Abstract
Obstruction of the ureteropelvic junction (UPJ) is a common congenital anomaly frequently associated with ureteral defects. To study the molecular mechanisms that modulate ureteral development, we inactivated Smad4, the common Smad critical for transcriptional responses to TGF-β and Bmp signaling, in the ureteral and bladder mesenchyme during embryogenesis. Loss of canonical Smad signaling in these tissues caused bilateral UPJ obstruction and severe hydronephrosis beginning at embryonic day 17.5. Despite a reduction in quantity of ureteral smooth muscle, differentiation proceeded without Smad4, producing a less severe phenotype than Bmp4 mutants; this finding suggests that at least some Bmp4 functions in ureteral smooth muscle may be Smad-independent. The absence of canonical Smad signaling in the ureteral mesenchyme, but not in the urothelium itself, led to urothelial disorganization, highlighting the importance of mesenchymal support for epithelial development. Transcript profiling revealed altered expression in known Bmp targets, smooth muscle-specific genes, and extracellular matrix-related genes in mutant ureters before the onset of hydronephrosis. Expression of the Bmp target Id2 was significantly lower in Smad4 mutants, consistent with the observation that Id2 mutants develop UPJ obstruction. In summary, Smad4 deficiency reduces the number and contractility of ureteral smooth muscle cells, leading to abnormal pyeloureteral peristalsis and functional obstruction. The subsequent bending and luminal constriction of the ureter at the UPJ marks the transition from a functional obstruction to a more intractable physical obstruction, suggesting that early intervention for this disease may prevent more irreversible damage to the urinary tract.
Hydronephrosis is the most common prenatal genitourinary abnormality.1 Prenatal hydronephrosis has a high incidence of 1:100 to 1:500, with ureteropelvic junction (UPJ) obstruction being a major cause.2,3 A portion of the prenatal hydronephrosis cases cannot self-resolve and can disrupt kidney development, leading to irreversible damage of the kidneys. Although the exact cause of UPJ obstruction and prenatal hydronephrosis is difficult to determine in individual patients, genetic mutations and environmental disruptions during embryonic development have been shown to cause such defects in animal models.
Nephrogenesis in mice starts with the outgrowth of the ureteric bud (UB) from the Wolffian duct to invade the metanephric mesenchyme (MM). The reciprocal interaction between the UB and MM drives nephrogenesis.4 The proximal part of the UB is surrounded by MM and later gives rise to the collecting duct system. The distal UB, remaining outside the MM, further elongates and connects to the bladder, giving rise to the ureteral epithelia. Unlike the proximal ureter, the distal ureter is wrapped around by mesenchymal cells derived from the tail bud region.5 The ureter provides a conduit for active transfer of urine from the kidney to the bladder propelled by peristalsis. Although the urothelium and the ureteral smooth muscle (SM) have distinct embryonic progenitors, they are tightly intertwined during development. Any abnormality in one of these two compartments may affect the development of the other and ultimately impair the formation of a functional ureter, leading to urinary tract obstruction and hydronephrosis.
Although the molecular network regulating ureteral development has not yet been fully defined, recent studies have revealed a number of key players involved in ureteral development. Sonic hedgehog is expressed in the ureteral epithelium and known to induce the expression of Bone morphogenetic protein 4 (Bmp4), a member of the TGF-β superfamily, in the surrounding mesenchymal cells.6 Bmp4 is also involved in the differentiation of mesenchymal cells into SM layers around the ureteral epithelium.5
Apart from Bmp4, various other components of TGF-β superfamily are involved in nephrogenesis and the development of the urinary tract.7–10 Signals from different TGF-β ligands and receptors diverge and converge on different sets of R-Smads (Smad2 and -3 for TGF-β, Activin, and Nodal and Smad1, -5, and -8 for Bmps), producing distinct and sometimes opposing outcomes.11 All activated R-Smads translocate into the nucleus in complexes with the common Smad (Smad4) to regulate the transcription of downstream genes.12 Thus, Smad4 is at the core of the transcriptional responses in the canonical TGF-β signaling pathway. Recent advances have revealed that, besides the kinase activities of the TGF-β type I receptors, other kinases, such as mitogen activated protein kinase (MAPK), cyclin-dependent kinase, calcium calmodulin-dependent protein kinase 2, and G protein–coupled receptor kinase 2, can also phosphorylate Smads.11,13 In addition, Smad-independent TGF-β responses have been reported in Smad-deficient cell lines and animal models.11,13 Signal transduction from TGF-β ligands and receptors to Smads is complicated and nonlinear. Thus, to better understand the mechanism by which the urinary tract develops, it is necessary to investigate the precise role of Smad signaling in this context in addition to the studies of the TGF-β ligands and receptors.
In this study, we have specifically inactivated Smad4 in the ureteral and bladder mesenchyme. The loss of canonical Smad signaling in the lower urinary tract mesenchyme resulted in bilateral UPJ obstruction and severe hydronephrosis soon after urine production during embryogenesis. To our surprise, in light of the results from studies involving Bmp4 inactivation, ureteral mesenchyme was able to differentiate into SM layers, albeit in reduced quantity. The pathogenesis of UPJ obstruction is phasic in this model, progressing from initial molecular changes to multiple early developmental defects (especially the reduction in SM that affects ureteral peristalsis) and then from functional deficiency in ureteral urine transfer to more permanent structural obstruction later in development.
Results
Inactivation of Smad Signaling in the Mesenchyme of the Lower Urinary Tract Causes Hydronephrosis by UPJ Obstruction
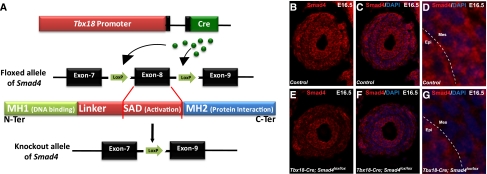
To investigate the role of Smad-dependent signaling in the development of the lower urinary tract, we have used a Tbx18-Cre transgene previously generated in our laboratory to drive Cre expression and subsequent deletion of Smad4 from a floxed-Smad4 allele specifically in the ureteral and bladder mesenchyme but not in the kidney proper or urothelium (Figure 1A).14 Smad4 immunostaining of ureter cross-sections confirms the absence of Smad4 in the mesenchymal region corresponding to the ureteral SM layers (Figure 1, B–G). Besides the epithelium, cells outside the SM layer remain positive for Smad4 in the mutants, a pattern consistent with the pattern of the Tbx18-Cre transgene expression.14
Figure 1.
