Abstract
The typical assumption is that patients with CKD will have progressive nephropathy. Methodological issues, such as measurement error and regression to the mean, have made it difficult to document whether kidney function might improve in some patients. Here, we used data from 12 years of follow-up in the African American Study of Kidney Disease and Hypertension to determine whether some patients with CKD can experience a sustained improvement in GFR. We calculated estimated GFR (eGFR) based on serum creatinine measurements during both the trial and cohort phases. We defined clearly improved patients as those with positive eGFR slopes that we could not explain by random measurement variation under Bayesian mixed-effects models. Of 949 patients with at least three follow-up eGFR measurements, 31 (3.3%) demonstrated clearly positive eGFR slopes. The mean slope among these patients was +1.06 (0.12) ml/min per 1.73 m2 per yr, compared with −2.45 (0.07) ml/min per 1.73 m2 per yr among the remaining patients. During the trial phase, 24 (77%) of these 31 patients also had clearly positive slopes of 125I-iothalamate–measured GFR during the trial phase. Low levels of proteinuria at baseline and randomization to the lower BP goal (mean arterial pressure ≤92 mmHg) associated with improved eGFR. In conclusion, the extended follow-up from this study provides strong evidence that kidney function can improve in some patients with hypertensive CKD.
CKD is typically characterized by progressive loss of renal function, although there is wide variability in the rate of progression. Proteinuria, hypertension, and black race have been identified as independent predictors for more rapid progression of CKD.1–3 Accelerated progression of CKD is thought to contribute to the disproportionate burden of ESRD among African Americans.4–6
Although the majority of CKD patients experience a progressive decline in renal function, several clinical trials with variable follow-up data have described a minority of patients with stable renal function during follow-up.1,7 Together with some histopathologic evidence,8 there are experimental data supporting potential for improved renal function.9 Clinical studies identifying patients with stable renal function have been short term (just 2–4 years) and predominantly included populations of European descent.
Documenting with certainty that kidney function actually improves is challenging. Because measures of kidney function such as the GFR are associated with random measurement error and because most studies use such measurements to identify individuals with reduced kidney function, the appearance of increased kidney function might merely reflect regression to the mean, especially in short-term studies. There is presently no convincing evidence that kidney function, as assessed by GFR, actually improves in patients with CKD. Identification of such individuals may provide insights into reparative mechanisms that may guide therapeutic approaches.
The African American Study of Kidney Disease and Hypertension (AASK), with up to 12 years of follow-up, provides a unique opportunity to observe CKD progression among African Americans with hypertensive CKD. The objective of this study was to determine if CKD can improve with sustained GFR increases, using data obtained over the course of extended follow-up in the AASK study.
Results
Demographic characteristics for the entire study cohort were previously published.10 Similar to the entire cohort, patients included in this analysis had a mean age at randomization of 55±11 years, average baseline iothalamate GFR (iGFR) of 49±13 ml/min per 1.73 m2, median number of estimated GFR (eGFR) measurement of 16 (interquartile range [IQR], 9–20), and mean protein excretion of 462±879 mg/d; 61% were male.
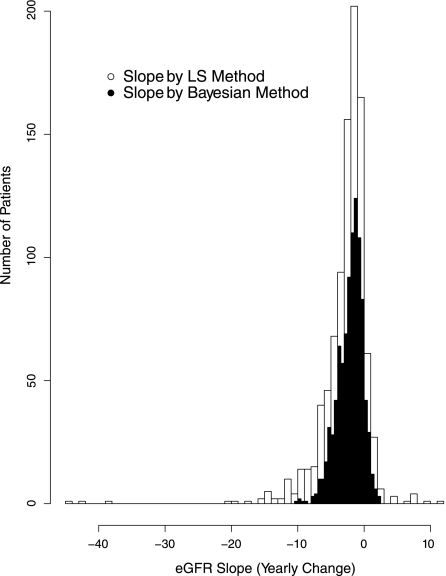
Figure 1 shows the distribution of the individual patient’s least-squares eGFR slopes over the follow-up period. A total of 104 of the 949 (11%) of analyzed patients had positive slopes. The figure also shows the distribution of the eGFR slopes as computed under the Bayesian linear mixed-effects model, in which 94 (10%) had positive slopes. Because the Bayesian model accounts for measurement error and other sources of short-term biologic variability, the Bayesian slope estimates display less variability than the least-squares slope estimates.
