Abstract
Excess soluble fms-like tyrosine kinase 1 (sFlt-1) of vascular endothelial growth factor receptor 1 secreted from the placenta causes pre-eclampsia–like features by antagonizing vascular endothelial growth factor signaling, which can lead to reduced endothelial nitric oxide synthase (eNOS) activity; the effect of this concomitant decrease in eNOS activity is unknown. We tested whether the decrease in nitric oxide occurring in female mice lacking eNOS aggravates the pre-eclampsia–like phenotype induced by increased sFlt-1. Untreated eNOS-deficient female mice had higher BP than wild-type mice. Adenovirus-mediated overexpression of sFlt-1 increased systolic BP by approximately 27 mmHg and led to severe loss of fenestration of glomerular capillary endothelial cells in both eNOS-deficient and wild-type mice. However, only the eNOS-deficient sFlt-1 mice exhibited severe foot process effacement. Compared with wild-type sFlt-1 mice, eNOS-deficient sFlt-1 mice also showed markedly higher urinary albumin excretion (467±74 versus 174±23 μg/d), lower creatinine clearance (126±29 versus 452±63 μl/min), and more severe endotheliosis. Expression of preproendothelin-1 (ET-1) and its ETA receptor in the kidney was higher in eNOS-deficient sFlt-1 mice than in wild-type sFlt-1 mice. Furthermore, the selective ETA receptor antagonist ambrisentan attenuated the increases in BP and urinary albumin excretion and ameliorated endotheliosis in both wild-type and eNOS-deficient sFlt-1 mice. Ambrisentan improved creatinine clearance and podocyte effacement in eNOS-deficient sFlt-1 mice. In conclusion, reduced maternal eNOS/nitric oxide exacerbates the sFlt1-related pre-eclampsia–like phenotype through activation of the endothelin system.
Pre-eclampsia is a pregnancy-related disease characterized by high BP and proteinuria, and affects 3%–5% of all pregnancies.1,2 Although the etiology of pre-eclampsia is not fully understood, it is widely accepted that placental hypoxia/ischemia increases the production by the placenta of soluble fms-like tyrosine kinase 1 (sFlt-1) and soluble endoglin, and likely other factors.1 Increased sFlt-1 is recognized as one of the most important causal factors for maternal symptoms of pre-eclampsia.1 sFlt-1 is a splice variant of vascular endothelial growth factor (VEGF) receptor-1 that lacks the cytoplasmic and transmembrane domains, but retains the ligand-binding domain. sFlt-1 decreases the chance of VEGF binding to its signaling receptor, and reduces the phosphorylation of Ser 1177 of endothelial nitric oxide synthase (eNOS) by VEGF and the activity of eNOS.3 Plasma concentration of sFlt-1 is higher in pregnant women with pre-eclampsia than in normal pregnant women, and is higher in severe pre-eclampsia than in mild pre-eclampsia.4 Both pregnant and nonpregnant rats overexpressing sFlt-1 protein have high BP, proteinuria, and endotheliosis, the hallmarks of pre-eclampsia.5
Although pre-eclampsia is a placenta-related disease, not all pregnant women with low placental perfusion develop pre-eclampsia,6 and women who have had pre-eclampsia often develop cardiovascular diseases later in life,7,8 suggesting that maternal factors can predispose to pre-eclampsia.8,9 Pregnant women with pre-existing microvascular diseases, such as hypertension, diabetes, and insulin resistance, have an increased risk of pre-eclampsia.8,10,11 Mice lacking eNOS have high BP, insulin resistance, hyperlipidemia, and decreased production of nitric oxide (NO).12 Some, but not all, investigators report that NOS3 polymorphisms leading to lower NO production are associated with hypertension13 and/or pre-eclampsia.14,15 Thus, it is likely but not clear whether reduced eNOS/NO causes an exacerbation of pre-eclampsia. Here, we demonstrate that lack of eNOS aggravates the pre-eclampsia–like phenotype induced by increased sFlt-1 in nonpregnant female mice. We also show that upregulation of the ET system is responsible for the pre-eclampsia–like phenotype induced by increased sFlt-1 in mice.
Results
General Effects of Increased sFlt-1 in eNOS−/− Mice
Adenovirus (Ad sFlt-1 or Ad Fc, 3×109 PFU) was injected into nonpregnant female eNOS−/− and wild-type (WT) mice. Starting 4 days after administration, individual mice were housed in metabolic cages, and body weight, daily food and water intake, and urine output were measured every 24 hours for 2 days. Overexpressing sFlt-1 did not significantly affect body weight and water intake (Table 1). Food intake of eNOS−/− sFlt-1 mice was lower than that of WT sFlt-1 mice, although it did not reach statistical significance. eNOS−/− sFlt-1 mice had significantly less urine volume than WT sFlt-1 mice, although the levels of plasma sFlt-1 were similar between the two genotypes (Table 1).
Table 1.
