Abstract
Injection of amniotic fluid stem cells ameliorates the acute phase of acute tubular necrosis in animals by promoting proliferation of injured tubular cells and decreasing apoptosis, but whether these stem cells could be of benefit in CKD is unknown. Here, we used a mouse model of Alport syndrome, Col4a5−/− mice, to determine whether amniotic fluid stem cells could modify the course of progressive renal fibrosis. Intracardiac administration of amniotic fluid stem cells before the onset of proteinuria delayed interstitial fibrosis and progression of glomerular sclerosis, prolonged animal survival, and ameliorated the decline in kidney function. Treated animals exhibited decreased recruitment and activation of M1-type macrophages and a higher proportion of M2-type macrophages, which promote tissue remodeling. Amniotic fluid stem cells did not differentiate into podocyte-like cells and did not stimulate production of the collagen IVa5 needed for normal formation and function of the glomerular basement membrane. Instead, the mechanism of renal protection was probably the paracrine/endocrine modulation of both profibrotic cytokine expression and recruitment of macrophages to the interstitial space. Furthermore, injected mice retained a normal number of podocytes and had better integrity of the glomerular basement membrane compared with untreated Col4a5−/− mice. Inhibition of the renin-angiotensin system by amniotic fluid stem cells may contribute to these beneficial effects. In conclusion, treatment with amniotic fluid stem cells may be beneficial in kidney diseases characterized by progressive renal fibrosis.
Stem cells, which are derived from several sources, have been proposed as possible therapeutic agents for many different diseases. In recent years, investigators have focused their efforts on using stem cells, mainly mesenchymal stem cells derived from bone marrow (MSC), to treat both acute kidney disease and CKD. MSC injected into an acutely damaged kidney are able to restore renal functional and structural measures to normal.1–3 One possible mechanism of action involves an autocrine- or paracrine-mediated release of different cytokines into the local microenvironment, which then promotes repair by endogenous cells.2 Although more extensive work has been performed in acute models of kidney injury, the application of stem cells to treat CKD is still a relatively new area of investigation.
Alport syndrome is a genetic form of CKD in which chronic fibrosis and inflammation eventually lead to complete kidney failure.4,5 Some groups have already investigated stem cells for the treatment of CKD in animal models of the syndrome. Prodromidi et al.6 and Sugimoto et al.7 showed reduced glomerular scarring and interstitial fibrosis with improved renal function after injection of MSC. Le Bleu et al.8 demonstrated that stem cell therapies ameliorate murine Alport syndrome, regardless of stem cell type and modality of injection. Nevertheless, the mechanisms of renal protection induced by stem cells in animal models of CKD are still not completely understood.
We investigated the possibility of using amniotic fluid stem cells (AFSCs) as a treatment for Alport syndrome. AFSCs are pluripotent cells9,10 derived from amniotic fluid through c-kit–positive selection.11,12 Our group has previously shown that injection of AFSCs ameliorates the acute phase in animals with acute tubular necrosis, promoting proliferation of damaged tubular cells and decreasing apoptosis in a setting of substantial immunomodulatory effects.13
In this investigation we demonstrate that a single injection of AFSCs into mice with Alport syndrome (B6.Cg-Col4α5tm1Yseg/J) during the very early phase of disease can ameliorate physiologic and morphologic renal damage by reducing interstitial fibrosis and recruitment and activation of macrophages within the interstitial space. We show that the expression of genes promoting fibrosis and stimulating new extracellular matrix is decreased after stem cell injection. In addition, we show that injection of AFSCs helps to preserve glomerular podocyte number. In vitro experiments suggested that AFSCs may protect podocytes by downregulation of the angiotensin II signaling pathway, probably favoring their survival. Even though a single injection of AFSCs does not completely reverse renal injury due to Alport syndrome in treated mice, our findings suggest a possible mechanism whereby AFSCs lead to substantial reductions in disease progression, which may ultimately suggest changes in therapy and lead to prolonged survival in treated animals.