Figure 1.
Distribution of estimated eGFR slopes (least-squares versus Bayesian) of study participants. This graph shows the histograms that compare the distribution of the least-squares eGFR slopes obtained by applying linear regression to individual participant with the Bayesian estimates obtained from the Bayesian linear mixed models.
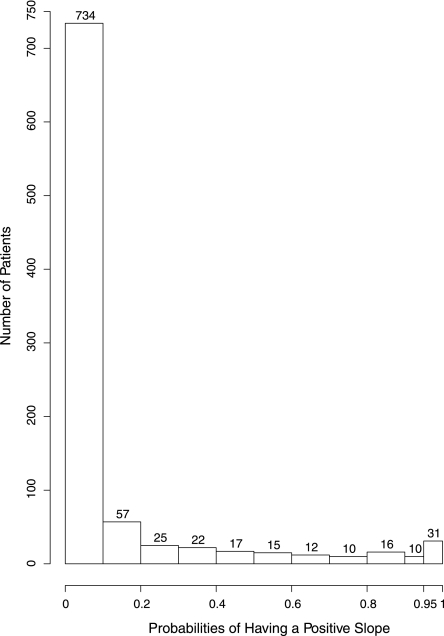
The Bayesian model is able to provide the probability that each patient’s true underlying slope is greater than zero. The distribution of these probabilities across the 949 patients is displayed in Figure 2. As shown, 31 patients had a probability of at least 0.95 of having a positive slope, 41 had a probability of at least 0.90 of having a positive slope, and 94 patients had a probability of at least 0.50 of having a positive slope.
Figure 2.
Probabilities of having a positive slope (being an improver). This graph shows the histogram of probabilities of having a positive slope (being an improver) for all participants. The height of each vertical bar represents the number of participants in that category. There are 31 patients with P≥0.95, and 94 patients with P≥0.5.
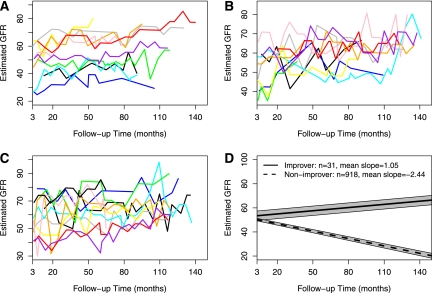
The individual eGFR trajectories and the mean eGFR linear regression line of these 31 participants are displayed in Figure 3, along with the mean eGFR regression line derived from the remaining cohort. The median follow-up time was 9.8 years (IQR, 7.6–10.7) for the 31 improvers. Baseline eGFR values were comparable for improvers and nonimprovers (53.2 versus 50.9 ml/min per 1.73 m2; P=0.53), but the eGFR slopes differed significantly during follow-up (1.06 versus −2.45 ml/min per 1.73 m2 per yr; P<0.001) (Table 1). Notably, this pattern was also evident during the trial phase as demonstrated by mean iGFR slope of 1.21 ml/min per 1.73 m2 per yr for improvers and −2.03 ml/min per 1.73m2 per yr for other participants (P<0.001). Baseline serum creatinine and changes followed a similar pattern as eGFR and iGFR. Improvers also had a smaller yearly change of proteinuria (trial phase data) than nonimprovers (1.1%/yr versus 13.8%/yr; P=0.01). The mean weight change was −0.33 kg/yr for improvers, compared with −0.15 kg/yr for nonimprovers (P=0.81).
Figure 3.
Estimated GFR (eGFR) trajectories of the 31 improvers. (A–C) eGFR trajectories of the 31 improvers are shown. (D) The solid straight line shows the mean progression pattern of the improvers, whereas the dashed straight line represents the mean progression pattern of the nonimprovers. Gray regions represent the confidence bands.
Table 1.
Mean slopes (yearly changes) of estimated GFR, iothalamate GFR, serum creatinine, and proteinuria by degrees of progression
| Estimate (SD) | ||
|---|---|---|
| Improvers (n=31) | Nonimprovers (n=918) | |
| Estimated GFR (ml/min per 1.73 m2 per yr) | 1.06 (0.12) | −2.45 (0.07) |
| Iothalamate GFRa (ml/min per 1.73 m2 per yr) | 1.21 (0.44) | −2.03 (0.11) |
| Serum creatinine (mg/dl per yr) | −0.04 (0.01) | 0.29 (0.02) |
| Urine protein/urine creatinine (%/yr)a | 1.1% (4.4) | 13.8% (0.9) |
| Weight (kg/yr) | −0.33 (0.67) | −0.15 (0.14) |
Data only available in the first 5 years.