Smad4 inactivation in the mesenchyme of the lower urinary tract. (A) Tbx18-Cre transgene drives Cre-mediated loxP recombination of a floxed allele of Smad4, resulting in the Smad4 knockout allele lacking exon 8. (B–G) Immunostaining with an anti-Smad4 antibody that detects the full-length Smad4 protein shows the lack of full-length Smad4 protein in the mesenchyme of mutants (E–G) compared with control sections (B–D). Higher magnification of Smad4 immunostaining of control and mutant is shown in (D) and (G), respectively. Epi, epithelium; Mes, mesenchyme; MH1, MAD (mothers against decapentaplegic)-homology 1 domain; MH2, MAD-homology 2 domain; SAD, Smad activation domain.
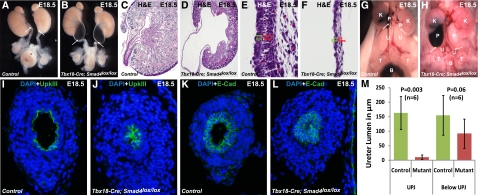
Disruption of canonical Smad signaling in mesenchyme from the pelvis down to the bladder resulted in hydronephrosis that takes place as early as embryonic day (E) 17.5, not long after the initiation of urine production. Over 95% (n=48) of the mutants showed bilateral severe hydronephrosis at E17.5 and onward. Severe renal pelvic expansion and the relatively normal appearance of the ureter below the UPJ resembles UPJ obstruction in human patients.15 Ureter kinking at the UPJ was also observed (Figure 2, A and B). Histologic analysis of sagittal sections from the kidneys of Smad4 mutants revealed that severe dilation of the renal pelvis in these mutants resulted in thinning of renal pelvic urothelium and SM layers compared with the sections of the wild-type samples (Figure 2, C–F).
Figure 2.
Smad4 inactivation in the ureteral mesenchyme leads to hydronephrosis and UPJ obstruction. (A and B) Smad4 inactivation in ureteral mesenchyme results in severe bilateral hydronephrotic kidneys having kinked ureter at UPJ in mutants compared with normal ureter phenotype in controls. (C and D) Severe renal pelvis retention and fluid pressure resulted in decreased thickness of epithelium and smooth muscle layer at the UPJ of mutants compared with wild type. A higher magnification of the sagittal section of the pelvis shows a thinner layer of epithelium and mesenchyme in (F) mutant compared with (E) control. Ink is injected in the pelvis of right kidney of control and mutant mice. (G) Ink injected in the pelvis of control kidney reaches to the bladder (evidenced by accumulation of ink in the bladder), whereas (H) ink cannot pass through the UPJ in mutants and reach to the bladder (shown by the absence of ink in the ureter and bladder). White arrows indicate the UPJ. Immunostaining of control and mutant sections at UPJ by anti-UpkIII and anti–E-Cad antibodies shows narrow ureteral lumen in (J and L) mutants compared with (I and K) normal lumen in controls. (M) Statistical measurement (two-tailed t test) of lumen diameter in the mutants versus controls shows more severe constriction of ureter lumen at UPJ (P=0.003, n=6) than below UPJ (P=0.06, n=6). B, bladder; Epi, epithelium; K, kidney; Mes, mesenchyme; P, pelvis; T, testis; U, ureter.
To further ascertain the etiology of hydronephrosis, we injected ink into the renal pelvis of mutant and control mice. The injected ink was able to reach the bladder in the controls but failed to pass through the UPJ in mutants (Figure 2, G and H), indicating UPJ obstruction. The presence of UPJ obstruction is also consistent with the absence of significant amounts of urine in mutant bladders. Immunostaining of Uroplakin-3 (UpkIII) and E-Cadherin (E-Cad) revealed a reduced ureteral lumen at the UPJ in mutants (Figure 2, I–L, respectively). The lumen reduction, however, was not as significant in the mutant ureters at levels lower than the UPJ (Figure 2M). UpkIII, along with other Uroplakins, forms crystals covering the mature urothelium in the lower urinary tract. The presence of UpkIII suggests that the absence of canonical Smad signaling in the mesenchyme did not alter the differentiation of the urothelium.
Smad4 Inactivation Leads to Structural and Functional Defects of SM Layers
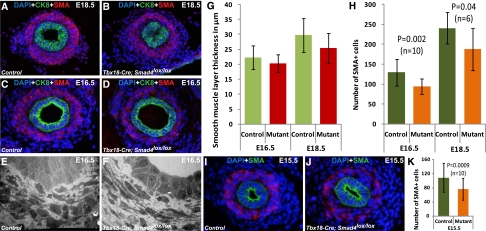
We further examined the ureteral SMs because of their crucial role in ureteral peristalsis. To our surprise, unlike the previous studies on Bmp4 disruption,16,17 Smad4 mutants have SM differentiation and formation of SM layers around the epithelium (Figure 3, A–D). We investigated the SM layer thickness in mutant and control ureters at E18.5, when hydronephrosis has occurred, and E16.5, just before the onset of hydronephrosis. The thickness of SM layers was not significantly changed between mutants and controls at E16.5 and E18.5 at levels below the UPJ (Figure 3G). However, the number of α-smooth muscle actin (αSMA) -positive SM cells (SMCs) was fewer in mutant ureters, resulting in fewer SMCs per unit area (Figure 3H). This observation was further confirmed by electron microscopy images at E16.5 showing lower cell density in the SM layers in the mutants (Figure 3, E and F). At E16.5, there is no difference in apoptosis or cell proliferation of SMCs between mutants and controls. At E15.5, the earliest time point for us to reliably quantify the ureteral SMCs, we observed fewer of them in the mutants (Figure 3, I–K). The decrease in the SMC number would, in part, affect the ureteral contractile ability and coordination, leading to the functional obstruction of the urinary tract at an earlier stage of ureteral development and maturation. We, thus, investigated if ureteral peristalsis is really defective in the mutant ureter. Video recording of isolated newborn ureters in culture dishes showed that the mutant ureters retain the ability to contract. However, the mutant ureters lacked the coordination observed in the peristalsis of control ureters (Supplemental Videos 1 and 2). Such defects would affect efficient urine transport and may lead to urinary tract obstruction.