Characteristics of eNOS−/− and WT mice with Ad sFlt-1 or Ad Fc
| Body Weight (g) | Food Intake (g/d) | Water Intake (ml/d) | Urine Volume (ml/d) | Plasma sFlt-1 (ng/ml) | Plasma Cystatin C (μg/ml) | Plasma Albumin (g/dl) | Plasma Cholesterol (mg/dl) | Hematocrit (%) | |
|---|---|---|---|---|---|---|---|---|---|
| WT | 22.7±1.3 | 4.0±0.4 | 3.6±0.5 | 1.5±0.2 | 3.7±0.8 | Undetectablea | 2.68±0.05 | 104.4±3.5 | 45.2±1.3 |
| eNOS−/− | 20.1±1.2 | 4.2±0.2 | 4.4±0.2 | 1.0±0.1 | 3.8±1.3 | Undetectablea | 2.74±0.07 | 119.0±6.8 | 47.2±0.4 |
| WT sFlt-1 | 21.3±1.1 | 3.5±0.4 | 2.5±0.7 | 0.8±0.3b | 48.0±0.7b | 0.16±0.03 | 1.94±0.26b | 197.9±23.3b | 47.8±4.5 |
| eNOS−/− sFlt-1 | 21.2±0.8 | 2.5±0.5 | 2.5±0.6 | 0.2±0.1b,c | 47.9±0.8b | 0.77±0.24c | 1.10±0.07b,c | 242.5±7.5b,c | 60.0±2.3b,c |
WT sFlt-1 and eNOS−/− sFlt-1, WT and eNOS−/− mice received adenovirus (3×109 PFU) to overexpress sFlt-1; WT and eNOS−/−, WT and eNOS mice received Ad Fc. Body weight, food intake, water intake, and urine volume were obtained 6 days after administration of adenovirus. The other data were obtained from the blood samples at sacrifice after metabolic cage study. Data are expressed as mean ± SEM (n=6–8).
Concentrations below detectable levels (<0.05 μg/ml).
P<0.05 versus the same genotype that received Ad Fc.
P<0.05 versus WT sFlt-1.
Because hypertension is one of the diagnostic criteria of pre-eclampsia, we used telemetry to measure BP at the aortic arch. Nonpregnant female eNOS−/− mice had 18 mmHg higher systolic BP (SBP) and diastolic BP (DBP) than WT mice (144.2±3.3 versus 126.0±2.6 mmHg for SBP, P=0.003; and 104.3±3.5 versus 97.1±1.9 mmHg for DBP, P=0.07). sFlt-1 virus increased SBP of both eNOS−/− and WT mice by 27 mmHg (171.5±7.9 and 153.2±5.2 mmHg, P=0.03) (Figure 1A). Ad Fc did not affect BP (not shown). We conclude that sFlt-1 overexpression increases BP similarly in WT and eNOS−/− mice.
Figure 1.
Increased sFlt-1 expression increases BP and UAE in WT and eNOS−/− nonpregnant mice, and decreases CCr eNOS−/− nonpregnant mice. (A) SBP and DBP after administration of Ad sFlt-1 in both WT and eNOS−/− mice. *Significantly (P<0.05) higher by Dunnett’s test than mean of BP during the control period (before Ad virus administration). (B and C) UAE and CCr 6 days after administration of Ad Fc or Ad sFlt-1. Data are expressed as mean ± SEM in this and all subsequent figures, unless indicated otherwise. n≥5 for each group.
eNOS−/− sFlt-1 Mice Have Higher Urinary Albumin Excretion and Lower Creatinine Clearance
We next tested the effects of increased sFlt-1 on urinary albumin excretion (UAE) and creatinine clearance (CCr). Control virus (Ad Fc) did not change daily UAE in mice of each genotype. sFlt-1 virus increased UAE almost 6 times in WT mice (from 27±4 to 174±23 μg/d, P<0.0001), and about 17 times in eNOS−/− mice (from 28±5 to 467±74 μg/d, P<0.0001) (Figure 1B). The increased sFlt-1 and lack of eNOS synergistically increased UAE (P<0.0001 for interaction) (Supplemental Table S1). Increased sFlt-1 decreased CCr only in the eNOS−/− mice (from 410±48 to 126±29 μl/min, P=0.003) (Figure 1C). Again, there was synergistic interaction between sFlt-1 and lack of eNOS (P=0.014 for interaction) (Supplemental Table S1). Plasma concentrations of cystatin C were below detectable levels (<0.05 μg/ml) in mice without sFlt-1 overexpression, but eNOS−/− sFlt-1 mice had significantly higher plasma cystatin C levels than WT sFlt-1 mice (Table 1). An increase in sFlt-1 also decreased plasma concentration of albumin and increased that of total cholesterol in both WT and eNOS−/− mice, to a greater extent in eNOS−/− mice (Table 1). Hematocrit increased only in eNOS−/− sFlt-1 mice (Table 1). We conclude that lack of eNOS and sFlt-1 synergistically exacerbates the increase in UAE and the decrease in GFR in nonpregnant female mice.