The Bayesian mixed-effects model was also applied to the iGFR data available in the trial phase only. Of the 31 improvers identified from eGFR data, 24 (77%) also had >0.95 probability of having a positive slope in iGFR. The mean iGFR slope of these 24 improvers was 2.12 ml/min per 1.73 m2 per yr, compared with −2.02 ml/min per 1.73m2 per yr for the rest of the cohort (P<0.001).
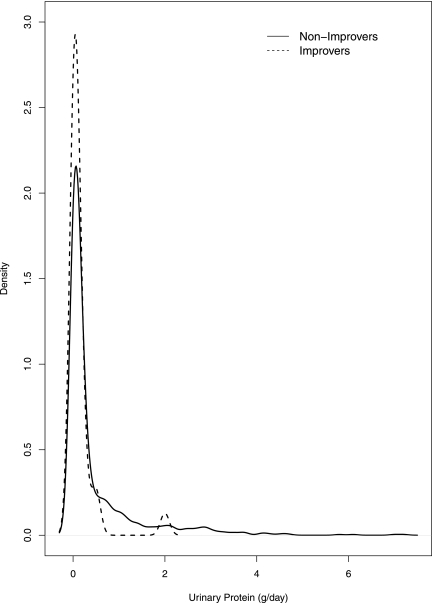
Baseline urinary protein, measured by spot and timed collection, was lower (median 0.10 g/d; IQR, 0–0.1; range, 0–2.0) among improvers than nonimprovers (median 0.10 g/d; IQR, 0–0.4; range. 0–7.2) (Table 2). The actual distributions of urinary protein are displayed in Figure 4. Baseline proteinuria, measured by the urinary protein/urinary creatinine ratio, was also lower among improvers than nonimprovers. Income and education level, BP, and biochemical measurements were similar in both groups. Baseline weight and randomized medications did not differ significantly, but a larger percentage of improvers received low BP control than nonimprovers (P=0.002) at randomization. There was a slight trend toward younger age in improvers compared with nonimprovers. The best-fitting multivariate model, as determined by Akaike Information Criterion (backward selection), included the randomization group and the following predictors: age, sex, BMI, weight, total cholesterol, and proteinuria. Lower age and proteinuria were statistically significantly associated with being an improver (odds ratio, 1.04; 95% confidence interval [95% CI], 1.00–1.09; P=0.05 per 1 year younger; and 1.36; 95% CI, 1.05–1.75; P=0.02 per 50% lower proteinuria).
Table 2.
Comparison of baseline characteristics between improvers and nonimprovers
| Characteristic | Nonimprovers (n=918) | Improvers (n=31) | P Value |
|---|---|---|---|
| Age (yr) | 54.82 (10.61) | 51.11 (10.61) | 0.07 |
| Female | 361 (39.3) | 9 (29) | 0.25 |
| Smoking status | |||
| current | 260 (28.3) | 12 (38.7) | 0.26 |
| past | 261 (28.4) | 10 (32.3) | |
| never | 397 (43.2) | 9 (29) | |
| Alcohol use | 248 (27.1) | 11 (35.5) | 0.31 |
| Income level ($) | |||
| <15,000 | 433 (47.2) | 16 (51.6) | 0.84 |
| >15,000 | 313 (34.1) | 9 (29) | |
| decline | 172 (18.7) | 6 (19.4) | |
| Education | |||
| less than high school diploma | 378 (41.2) | 12 (38.7) | 0.95 |
| high school diploma | 264 (28.8) | 9 (29) | |
| more than high school diploma | 275 (30) | 10 (32.3) | |
| History of heart disease | 466 (50.8) | 17 (54.8) | 0.66 |
| Duration of hypertension (yr) | 13.98 (10.05) | 14.32 (11.62) | 0.87 |
| Iothalamate GFR (ml/min per 1.73 m2) | 47.5 (13.5) | 50.0 (9.6) | 0.17 |
| Estimated GFR (ml/min per 1.73 m2) | 47.8 (14.0) | 50.6 (10.9) | 0.16 |
| Serum creatinine (mg/dl) | 1.97 (0.66) | 1.87 (0.49) | 0.27 |
| Systolic BP (mmHg) | 149.45 (23.53) | 152.68 (27.83) | 0.53 |
| Diastolic BP (mmHg) | 95.09 (14.01) | 97.61 (20.07) | 0.49 |
| BMI (kg/m2) | 30.65 (6.55) | 29.85 (5.76) | 0.46 |
| Weight (kg) | 89.69 (20.54) | 88.19 (19.96) | 0.68 |
| Total cholesterol (mg/dl) | 211.93 (44.12) | 198.97 (45.19) | 0.13 |