Figure 3.
Deletion of Smad4 in the ureter mesenchyme causes smooth muscle layer defects. Immunostaining by anti–α-SMA antibody shows formation of a smooth muscle layer around urothelium (stained by anti-CK8 antibody) in (B and D) mutant ureters similar to (A and C) controls at E16.5 and E18.5. There is no significant difference in the thickness of smooth muscle layer between mutant and control (two-tailed t test). (G) Smooth muscle layer thickness (in micrometers) at E16.5 was controls, 22.25±3.96 (n=8); mutants, 20.30±3.03 (n=8); P=0.24. Smooth muscle layer thickness (in micrometers) at E18.5 was controls, 29.81±5.67 (n=6); mutants, 25.45±5.07 (n=6); P=0.12. Lower numbers of α-SMA–positive cells were observed in mutant ureter compared with controls (two-tailed t test). (H) Number of α-SMA–positive cells at E16.5 was controls, 129.29±33.52 (n=10); mutants: 93.79±19.39 (n=10); P=0.002. Number of α-SMA–positive cells at E18.5 was controls, 240.44±39.44 (n=6); mutants, 187.25±52.32 (n=6); P=0.04. Electron microscopy imaging that identifies individual cells in mutant and control ureters shows fewer cells in (F) the mutant ureter section compared with (E) the control ureter section. (I–K) Significantly fewer SMA-positive cells were observed in mutant compared with control at E15.5. Average number of α-SMA–positive cells at E15.5 was controls, 108.59±41 (n=10); mutants, 76.3±31.4 (n=10); P=0.0009.
Because the Tbx18-Cre transgene is expressed in both the ureteral and bladder mesenchymes, we decided to investigate the bladders but could not see any obvious defects in either the epithelial or mesenchymal derivatives (Supplemental Figure 1). We have also never detected any significant functional differences between the control and mutant bladders. However, it is still unclear if the mutant bladders are fully functional, because bilateral UPJ obstruction can prevent significant amounts of urine from reaching the bladder, leaving it unchallenged.
Inactivation of Smad Signaling in the Mesenchyme Causes Irregular Ureteral Epithelium Along the Proximal–Distal Axis and Secondary Epithelial Blockage of Ureteral Lumen at UPJ
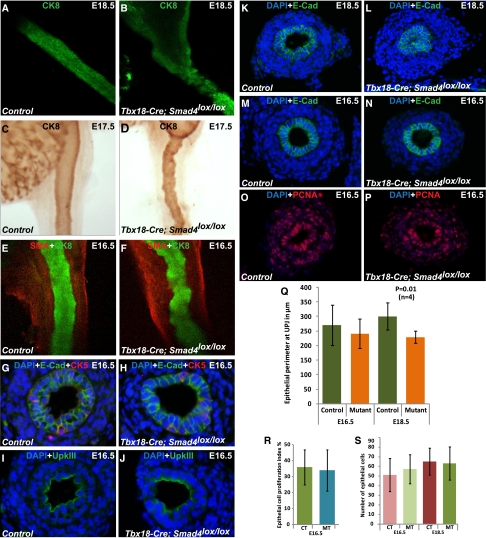
The urothelial lining along the longitudinal axis of the ureter, which was highlighted by the Cytokeratin 8 (CK8) staining, was irregular in Smad4 mutants compared with the smooth profile of the urothelium in the control ureter (Figure 4, A and B). We noted urothelial irregularity at E17.5, the earliest stage to notice hydronephrosis (Figure 4, C and D), and even at E16.5 (Figure 4, E and F) before the onset of hydronephrosis. We also used cytokeratin 5 (CK5), E-Cad, and UpkIII antibodies to investigate the differentiation of the basal (CK5+, UpkIII−, and E-Cad+), intermediate (CK5−, UpkIII−, and E-Cad+), and luminal (CK5−, UpkIII+, and E-Cad+) layers of the ureteral epithelial. Compared with the adult bladder, the embryonic ureter has fewer layers of urothelium and a developing basal layer.18 Nevertheless, we have not detected any significant difference in the composition of the basal, intermediate, and luminal layers of the ureteral epithelium on cross-sections at E16.5 (Figure 4, G–J). These data suggest that, although the longitudinal organization of the ureteral epithelium is affected in the mutants, the differentiation of the cell layers along the radial axis is not significantly affected before the onset of hydronephrosis. The presence of urothelial irregularity along the longitudinal axis before the onset of hydronephrosis suggests that disorganization of the urothelium is not a result of hydronephrosis but is an early developmental change associated with Smad4 inactivation in the ureteral mesenchyme. Thus, although Smad signaling in the ureteral mesenchyme is dispensable for the differentiation of the ureteral epithelium, it is required for the proper organization of the epithelium. It is still unclear whether the alteration in epithelial organization is an indirect result of corresponding disorganization of the mesenchyme or changes in the developmental cues provided to the epithelium by the mesenchymal cells. Interestingly, based on our own study (data not shown) and the previous report by Oxburgh et al.,19 Hoxb7-Cre–mediated inactivation of Smad4 in the urothelium did not cause overt organization defects in the ureter. Besides revealing a selective indispensable functional role of Smad signaling in the ureteral mesenchyme, these findings also suggest that the ureteral mesenchyme is important in providing signaling cues and/or physical support for the normal development of the ureteral epithelia.
Figure 4.
