an attenuated DTA was irreversibly activated upon Cre recombination within the target cells.
Results
Inducible Expression of DTA in Parietal Cells
To ablate PECs in vivo, triple transgenic PEC-rtTA/LC1/ROSA26DTA176 mice5,15 were generated by intercrossing (Figure 1A). In these mice, Cre recombinase was expressed transiently upon administration of doxycycline. This activated irreversible expression of an attenuated DTA (ROSA26DTA176 transgene15) in PECs. The PEC-rtTA transgene is active predominantly within PECs, but also within the loop of Henle and the thick ascending limp (Figure 1B, as indicated with an X).5
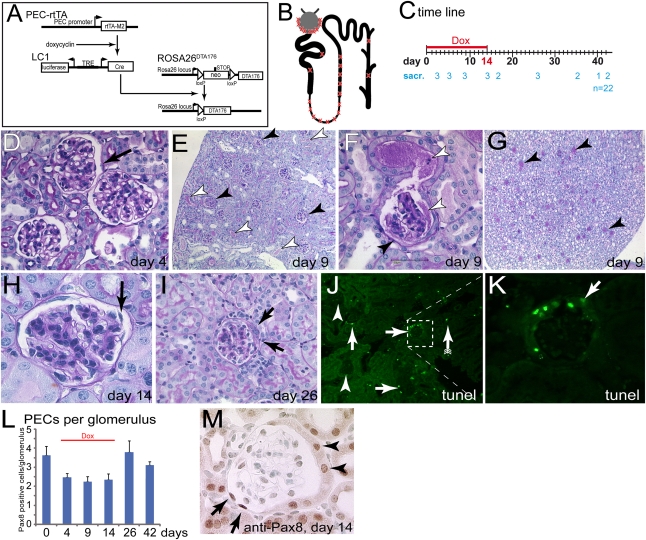
Figure 1.
Transgenic model for inducible ablation of PECs. (A) Schematic of the transgenes. The reverse tetracycline-inducible transactivator (PEC-rtTA) is expressed in PECs. Upon administration of doxycycline, Cre recombinase is transiently expressed from the second transgene (LC1). This activates expression of an attenuated DTA (DTA176). (B) Schematic of expected cellular ablation within the nephron. The transgenic PEC-rtTA is active in PECs, the loop of Henle, and the thick ascending limp (as indicated with an X). In addition, single scattered cells are targeted within the proximal tubule and the collecting duct. (C) Study timeline. Animals were treated with doxycycline for 14 days and killed at the indicated time points (numerals indicate the number of sacrificed animals per time point). (D through I) PAS-stained paraffin section of the kidney at different time points. (D) On day 4, no histomorphological abnormalities were observed in PECs (arrow) or elsewhere within the kidney (female). (E) On day 9, protein was observed within the Bowman’s space of a fraction of the glomeruli (black arrowheads). Protein-filled tubuli are marked by white arrowheads. (F) Protein has leaked into the primary urine (white arrowheads). Parts of the inner aspect of Bowman’s capsule are covered by fuzzy material (black arrowhead). (G) Within the renal papilla, a fraction of the tubules contain protein (arrowheads; mice in E through G are male). (H) On day 14, PECs appear partly detached (arrow, female). (I) Late time points are characterized by extracapillary proliferations (i.e., cellular crescents, arrows, 10-week-old female mice). (J) TUNEL assay 9 days after induction. A fraction of the glomeruli contain TUNEL-positive PECs (arrows), whereas other glomeruli do not show any TUNEL-positive cells (arrowheads). Scattered tubular TUNEL-positive cells could be observed (arrow with tails). (K) Higher magnification of inset in J. Cells lining the inner aspect of Bowman’s capsule contained DNA fragments. Negative control not shown. (L) Pax8-positive cells quantified per glomerulus at different time points (n=3 per time point, 50 glomeruli per mouse). Overall, approximately 30% of all PECs were ablated. (M) Representative image of a glomerulus. Pax8-positive oval nuclei of cells with a flat cytoplasm were counted as PECs. Tubular cells lining Bowman’s capsule were not counted as PECs (arrowheads, male). Dox, doxycycline; sacr, sacrificed.
To induce PEC ablation, mice received 2 mg/ml doxycycline within their drinking water for 14 days. Fifty percent Ringer solution was used as drinking water during induction to substitute potential salt losses due to tubular injury. Mice were sacrificed from day 4 up to 3 months after induction as indicated (Figure 1C).
The renal histology was examined at various time points in paraffin sections stained with periodic acid–Schiff (PAS) (Figure 1, D–I). DTA arrests translation within 3–4 days by inactivating polypeptide elongation factor 2.16,17 After 4 days of doxycycline administration, no significant abnormalities were observed (Figure 1D).
On day 9, pathologic changes were observed in approximately 30% of all glomeruli (Figure 1E, black arrowheads). As observed by light microscopy, affected glomeruli showed significant proteinuria within Bowman’s space and also within adjacent tubuli (Figure 1, E and F, white arrowheads). PECs appeared swollen or foamy or could no longer be distinguished (Figure 1F, black arrowhead). Within the renal papilla, a fraction of the tubules were filled with protein, confirming the focal nature of the pathologic findings described above (Figure 1G). On day 14, PECs appeared disorganized or even detached in approximately 30% of all glomeruli (Figure 1H, arrow). Protein accumulation in Bowman’s space and in tubuli was no longer visible. Later time points (days 14–42) were characterized by extracapillary proliferations in affected glomeruli (Figure 1I, arrows; see below).
To verify cellular ablation of PECs in an alternative approach, fragmentation of DNA, which is typically observed in DTA-treated cells, was visualized by terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) staining.16,17 A subpopulation of glomeruli stained TUNEL positive (Figure 1, J–L), indicating significant DNA fragmentation specifically within the nuclei of PECs (Figure 1K).
To investigate whether the total number of PECs was decreased after induction of PEC ablation, Pax8-positive nuclei per glomerulus were counted (Figure 1, L and M) (n=3 per time point, 50 glomeruli per mouse). Pax8 is a transcription factor expressed in parietal and tubular cells. These two cell types could be distinguished by light microscopy (tubular cells on Bowman’s capsule were not counted). Compared with controls (day 0), the total number of PECs was decreased by approximately 30% upon administration of doxycycline. Considering that approximately 30% of all glomeruli were affected, a significantly higher proportion of the PECs were ablated within the affected glomeruli (50%–90% ablation). After doxycycline administration was terminated on day 14, PEC numbers increased. The increase in the number of PECs per glomerulus was in part due to extracapillary proliferations (cellular crescents).