eNOS−/− sFlt-1 Mice Have Severe Endotheliosis and Foot Process Effacement
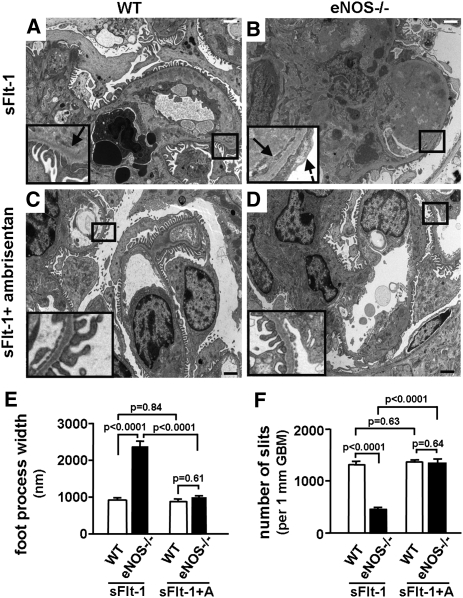
Glomerular endotheliosis is a characteristic feature in biopsies of pre-eclamptic kidneys.16 Light microscopy showed that eNOS−/− and WT mice that received Ad Fc virus did not show any endotheliosis, and did not differ in glomerular open capillary volume (Figure 2, A, B, and E). In contrast, mice receiving the Ad sFlt-1 virus developed endotheliosis. Endotheliosis of eNOS−/− sFlt-1 mice was markedly more severe than that of WT sFlt-1 mice (glomerular open capillary volume: 13.1%±1.8% versus 26.6%±1.6%, P=0.0003) (Figure 2, C through E). There was strong synergism between sFlt-1 and lack of eNOS (P<0.0001 for interaction) (Supplemental Table S1). Electron microscopy confirmed the swelling and loss of fenestrae of glomerular capillary endothelial cells in both WT and eNOS−/− mice with increased sFlt-1 (Figure 3). Strikingly, eNOS−/− sFlt-1 mice also had severe foot process effacement, as shown by the increase in foot process width (FPW) and the decrease in number of slits in glomerular basement membrane (GBM) (FPW: 2334±233 in eNOS−/− sFlt-1 mice versus 764±37 nm in WT sFlt-1 mice, P<0.0001; number of slits: 455±46/mm GBM in eNOS−/− sFlt-1 mice versus 1328±68 in WT sFlt-1 mice, P<0.0001) (Figure 3, C through F). There was strong synergism between lack of eNOS and sFlt-1 (P=0.0017 and P=0.0099, respectively, for interaction) (Supplemental Table S1). We conclude that the absence of eNOS markedly and synergistically aggravates structural changes in glomeruli induced by increased sFlt-1, particularly those involving podocytes.
Figure 2.
eNOS−/− mice with increased sFlt-1 show more severe endotheliosis than WT sFlt-1 mice. Representative glomeruli from (A) WT Fc mice, (B) eNOS−/− Fc mice, (C) WT sFlt-1 mice, and (D) eNOS−/− sFlt-1 mice. Periodic acid–Schiff stain. (E) Quantification of glomerular open capillary volume (index of endotheliosis). n≥5 for each group. Bars, 20 μm.
Figure 3.
Both WT and eNOS−/− mice with increased sFlt-1 show loss of glomerular endothelial fenestration, and eNOS−/− sFlt-1 mice have effacement of foot processes. Electron micrographs of glomeruli from (A) WT Fc mice, (B) eNOS−/− Fc mice, (C) WT sFlt-1 mice, and (D) eNOS sFlt-1 mice. Arrow: the loss of glomerular endothelial fenestration; broken arrows: effacement of foot process. Insets: higher magnification of demarcated boxes showing podocytes, endothelial cells, and GBM. (E) FPW. (F) Number of slits in 1-mm GBM. Data were obtained from electron micrographs and calculated as described in the Concise Methods. n≥5 for each group. Bars, 2 μm.
Upregulation of PreproET-1 and ETA Receptor in eNOS−/− sFlt-1 Mice
Women with pre-eclampsia have elevated levels of plasma ET-1, which suggests that the ET system is involved in the pathogenesis of pre-eclampsia.17–19 Because NO inhibits ET-1 expression,20,21 this suggests that decreased eNOS/NO might exacerbate pre-eclampsia by upregulating ET-1. To test this, we first determined the level of preproET-1 mRNA expression in the kidneys, which did not differ in mice with Ad Fc virus (Figure 4A). sFlt-1 virus increased preproET-1 mRNA expression 2.7 times in WT mice (from 1.00±0.09 to 2.74±0.31, P=0.01) and about 6 times in eNOS−/− mice (from 0.82±0.04-fold to 5.00±0.89-fold, P<0.0001) (Figure 4A). The increased sFlt-1 and lack of eNOS acted synergistically on increasing preproET-1 mRNA expression (P=0.01 for interaction) (Supplemental Table S1). The expression of ETAR did not differ in mice with Ad Fc virus (Figure 4B). Elevated sFlt-1 increased ETAR mRNA expression 3.4 times in WT mice (from 1.00±0.17 to 3.44±0.65, P=0.01) and about 3.7 times in eNOS−/− mice (from 1.65±0.35-fold to 6.04±1.13-fold, P<0.0001) (Figure 4B). We conclude that absence of eNOS and elevated sFlt-1 increase the expression of preproET-1 synergistically and that of ETAR additionally in the kidney (Supplemental Table S1).