Loss of Smad signaling specifically in the ureteral mesenchyme also affects ureteral epithelium. (A–F) Staining of control and mutant ureteral epithelium by anti-CK8 antibody shows (B, D, and F) irregular epithelium throughout the length of the mutant ureter compared with (A, C, and E) normal smooth outlined epithelium in control ureters. Immunostaining of ureter epithelium by CK5 and UpkIII to label the epithelial cells in the basal layer and on the luminal surface, respectively. In addition, E-Cad stains all the epithelial cells. (G–J) No significant difference in the cellular composition between mutants and controls was found. Immunostaining by an anti–E-Cad antibody targeting the epithelium shows narrow ureter lumen at the (L) mutant UPJ compared with (K) normal ureteral lumen of control at E18.5, which is not observed at (M and N) E16.5. (Q) Epithelial perimeter (in micrometers) at E16.5 was controls, 270.35±69.06 (n=4); mutants, 241.55±50.78 (n=4); P=0.45. Epithelial perimeter (in micrometers) at E18.5 was controls, 301.31±46.28 (n=4); mutants: 229.7±20.08 (n=4); P=0.01. Immunostaining of (O) control and (P) mutant cross-sections by antiproliferation cell nuclear antigen (PCNA) antibody indicates no difference in the cellular proliferation. (R) Metric data analysis (two-tailed t test) also indicates no difference in the proliferation index of epithelial cells of mutant (34±13, n=4) and control (36±11, n=4) ureters at E16.5 (two-tailed t test, P=0.83) and (S) consequently, no difference in the epithelial cell number at E16.5 (controls, 51.33±17.18 [n=4]; mutants, 57.38±15.13 [n=4]; P=0.06) and E18.5 (controls, 65.22±13.9 [n=4]; mutants, 63.33±17.22 [n=4]; P=0.50). CT, control; MT, mutant.
In addition, urothelial blockage of the ureteral lumen at UPJ was seen at E18.5 but not at E16.5 in the mutants (Figure 4, K–N and Q). Consistent with luminal constriction, the outer perimeter of the urothelium at the UPJ was also significantly decreased at E18.5 in the mutants (Figure 4, K–L and Q). At E16.5, however, the lumen at the UPJ was not restricted, and the outer perimeter of the ureteral epithelium was not significantly different in the mutants (Figure 4, M, N, and Q). We also showed that the proliferation index of the epithelium at the UPJ was similar between control and mutant mice at E16.5 (Figure 4, O, P, and R). Consequently, the number of epithelial cells per cross-section was also not significantly different between mutants and controls at E16.5 and E18.5 (Figure 4S). These results argue against the possibility of epithelial overproliferation at the epithelial blockage site of the ureteral lumen at the UPJ in the mutants. The blockage of the lumen at E18.5 and onward conceivably contributes to the physical obstruction at UPJ in the mutants. However, because luminal blockage by the urothelium occurs later than E16.5, it may not be the result of early molecular and cellular changes in the ureter. Besides changes in the mesenchyme throughout the ureter, the UPJ area may be additionally influenced by factors specific to its anatomic location, such as the tendency of kinking when the renal pelvis is expanded. Such changes may amplify the earlier dysfunction and lead to more severe and permanent obstruction.
Whole-Genome Expression Analysis Reveals Altered Expression of Muscle-Related Genes, Basement Membrane Components, and Known Targets of Bmp4
To investigate the molecular changes responsible for various cellular changes in the Smad4 mutants, we carried out whole-genome transcript profiling of mutant and control ureters at E16.5 before the onset of hydronephrosis (Supplemental Table 1). Genes with significantly different levels of transcription in the mutant ureter at E16.5 were grouped into four categories (Table 1). Changes in the expression of muscle-related genes, such as Myosin, heavy polypeptide 3; Cadherin 15; Calsequestrin 1; Tropomyosin 2; Actin, γ2; Actinin α2; ATPase, Ca2+ transp, fast twitch 1; and Myomesin 2, may contribute to the abnormal organization and defective peristalsis of the SM layer. Higher expression of several extracellular matrix genes, such as Collagen, type VII, α1; Procollagen, type VI, α2; Collagen, type XX, α1; Laminin α1; and Tetraspanin 2, suggests possible basement membrane alterations that may potentially restrict epithelial expansion, disrupt the normal interactions between the SM layer and urothelium, and even affect normal peristalsis. Furthermore, Id2, a known downstream target of Bmp4, is downregulated in the mutants. It is worth noting that mice carrying an Id2 deletion allele have high incidence of hydronephrosis.20 However, Bmp4 itself was upregulated in the Smad4 mutants, likely reflecting a futile feedback mechanism.
Table 1.
Transcriptional alterations in Smad4 mutant ureters
| Target | Definition | Array Fold Change | qPCR Fold Change |
|---|---|---|---|
| Upregulated in mutants | |||
| muscle-related genes | |||
| Myh3 | Myosin, heavy polypeptide 3 | 4.896 | 3.603 |
| Cdh15 | Cadherin 15 | 3.584 | n.d. |
| Tpm2 | Tropomyosin 2, β | 3.022 | 1.827 |
| Actn2 | Actinin α2 | 2.577 | 1.04 |
| Chodl | Chondrolectin | 2.150 | n.d. |
| Atp2a1 | ATPase, Ca2+ transp, fast twitch 1 | 2.140 | n.d. |
| Myo18b | Myosin XVIIIb | 2.072 | 1.69 |
| Myom2 | Myomesin 2 | 2.013 | 5.45 |
| extracellular matrix-related genes | |||
| Col7a1 | Collagen, type VII, α1 | 3.004 | 2.90 |
| Cntnap4 | Contactin associated protein-like 4 | 2.672 | 1.661 |
| Lama1 | Laminin, α1 | 2.419 | 3.115 |
| Col20a1 | Collagen, type XX, α1 | 2.152 | 1.015 |
| Col6a2 | Procollagen, type VI, α2 | 2.079 | 1.479 |
| kidney/ureter-related genes | |||
| Etv4 | Ets variant gene 4 | 2.550 | n.d. |
| Robo1 | Roundabout homolog 1 | 2.133 | 1.74 |
| Kif26a | Kinesin family member 26A | 2.092 | 1.096 |
| other important genes | |||
| BMP4 | Bone morphogenetic protein 4 | 2.290 | 2.005 |
| OSR2 | Odd-skipped related 2 | 5.726 | 8.706 |
| Downregulated in mutants | |||
| muscle-related genes | |||
| Hopx | HOP homeobox | −2.049 | −1.69 |
| Casq1 | Calsequestrin 1 | −2.073 | −3.05 |
| Actg2 | Actin, γ2, smooth muscle | −2.177 | −4.110 |
| Myom1 | Myomesin 1 | −2.199 | −8.801 |
| Lin7a | Lin-7 homolog A | −3.726 | n.d. |
| extracellular matrix-related genes | |||
| Tspan2 | Tetraspanin 2 | −2.038 | −1.59 |
| Cav2 | Caveolin 2 | −2.108 | −1.53 |
| kidney/ureter-related genes | |||
| Lmcd1 | LIM and cysteine-rich domains 1 | −2.308 | n.d. |
| Cyp2e1 | Cytochrome P450, 2e, polypeptide | −2.389 | n.d. |
| Id2 | Inhibitor of DNA binding 2 | −2.594 | −3.049 |
| other important genes | |||
| STAC | Src homology3 and cysteine-rich domain | −3.189 | −1.721 |
| PCP4L1 | Purkinje cell protein 4-like 1 | −3.363 | −3.277 |
qPCR, quantitative PCR; n.d., not determined.