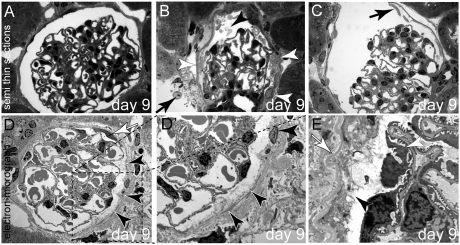
Ultrastructural analyses were performed on days 9, 14, and 26. On semi-thin sections, most glomeruli appeared unaffected (Figure 2A). However, in approximately 30% of the glomeruli, proteinuria and/or significant PEC abnormalities were observed. PECs appeared detached or fuzzy in part (Figure 2, B and C) and Bowman’s space contained debris. The glomerular tuft was normal. Transmission electron microscopy (TEM) showed that PECs were found to disintegrate within affected glomeruli (Figure 2D, black arrowheads). PECs had lost the apical plasma membrane; however, most of the organelles were still visible, including the nuclei. Cytosolic components were in direct contact with Bowman’s space (Figure 2, D′ and E, black arrowheads). The Bowman’s capsule (parietal basement membrane) appeared wrinkled (Figure 2E, white arrow).
Figure 2.
Ultrastructural analysis of subtotal PEC ablation. (A) Normal glomerulus 9 days after induction. (B) Cellular debris (black arrowhead) and protein within Bowman’s space on day 9. Remnants of PECs line Bowman’s capsule (white arrowheads), also within an adjacent glomerulus (arrow). (C) PECs have partially detached from Bowman’s capsule (arrow) (A through C, semi-thin sections). (D and D′) TEM images of a glomerulus 9 days after induction of PEC ablation. A viable PEC is marked by a white arrow. The remaining PECs are disintegrating (black arrowheads; D′ shows a higher magnification of the inset in D). (E) In this higher magnification, the apical plasma membrane of the PEC has disappeared. The cellular contents including the organelles are in direct contact with Bowman’s space (arrowhead). Bowman’s capsule (i.e., the PEC basement membrane) is wrinkled but still intact (white arrow, all animals aged 8 weeks; female mice are shown in A, C, and D, and male mice are shown in B and E).
PEC Ablation Is Associated with Proteinuria
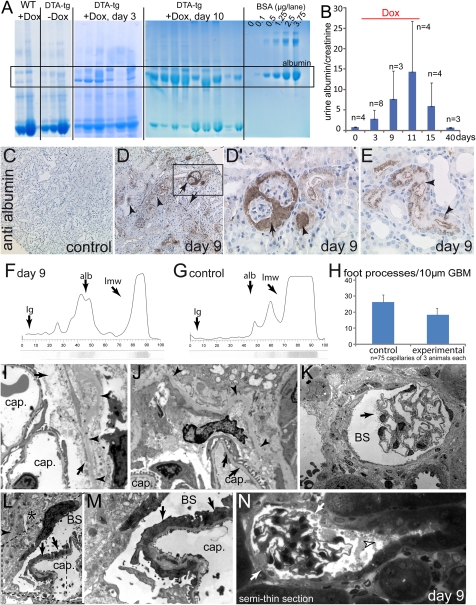
To investigate if ablation of PECs affected the integrity of the glomerular filtration barrier, urine from experimental animals was collected at different points and subjected to SDS-PAGE (Figure 3A). No albuminuria was observed in control animals (wild-type mice treated with doxycycline for 14 days [WT+Dox] or in triple transgenic DTA mice without doxycycline treatment [DTA-tg − Dox]). Moderate to significant albuminuria was observed 3 or 10 days after induction with doxycycline, respectively. To quantify albuminuria, albumin/creatinine ratios were measured within the urine at different time points (Figure 3B). Albuminuria increased during the 14 days of induction by administration of doxycycline. When doxycycline administration was discontinued, albuminuria reverted to normal levels. These results suggested a correlation between ablation of PECs and albuminuria.
Figure 3.
Ablation of PECs induces acute permeability defects of the glomerular filtration barrier. (A) Five microliters of urine from different experimental animals was run on SDS-PAGE and stained with Coomassie. Significant progressive albuminuria was observed exclusively in mice subjected to acute PEC ablation (see BSA standards for comparison). (B) The albumin/creatinine ratios were determined in 17-hour urine collections at the time points indicated. Progressive albuminuria was observed during the PEC ablation period (doxycycline administration), which was fully reversible when doxycycline was discontinued. (C–E) Immunohistologic staining for mouse albumin (hematoxylin counterstain) in control and experimental animals on day 9 (male). (D and D′) Significant staining was observed in Bowman’s space and associated tubuli (arrows). Significantly more albumin reabsorption droplets were detected in other tubules (arrowheads). (F and G) SDS-PAGE with subsequent silver stain (lower part) and densitometric analysis (graph) of a proteinuric animal (day 9) and a healthy control animal. The expected position of Ig, albumin (alb), and low molecular weight proteins (lmw, which are abundant in mice) are indicated. (H) The number of foot processes was evaluated in 25 capillary circumferences per length of GBM of three experimental or control animals each (n=75). (I and J) Normal foot processes on capillary loops (cap) within the immediate vicinity of disintegrating PECs (arrows). The PEC basement membrane is wrinkled but intact (arrowheads). (K) Focal microvillous transformation of a single podocyte (arrow) projecting into Bowman’s space (BS). (L and M) Focal effacement of podocyte foot processes (arrows) on a segment of a capillary loop. An electron-dense viable PEC can be seen extending onto the cellular debris (asterisk). The intact Bowman’s capsule is marked by arrowheads (G shows a higher magnification of the inset in F). (H) Representative example of semi-thin serial sections of an affected glomerulus (see Supplemental Figure 2 for the entire series). Bowman’s space is filled with cellular debris (arrowhead); however, the tubular outflow is not blocked. PECs are partially disintegrated (arrows) (mice in I–N are male).