Figure 4.
Upregulation of renal preproET-1 and ETA receptor in the eNOS−/− mice with excess sFlt-1. mRNA levels of (A) preproET-1 and (B) ETA receptor in the kidney of WT and eNOS−/− mice administered with Ad Fc or Ad sFlt-1. RNA was prepared from the whole kidney. n≥5 for each group.
Role of ET-1 and ETAR in Pathogenesis of Pre-Eclampsia
To investigate the role of ET-1 in the sFlt-1–induced pre-eclampsia–like phenotype, low-dose ET-1 (5 pmol/kg per min) that does not affect systemic BP was administered to nonpregnant WT female sFlt-1 mice. ET-1 increased UAE about two times, decreased CCr, and glomerular open capillary volume (16.9%±1.2%) to the levels of eNOS−/− sFlt-1 mice, and caused podocyte foot process effacement (Supplemental Figures 1 and 2). ET-1 increased FPW (3290±1150 nm) and decreased the number of slits (442±154/mm GBM), which are indistinguishable from those of eNOS−/− sFlt-1 mice. To investigate the role of ETAR in pre-eclampsia, mice were treated with a selective ETAR antagonist, ambrisentan, starting 1 day before adenovirus administration and continuing until the end of the experiment. Ambrisentan reduced the increase in BP caused by sFlt-1 (Figure 5A). As shown in Figure 1, Ad sFlt-1 increased UAE about 6 times in WT mice and almost 17 times in eNOS−/− mice. Starting from these higher levels, ambrisentan decreased to 33% UAE in WT sFlt-1 mice (from 174±23 to 58±20 μg/d, P=0.01), and to 45% UAE in eNOS sFlt-1 mice (from 467±74 to 212±78 μg/d, P<0.0001) (Figure 5B). Ambrisentan corrected the reduced CCr in eNOS−/− sFlt-1 mice (from 126±29 to 368±53 μl/min, P=0.004) (Figure 5C). Morphologic studies showed that ambrisentan improved endotheliosis in both WT sFlt-1 (glomerular open capillary volume increased from 26.6%±1.6% to 35.1%±1.8%, P<0.001) and eNOS sFlt-1 mice (from 13.1%±2.9% to 24.8%±1.9%, P<0.001) (Figure 6). Electron microscopy showed that ambrisentan corrected foot process effacement, FPW, and the number of slits in eNOS−/− sFlt-1 mice (Figure 7). We conclude that ET-1 is responsible for the exacerbation of pre-eclampsia–like phenotype in both WT and eNOS−/− mice, and that inhibiting ETAR improves pathologic changes caused by increased sFlt-1.
Figure 5.
Effects of a selective ETA receptor antagonist ambrisentan on SBP, UAE, and CCr. (A) Ambrisentan significantly decreases SBP of WT sFlt-1 and eNOS−/− sFlt-1 mice. (B) Ambrisentan decreases daily UAE more in eNOS−/− sFlt-1 mice than in WT sFlt-1 mice. (C) Ambrisentan increases CCr in eNOS−/− sFlt-1 mice. n≥5 for each group.
Figure 6.
Ambrisentan improves endotheliosis in WT sFlt-1 and eNOS−/− sFlt-1 mice. (Upper panels) Representative photographs of glomeruli from WT sFlt-1 and eNOS−/− sFlt-1 mice with and without ambrisentan treatment. Periodic acid–Schiff stain. (Lower panel) Quantification of glomerular open capillary volume. n≥5 for each group. Bars, 20 μm.
Figure 7.
Ambrisentan prevents loss of endothelial fenestration and effacement of podocyte foot processes. (A–D) Representative electron micrographs of glomeruli from WT sFlt-1 and eNOS−/− sFlt-1 mice with and without ambrisentan treatment. Arrow: the loss of endothelial fenestration; broken arrows: effacement of foot process. Insets: higher magnification of demarcated boxes showing podocytes, endothelial cells and GBM. (E) FPW. (F) Number of slits in 1-mm GBM. Data were obtained from electron micrographs and calculated as described in the Concise Methods. n≥5 for each group. Bars, 2 μm.
Discussion
In several animal models, sFlt-1 induces a pre-eclampsia–like phenotype, which includes endothelial dysfunction, elevated BP, and proteinuria.5,22 Our present study demonstrates that increased sFlt-1 causes a more severe pre-eclampsia–like phenotype in mice that are also deficient in eNOS than in WT mice. We show that increased sFlt-1 combined with absence of eNOS synergistically aggravates UAE, decreases CCr, and causes more severe endotheliosis. In addition, the synergism between increased sFlt-1 and absence of eNOS causes foot process effacement in eNOS−/− sFlt-1 mice not seen in WT sFlt-1 mice. Elevated sFlt-1 increases the renal expression of the ET system more in the eNOS−/− mice than in WT mice. ET-1 exacerbates and ambrisentan improves the pathologic changes caused by increased sFlt-1.