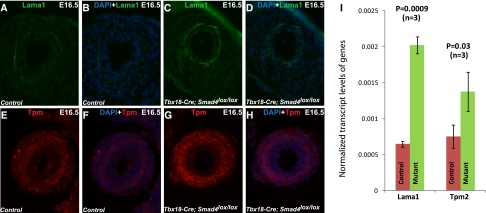
In addition to higher Laminin α1 transcript levels revealed by microarray, immunostaining also showed higher levels of Laminin α1 proteins in the basement membrane of the mutant ureteral epithelia (Figure 5, A–D). Furthermore, higher transcript levels of Tropomyosin 2 led to higher Tropomyosin protein levels in the mutant SM layers (Figure 5, E–H). Tropomyosin is an actin-binding protein that regulates muscle contraction by modulating the actin–myosin interaction.21 Although it is unclear if the upregulation of Tropomyosin is a direct result of Smad4 inactivation, the alteration in Tropomyosin may affect muscle contraction and peristalsis in mutant ureters.
Figure 5.
Change in the transcript levels of genes involved in muscle contractility and extracellular matrix in ureters of the Smad4 mutants. Immunostaining by an anti-Laminin α1 antibody shows higher expression of Laminin α1 in the basement membrane of (C and D) mutant urothelium compared with (A and B) control. Tropomyosin immunostaining indicates its higher expression in (G and H) muscle layer of mutant ureter compared with (E and F) controls. (I) Transcript levels of Laminin α1 (controls, 0.000649±0.00004 [n=3]; mutants, 0.002022±0.00012 [n=3]; P=0.0009) and Tropomyosin 2 (controls, 0.000755±0.00016 [n=3]; mutants, 0.001378±0.00027 [n=3]; P=0.04) normalized to Gapdh using the 2−ΔΔCT method. Lama1, laminin α1; Tpm, tropomyosin 2.
Discussion
The TGF-β superfamily signaling plays an important role in normal development of the urogenital system and the pathogenesis of urinary tract defects.7,10,19,22,23 Previous studies have shown that inactivation of Bmp4 disrupts the structural and functional organization of the kidney and lower urinary tract.16 To specifically investigate the role of canonical Smad-dependent TGF-β superfamily signaling in the development of the lower urinary tract, we have used the Tbx18-Cre transgene to mediate the inactivation of Smad4, the common Smad indispensible for the transcriptional responses of TGF-β superfamily signaling, in the ureteral mesenchyme. Loss of canonical Smad signaling in the mesenchyme led to UPJ obstruction and severe hydronephrosis. In a parallel study, we have found no urinary tract defects in mice with Smad4 inactivation in the ureteral epithelium mediated by a Hoxb7-Cre transgene (data not shown). Thus, it seems that Smad signaling is indispensable in the ureteral mesenchyme but dispensable in the urothelium for the normal developing of the ureter. Similarly, it has been found that Smad signaling is essential in the metanephric mesenchyme but not the UB derivative, including the collecting duct system in the kidney proper.19 It is still unclear whether the alteration in epithelial organization along the longitudinal axis is an indirect result of corresponding disorganization of the mesenchyme or changes in the developmental cues provided to the epithelium by the mesenchymal cells. Nevertheless, this finding further indicates that the ureteral mesenchyme is important in providing signaling cues and/or physical support for the normal development of the ureteral epithelia.
A number of genes related to TGF-β super family are involved in the pathogenesis of hydronephrosis, including Bmp4,24 Bmp5,25 and Id2.20 Decreased Bmp4 expression in the ureteral mesenchyme of the Tbx18 mutants indicates that Bmp4 functions downstream of Tbx18.17 Id2 is a known downstream target of Bmp4, and the two have similar expression domains in the ureteral mesenchyme.20 A previous study showed that Id2 mutants have hydronephrosis.20 The decreased expression of Id2 in Smad4 mutants could be involved in the initial SM defects that eventually led to UPJ obstruction and hydronephrosis. The upregulation of Bmp4 expression observed in Smad4 mutants is similar to the upregulation reported for the Id2 mutants,20 suggesting a possible feedback mechanism in both models. It seems that the Bmp4–Smad–Id2 axis is critical for normal urinary tract development, and any disturbance may lead to defects such as UPJ obstruction.