Results
AFSC Characterization
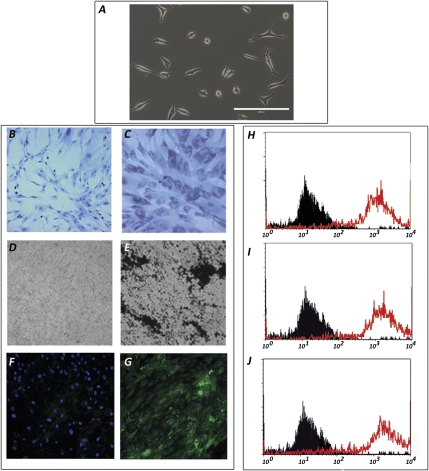
A clonal population of AFSCs between passages 15 and 20 was used in all experiments. These cells presented a fibroblastic shape (Figure 1A) and differentiated into adipocyte-like cells staining positive for oil-red-O (Figure 1C), into osteoblast-like cells expressing alkaline phosphatase (Figure 1E), and into skeletal muscle–like cells expressing tropomyosin (Figure 1G). AFSCs are positive for OCT-4, alkaline phosphatase, and Thy-1 as determined by FACS analysis (Figure 1, H–J), thus indicating that our clonal population has a broad differentiation potential.
Figure 1.
AFSC characterization and pluripotential capacity. After 19 passages, AFSCs present a fibroblastoid appearance under bright field microscopy (A, ×20). AFSCs, under appropriate stimuli, are able to differentiate into adipocyte-like cells, as shown by oil red-O staining (C, ×10); into osteoblast-like cells, as indicated by alkaline phosphates activity. (E, ×10); and into myocyte-like cells, as shown by expression of tropomyosin (G, ×10), compared with controls (B, D, and F, ×10). In addition, AFSCs express mesenchymal and embryonic stem cell markers such as OCT-4 (H), alkaline phosphatase (I), and THY-1 (J), as shown by FACS analysis of undifferentiated cells.
Animal Model: Effects of AFSCs on Lifespan, Serum Creatinine Levels, Proteinuria, and BUN
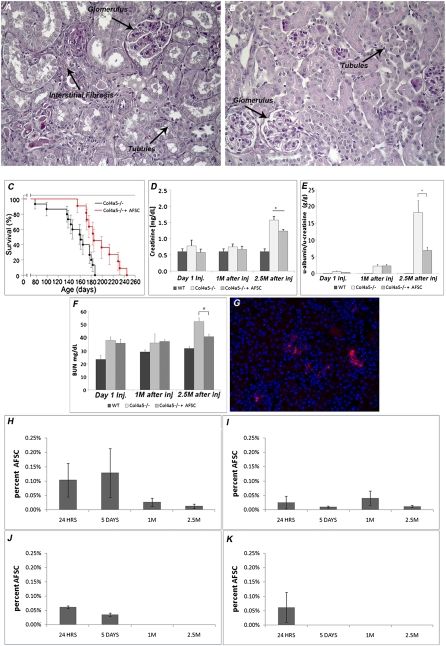
Col4a5−/− mice develop abnormal glomerular morphology, with increased interstitial fibrosis when compared with C57BL/6 WT (Figure 2, A and B). Injection of AFSC into Col4a5−/− mice at 1.5 months of age increased their mean survival by 20% (Figure 2C) and significantly reduced serum creatinine levels (Figure 2D), proteinuria (Figure 2E), and BUN levels (Figure 2F) compared with their nontreated siblings.
Figure 2.
AFSC injections prolong animal survival and improve renal function. The progression of Alport syndrome leads to expanded fibrosis both in glomerular and interstitial spaces (A and B). Wild-type mice present normal renal morphology without any abnormalities (A, ×20) compared with Col4a5−/− mice that exhibit progressive kidney disease (B, ×20), as shown in representative periodic acid-Schiff staining pictures of kidney sections from mice killed at 5 months of age. Black arrows point to globally sclerotic glomeruli, widespread tubular cast formation, diffuse interstitial fibrosis, and abundant infiltration of inflammatory cells. Injection of AFSC increased the lifespan of treated mice and ameliorated serum creatinine, proteinuria, and BUN when compared with controls (C–F). Survival analysis of Col4a5−/− mice injected with AFSC at 1.5 months after birth (black, n=11) versus noninjected Col4a5−/− mice (red, n=15) demonstrates that injected mice had a mean increase in lifespan of 20% (C). Comparison of serum creatinine levels in Col4a5−/− mice injected with AFSCs (n=15), noninjected Col4a5−/− mice (n=15), and wild-type (WT) controls show significantly lower serum creatinine levels at 2.5 months after AFSC injection in treated mice compared with their nontreated controls (D). Furthermore, significant amelioration in proteinuria (E) and BUN (F) was also detected in mice at 2.5 months after injection. Injected cells were traceable after 5 days and could be detected by fluorescence (red, Qdot label) within the interstitial space and the glomeruli (G, ×20). Presence of these cells in kidney, lung, liver and heart, as detected by FACS, was demonstrated at 24 hours, 5 days, 1 month, and 2.5 months after injection (H–K). At 24 hours AFSC were present in all four organs, with the majority being localized in the kidney, accounting for an average of 0.1% of the cells (H). At the later time points, no cells were present in the liver (J) and heart (K). After 2.5 months, 0.01% AFSCs were detected in the kidney as well as in the lung. All values are presented as mean ± SEM (*P<0.05).