Elevation of BP is the key feature of pre-eclampsia.23 In our study, sFlt-1 increased BP of both WT and eNOS−/− mice by keeping the difference in SBP similar before and after increases in sFlt-1 (Figure 1A). ET-1 is one of the most potent vasoconstrictors and is associated with pre-eclampsia.17–19 sFlt-1 and lack of eNOS both increase the expression of preproET-1 and ETAR in the kidney, and eNOS−/− sFlt-1 mice have the highest expression of these (Figure 4). A selective ETAR antagonist ambrisentan abolished about two-thirds of elevated BP by sFlt-1 in both WT and eNOS−/− mice, indicating that ET plays a major role in increases in BP of pre-eclampsia (Figure 5A), but that the difference in BP between WT and eNOS−/− mice is ET independent. Our data are consistent with the report from Murphy et al. showing that blockade of ETAR using ABT-627/atrasentan (5 mg/kg per d via drinking water) abolished the increase in BP by sFlt-1 administration in pregnant rats.24
Proteinuria is another key feature of pre-eclampsia.23 We have previously proposed a theory that the decrease in GFR causes an increase in albuminuria.25 In this study, WT sFlt-1 mice excreted approximately 6 times WT Fc control levels of albuminuria without affecting CCr, whereas eNOS−/− sFlt-1 mice excreted about 17 times eNOS−/− Fc levels of albuminuria with 4 times the decrease in CCr (Figure 1, B and C). Although the glomerular damage in WT sFlt-1 mice was not sufficient to decrease CCr, plasma cystatin C levels of WT sFlt-1 mice were at least three times higher than those of WT mice without increased sFlt-1 (Table 1), suggesting that increased sFlt-1 decreases GFR of WT mice, probably due to endotheliosis and loss of glomerular endothelial fenestrae (Figures 2 and 3).
When an increase in sFlt-1 is combined with the absence of eNOS, CCr is now decreased, plasma cystatin C is increased to at least 15 times of mice without an increase in sFlt-1, and UAE is markedly increased (Table 1, Figure 1). In eNOS−/− sFlt-1 mice, endotheliosis is enhanced and foot process effacement is remarkable (Figures 2 and 3). Loss of fenestrae and foot process effacement decrease the flow of water and small molecules through glomerular capillary fenestrae and slit diaphragm, thereby decreasing GFR.25 The severe albuminuria leads to decreased plasma albumin levels and increased plasma total cholesterol levels, leading to volume contraction as indicated by increased Hct. Accordingly, severe renal failure of eNOS−/− sFlt-1 mice as depicted by low daily urine volume without a decrease in water intake compared with WT sFlt-1 mice (Table 1) is partly prerenal.
Treatment with ambrisentan decreases UAE and ameliorates endotheliosis in both WT sFlt-1 and eNOS−/− sFlt-1 mice, and corrects CCr and foot process effacement in eNOS−/− sFlt-1 mice (Figures 5 through 7). Moreover, WT sFlt-1 mice treated with ET-1 develop renal phenotypes similar to eNOS−/− sFlt-1 mice, evidenced by more severe albuminuria, decreased CCr, severe endotheliosis, and foot process effacement (Supplemental Figures S1 and S2). Our results are consistent with studies showing the association of ET-1 with pre-eclampsia17–19 and with a recent study showing that sFlt-1 increases ET-1 expression in glomerular endothelial cells,26 that ET-1 binds to its receptor in podocytes and causes nephrin shedding and proteinuria, and that an ETA receptor antagonist N-acetyl-[D-TRP16]-ET1 fragment 16-21 abolishes these serial effects.26 Taken together, our results indicate that the activated ET system is responsible for pre-eclampsia–like phenotypes observed in WT sFlt-1 and eNOS−/− sFlt-1 mice. Because ambrisentan decreases the increase in BP by sFlt-1, it cannot be ruled out that the enhanced effect of sFlt-1 on albuminuria observed in the eNOS−/− mice could, in part, be due to their higher BP. Our findings are also consistent with recent reports of the ET-1 pathway being a critical downstream mediator of sunitinib (VEGF inhibitor)–related vascular disease.27
In humans with pre-eclampsia, although a majority of patients get better after delivery, focal glomerulosclerosis leading to end stage kidney disease is noted in some patients with severe disease.28,29 Our findings of foot process effacement and impaired renal function in eNOS−/− sFlt-1 mice suggest that pre-existing vascular endothelial dysfunction may be an important determinant of CKD in women with pre-eclampsia. Elsewhere, we have presented calculations leading to the conclusion that the transport of albumin across GBM is predominantly by diffusion rather than by flow, whereas the transport of water and small molecules is entirely by flow.25 It follows from this dichotomy that as the amount of fluid crossing a glomerulus decreases, the concentration of albumin in the glomerular effluent will increase. Endotheliosis and foot process effacement can separately or in combination cause substantial decreases in single nephron GFR30 and, according to our hypothesis, lead to albuminuria. The data from current experiments are compatible with this hypothesis. Thus, as shown in Figures 1 through 3, albuminuria in WT sFlt-1 mice is observed when there is endotheliosis unaccompanied by foot process effacement. In eNOS−/− sFlt-1 mice, the endotheliosis is substantially more severe and is now accompanied by foot process effacement. Together, these decrease GFR and worsen albuminuria (Figures 1 through 3).