The lack of Smad-dependent TGF-β signaling in the mutant ureteral mesenchyme did not affect the proliferation and differentiation of the epithelial cells in Smad4 mutants, at least from E16.5 onward (Figures 2 and 4). However, the wavy basement membrane of the ureteral epithelium in Smad4 mutants, even before the onset of hydronephrosis, indicates a critical role of the mesenchyme in providing signaling cues and/or physical support for the normal development of the urothelium. Whole-genome expression analysis revealed upregulation of various extracellular matrix genes, including Laminin α1. In addition to structural roles, Laminin α-chains are important in signaling because of their ability to interact with Integrins. Laminin α1 is predominantly expressed in embryonic development and has very low expression in the basement membrane of adult ureteral epithelium in humans.26 Higher expression of Laminin α1 in the basement membrane of ureteral epithelium in Smad4 mutants may alter the basement membrane, making it less accommodating to epithelial development and pyeloureteral peristalsis. Similar to our results, previous studies of early embryogenesis in Smad4 mutants have shown increased deposition of extracellular basement membrane.27
Apart from the quantitative reduction of cell numbers, SM layer is affected qualitatively in the Smad4 mutants as well. Tropomyosin, along with Troponin, competes with Myosin for inhibitory binding sites on Actin and thus, regulates muscle contraction. In the presence of calcium, the Tropomyosin–Actin complexes dissociate to permit the Actin–Myosin interaction and the subsequent muscle contraction.28 Upregulation of Tpm2 in the mesenchyme of Smad4 mutant ureters may alter the SM contractile property and peristalsis. Smad4 mutants have decreased expression of Calsequestrin 1, encoding a calcium binding protein in the sarcoplasmic reticulum that controls the trafficking of calcium ions. Previous studies have shown that the release of calcium into the cytoplasm is greatly reduced in Calsequestrin 1 mutants.29 Mutations in ATPase, Ca2+ transporting, cardiac muscle, fast twitch 1 (Apt2a1) result in reduced Ca2+ uptake by the sarcoplasmic reticulum. Apt2a1 mutations are associated with Brody disease and pseudomyotonia, both of which involve defects in muscle contraction and relaxation.30,31 In Smad4 mutants, where Apt2a1 expression is upregulated, less Ca2+ is expected in the cytoplasm, which is opposite of the Ca2+ found in Apt2a1 loss of function mutants. It is possible that multitudes of alterations in the SM layers of the Smad4 mutants have additive or synergistic effects. The cumulative effects of alterations in transcript levels of Calsequestrin 1, Apt2a1, and Tropomyosin 2, along with decreased SMCs, can have major influence on the contractile ability and coordination of peristalsis in the mutant ureters, leading to functional deficiency in urine transfer. Although such SM defects occurred throughout the length of the ureter, fluid accretion tends to occur upstream of the first point of impediment (at the top of the ureter or UPJ level in this case), leading to fluid accumulation above the UPJ. UPJ bends under the pressure of fluid accumulation and the dilatation of the renal pelvis, reinforcing a downward spiral into more severe and persistent structural obstruction at UPJ. The jamming of the lumen by the urothelium constitutes at least part of the physical blockage of urine passage at the UPJ.
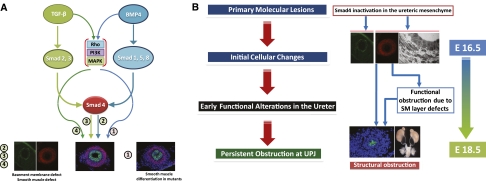
Although the ureteral SMs showed a number of alterations, SM development in Smad4 mutants seems to be much more advanced than in mutants with loss of Bmp4.16,17 The TGF-β superfamily seems to also have an Smad-independent noncanonical pathway by ρ-GTPase, phosphatidylinositol 3′ kinase, and MAPK.32 Because less-severe SM defects are observed in Smad4 mutants (this study) compared with the Bmp4 mutants previously reported, Bmp4 effects in ureteral SM development, such as the differentiation of the mesenchymal cells into SMCs, may be partially Smad-independent. Conversely, other events, such as the organization of the SM layers and the basement membrane, seem to be regulated by Smad-dependent transcriptional responses of TGF-β and Bmp4 (Figure 6A).
Figure 6.
Pathogenesis of UPJ obstruction in ureters of the Smad4 mutants. Because the SM layer development seems less affected in Smad4 mutants than Bmp4 mutants16 and TGF-β superfamily signaling may have Smad4-independent downstream pathways mediated by ρ-GTPase, phosphatidylinositol 3′ kinase, and MAPK, it is possible that the part of Bmp4 signaling in ureteral SM development may be Smad4-independent. (A) At the same time, other phenotypes in Smad4 mutants could be attributed to the lack of TGF-β/Bmp4 signaling by Smad4. Although the series of sequential and parallel events intertwine, the pathogenesis of UPJ obstruction in the Smad4 mutants seems to go through a few distinct phases. The primary molecular lesion is Smad4 inactivation in the ureteral mesenchyme. This inactivation causes initial cellular changes, including the reduction of SMC number and the change of contractility related proteins. These and other early changes lead to functional obstruction of the ureter. (B) Functional obstruction causes subsequent bending and luminal constriction of the ureter at UPJ, marking the transition from the earlier dysfunction to later, more intractable structural obstruction.
Sequential and parallel developmental events/alterations observed in the present study are useful in deducing the pathogenic progression of urinary tract obstruction (Figure 6B). The initial molecular lesion (deletion of Smad4 in the ureteral mesenchyme) affects the SMC number and contractility as well as the urothelial basement membrane at stages before overt urinary tract obstruction. Such earlier deficiencies affect efficient pyeloureteral peristalsis, leading to functional obstruction. These earlier defects became more advanced at later stages (E18.5 onward) at least partially as a result of the functional obstruction itself, and they also result in physical obstruction, such as the severe kinking of the ureter at the UPJ and the constriction of the ureteral lumen by epithelial crowding. Thus, functional obstruction may transition into more damaging and more permanent physical obstruction. This may be one more reason to correct the early and usually milder functional alterations before they lead to more irreversible damages to the urinary tract.
Concise Methods
Mouse (Mus musculus) Strains and Sample Collection
All animal studies have been approved by the Institutional Animal Care and Use Committee at Washington University School of Medicine and have been conducted according to relevant National Institutes of Health guidelines. The generation of the floxed-Smad4 allele (Smad4lox) and Tbx18-Cre was described previously.14,33 Mice carrying floxed-Smad4 allele were crossed with the Tbx18-Cre transgenic mice to produce Tbx18-Cre;Smad4lox/lox embryos that would have homozygous deletion of Smad4 (exon 8) in mesenchyme of lower urinary tract (Figure 1A). Although only exon 8 of Smad4 is removed by Cre-mediated LoxP recombination because of the essential role of exon 8 in Smad4 function, the resulting Smad4 deletion allele has been reported to behave as a null or at least, a severe hypomorphic allele.33 The Tbx18-Cre transgene has a high level of expression in the ureteral and bladder mesenchyme.14 Tbx18-Cre;Smad4lox/lox embryos are designated as mutants in this study. Their littermates with no homozygous deletion of Smad4 in any cells are considered controls. Direct comparison was made between littermates or age-matched controls. All experiments were repeated at least three times.