| HDL (mg/dl) | 48.71 (15.83) | 46.13 (13.09) | 0.29 |
| non-HDL (mg/dl) | 163.27 (43.64) | 152.7 (44.6) | 0.21 |
| triglyceride (mg/dl) | 135.76 (70.55) | 118.43 (46.93) | 0.10 |
| Albumin (g/dl) | 4.27 (0.31) | 4.28 (0.37) | 0.86 |
| Uric acid (mg/dl) | 8.23 (1.84) | 8.02 (1.93) | 0.54 |
| Phosphorus (mg/dl) | 3.51 (0.53) | 3.73 (0.97) | 0.22 |
| Urine protein (g/d)a | 0.10 (0–0.40) | 0.10 (0–0.10) | 0.01 |
| Ratio of urine protein/urine creatininea | 0.07 (0.03–0.29) | 0.04 (0.02–0.07) | 0.01 |
| Antihypertensive intervention | |||
| ACE inhibitor | 361 (39.3) | 14 (45.2) | 0.35 |
| β blocker | 382 (41.6) | 9 (29) | |
| calcium channel blocker | 175 (19.1) | 8 (25.8) | |
| BP goal | |||
| low (≤92 mmHg) | 449 (48.9) | 24 (77.4) | 0.002 |
| usual (102–108 mmHg) | 469 (51.1) | 7 (22.6) |
Mean (SD) or median (interquartile range) is provided for each continuous variable, and n (%) is provided for each categorical variable.
Wilcoxon rank-sum test was used.
Figure 4.
Distribution of urinary protein by improvers and nonimprovers. The solid line is the density curve of urinary protein for nonimprovers, whereas the dashed line is for improvers. Density curves are obtained using kernel-based smoothing method.
After exclusion of a single patient with an amputation below the knee (see Concise Methods), comparison of the remaining 30 improvers with the remaining cohort provided similar results to those described above.
Discussion
We observed that 10% of individuals (posterior probability of having positive eGFR slopes >0.50) in the cohort did not develop progressive nephropathy and 3% (posterior probability of having positive eGFR slopes >0.95) demonstrated clear clinical evidence of CKD improvement with extended follow-up of renal function in AASK participants. Furthermore, in this population with characteristically low-grade proteinuria, we determined that low urinary protein excretion at baseline was a significant predictor of improved kidney function (Table 2). The percentage of improvers was significantly higher for the low BP control group than the usual BP group. This result stands in contrast to the trial’s primary and main prespecified secondary analyses which showed no significant benefits in the full AASK cohort of the lower BP goal on GFR slope or on clinical events defined by ESRD, death, and either designated increases in serum creatinine or designated decreases in iothalamate GFR. The association between assignment to the lower BP goal and the proportion of improvers suggests that although the low BP control failed to slow progression in the full AASK cohort,11,12 it may benefit low-grade CKD patients. This post hoc finding, however, must be interpreted as exploratory and deserves further validation. Interestingly, other factors such as baseline GFR, BP, and socioeconomic indicators were not predictive of improvement in eGFR. On the basis of long-term follow-up of AASK participants, these results, in aggregate, provide strong evidence that kidney function can improve in some patients with hypertensive CKD.