In Vivo Tracking of AFSCs
Qdot-labeled AFSCs were detectable within the kidney both in the interstitial space and in glomeruli (Figure 3E), as shown in a representative picture at 5 days (Figure 2G). By FACS at different time points (24 hours, 5 days, 1 month, and 2.5 months after injection), AFSCs are present in greater numbers in the kidney. No cells were detectable in the heart or liver at later time points (Figure 2, H–K).
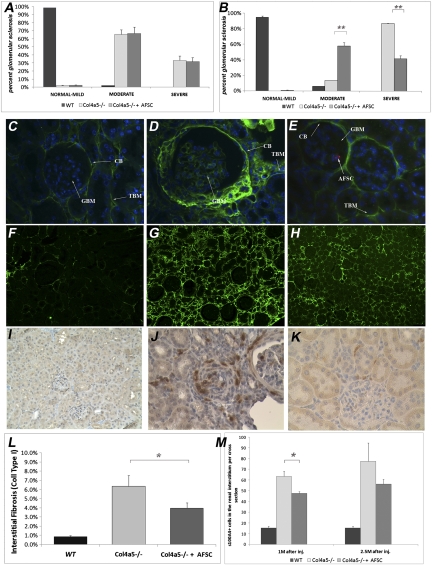
Figure 3.
AFSC injections significantly ameliorate glomerular and interstitial fibrosis in AS mice. AFSC injection ameliorated glomerular sclerosis (A and B). Morphometric analysis of percentage of sclerotic glomeruli in Col4a5−/− mice injected with AFSCs (n=10) at 1.5 months after birth and killed at 1 (A) and 2.5 months (B) after injection and similarly for noninjected Col4a5−/− mice (n=10) is shown. Using periodic acid-Schiff staining, 50 randomly selected glomeruli per mouse were scored according to the severity of fibrosis as follows: normal-mild (0%–33%), moderate (33%–66%), and severe (66%–100%). As shown by graphs, the injected mice present less sclerotic glomeruli than their noninjected siblings at 2.5 months after injection. AFSCs reduced COL4α1 deposition within the glomeruli (C–E). Injected mice showed less glomerular deposition of COL4α1 compared with noninjected mice. Representative pictures show COL4α1 distribution (green) in wild-type mouse (C, ×40), noninjected Col4a5−/− mouse killed at 4 months after birth (D, ×40), and Col4a5−/− mouse injected with AFSC at 1.5 months after birth and killed at 2.5 months after injection (E, ×40). Rare AFSC labeled red with CM-DiI were seen inside the glomeruli of injected mice (arrow, E) (BC, Bowman capsule; TBM, basal membrane of the tubules; GBM, glomerular basement membrane). Injection of AFSC diminished the deposition of extracellular matrix in the interstitium, as shown by quantification of collagen I (F–H and L). In the representative picture of injected mice (H, ×20; n=10) the presence of collagen I is clearly less than in the noninjected mice (G, ×20; n=10) and is much more similar to that of the wild type (F, ×20; n=10). These data were confirmed by quantification of staining. Graph in L compares collagen I accumulation per cross-section in all experimental groups analyzed using HistoQuest software, revealing a statistically significant difference at 2.5 months after injection. Injection of AFSC also reduced the number of S100A4-positive cells (I–K and M). This marker is mostly expressed by cells undergoing myofibroblast transformation and by cells actively producing collagen during the progression of fibrosis, including endothelial cells and macrophages. In treated mice (I, ×20), the number of S100A4-positive cells in the interstitial space is reduced compared with their nontreated siblings (J, ×20). The graph in M represents the change in number of S100A4-positive cells per cross-section in mice injected (n=10) at 1.5 months after birth and killed at 2.5 and 4 months of age versus noninjected (n=10) mice of the same age. All values are presented as mean ± SEM (*P<0.05). WT, wild type.