Histologic Analysis and Immunohistochemistry
For histologic analyses, embryos were either fixed with 4% paraformaldehyde and embedded in paraffin or cryopreserved in Optimal Cutting Temperature compound (Sakura Finetek USA, Torrance, CA); 7-µm paraffin sections or 16-µm cryosections were prepared. Sections were stained by hematoxylin and eosin using standard protocol.34 Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling analysis was performed on paraffin-embedded sections by using the ApopTag plus peroxidase in situ apoptosis detection kit (Roche, NutleY, NJ).35 Paraffin or cryosections were stained with a mouse monoclonal anti–E-Cad antibody (610181, 1:200; BD Transduction), mouse monoclonal anti-αSMA antibody (A2547, 1:200; Sigma), rat anti-CK8 (TROMA-1; Developmental Studies Hybridoma Bank, Iowa City, IA), mouse antitropomyosin (CH1; Developmental Studies Hybridoma Bank, Iowa City, IA), mouse monoclonal anti-UpkIII antibody (03-610108, 1:10; American Research Products), or rabbit polyclonal anti-proliferating cell nuclear antigen (PCNA) antibody (IHC-00012; Bethyl Laboratories). Smad4 was detected by a mouse monoclonal anti-Smad4 antibody (Sc-7966; Santa Cruz) raised against amino acids 1–552 representing the full-length Smad4 human protein as described earlier.36 Laminin α1 antibody was provided by Jeffrey H. Miner (Washington University, St. Louis, MO). Appropriate AlexaFlour488- or -555–conjugated secondary antibodies (1:1000; Molecular Probe) were used to detect the corresponding primary antibodies. Whole-mount immunostaining was carried out with the rat anti-CK8 antibody and/or the mouse monoclonal anti-αSMA antibody as described.37 The proliferation index was determined by the percentage of PCNA-positive cells on sections immunostained with the PCNA antibody and either the CK8 or E-Cad antibody.
Ink Injection Experiments and Transmission Electron Microscopy
To visualize the ureteral lumen, India ink was injected into the pelvis of E18.5 mutant and control kidneys using drawn-out glass capillary tubes connected to mouth pipettes. Images were taken by a Nikon SMZ1500 stereoscope (Nikon, Melville, NY) fitted with a MicroPublisher digital imaging system (QImaging, Burnaby, BC, Canada). Transmission electron microscopy was performed according to standard procedures.38
Video Recording of Peristalsis Movement
For video capturing of the pyeloureteral peristalsis, we dissected out the newborn ureters attached to the kidney and bladder in PBS. These samples were cultured at 37°C in DMEM with 10% FCS for about 1 hour before video capturing with the MicroPublisher imaging system using StreamPix software (NorPix Inc., Montreal, QC, Canada).
Microarray Gene Expression Analysis
We used Illumina MouseWG-6 v2.0 Expression BeadChips (Illumina Inc., San Diego, CA) for whole-genome expression profiling. Ureters were collected, excluding kidney proper and bladder, from six mutant and six control E16.5 mouse embryos. Ureters from two mice of the same genotype were pooled to make one mutant or control sample. Expression profiling of three mutant and three control samples was done at the Genome Center at Washington University in St. Louis, MO. The differential expressions of genes were analyzed using the Partek Genomics Suite software (Partek Incorporated, St. Louis, MO). Genes of interests were selected from the pool with more than or equal to a twofold change and significant difference (P<0.05). These genes of interests were further validated by quantitative PCR. cDNA was generated with oligo-dT primers using the Superscript III Kit (Invitrogen by Life Technologies, Carlsbad, CA). Real-time quantification was done using SYBRGreen PCR reaction (Applied Biosystems by Life Technologies, Carlsbad, CA). Relative gene expression was deduced using the ΔΔCt method.39
Disclosures
None.
Acknowledgments
We thank the George M. O'Brien Washington University Center for Kidney Disease Research (National Institutes of Health Grant P30DK079333) for assistance in phenotypic analyses. We thank Dr. Chuxia Deng for providing the floxed-Smad4 allele, Dr. Masato Hoshi for technical assistance on the confocal microscope, Jeanette Cunningham for electron microscopy, and Dr. Sanjay Jain for helpful comments on the manuscript. We thank Dr. Indira Mysorekar for Cytokeratin 5 antibody and helpful suggestions about the experiments.
F.C. has been supported, in part, by National Institutes of Health Grants R01 DK081592 and R01 DK087960.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011060566/-/DCSupplemental.