PECs do not participate in the formation of the glomerular filtration barrier; therefore, an effort was undertaken to resolve the nature of the injury in more detail. To verify that the proteinuria originated from the glomerulus, mouse albumin was visualized on immunohistologic staining (Figure 3, C–E). As observed in our PAS stainings, albumin was present within Bowman’s space in a subfraction of the glomeruli and within the lumen of the associated tubules (Figure 3, D and D′, arrowhead). Significant albumin resorption droplets were observed in other tubules, indicating that tubular protein uptake was preserved. Similarly, megalin staining was preserved throughout the tubular system on day 9 (Supplemental Figure 1, A–D). On Coomassie stainings, the loss of protein into the urine seemed to be predominantly restricted to albumin (“selective albuminuria”) (Figure 3A). To confirm this finding, a diagnostic urinary electrophoresis was performed with a subsequent silver staining (representative examples in Figure 3, F and G).18 Indeed, no significant high-molecular proteins (e.g., Ig) were detected in proteinuric samples (Figure 3F) (day 9), confirming that the proteinuria was selective. In an effort to identify the most likely cause for selective glomerular proteinuria, the extent of focal foot process effacement was quantified by electron microscopy. The number of foot processes per 10-μm glomerular basement membrane (GBM) was counted in a total of 25 capillary circumferences of three experimental (day 9) or three control animals (Figure 3H and Supplemental Figure 1, E–H). Indeed, focal foot process effacement was observed in the experimental animals. As shown in the exemplary TEM images, focal podocyte foot process effacement was the major pathologic finding of the glomerular filtration barrier, which was otherwise normal (Figure 3, I–M). Localized changes of podocytes were also observed, such as microvillous transformation (Figure 3K, arrow).
To exclude acute obstruction of the tubular outflow as the underlying cause for albuminuria, serial semi-thin sections were evaluated after 9 days of doxycycline administration. As described above, cellular debris was observed within the Bowman’s space of the affected glomeruli (Figure 3N and Supplemental Figure 2). The tubular orifice remained open in all of the evaluated glomeruli. Within the tubular system, ablation of tubular cells was observed particularly within the thick ascending limp. Consistently, drinking volume per day was increased in the experimental mice, indicating a concentration defect (Supplemental Figure 3). Tubular lumina contained cellular debris during the induction period with doxycycline. However, this was accompanied by a rapid proliferative response and restitution of the normal architecture (Supplemental Figure 4). At early time points, proximal tubular cells did not show signs of degeneration as it is typically observed as a result of a disconnection of the glomerulus from its tubule.19 At later time points, segmental areas of interstitial fibrosis were observed. For this reason and as outlined below, these were interpreted not as the direct result of tubular necrosis but as secondary degeneration due to glomerular crescent formation.
To confirm the patency of the tubular system in an alternative experimental approach, mice were injected with gold-labeled BSA after 4 or 9 days of doxycycline administration. Mice were killed 15 minutes after the injection. From these kidneys, Epon-embedded thick sections were prepared to examine the gold-labeled BSA distribution within the tissue. On day 4, low amounts of the tracer had passed into the primary urine in all glomeruli, similarly to control animals (Figure 4, A and B). Gold-labeled BSA was reabsorbed within the proximal part of proximal tubules resulting in a brown staining (arrows). On day 9, gold-labeled BSA tracer was reabsorbed in all proximal tubules throughout the renal cortex (Figure 4C). Gold-labeled BSA tracer was also observed within the lumen of the more distal parts of the tubular system (Figure 4C, white arrows). As a positive control, transgenic Thy1.1 mice20 were injected with gold-labeled BSA 1 day after induction of proteinuria with Thy1.1 antiserum (Figure 4D). On thick sections, gold-labeled BSA tubuli (black arrows) were observed next to tubules without any labeling (white arrows), indicating tubular obstruction by protein casts. These findings were confirmed on semi-thin sections (Figure 4, E and F). In controls or experimental DTA-transgenic animals, the capillary lumen stained yellow, indicating that gold-labeled BSA was still present within the circulation (black arrowhead). Reabsorbed gold-labeled BSA tracer was observed within proximal tubuli as a brown color (white arrows). In contrast, gold-labeled BSA tubuli (white arrows) were located next to unlabeled tubuli in Thy1.1 transgenic mice (Figure 4F, black arrows). Lumina of unlabeled tubuli appeared widened and protein casts were observed blocking the tubular lumen entirely. Overall, most tubular cells contained multiple protein resorption droplets. From these data, it was concluded that tubular obstruction did not occur in the triple transgenic PEC-rtTA/LC1/ROSA26DTA176 mice 4 or 9 days after induction with doxycycline.
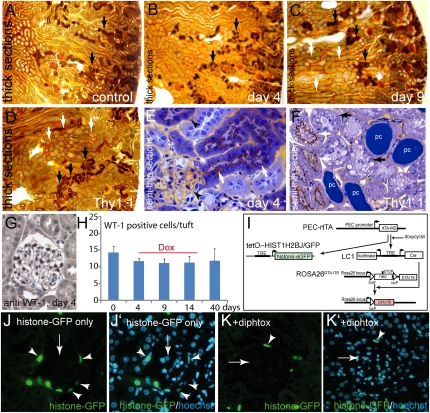
Figure 4.
No evidence for tubular obstruction or ectopic cell ablation in the glomerulus. (A–F) To test if tubular obstruction occurs as a result of PEC ablation, experimental animals were injected with gold-labeled BSA 15 minutes before sacrifice (A–D, 9-µm thick sections). In control animals, gold-labeled BSA reabsorption occurred in the proximal part of proximal tubules throughout the entire cortex (A, arrows). (B) The same was true for experimental animals 4 days after induction with doxycycline. (C) On day 9, gold-labeled BSA was observed as a brown color in proximal tubules throughout the entire cortex similar to controls (black arrows). Because of proteinuria, gold-labeled BSA was also observed as a gray color in other parts of the tubular system (white arrows). (D) As a positive control for focal tubular obstruction by protein casts, a Thy1.1 transgenic mouse is shown 1 day after injection of Thy1.1 antiserum, which induces nephrotic-range proteinuria within minutes. Reabsorption of gold-labeled BSA can only be seen in some proximal tubuli (black arrow), whereas other tubuli are unstained (white arrows). (E) On semi-thin sections, gold-labeled BSA was still present within the capillary lumen (arrowheads) and reabsorbed in proximal tubuli (arrows). (F) In the Thy1.1 transgenic mouse with tubular obstruction, gold-labeled BSA–containing tubules (white arrow) were found next to protein-filled tubules without any gold-labeled BSA but instead with signs of cellular degeneration (black arrow). Some tubular lumina were blocked by protein casts (pc). All animals in A–F were 16-week-old male mice. (G and H) Absolute podocyte number per glomerular cross-section (n=150 in three animals of each time point as indicated; G, female). (I) To test whether leaky expression of the PEC-rtTA transgene occurs, an additional transgene tetO7-HIST1H2BJ/GFP was introduced that expresses histone-eGFP in a doxycycline-inducible fashion. (J and J′) When doxycycline was administered in triple transgenic mice that only lacked the ROSADTA176 transgene (suicide transgene), histone-eGFP was expressed specifically in almost all PECs (arrowheads) and no trace was observed elsewhere in the glomerulus (arrow). (K and K′) When doxycycline was administered in quadruple transgenic mice, histone-eGFP labeled PECs were observed much less frequently (arrowhead, 5 days after induction). No labeling of other glomerular cells, specifically of podocytes was observed (mice in J–K′ are male).