Effects of AFSCs on Renal Morphology
Col4a5−/− mice injected with AFSCs did not exhibit a statistically significant change in the fraction of glomeruli with moderate or severe fibrosis after 1 month of injection when compared with mice that were not injected (Figure 3A). In contrast, littermate Col4a5−/− untreated mice demonstrated a progressive increase in the number of severely sclerotic glomeruli when compared with their injected siblings (Figure 3B). These results indicated that in treated mice, the progression of glomerular sclerosis is less aggressive.
In wild-type mice, COL4α1 is expressed in the Bowman capsule and in the basement membrane of tubules, whereas it is absent from the glomerular basement membrane (GBM) (Figure 3C). At 2.5 months after treatment, noninjected Col4a5−/− mice have a strong accumulation of COL4α1 in the GBM, as well as in the Bowman capsule (Figure 3D). In contrast, Col4a5−/− mice that received AFSC treatment demonstrated a more nearly normal expression of COL4α1 in both the GBM and Bowman capsule (Figure 3E). AFSCs were present in small numbers in the glomeruli of treated mice at 2.5 months after injection (Figure 3E, arrow).
To determine the deposition of new extracellular matrix as a sign of fibrosis, we compared the presence of collagen I (Figure 3, F–H) and myofibroblasts (Figure 3, I–K) within the kidneys of the experimental groups. Treated mice showed a statistically significant reduction in collagen I staining after 2.5 months (Figure 3L) and less presence of myofibroblasts within the renal interstitial space (Figure 3M). We also evaluated the change in expression of important regulators of the TGFβ/bone morphogenetic protein (BMP) and epithelial-mesenchymal transition pathways. The injected mice showed a significant downregulation of important transcription factors of the TGFβ pathway as well as lower abundance of proteins involved in matrix deposition, as shown by protein array and WB (Supplemental Figures 1 and 2). These findings further support the antifibrotic role of AFSCs observed in the renal interstitial space.
Effects of AFSCs on Macrophage Recruitment and Phenotype Activation
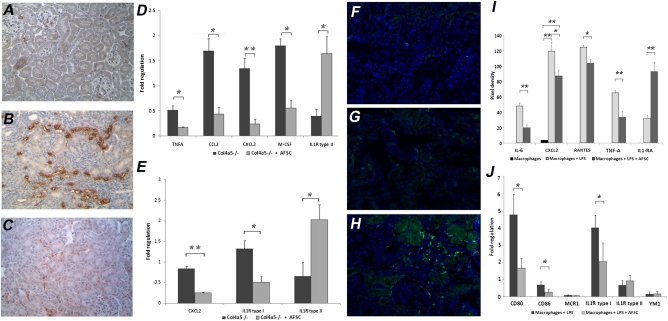
Col4a5−/− mice that received AFSC injections had fewer macrophages in the interstitial space at 2.5 months after treatment compared with their nontreated siblings (Figure 4, B and C). Expression analysis of M1 and M2 macrophage activation phase genes evaluated 5 days after AFSC treatment revealed a lower expression for TNFα, CCL2, and CXCL2 (M1 markers) and an elevated expression for IL1-RII (M2 marker) in treated mice. Moreover, macrophage colony-stimulating factor (M-CSF), a chemoattractant for macrophages, was downregulated in the injected kidneys (Figure 4D). At 1 month after injection, CXCL2 and IL1-RI were upregulated in nontreated mice and IL-1RII was still upregulated in treated mice (Figure 4E). The presence of M2 macrophages, recognized to promote tissue remodeling versus progression of fibrosis, was increased in the interstitial space of treated mice, as demonstrated by CD150 immunofluorescence staining (Figure 4H). The number of M1 macrophages is decreased in injected mice, as shown by double staining for macrophage/L1 protein and CD80 (Supplemental Figure 3). To confirm that AFSCs might influence the recruitment and activation of macrophages, we performed an in vitro co-culture assay of AFSCs with macrophages activated with lipopolysaccharide. As seen in Figure 4I, a protein array of the co-culture supernatant demonstrated that cytokines such as IL-6, CCL5, RANTES, and TNFα, which are increased in CKD and induce macrophage recruitment, are decreased in the presence of AFSCs. Furthermore, markers such as CD80, CD86, and IL1-RI (specific of M1-type macrophages) are decreased (Figure 4J). Taken together, these data indicate decreased signaling for recruitment of macrophages to the kidney and a phenotype switch of macrophages toward a more regenerative M2 activated state when in the presence of AFSCs.