Numerous studies over the last 2 decades have focused on predictors of CKD progression and interventions that slow the disease course.1,3,7,13–16 Among these studies, however, there is limited, if any, information characterizing subsets of study populations with improved renal function. The first unequivocal clinical evidence supporting the potential for CKD regression was demonstrated in patients with type 1 diabetes after pancreas transplantation. With correction of the metabolic milieu, proteinuria and histopathologic findings consistent with diabetic nephrosclerosis improved over 10 years. Renal function measured by creatinine clearances, however, did not improve. These results, however, are likely confounded by immune suppression therapy. In our study, we do not have renal biopsy data to document pathologic evidence of CKD regression. However, the average positive eGFR slope of approximately 1 ml/min per 1.73 m2 per yr, evaluated over 8–12 years and confirmed by measured iGFR in the trial phase, supports sustained improvement in GFR in a small subset of our participants. The course of renal function in this group is distinctly different from the remainder of the cohort, which progressed at an average rate of approximately −2.5 ml/min per 1.73 m2 per yr.
Overall, it is challenging to compare the percentage of AASK patients with nonprogressive disease to results described in other studies. The Modified Diet in Renal Disease (MDRD) study described 15% of the patients with stable or improved renal function as defined by least-squares GFR slopes that were ≥0. However, these findings were based on a relatively short follow-up period of 2.2 years, and did not account for the uncertainty in the estimated slopes due to measurement error and short-term biologic fluctuation. In the Ramipril Efficacy in Nephropathy follow-up trial with a mean follow-up of 28 months,17 the investigators demonstrated a positive GFR slope in 10 of 26 patients receiving prolonged ramipril therapy among patients with chronic nondiabetic glomerulopathies. Similarly, they also observed a significant reduction in proteinuria in parallel with the improvement in GFR. In this trial, however, improvement in GFR was delayed in most individuals by approximately 1 to 4 years after randomization. In addition, participants switched to ramipril therapy after the 36-month trial did not show improvement in GFR. In contrast, we did not observe a delay in positive GFR slope (Figure 3) and improvement in GFR was not linked to length of time on ramipril therapy (P=0.76). To our knowledge, there are no studies among patients with hypertensive nephrosclerosis to accurately compare the course of renal disease in African Americans versus other racial/ethnic groups. Potentially, the Chronic Renal Insufficiency Cohort Study,18 an on-going observational cohort study of over 3900 racially diverse patients with CKD including hypertensive nephrosclerosis, may help to further characterize patients with stabilization and regression of renal disease.
The renoprotective effects of ACE inhibitor therapy have been well described.3,13–16 Experimental data show that renin-angiotensin-aldosterone system blockade enhances repair mechanisms subsequently leading to histologic and functional regression of chronic renal injury.9,19,20 However, regression of CKD with these agents has not been well supported in clinical trials. The reduction in GFR from hemodynamic effects of renin-angiotensin-aldosterone system blockade may mask functional improvements. In addition, the duration and extent of chronic irreversible damage in disease manifested clinically may limit the potential for renal regeneration. In that regard, it is notable that there was a trend (P=0.07) toward younger age as a predictor of positive eGFR slope in the AASK cohort. This age-related trend may be due to a number of factors, including the possibility that younger individuals have less risk factor exposure leading to less irreversible damage and also greater regenerative potential.
Our findings link low baseline protein excretion in a non-glomerular disease population to CKD regression. Furthermore, we demonstrate that the percentages change in proteinuria is associated with improvement in eGFR (Table 1). This is an interesting finding as clinical trials show that the extent of reduction in proteinuria among patients with glomerulopathies is predictive of slower disease progression.3,14,17 Experimentally, proteinuria induces interstitial inflammation and fibrosis through multiple mechanisms and is considered an important mediator of progressive damage.21 However, the beneficial effects of reducing already low-grade protein excretion in nonglomerular diseases like hypertensive nephropathy may be limited.
Our study has limitations. First, we do not have renal biopsy confirmation that would link improvement in eGFR to histopathologic regression of CKD. In addition, we do not have measured GFR for the entire length of the study to confirm these results. However, measured GFR limited to the trial phase confirmed the improvement in eGFR for the majority of patients. Another potential alternate explanation for the apparent increase in kidney function is a systematic bias in the estimate of kidney function based on serum creatinine due to changes in body composition or diet. As far as we can discern, however, anthropomorphic and nutritional factors that may lead to changes in serum creatinine unrelated to renal function seem to be constant among individuals. To reduce any such bias, we reviewed serial weight measurements and excluded one participant with an amputation during the study. Although our statistical methods reduced the potential for random fluctuations to be considered a meaningful improvement in kidney function, we cannot wholly discount the possibility that some participants had a pattern of random errors that met our definition of “improving.” Lastly, as previously stated, the study population was limited to African Americans, and these results may not be generalized to other racial groups.