Finally, an effort was undertaken to test the possibility that the PEC-rtTA transgene might also mediate ectopic cell ablation in podocytes. First, the total number of WT-1–positive cells on the glomerular tuft (presumptive podocytes) was evaluated in three animals of each time point (50 glomeruli per animal) (Figure 4, G and H). Surprisingly, a slight reduction in podocytes was noted 4 days after induction of the model. To test if the PEC-rtTA transgene might be active not only in PECs but also in podocytes, the tetO7-HIST1H2BJ/GFP transgene21 was added to our triple transgenic mice by intercrossing. This transgene expresses histone coupled to enhanced green fluorescent protein (histone-eGFP) upon administration of doxycycline (Figure 4I). Histone-eGFP accumulates within the nucleus, where it persists due to its long half-life. This system is capable of detecting even very low background activity because the gene product accumulates over time and expression can be upregulated in a linear fashion (not “on” or “off” as is the case for the ROSA26DTA176 transgene). When inducing PEC-rtTA/LC1/tetO7-HIST1H2BJ/GFP, which lacks ROSA26DTA176, histone-eGFP was accumulated within the nucleus virtually of every PEC (Figure 4, J and J′). No cell within the glomerulus showed even traces of histone-eGFP. Some tubular epithelial cells stained positive, as described above. When inducing PEC-rtTA/LC1/ROSA26DTA176/tetO7-HIST1H2BJ/GFP for 2 or 5 days, still no trace of histone-eGFP could be detected within podocytes or any other glomerular cell, other than remaining PECs. Thus, no ectopic activity of the PEC-rtTA transgene was observed in podocytes in this model.
Activation of PECs after Subtotal Ablation
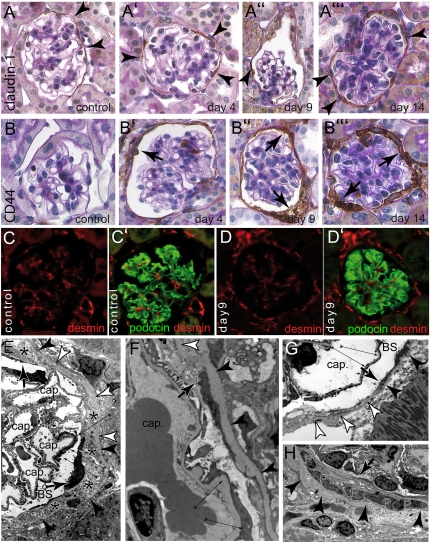
To analyze the remaining PECs after subtotal ablation in more detail, immunohistologic stainings were performed. Staining for the PEC marker claudin-1 showed that claudin-1–positive PECs could still be identified in most glomeruli at all time points (Figure 5, A through A′′′). A de novo expression of CD44, a marker for activated PECs, was observed in >50% of the glomeruli after induction of PEC ablation (Figure 5, B through B′′′). There was no specific anatomic preference relative to the vascular pole for the occurrence of activated PECs. CD44 expression increased in intensity over time, indicating progressive activation of PECs in this model. No CD44 was expressed in PECs of mice that had not received doxycycline or that did not carry the ROSA26DTA176 transgene (Figure 5B, control). These findings were confirmed with immunofluorescence (Supplemental Figure 5).
Figure 5.
Activation of PECs after subtotal ablation. Immunohistologic stainings for PEC marker claudin-1 (A–A′′′) or PEC activation marker CD44 (B–B′′′) combined with PAS counterstainings were performed at different time points as indicated. In controls, PECs stained only for the PEC marker claudin-1, confirming that PECs are not activated under physiologic conditions (A and B, arrowheads). Upon induction of PEC ablation, PECs (arrowheads) expressed CD44 progressively (arrows) (mice in A′, A′′, B, and B′ are female; mice in A, A′′′, B′′, and B′′′ are male). (C and D) To analyze podocytes, double stainings for podocyte-marker podocin (green) and podocyte activation marker desmin (red) were performed in control animals and 9 days after induction. In both groups, a similar expression pattern was observed. Podocytes stained positive for podocin. Desmin was expressed predominantly within the mesangium but also in endothelial cells (female). (E) Viable electron-dense PECs (arrows) extended long thin processes across areas of Bowman’s capsule covered by cellular debris (asterisks). In addition, focal podocyte foot process effacement can be seen (thin arrows). Bowman’s capsule remains intact (arrowheads) even in areas with no viable PECs (white arrowheads). (F and G) Higher magnification of a cellular extension of a PEC (arrow points to the tip of the extension, white arrowhead point to the denuded area of Bowman’s capsule). Thin arrows point to focal podocyte foot process effacement (cap, capillary lumen; BS, Bowman’s space). (H) An accumulation of PECs (P) is shown within cellular debris. Arrowheads point to Bowman’s capsule. A cellular extension is seen emerging from the right lining the inner aspect of Bowman’s capsule and forming a multilayered crescent (arrow).
To test for podocyte activation or dedifferentiation, immunofluorescence for the marker proteins desmin and podocin were performed (Figure 5, C–D′). In control mice, podocytes did not express desmin, a marker for podocyte activation.22 A robust expression of podocin, a marker for fully differentiated podocytes, was observed (Figure 5, C and C′). Nine days after induction of PEC ablation, no change of this staining pattern was observed in podocytes, indicating that podocytes were not significantly affected at least as judged by expression of these two markers (Figure 5, D and D′). These results were consistent with our ultrastructural analyses (see above).