In summary, our data demonstrate that absence of eNOS aggravates the pre-eclampsia–like phenotype induced by increased sFlt-1 in nonpregnant female mice. eNOS−/− sFlt-1 mice have elevated expression of preproET-1 and ETAR in the kidney. Ambrisentan reduces the pathologic changes that occur in both WT sFlt-1 and eNOS−/− sFlt-1 mice. A similar interaction between ET and NO is expected in pregnant mice treated with sFlt-1. Early screening of pregnant women for polymorphisms of NOS3 that lead to lower NO production may help identify women at high risk for pre-eclampsia. The use of ETAR antagonists that cross the placenta is not recommended for treatment of pre-eclampsia because of their teratogenicity.31,32 However, future studies may reveal targets downstream of ET receptor that could be harnessed for the treatment of pre-eclampsia.
Concise Methods
Animals
All animal experiments were conducted at the University of North Carolina at Chapel Hill in accordance with the International Animal Care and Use Committee guidelines. Because increased sFlt-1 recapitulates the phenotype of pre-eclampsia in females regardless of pregnancy,5 this study used nonpregnant female eNOS−/− mice,33 backcrossed at least 10 times to C57BL/6J. Littermates (WT and eNOS−/−, aged 3–5 months) were injected into the tail veins with 3×109 PFU of adenovirus to overexpress sFlt-1 (Ad sFlt-1) or adenovirus encoding murine Fc protein (Ad Fc) at equivalent doses.5 Ad Fc was used as a control to rule out nonspecific effects of adenovirus. These adenoviruses have been described previously.34 Briefly, sFlt-1 cDNA is inserted into the E1 region of E3-deleted adenovirus type 5. Before and 4 days after adenovirus administration, the individual mice were placed in metabolic cages for 2 days. Body weight, food and water intake, and urine volume were measured every 24 hours, and the samples collected on the second day (6 days after injection) were subjected to analyses.
GFR Estimated by CCr
CCr was determined by measuring plasma and urinary creatinine concentration with the method we developed using tandem liquid chromatography–mass spectrometry.35
Biochemical Measurements in Blood and Urine
Urinary albumin was determined using Albuwell-M kits (Exocell Inc, Philadelphia, PA). ELISA kits were used to measure plasma sFlt-1 (R&D Systems Inc, Minneapolis, MN) and cystatin C (NovaTeinBio Inc, Cambridge, MA). Plasma levels of albumin and total cholesterol were measured by the Vitros Chemistry System 350 (Ortho-Clinical Diagnostics, a Johnson & Johnson Company, Raritan, NJ).
Quantitative Reverse Transcription-PCR
Total RNA from the whole kidneys was extracted using Trizol (Life Technologies, St. Paul, MN), and mRNA was quantified with TaqMan real-time quantitative reverse transcription-PCR, with β-actin as a reference gene in each reaction.36 The sequences of primers and probes are available upon request.
Morphometric Studies
Cross paraffin sections of kidneys (4 µm thick) were stained with periodic acid–Schiff and Masson’s trichrome methods, and scanned using a ScanScope CS (Aperio, Vista, CA). Glomerular open capillary volume was measured by Image J software, and expressed as a percentage of glomerular tuft area. The FPW was measured as previously described by Koop et al.37 with minor modifications. Briefly, transmission electron micrographs at ×5000 magnification were printed, and the length of the GBM of the free capillary wall was measured and the number of slit pores overlying this GBM length was counted. The mean FPW was calculated as the total length of GBM divided by total numbers of slits.
Measurement of BP
Because BP of mice with increased sFlt-1 was not measureable using a computerized tail-cuff,38 BP was measured using telemetry.39
Effects of an Endothelin ETA Receptor Antagonist, Ambrisentan
Mice were treated with ambrisentan (10 mg/kg per d; GlaxoSmithKline, Middlesex, UK)40 via oral gavage starting 1 day before they were injected with 3×109 PFU of Ad sFlt-1. Four days after virus administration, mice were put in metabolic cages, and the experiments were conducted as described above.
Effects of ET-1 on Renal Phenotypes of Pre-Eclampsia
WT female nonpregnant mice aged 3–5 months were injected with 3×109 PFU of Ad sFlt-1. Half of the mice received low-dose ET-1 (5 pmol/kg per min; Sigma, St. Louis, MO) and the other half received saline via osmotic minipump (Alzet mode 1002) implanted subcutaneously. Four days after virus administration, individual mice were put in metabolic cages, and the experiments were conducted as described above.