Figure 4.
Injected mice present less macrophage recruitment and relative increase in M2 macrophages, further supported by in vitro assays. The representative distribution of macrophages by immunohistochemistry between wild-type (A, ×10) and noninjected (B, ×10) and injected Alport syndrome mice (C, ×10) at 2.5 months after AFSC administration demonstrate that injected mice had less macrophage infiltration in the interstitial space (identified by L1 staining). Gene expression analysis of M1 and M2 activation phase and recruitment genes was performed in both treated (n=6) and nontreated (n=6) mice after 5 days and 1 month of AFSC injection. Real-time PCR analysis revealed significant downregulation in TNFα, CCL2, CXCL2, and M-CSF, (involved in the signaling pathway of M1 macrophages) in treated mice together with an increase in IL1-RII, expressed by M2 macrophages, at 5 days (D). Downregulation of CXCL2 and IL1-RI and an upregulation of IL1-RII were also present at 1 month in treated mice, which may indicate a balance toward tissue remodeling due to M2 phenotype macrophages (E). CD150 staining at 2.5 months confirmed the presence of M2-type macrophages in the interstitial space of injected mice (H, ×20), whereas wild-type (F) and untreated (G) mice were mostly negative. In vitro co-culture of activated macrophages and AFSC demonstrated that AFSCs are able to decrease the expression of IL-6, CXCL2, RANTES, and TNFα, important modulators of macrophages recruitment (I). Treatment of AFSC with lipopolysaccharide or analysis of AFSCs with no stimulation produced no detectable signal for any of the above-mentioned markers (data not shown), suggesting that these cytokines are of macrophage origin. In addition CD80, CD86, and IL-RI were significantly downregulated, as shown by real-time PCR, which suggests that AFSCs are able to induce an M1-to-M2 phenotype switch in macrophages (J). All values are presented as mean ± SEM (*P<0.05; **P<0.01).
Effects of AFSCs on Glomerular Cells and GBM
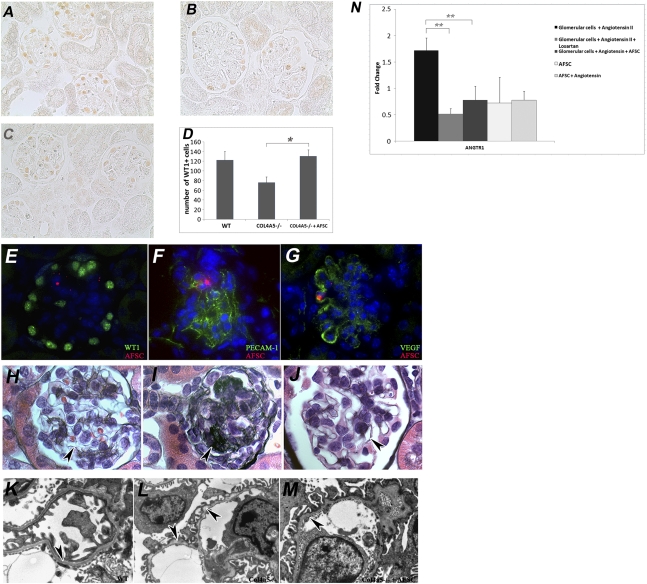
To test whether the beneficial outcome from AFSC injection may be attributed to preservation of podocyte number, we counted WT-1–positive cells within the glomeruli of nontreated mice with Alport syndrome (Figure 5B) and treated mice (Figure 5C). Our analysis demonstrated a significant difference (P< 0.05) between treated and nontreated Col4a5−/− mice at 2.5 months after injection; the number of WT-1–positive cells was similar between wild-type and treated mice, whereas the nontreated Col4a5−/− animals demonstrated a significant decrease in their number of WT-1–positive cells relative to the other two groups (Figure 5D). Because the count of podocytes seemed to be preserved in injected mice, we further sought to determine whether this observation may include differentiation of AFSCs into podocyte-like cells. Double staining for WT-1 and dye tracking revealed no podocyte differentiation of AFSCs at 2.5 months after injection (Figure 5E), whereas double staining with vascular endothelial growth (VEGF) factor demonstrated that AFSCs can integrate into the glomeruli and possibly differentiate into VEGF-expressing cells, probably endothelial-like cells (Figure 5G). Consistent with a lack of differentiation of AFSCs into podocytes, we did not observe any new production of COL4α5 (Supplemental Figures 4E, 5Q, and 6E).