Our study also has several strengths. First, improvers were identified from eGFR data over an extended follow-up period up to 12 years. Second, longitudinal iGFR and proteinuria data likewise documented improvement. Third, a Bayesian method was used to produce slope estimates that have much smaller variability than the crude least-squares estimates used in other studies. The Bayesian method provides a more complete assessment of each patient’s slope by providing a full (posterior) probability distribution that accounts for the uncertainty in the slope estimate.
In conclusion, our study with long-term follow-up provides strong evidence that kidney function can improve in some patients with hypertensive CKD.
Concise Methods
Study Population
This was a multicenter, randomized clinical trial of African American individuals who were aged 18–70 years, and had a GFR between 20 and 65 ml/min per 1.73 m2 at enrollment. The trial enrolled 1094 participants who were randomly assigned according to a 3×2 factorial design to one of three initial drug therapies for hypertension (ramipril, amlodipine, or metoprolol), and to one of two levels of BP control (mean arterial pressure ≤92 mmHg or 102–107 mmHg). The trial ended in September 2001, and the main results have been published elsewhere.11,13 From 787 participants alive and not on dialysis at the end of trial, 691 enrolled in the cohort study for an additional follow-up of 5 years.22 Institutional review boards at each center approved the protocol and procedures, and all participants gave written informed consent.
Measured and eGFR
GFR was assessed by renal clearance of 125I-iothalamate twice at baseline, and at 3, 6 months, and then every 6 months thereafter up to 5 years.23 From measured GFR data, an equation was derived to estimate GFR using serum creatinine24: eGFR = 329 × (serum creatinine)−1.096 × (age)−0.294 × (0.736 for female). For study participants, this equation was more accurate than the MDRD formula. The eGFR formula was then used for longitudinal assessment of renal function across both the trial and cohort phases of AASK.
To avoid the acute, hemodynamic changes in GFR related to drug intervention,11,13 we focused on chronic eGFR slopes, which were calculated from 3 months postrandomization onward. Those AASK participants with <3 eGFR measurements after the first 3-month follow-up visit were excluded (n=145). Therefore, 949 of 1094 participants were included in this analysis, with a median follow-up of 8.8 years.
Statistical Analyses
The standard method for estimating the eGFR slope of an individual patient is to determine the regression line that is closest to that patient’s eGFR measurements in the sense of minimizing the sum of the squared deviations of the individual eGFR measurements from the line. Slopes computed in this way are called least-squares slopes. Due to measurement error and other extraneous short-term biologic variation, the least-squares slopes tend to be more variable over a population than the underlying true slopes that they are intended to estimate. Hence, direct evaluation of the fraction of patients with positive least-squares slopes will overestimate the proportion of patients whose true long-term slopes are positive. To control for this bias, we applied Bayesian linear mixed-effects models to estimate the distributions of the underlying “true” eGFR slopes of the participants after accounting for this extraneous short-term variability. We estimated a “posterior” mean slope for each patient on the basis of the Bayesian model described below, which can be interpreted as the mean of the slopes that are compatible with the data under our assumed model, as well as the posterior probability that the true underlying slope is >0. We classified patients as having a “clear positive slope” if the posterior probability that their true slope is >0 exceeded 0.95.
Technically, for each participant, the eGFR trajectory was modeled as a linear function of time with a patient-specific intercept and slope. The residual variance of the individual eGFR values about the patient’s regression lines were assumed to be proportional to the mean eGFR level, to account for greater variability at higher eGFR levels.25 A hierarchical prior with two levels was specified for the patient-specific intercept and slope. At the first level, the patient-specific intercept and slope were assumed to follow a bivariate normal distribution. At the second level, the mean intercept and slope were assumed to follow noninformative uniform distributions in order to assure that our results reflect the data rather that our prior assumptions. The covariance matrix of the bivariate normal distribution follows a Wishart prior distribution with two degrees of freedom. Other parameters in the mixed model were assumed to follow independently improper uniform prior distributions. On the basis of this Bayesian model, a Gibbs sampling algorithm was used to simulate posterior samples of the eGFR slopes from the joint poster distribution. The posterior means and the probabilities of having positive slopes were estimated based on these posterior samples.