Next, the ultrastructure of the remaining PECs was evaluated after induction of DTA PEC ablation. Viable PECs were observed as electron-dense cells on the inner aspect of Bowman’s capsule (Figure 5E). These PECs were residing either directly on the parietal basement membrane (i.e., Bowman’s capsule) or directly on top of cellular debris of former PECs (Figure 5E, asterisks). Remaining viable PECs extended thin cellular projections covering the cellular debris with a novel basement membrane (Figure 5, E–H, arrows). PECs were also identified in dense accumulations and/or in several layers (i.e., the equivalent of early cellular crescents) (Figure 5H). In summary, these observations suggest that PECs are activated to spread and cover the denuded inner aspect of Bowman’s capsule.
Activated PECs Proliferate and Form Typical Cellular Crescents
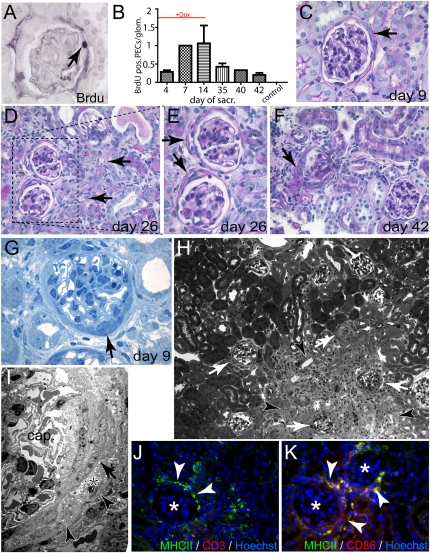
To test if PECs engage in cellular proliferation after subtotal ablation, experimental animals received bromodeoxyuridine (BrdU) injection 1 day before sacrifice at different time points. Proliferation activity of PECs was evaluated on histologic sections stained for BrdU (Figure 6, A and B). A significant increase of BrdU-positive PECs was observed after induction of the model. After administration of doxycycline was discontinued, proliferation of PECs decreased but remained elevated until the end of the observation period. Sustained proliferation of PECs resulted in the formation of cellular crescents (i.e., multilayered extracapillary cellular proliferates within Bowman’s space) (Figure 5, C–F). At earlier time points (day 9) (Figure 6C), glomeruli with cellular crescents were observed amid healthy looking tubuli. At later time points (day 26–40), affected glomeruli were associated predominantly with areas of degenerating tubules and focal areas of interstitial fibrosis (Figure 6, D–F), suggesting that tubular degeneration occurred as a result of glomerular crescent formation and subsequent blockage of the tubular outflow of the primary urine.19 Focal degeneration of nephrons and interstitial fibrosis did not significantly impair renal function, as determined by serum creatinine or urea levels (not shown).
Figure 6.
Proliferation of PECs results in formation of cellular crescents. (A and B) To determine if PECs engage in cellular proliferation, the number of BrdU-positive PECs per glomerulus was determined using immunohistochemistry at the indicated time points (A, arrow indicates a BrdU-positive PEC, female). Proliferation of PECs increased progressively during the administration of doxycycline and remained elevated even after 40 days. (C–F) PAS-stained paraffin sections at different time points. At early time points, cellular crescents can be seen in glomeruli without significant tubular damage (C, arrow). At later time points, glomeruli with cellular crescents are found next to or within areas of tubular degeneration (D, arrows; E shows a higher magnification of inset in D). Cellular crescents show the typical morphology of multilayered extracapillary proliferations (E and F, arrows; mice in C through F are female). (G) Semi-thin section of a cellular crescent (arrow) with the typical morphology. No capillary necrosis could be seen (male). (H) Semi-thin section of an area with significant tubular degeneration (arrowheads) and associated glomeruli with cellular crescents (white arrows). (I) TEM of a cellular crescent with multiple layers of PECs (arrow), cellular debris (asterisk), and an intact Bowman’s capsule (arrowheads). The capillary tuft (cap.) shows normal capillary loops. (J and K) Glomeruli with extracapillary proliferations (asterisk) recruited a typical periglomerular infiltrate of MHCII-positive, CD3-negative, CD86-positive cells, representing activated dendritic cells (arrowheads).
Formation of cellular crescents was corroborated on semi-thin sections. Typical multilayered extracellular proliferations (i.e., cellular crescents) could be observed as early as 9 days after induction of subtotal ablation of PECs (Figure 6G). Again, glomeruli containing cellular proliferations were localized next to or within areas of degenerating tubules (Figure 6H, arrowheads). TEM showed that the multilayered structures contained layers of PECs (Figure 6I, arrow) and cellular debris (asterisk). Bowman’s capsule was not breached (arrowheads). Similarly, we did not observe a break of the GBM (not shown). Glomeruli, which were affected by extracapillary proliferations, recruited a typical periglomerular infiltrate. This infiltrate consisted primarily of MHCII, CD86, CD11b-positive activated dendritic cells, and/or macrophages (Figure 6, J and K arrowheads). CD3-positive T cells were not recruited.
An effort was undertaken to investigate why activation of PECs did not result in formation of segmental sclerotic lesions after subtotal ablation (i.e., secondary FSGS). Adhesions between Bowman’s capsule and the glomerular tuft have been described as the “crucial point of no return,” (ref. 23, p 1368) which serves as an entry site for activated PECs to migrate and destroy segments of the glomerular tuft.4,23 TEM was used to screen experimental animals screened for adhesions 9 or 14 days after induction of subtotal PEC ablation (Supplemental Figure 6). No case of an adhesion between activated PECs on Bowman’s capsule and podocytes on the glomerular tuft was observed.
Discussion
In this study, four major observations were made. First, our results document that the transactivator mouse line PEC-rtTA can be used to ablate specific cell populations in an inducible fashion. The promoter of the PEC-rtTA mouse was derived from the podocalyxin gene (a hybrid of the rabbit and human PODXL1 gene). The endogenous PODXL1 gene is active predominantly in podocytes, whereas the hybrid promoter fragment (PEC promoter) is active only in PECs and not in podocytes. In this study, we ruled out ectopic activity of the PEC-rtTA transgenic mouse line, using a very sensitive approach of the accumulating histone-eGFP reporter transgene. Within the kidney, cellular ablation occurred also within the tubular system, as described previously.5 However, these changes were fully reversible and did not result in tubular obstruction. Mice were viable throughout the induction of cellular ablation consistent with our initial characterization of the PEC-rtTA mouse line.5 This finding argues against the possibility that the PEC-rtTA mouse line mediates significant transcriptional activity within extrarenal tissues.