Figure 5.
Injection of AFSC preserves GBM structure and podocyte number in treated mice. Injection of AFSC preserved podocyte number (A–D). Count of distribution of WT-1–positive cells per 50 randomly selected glomeruli per mouse (n=10 mice per group) demonstrated that the number of WT-1–positive cells is preserved in treated mice (D). Representative pictures show immunohistochemistry findings for WT-1 in wild-type (WT) mice (A, ×20) and in Col4a5−/− mice treated with AFSC at 1.5 months after birth and killed at 2.5 months after injection (C, ×20) versus noninjected Col4a5−/− mice killed at 4 months after birth (B, ×20). AFSC did not differentiate into podocyte-like cells after 2.5 months (E, ×63), as shown by double staining of AFSC labeled with CM-DiI (red) and WT-1 (green). CM-DiI–labeled AFSC (red) showed mild co-localization with PECAM-1 (green) (F, ×63) and VEGF (green) (G, ×63), indicating the possibility of AFSCs differentiating into VEGF-producing–like cells. Mice that received AFSC infusion demonstrated an improved appearance of the GBM (H–J). Representative pictures of Jones staining demonstrated that wild-type mice had a normal deposition of GBM, shown by the black arrows (H, ×100), whereas the noninjected Col4a5−/− mice (I, ×100) had abundant matrix accumulation compared with their injected siblings (black arrow in J, ×100). In addition, AFSC injection helped the preservation of GBM architecture (K–M). Representative electron transmission microscopy images showed severe thinning and splitting of GBM in Col4a5−/− mice (black arrow, L) compared with their littermate wild-type mice (black arrow, K), whereas treated mice presented less thinning and splitting of the GBM at 2.5 months after injection (black arrow, M). AFSCs blocked expression of angiotensin II receptor 1 (ANGTR1). In vitro glomerular cells, stimulated with angiotensin II, showed increased ANGTR1 expression, whereas losartan (an angiotensin II receptor antagonist) blocked ANGTR1 expression (N). Similar to losartan, AFSC downregulated ANGTR1 expression, thus indicating their capacity to interfere with the angiotensin II signaling pathway. In addition, AFSCs exposed to angiotensin II did not manifest a change in ANGTR1 expression level (N). (*P<0.05; **P<0.01)
The expression of mRNA levels for all the α chains demonstrated that in our animal model, injected and noninjected mice presented expression of Col4α1 and Col4α2, Col4α3, and Col4α4 that increases over time, whereas Col4α6 and Col4α5 are strongly downregulated when compared with the wild type (Supplemental Figure 4). These data were also confirmed by histology at 2.5 months after injection (Supplemental Figure 5), where it is notable that the expression of Col4α3 and Col4α4 is minimal and irregular. Nevertheless, if protein levels for Col4α2 are normalized against β-actin expression, injected mice show lower protein levels at 2.5 months, as shown in Supplemental Figure 5C. This finding suggests a possible difference in regulation of α chain expression in the GBM of treated mice.
We further investigated whether the GBM of injected mice was protected from splitting, which is typical of GBM in Alport syndrome. Figure 5, H–G, shows that, under light microscopy, the GBM in injected mice seems to be less damaged; this preservation was also confirmed by transmission electron microscopy (Figure 5, K–M).
To test whether the preservation of glomerular cells by AFSC was correlated with modulation of specific molecular pathways, we performed an in vitro co-culture experiment with glomerular cells stimulated by angiotensin II and AFSCs. Our characterization of the cells obtained by plating isolated mouse glomeruli suggests that the majority of “early outgrowth” cells were podocytes (WT-1–positive),14 with a smaller number representing mesangial cells (α-SMA–positive); no endothelial cells appeared to be present (Supplemental Figure 7). ANGTR1 (angiotensin II receptor) was upregulated in the presence of angiotensin II and was significantly downregulated in the presence of losartan, an angiotensin II receptor antagonist as shown by real-time PCR. When AFSCs were added to the co-culture, the expression of ANGTR1 was similar to that with losartan, suggesting that AFSCs also decrease the expression of ANGTR1, antagonizing angiotensin II signaling and thus favoring podocyte survival (Figure 5N).