References
- 1.Johnson CE, Elder JS, Judge NE, Adeeb FN, Grisoni ER, Fattlar DC: The accuracy of antenatal ultrasonography in identifying renal abnormalities. Am J Dis Child 146: 1181–1184, 1992 [DOI] [PubMed] [Google Scholar]
- 2.Roth JA, Diamond DA: Prenatal hydronephrosis. Curr Opin Pediatr 13: 138–141, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Woodward M, Frank D: Postnatal management of antenatal hydronephrosis. BJU Int 89: 149–156, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Dressler GR: The cellular basis of kidney development. Annu Rev Cell Dev Biol 22: 509–529, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Brenner-Anantharam A, Cebrian C, Guillaume R, Hurtado R, Sun TT, Herzlinger D: Tailbud-derived mesenchyme promotes urinary tract segmentation via BMP4 signaling. Development 134: 1967–1975, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Yu J, Carroll TJ, McMahon AP: Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development 129: 5301–5312, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Bush KT, Sakurai H, Steer DL, Leonard MO, Sampogna RV, Meyer TN, Schwesinger C, Qiao J, Nigam SK: TGF-beta superfamily members modulate growth, branching, shaping, and patterning of the ureteric bud. Dev Biol 266: 285–298, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Hu MC, Piscione TD, Rosenblum ND: Elevated SMAD1/beta-catenin molecular complexes and renal medullary cystic dysplasia in ALK3 transgenic mice. Development 130: 2753–2766, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Gupta IR, Macias-Silva M, Kim S, Zhou X, Piscione TD, Whiteside C, Wrana JL, Rosenblum ND: BMP-2/ALK3 and HGF signal in parallel to regulate renal collecting duct morphogenesis. J Cell Sci 113: 269–278, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Hartwig S, Bridgewater D, Di Giovanni V, Cain J, Mishina Y, Rosenblum ND: BMP receptor ALK3 controls collecting system development. J Am Soc Nephrol 19: 117–124, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massagué J, Seoane J, Wotton D: Smad transcription factors. Genes Dev 19: 2783–2810, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Schmierer B, Hill CS: TGFbeta-SMAD signal transduction: Molecular specificity and functional flexibility. Nat Rev Mol Cell Biol 8: 970–982, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Xu L: Regulation of Smad activities. Biochim Biophys Acta 1759: 503–513, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Tripathi P, Guo Q, Coussens M, Ma L, Chen F: Cre/lox recombination in the lower urinary tract. Genesis 47: 409–413, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kern WF: Atlas of Renal Pathology, Philadelphia, Saunders, 1999 [Google Scholar]
- 16.Wang GJ, Brenner-Anantharam A, Vaughan ED, Herzlinger D: Antagonism of BMP4 signaling disrupts smooth muscle investment of the ureter and ureteropelvic junction. J Urol 181: 401–407, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Airik R, Bussen M, Singh MK, Petry M, Kispert A: Tbx18 regulates the development of the ureteral mesenchyme. J Clin Invest 116: 663–674, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin K, Lee J, Guo N, Kim J, Lim A, Qu L, Mysorekar IU, Beachy PA: Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature 472: 110–114, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oxburgh L, Chu GC, Michael SK, Robertson EJ: TGFbeta superfamily signals are required for morphogenesis of the kidney mesenchyme progenitor population. Development 131: 4593–4605, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Aoki Y, Mori S, Kitajima K, Yokoyama O, Kanamaru H, Okada K, Yokota Y: Id2 haploinsufficiency in mice leads to congenital hydronephrosis resembling that in humans. Genes Cells 9: 1287–1296, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Higashi-Fujime S, Hozumi T: Restoration of defective mechanochemical properties of cleaved actins by native tropomyosin: Involvement of the 40-50 loop in subdomain 2 of actin in interaction with myosin and tropomyosin. Biochem Biophys Res Commun 237: 121–125, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Sims-Lucas S, Caruana G, Dowling J, Kett MM, Bertram JF: Augmented and accelerated nephrogenesis in TGF-beta2 heterozygous mutant mice. Pediatr Res 63: 607–612, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Fujita H, Hida M, Kanemoto K, Fukuda K, Nagata M, Awazu M: Cyclic stretch induces proliferation and TGF-beta1-mediated apoptosis via p38 and ERK in ureteric bud cells. Am J Physiol Renal Physiol 299: F648–F655, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Miyazaki Y, Oshima K, Fogo A, Hogan BL, Ichikawa I: Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J Clin Invest 105: 863–873, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King JA, Marker PC, Seung KJ, Kingsley DM: BMP5 and the molecular, skeletal, and soft-tissue alterations in short ear mice. Dev Biol 166: 112–122, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Hattori K, Mabuchi R, Fujiwara H, Sanzen N, Sekiguchi K, Kawai K, Akaza H: Laminin expression patterns in human ureteral tissue. J Urol 170: 2040–2043, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Costello I, Biondi CA, Taylor JM, Bikoff EK, Robertson EJ: Smad4-dependent pathways control basement membrane deposition and endodermal cell migration at early stages of mouse development. BMC Dev Biol 9: 54, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ochala J: Thin filament proteins mutations associated with skeletal myopathies: Defective regulation of muscle contraction. J Mol Med (Berl) 86: 1197–1204, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Paolini C, Quarta M, Nori A, Boncompagni S, Canato M, Volpe P, Allen PD, Reggiani C, Protasi F: Reorganized stores and impaired calcium handling in skeletal muscle of mice lacking calsequestrin-1. J Physiol 583: 767–784, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Odermatt A, Taschner PE, Khanna VK, Busch HF, Karpati G, Jablecki CK, Breuning MH, MacLennan DH: Mutations in the gene-encoding SERCA1, the fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+ ATPase, are associated with Brody disease. Nat Genet 14: 191–194, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Drögemüller C, Drögemüller M, Leeb T, Mascarello F, Testoni S, Rossi M, Gentile A, Damiani E, Sacchetto R: Identification of a missense mutation in the bovine ATP2A1 gene in congenital pseudomyotonia of Chianina cattle: An animal model of human Brody disease. Genomics 92: 474–477, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Moustakas A, Heldin CH: Non-Smad TGF-beta signals. J Cell Sci 118: 3573–3584, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Yang X, Li C, Herrera PL, Deng CX: Generation of Smad4/Dpc4 conditional knockout mice. Genesis 32: 80–81, 2002 [DOI] [PubMed] [Google Scholar]
- 34.McDill BW, Li SZ, Kovach PA, Ding L, Chen F: Congenital progressive hydronephrosis (cph) is caused by an S256L mutation in aquaporin-2 that affects its phosphorylation and apical membrane accumulation. Proc Natl Acad Sci USA 103: 6952–6957, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jia Q, McDill BW, Sankarapandian B, Wu S, Liapis H, Holzman LB, Capecchi MR, Miner JH, Chen F: Ablation of developing podocytes disrupts cellular interactions and nephrogenesis both inside and outside the glomerulus. Am J Physiol Renal Physiol 295: F1790–F1798, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu X, Han J, Ito Y, Bringas P, Jr, Deng C, Chai Y: Ectodermal Smad4 and p38 MAPK are functionally redundant in mediating TGF-beta/BMP signaling during tooth and palate development. Dev Cell 15: 322–329, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang CP, McDill BW, Neilson JR, Joist HE, Epstein JA, Crabtree GR, Chen F: Calcineurin is required in urinary tract mesenchyme for the development of the pyeloureteral peristaltic machinery. J Clin Invest 113: 1051–1058, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Jarad G, Tripathi P, Pan M, Cunningham J, Martin DR, Liapis H, Miner JH, Chen F: Activation of NFAT signaling in podocytes causes glomerulosclerosis. J Am Soc Nephrol 21: 1657–1666, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Livak KJ, Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]