These results further support that the PEC promoter is a valuable tool because no endogenous gene product has thus far been identified that targets PECs with similar specificity. Interestingly, a significant pathology was observed only in approximately 30% of all glomeruli after induction of cellular ablation. Two explanations might account for this finding. Mosaic expression by the PEC-rtTA mouse has already described in our previous work.3–5 First, it is possible that either ablation of only a few PECs in a single glomerulus is easily handled or repaired by the remaining PECs or that it was missed by our analysis because it was not sufficient to induce proteinuria. Second, ablation of PECs might have increased transcriptional activity of the PEC-rtTA mouse in the neighboring PECs and thus might have further increased PEC ablation only in the affected glomeruli.
The second major finding was that acute ablation of PECs was associated with albuminuria, (i.e., a partial loss of the integrity of the glomerular filtration barrier). PECs are not a direct component of the glomerular filtration barrier and this is the first observation in which a specific insult to a cell population outside the filter impairs the integrity of the glomerular filtration barrier. In this study, we provide definite immunohistologic proof that albuminuria originated from the glomerulus. Proteinuria was selective (i.e., it consisted predominantly of albumin, a characteristic feature for a minor loss of the selectivity). No evidence for tubular obstruction was observed as a possible reason for any of the glomerular pathologic changes. In addition, a tubular reabsorption defect of albumin was ruled out. Our major observation was focal foot process effacement, which represents the most likely cause for transient proteinuria. This is also consistent with the observation that proteinuria was transient. As soon as doxycycline administration and ablation of PECs were discontinued, proteinuria was fully reversible. This suggests that disintegrating PECs generated a signal that resulted in an injury to the glomerular filter. Activation and proliferation of PECs persisted beyond the induction of the model but were not associated with proteinuria, arguing against a specific signal released from activated PECs to the glomerular filtration barrier. On the other hand, a danger signal could be transmitted via cellular components released from the disintegrating PECs (nucleic acids, ATP, uric acid, etc.). These components then bind and activate specific receptors that are known to be expressed on podocytes, such as Toll-like receptor 2 or potentially the inflammasome.24
The third major finding of this study was that subtotal ablation of PECs resulted in activation of the remaining PECs, which expressed the CD44 activation marker de novo and proliferated. The transgenic system to ablate PECs with an attenuated mutant of the A chain of DTA is highly specific and cannot be transmitted from one cell to the next, even after lysis of the affected cell.15,17 Because expression of the transgene was activated by irreversible Cre recombination, cellular ablation of PECs occurred in a “yes” or “no” pattern. Therefore, activation of PECs was not a direct result of a minor insult by transgenic cell ablation system. Nevertheless, we observed a slight decrease of the absolute podocyte number during the first 4 days of induction. We propose that this is most likely a consequence of the ablation of “transitional cells,” which are localized along the efferent arteriole at the interface between the PECs and fully differentiated podocytes.5 Transitional cells express PEC and podocytes marker proteins (e.g., WT-1) simultaneously, and the PEC-rtTA transgenic mouse line also mediates expression in these cells.5
Apart from the mechanisms described above, activation of PECs might have occurred as a physiologic reaction to regenerate an acute glomerular injury. In previous work, it has been proposed that PECs might regenerate not only themselves by cellular proliferation25 but might also regenerate tubular cells7 and podocytes.5,6 In this study, we found that the remaining PECs formed cellular extensions to cover the cellular debris and/or the denuded areas of the Bowman’s capsule. Therefore, activation of PECs is potentially part of the normal regenerative process, at least to repair injuries inflicted upon PECs themselves.
The forth major finding of this study is that subtotal ablation of PECs ultimately resulted in the formation of typical cellular crescents as seen in RPGN. Interestingly, cellular crescents were not formed as a result of an acute inflammatory process in our model. Cellular crescents without an immunologic pathogenesis have been observed previously in a mouse model with podocyte-specific inactivation of Vhlh2 or with overexpression of platelet-derived growth factor.26 It is noteworthy that we did not observe a single cellular adhesion between the capillary tuft and the cellular crescents (extracapillary proliferations). This observation strongly supports the notion that these cellular crescents formed without the participation of activated podocytes. In our previous work, we analyzed the cellular composition of the periglomerular infiltrate, which is normally observed only next to typical cellular crescents.27 In this study, cellular crescents also recruited a typical periglomerular infiltrate, which consisted primarily of activated dendritic cells. From these findings, we concluded that subtotal ablation of PECs can induce typical cellular crescents.
As shown in our recent work, activation of PECs may occur in at least two different major pathologic entities: in FSGS or in the formation of cellular crescents (e.g., in RPGN).3,4 In this context, we will reexamine previous studies on the consequences of transgenic ablation of podocytes.12–14 In these studies, the loss of podocytes progressed over time even after transgenic depletion of podocytes was terminated,28 ultimately resulting in the formation of classic sclerotic lesions (FSGS). Therefore, we hypothesize that PECs were also activated as a result of transgenic ablation of podocytes. Activated PECs invaded the glomerular tuft via an adhesion in which they deposited matrix and displaced the remaining podocytes, causing progressive loss of podocytes.28 We recently described the pathomechanism for the formation of sclerotic lesions that is common to all sclerotic lesions.4
Remarkably, in this model of PEC ablation, activation of PECs did not result in FSGS-like lesions. A possible explanation for this finding is that cellular adhesions between Bowman’s capsule and the glomerular tuft did not form as a prerequisite for the formation of a segmental sclerotic lesion. These findings support the notion that an additional injury to the podocyte may be required for the formation of an adhesion, such as an acute loss of podocytes and/or a partially denuded GBM.12,13,23
Concise Methods
Transgenic Mice
ROSADTA176 mice15 were kindly provided by Dr. M.R. Capecchi (University of Utah, Salt Lake City, UT). Mice were housed in the facility of the Aachen University Hospital under specific pathogen free conditions. Animals received regular food and water ad libitum. All animal studies were approved by Landesamt für Natur, Umwelt und Verbraucherschutz NRW Cologne (#8.87-51.04.20.09.314). Unless indicated otherwise, all mice were 8 weeks old when included in this study.
PEC-rtTA/LC1/ROSADTA176 mice received doxycycline hydrochloride via the drinking water ad libitum for 14 days (5% sucrose and 1 mg/ml of doxycycline per ml in 50% Ringer solution, protected from light), which was exchanged every 2 days.