Statistical Analyses
Data are expressed as mean ± SEM. To compare the effects of sFlt-1, eNOS, and their combinations, we used multifactorial ANOVA, Dunnett’s test, and Tukey Kramer’s test with JMP 8.0 software (SAS Institute Inc., Cary, NC). Differences were considered to be statistically significant with P<0.05.
Disclosures
S.A.K. is a co-inventor on multiple patents held by the Beth Israel Deaconess Medical Center that are related to angiogenic factors for the use in diagnosis and treatment of pre-eclampsia. S.A.K. discloses financial interest in Aggamin LLC.
Acknowledgments
We thank Mr. Longquan Xu, Mrs. Victoria J. Madden, Mr. Leonard Collins, Mrs. Gayle C. McGhee, and Dr. Howard M. Reisner for technical assistance, and Dr. Hiroshi Sato for critical reading of the manuscript.
This work was supported by grants from the American Heart Association (0265464U, 0855335E), the National Institutes of Health (HL049277, U01 DK076131, HL077145, P30DK56350, P30 ES10126), and a grant-in-aid from the Japan Society of Promotion of Science (JSPS, 22890016), as well as funds from the Department of Pathology and Laboratory Medicine at the University of North Carolina at Chapel Hill, a committee grant from the Japanese Society of Nephrology, a grant-in-aid from the Research Group on Progressive Renal Disease from the Ministry of Health, Labor and Welfare, Japan, the Miyagi Association of Kidney Disease, and the Banyu Life Science Foundation International. S.A.K. is supported by the Howard Hughes Medical Institute.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011040369/-/DCSupplemental.
References
- 1.Wang A, Rana S, Karumanchi SA: Preeclampsia: The role of angiogenic factors in its pathogenesis. Physiology (Bethesda) 24: 147–158, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Young BC, Levine RJ, Karumanchi SA: Pathogenesis of preeclampsia. Annu Rev Pathol 5: 173–192, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Gélinas DS, Bernatchez PN, Rollin S, Bazan NG, Sirois MG: Immediate and delayed VEGF-mediated NO synthesis in endothelial cells: Role of PI3K, PKC and PLC pathways. Br J Pharmacol 137: 1021–1030, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA: Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350: 672–683, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA: Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111: 649–658, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sibai B, Dekker G, Kupferminc M: Pre-eclampsia. Lancet 365: 785–799, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Irgens HU, Reisaeter L, Irgens LM, Lie RT: Long term mortality of mothers and fathers after pre-eclampsia: Population based cohort study. BMJ 323: 1213–1217, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barden A: Pre-eclampsia: Contribution of maternal constitutional factors and the consequences for cardiovascular health. Clin Exp Pharmacol Physiol 33: 826–830, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Myatt L, Webster RP: Vascular biology of preeclampsia. J Thromb Haemost 7: 375–384, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Rey E, Couturier A: The prognosis of pregnancy in women with chronic hypertension. Am J Obstet Gynecol 171: 410–416, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Ramsay JE, Stewart F, Greer IA, Sattar N: Microvascular dysfunction: A link between pre-eclampsia and maternal coronary heart disease. BJOG 110: 1029–1031, 2003 [PubMed] [Google Scholar]
- 12.Duplain H, Burcelin R, Sartori C, Cook S, Egli M, Lepori M, Vollenweider P, Pedrazzini T, Nicod P, Thorens B, Scherrer U: Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation 104: 342–345, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Niu W, Qi Y: An updated meta-analysis of endothelial nitric oxide synthase gene: Three well-characterized polymorphisms with hypertension. PLoS ONE 6: e24266, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serrano NC, Casas JP, Díaz LA, Páez C, Mesa CM, Cifuentes R, Monterrosa A, Bautista A, Hawe E, Hingorani AD, Vallance P, López-Jaramillo P: Endothelial NO synthase genotype and risk of preeclampsia: A multicenter case-control study. Hypertension 44: 702–707, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Fatini C, Sticchi E, Gensini F, Genuardi M, Tondi F, Gensini GF, Riviello C, Parretti E, Mello G, Abbate R: Endothelial nitric oxide synthase gene influences the risk of pre-eclampsia, the recurrence of negative pregnancy events, and the maternal-fetal flow. J Hypertens 24: 1823–1829, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Stillman IE, Karumanchi SA: The glomerular injury of preeclampsia. J Am Soc Nephrol 18: 2281–2284, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Kamoi K, Sudo N, Ishibashi M, Yamaji T: Plasma endothelin-1 levels in patients with pregnancy-induced hypertension. N Engl J Med 323: 1486–1487, 1990 [DOI] [PubMed] [Google Scholar]