Study clinicians reviewed a summary including primary and secondary causes of hospitalizations and serial measurements of weight, BP, total cholesterol and serum albumin to determine if any of the patients with clear positive eGFR slopes had clinical events that were likely to invalidate the creatinine-based estimates of GFR. This review led to the designation of one patient with a below-the-knee amputation as having potentially problematic eGFR values.
Baseline clinical and demographic variables were summarized as means and SDs (or medians and interquartile ranges) for continuous variables and as frequencies and percentages for categorical variables. Each variable was then compared between improvers and nonimprovers by using t tests, Wilcoxon rank-sum tests or chi-squared tests as appropriate. Linear mixed-effects models were used to assess and compare the changes of other biomarkers that were measured longitudinally. All statistical analyses were performed using SAS 9.1.3 (Cary, NC) and WinBUGS 1.4.3 software.
Disclosures
None.
Acknowledgments
A special acknowledgment is extended to the AASK participants for their time and commitment to the trial and cohort.
This study was supported by Grants U01 DK048652, UL1 RR024989, and R01 DK090046-01 from the National Institutes of Health (NIH). In addition to funding under a cooperative agreement from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), this work was supported in part by the following institutional General Clinical Research Center and other NIH grants: M01 RR-00080, 5M01 RR-00071, M0100032, P20-RR11145, M01 RR00827, M01 RR00052, 2P20 RR11104, and DK 2818-02. In addition, we gratefully acknowledge the donation of drug and some financial support to NIDDK by Pfizer, AstraZeneca, and King Pharmaceuticals.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Improvement in Kidney Function: A Real Occurrence,” on pages 575–577.
References
- 1.Hunsicker LG, Adler S, Caggiula A, England BK, Greene T, Kusek JW, Rogers NL, Teschan PE: Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int 51: 1908–1919, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, de Jong PE, de Zeeuw D, Shahinfar S, Toto R, Levey AS. AIPRD Study Group: Progression of chronic kidney disease: The role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: A patient-level meta-analysis. Ann Intern Med 139: 244–252, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Ruggenenti P, Perna A, Remuzzi G. Gruppo Italiano di Studi Epidemiologici in Nefrologia: ACE inhibitors to prevent end-stage renal disease: When to start and why possibly never to stop: A post hoc analysis of the REIN trial results. Ramipril Efficacy in Nephropathy. J Am Soc Nephrol 12: 2832–2837, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Hsu CY, Lin F, Vittinghoff E, Shlipak MG: Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol 14: 2902–2907, 2003 [DOI] [PubMed] [Google Scholar]
- 5.McClellan W, Warnock DG, McClure L, Campbell RC, Newsome BB, Howard V, Cushman M, Howard G: Racial differences in the prevalence of chronic kidney disease among participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Cohort Study. J Am Soc Nephrol 17: 1710–1715, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Tarver-Carr ME, Powe NR, Eberhardt MS, LaVeist TA, Kington RS, Coresh J, Brancati FL: Excess risk of chronic kidney disease among African-American versus white subjects in the United States: A population-based study of potential explanatory factors. J Am Soc Nephrol 13: 2363–2370, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Eriksen BO, Ingebretsen OC: The progression of chronic kidney disease: A 10-year population-based study of the effects of gender and age. Kidney Int 69: 375–382, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M: Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 339: 69–75, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Fogo AB: Progression and potential regression of glomerulosclerosis. Kidney Int 59: 804–819, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Sika M, Lewis J, Douglas J, Erlinger T, Dowie D, Lipkowitz M, Lash J, Cornish-Zirker D, Peterson G, Toto R, Kusek J, Appel L, Kendrick C, Gassman J; AASK group: Baseline characteristics of participants in the African American Study of Kidney Disease and Hypertension (AASK) Clinical Trial and Cohort Study. Am J Kidney Dis 50: 78–89, e1, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Wright JT, Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG. African American Study of Kidney Disease