Animal Experiments
One day before sacrifice, mice were injected twice with 200 µl BrdU (Sigma Aldrich, St. Louis, MO) at 4-hour intervals. Mice were anesthetized with ketamine/rompun and perfused with 3% paraformaldehyde in PBS (pH 7.4) via the left ventricle for 3 minutes. Pieces of both kidneys were snap frozen in Tissue-Tek (Miles Inc, Iowa City, IA), postfixed in 3% buffered formalin, and embedded in paraffin. For TEM, 2×2-mm pieces of the kidneys were postfixed in Karnovsky fixative, processed, and embedded in Epon using standard procedures.29 Semi-thin serial sections 1 µm in thickness were cut from these blocks, stained with methylene blue, and observed by light microscopy. Ultrathin sections were cut with a diamond knife, stained with lead citrate and uranyl acetate, and examined.
Gold-Labeled BSA
Mice were injected intravenously with 700 µl gold-labeled BSA (BSA Tracer Ultra Small, without azide, LOT 00913/1; Aurion, Wageningen, Netherlands). Fifteen minutes after injection, animals were sacrificed and kidneys were cut and fixed in 2.5% glutaraldehyde for 48 hours, washed in PBS for 30 minutes, and incubated in 2.3 M sucrose solution for 1 hour. Finally, tissues were snap frozen in liquid nitrogen and embedded in Epon. Thick sections and semi-thin sections (stained with methylene blue) were examined by light microscopy.
Immunofluorescence and immunohistochemical stainings were performed on kidneys fixed in 4% buffered formaldehyde for 24 hours and embedded in paraffin. For the single and double staining, 2- to 4-µm sections were rehydrated in PBS and subjected to microwave heating (3×5 minutes at 600W with antigen unmasking solution; Vector Laboratories, Burlingame, CA). Sections were incubated with the following primary antibodies: rabbit anti-claudin-1 (1:50; Abcam, Cambridge, UK), rat anti-CD44 (1:100; BD Biosciences Pharmingen, Mannheim, Germany), rabbit anti-THP and anti-WT-1, guinea pig anti-megalin (1:100, 1:50, 1:250; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-podocin (1:100; Sigma Aldrich, St. Louis, MO), mouse anti-desmin (1:500; Dako A/S, Glostrup, Denmark), horseradish peroxidase conjugated mouse anti-albumin (1:1000; Bethyl Laboratories Inc, Montgomery, TX), and Fluorescein Lotus Tetragonolobus Lectin (1:100; Vector Laboratories). The following secondary antibodies were used: DyeLight 488 anti-rabbit, DyeLight 549 anti-rat, DyeLight 549 anti-rabbit, and DyeLight 488 anti-mouse (1:200; Dianova Immundiagnostik, Hamburg, Germany) and biotinylated goat anti-guinea pig and horse anti-rabbit (1:250, 1:300; Vector Laboratories). For TUNEL staining, the Apoptosis Detection Kit S7111 was used (Chemicon International Inc, Temecula, CA). The following primary antibodies were used for immunofluorescence: biotinylated anti-CD86 (clone GL1; eBioscience, Frankfurt, Germany), anti-CD3e (clone 145-2C11; BD Biosciences Pharmingen), and anti-I-Ab (clone 25-9-17; BioLegend, Germany). The Vectastain ABC kit (Vector Laboratories) and Streptavidin Alexa 488 (Molecular Probes, Germany) or Streptavidin Alexa 568 (Invitrogen, Germany) was used to visualize the biotinylated antibodies. Slides were counterstained with Hoechst 33258 (Molecular Probes, Germany) to stain nuclei. Sections were evaluated with a Leica DMRX microscope. Images were collected with the Diskus software program (Diskus Kameras, Koenigswinter, Germany) and prepared for presentation with Adobe Photoshop CS5 and Illustrator CS5 software (Adobe Systems, Mountain View, CA).
For BrdU staining before the immunostaining, the sections were incubated in 2N HCl solution at 37°C for 30 minutes and subsequently neutralized in borate buffer. The mouse anti-BrdU (1:200; Millipore Corp, Billerica, MA) was used as primary antibody. For Pax8 staining, the goat anti-Pax8 (1:100; Abcam) was used. Biotinylated horse anti-mouse and biotinylated horse anti-goat (1:300; Vector Laboratories) were used as secondary antibodies.
For detection of albuminuria, urine collected in metabolic cages for 17 hours was subjected to SDS-PAGE (NuPage 4%–12% Bis-Tris Gel; Invitrogen, Carlsbad, CA) and stained using Coomassie SimplyBlue SafeStain (Invitrogen). For quantification, an albumin ELISA for urine was used (mouse albumin ELISA Quantitation Set; Bethyl Laboratories). Urine electrophoresis and densitometric analysis was performed in a diagnostic laboratory (Interneph, Aachen, Germany), as described previously.18
Disclosures
None.
Acknowledgments
This work was supported by the German Research Foundation (DFG) (TP17 SFB/Transregio 57 to J.F. and M.J.M.), the German Ministry for Science and Education (01 GN 0804 to M.J.M.), the Interdisciplinary Center for Clinical Research “BIOMAT” within the Faculty of Medicine at the Aachen University of Technology (RWTH-Aachen) (to. M.J.M.), the Genzyme Renal Innovation Program (to B.S.), the Netherlands Organization for Scientific Research (2007/09196/ALW to B.S.), and the NephCure Foundation (F001 to B.S. and M.J.M.).
C.K., T.O., J.F., and M.J.M. are members of the SFB/Transregio 57 DFG consortium on Mechanisms of Organ Fibrosis.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011050449/-/DCSupplemental.