- 18.Wolff K, Nisell H, Carlström K, Kublickiene K, Hemsén A, Lunell NO, Lindblom B: Endothelin-1 and big endothelin-1 levels in normal term pregnancy and in preeclampsia. Regul Pept 67: 211–216, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Baksu B, Davas I, Baksu A, Akyol A, Gulbaba G: Plasma nitric oxide, endothelin-1 and urinary nitric oxide and cyclic guanosine monophosphate levels in hypertensive pregnant women. Int J Gynaecol Obstet 90: 112–117, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Boulanger C, Lüscher TF: Release of endothelin from the porcine aorta. Inhibition by endothelium-derived nitric oxide. J Clin Invest 85: 587–590, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quaschning T, Voss F, Relle K, Kalk P, Vignon-Zellweger N, Pfab T, Bauer C, Theilig F, Bachmann S, Kraemer-Guth A, Wanner C, Theuring F, Galle J, Hocher B: Lack of endothelial nitric oxide synthase promotes endothelin-induced hypertension: Lessons from endothelin-1 transgenic/endothelial nitric oxide synthase knockout mice. J Am Soc Nephrol 18: 730–740, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Kumasawa K, Ikawa M, Kidoya H, Hasuwa H, Saito-Fujita T, Morioka Y, Takakura N, Kimura T, Okabe M: Pravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse model. Proc Natl Acad Sci USA 108: 1451–1455, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Redman CW, Sargent IL: Latest advances in understanding preeclampsia. Science 308: 1592–1594, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Murphy SR, LaMarca BB, Cockrell K, Granger JP: Role of endothelin in mediating soluble fms-like tyrosine kinase 1-induced hypertension in pregnant rats. Hypertension 55: 394–398, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smithies O: Why the kidney glomerulus does not clog: A gel permeation/diffusion hypothesis of renal function. Proc Natl Acad Sci USA 100: 4108–4113, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collino F, Bussolati B, Gerbaudo E, Marozio L, Pelissetto S, Benedetto C, Camussi G: Preeclamptic sera induce nephrin shedding from podocytes through endothelin-1 release by endothelial glomerular cells. Am J Physiol Renal Physiol 294: F1185–F1194, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Kappers MH, Smedts FM, Horn T, van Esch JH, Sleijfer S, Leijten F, Wesseling S, Strevens H, Jan Danser AH, van den Meiracker AH: The vascular endothelial growth factor receptor inhibitor sunitinib causes a preeclampsia-like syndrome with activation of the endothelin system. Hypertension 58: 295–302, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Vikse BE, Irgens LM, Leivestad T, Skjaerven R, Iversen BM: Preeclampsia and the risk of end-stage renal disease. N Engl J Med 359: 800–809, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Gaber LW, Spargo BH: Pregnancy-induced nephropathy: The significance of focal segmental glomerulosclerosis. Am J Kidney Dis 9: 317–323, 1987 [DOI] [PubMed] [Google Scholar]
- 30.Blantz RC, Gabbai FB: Glomerular hemodynamics in pathophysiologic conditions. Am J Hypertens 2: 208S–212S, 1989 [DOI] [PubMed] [Google Scholar]
- 31.Augustine-Rauch K, Zhang CX, Panzica-Kelly JM: In vitro developmental toxicology assays: A review of the state of the science of rodent and zebrafish whole embryo culture and embryonic stem cell assays. Birth Defects Res C Embryo Today 90: 87–98, 2010 [DOI] [PubMed] [Google Scholar]
- 32.George EM, Granger JP: Endothelin: Key mediator of hypertension in preeclampsia. Am J Hypertens 24: 964–969, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, Smithies O: Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci USA 93: 13176–13181, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuo CJ, Farnebo F, Yu EY, Christofferson R, Swearingen RA, Carter R, von Recum HA, Yuan J, Kamihara J, Flynn E, D’Amato R, Folkman J, Mulligan RC: Comparative evaluation of the antitumor activity of antiangiogenic proteins delivered by gene transfer. Proc Natl Acad Sci USA 98: 4605–4610, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi N, Boysen G, Li F, Li Y, Swenberg JA: Tandem mass spectrometry measurements of creatinine in mouse plasma and urine for determining glomerular filtration rate. Kidney Int 71: 266–271, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Takahashi N, Lopez ML, Cowhig JE, Jr, Taylor MA, Hatada T, Riggs E, Lee G, Gomez RA, Kim H-S, Smithies O: Ren1c homozygous null mice are hypotensive and polyuric, but heterozygotes are indistinguishable from wild-type. J Am Soc Nephrol 16: 125–132, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Koop K, Eikmans M, Baelde HJ, Kawachi H, De Heer E, Paul LC, Bruijn JA: Expression of podocyte-associated molecules in acquired human kidney diseases. J Am Soc Nephrol 14: 2063–2071, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Krege JH, Hodgin JB, Hagaman JR, Smithies O: A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension 25: 1111–1115, 1995 [DOI] [PubMed] [Google Scholar]
- 39.Caron K, Hagaman J, Nishikimi T, Kim HS, Smithies O: Adrenomedullin gene expression differences in mice do not affect blood pressure but modulate hypertension-induced pathology in males. Proc Natl Acad Sci USA 104: 3420–3425, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uchino T, Sanyal SN, Yamabe M, Kaku T, Takebayashi S, Shimaoka T, Shimada T, Noguchi T, Ono K: Rescue of pulmonary hypertension with an oral sulfonamide antibiotic sulfisoxazole by endothelin receptor antagonistic actions. Hypertens Res 31: 1781–1790, 2008 [DOI] [PubMed] [Google Scholar]