and Hypertension Study Group: Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: Results from the AASK trial. JAMA 288: 2421–2431, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Appel LJ, Wright JT, Jr, Greene T, Agodoa LY, Astor BC, Bakris GL, Cleveland WH, Charleston J, Contreras G, Faulkner ML, Gabbai FB, Gassman JJ, Hebert LA, Jamerson KA, Kopple JD, Kusek JW, Lash JP, Lea JP, Lewis JB, Lipkowitz MS, Massry SG, Miller ER, Norris K, Phillips RA, Pogue VA, Randall OS, Rostand SG, Smogorzewski MJ, Toto RD, Wang X. AASK Collaborative Research Group: Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 363: 918–929, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agodoa LY, Appel L, Bakris GL, Beck G, Bourgoignie J, Briggs JP, Charleston J, Cheek D, Cleveland W, Douglas JG, Douglas M, Dowie D, Faulkner M, Gabriel A, Gassman J, Greene T, Hall Y, Hebert L, Hiremath L, Jamerson K, Johnson CJ, Kopple J, Kusek J, Lash J, Lea J, Lewis JB, Lipkowitz M, Massry S, Middleton J, Miller ER, 3rd, Norris K, O’Connor D, Ojo A, Phillips RA, Pogue V, Rahman M, Randall OS, Rostand S, Schulman G, Smith W, Thornley-Brown D, Tisher CC, Toto RD, Wright JT, Jr, Xu S. African American Study of Kidney Disease and Hypertension (AASK) Study Group: Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: A randomized controlled trial. JAMA 285: 2719–2728, 2001 [DOI] [PubMed] [Google Scholar]
- 14.The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia) : Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet 349: 1857–1863, 1997 [PubMed] [Google Scholar]
- 15.Jafar TH, Schmid CH, Landa M, Giatras I, Toto R, Remuzzi G, Maschio G, Brenner BM, Kamper A, Zucchelli P, Becker G, Himmelmann A, Bannister K, Landais P, Shahinfar S, de Jong PE, de Zeeuw D, Lau J, Levey AS: Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med 135: 73–87, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. RENAAL Study Investigators: Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Ruggenenti P, Perna A, Benini R, Bertani T, Zoccali C, Maggiore Q, Salvadori M, Remuzzi G: In chronic nephropathies prolonged ACE inhibition can induce remission: Dynamics of time-dependent changes in GFR. Investigators of the GISEN Group. Gruppo Italiano Studi Epidemiologici in Nefrologia. J Am Soc Nephrol 10: 997–1006, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER, 3rd, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT. Chronic Renal Insufficiency Cohort (CRIC) Study Investigators: The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and methods. J Am Soc Nephrol 14[Suppl 2]: S148–S153, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Aldigier JC, Kanjanbuch T, Ma LJ, Brown NJ, Fogo AB: Regression of existing glomerulosclerosis by inhibition of aldosterone. J Am Soc Nephrol 16: 3306–3314, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Zoja C, Corna D, Camozzi D, Cattaneo D, Rottoli D, Batani C, Zanchi C, Abbate M, Remuzzi G: How to fully protect the kidney in a severe model of progressive nephropathy: A multidrug approach. J Am Soc Nephrol 13: 2898–2908, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Abbate M, Zoja C, Rottoli D, Corna D, Tomasoni S, Remuzzi G: Proximal tubular cells promote fibrogenesis by TGF-beta1-mediated induction of peritubular myofibroblasts. Kidney Int 61: 2066–2077, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Appel LJ, Middleton J, Miller ER, 3rd, Lipkowitz M, Norris K, Agodoa LY, Bakris G, Douglas JG, Charleston J, Gassman J, Greene T, Jamerson K, Kusek JW, Lewis JA, Phillips RA, Rostand SG, Wright JT: The rationale and design of the AASK cohort study. J Am Soc Nephrol 14[Suppl 2]: S166–S172, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Gassman JJ, Greene T, Wright JT, Jr, Agodoa L, Bakris G, Beck GJ, Douglas J, Jamerson K, Lewis J, Kutner M, Randall OS, Wang SR: Design and statistical aspects of the African American Study of Kidney Disease and Hypertension (AASK). J Am Soc Nephrol 14[Suppl 2]: S154–S165, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis J, Agodoa L, Cheek D, Greene T, Middleton J, O’Connor D, Ojo A, Phillips R, Sika M, Wright J., Jr African-American Study of Hypertension and Kidney Disease: Comparison of cross-sectional renal function measurements in African Americans with hypertensive nephrosclerosis and of primary formulas to estimate glomerular filtration rate. Am J Kidney Dis 38: 744–753, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Pinheiro J, Bates D: Mixed-Effects Models in S and S-PLUS, New York, Springer Verlag, 2000 [Google Scholar]