References
- 1.Moeller MJ, Soofi A, Hartmann I, Le Hir M, Wiggins R, Kriz W, Holzman LB: Podocytes populate cellular crescents in a murine model of inflammatory glomerulonephritis. J Am Soc Nephrol 15: 61–67, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Ding M, Cui S, Li C, Jothy S, Haase V, Steer BM, Marsden PA, Pippin J, Shankland S, Rastaldi MP, Cohen CD, Kretzler M, Quaggin SE: Loss of the tumor suppressor Vhlh leads to upregulation of Cxcr4 and rapidly progressive glomerulonephritis in mice. Nat Med 12: 1081–1087, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Smeets B, Uhlig S, Fuss A, Mooren F, Wetzels JF, Floege J, Moeller MJ: Tracing the origin of glomerular extracapillary lesions from parietal epithelial cells. J Am Soc Nephrol 20: 2604–2615, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smeets B, Kuppe C, Sicking EM, Fuss A, Jirak P, van Kuppelvelt TH, Endlich KH, Wetzels JF, Grone HJ, Floege J, Moeller MJ: Parietal epithelial cells participate in the formation of sclerotic lesions in focal and segmental glomerulosclerosis (FSGS). J Am Soc Nephrol 22: 1262-1274, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, Frye B, Elger M, Kriz W, Floege J, Moeller MJ: Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol 20: 333–343, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Maggi E, Serio M, Vannelli GB, Lasagni L, Romagnani S, Romagnani P: Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol 20: 322–332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lazzeri E, Crescioli C, Ronconi E, Mazzinghi B, Sagrinati C, Netti GS, Angelotti ML, Parente E, Ballerini L, Cosmi L, Maggi L, Gesualdo L, Rotondi M, Annunziato F, Maggi E, Lasagni L, Serio M, Romagnani S, Vannelli GB, Romagnani P: Regenerative potential of embryonic renal multipotent progenitors in acute renal failure. J Am Soc Nephrol 18: 3128–3138, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto T, Wilson CB: Quantitative and qualitative studies of antibody-induced mesangial cell damage in the rat. Kidney Int 32: 514–525, 1987 [DOI] [PubMed] [Google Scholar]
- 9.Clarke MC, Figg N, Maguire JJ, Davenport AP, Goddard M, Littlewood TD, Bennett MR: Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med 12: 1075–1080, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Miyake Y, Kaise H, Isono K, Koseki H, Kohno K, Tanaka M: Protective role of macrophages in noninflammatory lung injury caused by selective ablation of alveolar epithelial type II cells. J Immunol 178: 5001–5009, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, Hamann A, Wagner H, Huehn J, Sparwasser T: Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med 204: 57–63, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC: Podocyte depletion causes glomerulosclerosis: Diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Matsusaka T, Xin J, Niwa S, Kobayashi K, Akatsuka A, Hashizume H, Wang QC, Pastan I, Fogo AB, Ichikawa I: Genetic engineering of glomerular sclerosis in the mouse via control of onset and severity of podocyte-specific injury. J Am Soc Nephrol 16: 1013–1023, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Macary G, Rossert J, Bruneval P, Mandet C, Bélair MF, Houillier P, Duong Van Huyen JP: Transgenic mice expressing nitroreductase gene under the control of the podocin promoter: A new murine model of inductible glomerular injury. Virchows Arch 456: 325–337, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Wu S, Wu Y, Capecchi MR: Motoneurons and oligodendrocytes are sequentially generated from neural stem cells but do not appear to share common lineage-restricted progenitors in vivo. Development 133: 581–590, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Honjo T, Nishizuka Y, Hayaishi O: Diphtheria toxin-dependent adenosine diphosphate ribosylation of aminoacyl transferase II and inhibition of protein synthesis. J Biol Chem 243: 3553–3555, 1968 [PubMed] [Google Scholar]
- 17.Mitamura T, Higashiyama S, Taniguchi N, Klagsbrun M, Mekada E: Diphtheria toxin binds to the epidermal growth factor (EGF)-like domain of human heparin-binding EGF-like growth factor/diphtheria toxin receptor and inhibits specifically its mitogenic activity. J Biol Chem 270: 1015–1019, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Beckers G, Mann H, Melzer H, Bemelmans B, Jakse G, Rohrmann D: Urinary sodium dodecyl sulfate electrophoresis with silver staining: A noninvasive diagnostic tool for obstructive uropathy in children. J Urol 179: 703–707, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Le Hir M, Besse-Eschmann V: A novel mechanism of nephron loss in a murine model of crescentic glomerulonephritis. Kidney Int 63: 591–599, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Kollias G, Evans DJ, Ritter M, Beech J, Morris R, Grosveld F: Ectopic expression of Thy-1 in the kidneys of transgenic mice induces functional and proliferative abnormalities. Cell 51: 21–31, 1987 [DOI] [PubMed] [Google Scholar]
- 21.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E: Defining the epithelial stem cell niche in skin. Science 303: 359–363, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Floege J, Alpers CE, Sage EH, Pritzl P, Gordon K, Johnson RJ, Couser WG: Markers of complement-dependent and complement-independent glomerular visceral epithelial cell injury in vivo. Expression of antiadhesive proteins and cytoskeletal changes. Lab Invest 67: 486–497, 1992 [PubMed] [Google Scholar]
- 23.Elger M, Kriz W: Podocytes and the development of segmental glomerulosclerosis. Nephrol Dial Transplant 13: 1368–1373, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Anders HJ, Muruve DA: The inflammasomes in kidney disease. J Am Soc Nephrol 22: 1007–1018, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Pabst R, Sterzel RB: Cell renewal of glomerular cell types in normal rats. An autoradiographic analysis. Kidney Int 24: 626–631, 1983 [DOI] [PubMed] [Google Scholar]
- 26.van Roeyen CR, Eitner F, Boor P, Moeller MJ, Raffetseder U, Hanssen L, Bücher E, Villa L, Banas MC, Hudkins KL, Alpers CE, Ostendorf T, Floege J: Induction of progressive glomerulonephritis by podocyte-specific overexpression of platelet-derived growth factor-D. Kidney Int 80: 1292–1305, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Heymann F, Meyer-Schwesinger C, Hamilton-Williams EE, Hammerich L, Panzer U, Kaden S, Quaggin SE, Floege J, Gröne HJ, Kurts C: Kidney dendritic cell activation is required for progression of renal disease in a mouse model of glomerular injury. J Clin Invest 119: 1286–1297, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ichikawa I, Ma J, Motojima M, Matsusaka T: Podocyte damage damages podocytes: Autonomous vicious cycle that drives local spread of glomerular sclerosis. Curr Opin Nephrol Hypertens 14: 205–210, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Kriz W, Hartmann I, Hosser H, Hähnel B, Kränzlin B, Provoost A, Gretz N: Tracer studies in the rat demonstrate misdirected filtration and peritubular filtrate spreading in nephrons with segmental glomerulosclerosis. J Am Soc Nephrol 12: 496–506, 2001 [DOI] [PubMed] [Google Scholar]