Discussion
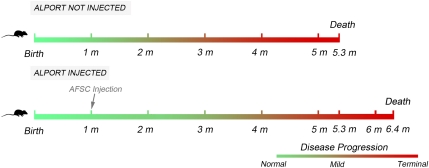
Alport syndrome is a genetic form of kidney disease in which a mutation in any of the genes coding for the three chains of type IV collagen (α3,α4,α5) results in a dysfunctional GBM, leading to deterioration of glomerular structure and function.4,15 Previously reported results obtained with injection of MSC, as well as with whole bone marrow transplantation have shown some degree of protection against progression of Alport syndrome. It has, however, been difficult to compare these studies because of diverse backgrounds in the mice investigated and experimental designs. Thus, the mechanism of renal protection by stem cells in this form of CKD is still unclear.7,8,16,17 The Alport syndrome mouse strain used in our experiments carries the mutation of colIVα5, the most common form of Alport syndrome in humans. This model was derived from C57BL/6 background, and no backcrossing between colonies was performed.18 In an effort to further optimize our results, we used a clonal population of AFSCs derived from C57BL/6 mice, and recipient mice were not irradiated because Katayama et al.19 demonstrated that irradiation itself is renoprotective in Alport mice.19 Timing of injection was in the “early phase” of disease, when structure and function of the kidney would be least compromised and possibly most amenable to repair. Our working hypothesis, therefore, was that a single early injection of AFSCs would be beneficial in prolonging the lifespan of Alport syndrome mice. Treated mice did survive 20% longer on average compared with their untreated siblings (Figure 6). Prolonged survival corresponded to a general amelioration in renal morphology, manifested in preservation of glomerular and interstitial structures with a decreased number of myofibroblasts, the cell type mainly responsible for deposition of new extracellular matrix.19,20 In addition, serum creatinine, proteinuria, and BUN were significantly improved relative to values in noninjected siblings.
Figure 6.
Schematic illustration comparing Alport disease progression between Col4a5−/− mice injected with AFSCs and their noninjected siblings. According to our results, injection of AFSCs slows down, but does not stop, ultimate progression of renal failure, prolonging median lifespan from 5.3 to 6.4 months in treated mice. In particular, modulation of gene expression appears to take place within the first days after injection and results in amelioration of both renal structure and function, evident at about 4 months of age in the treated mice compared with their noninjected siblings.
AFSCs possess pluripotential activity9,13 both in vitro and in vivo. We have previously demonstrated that these cells are renoprotective when injected directly into the kidney in a mouse model of acute kidney injury. AFSCs have the ability to secrete cytokines that can modulate the local immune/inflammatory response and support recovery of damaged tubular epithelial cells.13 As demonstrated in our previous work, integration of the injected cells and their differentiation into tubular epithelial cells was minimal. This was also confirmed in our current study of Alport syndrome, where AFSCs were detected 24 hours and 5 days after the injection and diminished over time, representing 0.01% of the kidney cells at 2.5 months after injection.
Contrary to findings reported by Kalluri and colleagues,7,8 we did not observe injected cells differentiating into podocyte-like cells expressing new collagen IV, in particular of COL4α5, at later time points. This is consistent with recent studies in both acute kidney disease1,21 and CKD models other than Alport syndrome,22 and in Alport mice treated with MSC.23 Nevertheless, the injected cells present within the glomeruli seem to express VEGF, probably indicating their ability to commit to endothelial-like cells. VEGF is an important signaling pathway during kidney disease progression because its expression is correlated with podocyte preservation; thus, we speculate that integrated cells may aid in recovery through VEGF modulation.24–26
Along with a lack of evidence of renoprotection by AFSCs in Alport syndrome mice by replacement of endogenous renal cells, our findings suggest three different mechanisms by which AFSCs may slow the progression of CKD: inhibition of fibrosis, modulation of secondary immune/inflammatory responses in the kidney, and preservation of podocytes, probably all through paracrine/endocrine mechanisms.
The dramatic changes in gene expression involved in the deposition of extracellular matrix and development of fibrosis (Supplemental Figure 1) at 5 days after injection confirms that AFSCs probably modulate the microenvironment in the damaged kidney, possibly through activation of SMAD1/5 signaling by alternative molecules of the TGFβ/BMP family, such as GDFs or AMH, rather than the BMPs.27,28 Zeisberg et al.29 demonstrated that in Alport syndrome, inhibition of MMP-2 and MMP-9 (critical matrix metalloproteinases in remodeling of extracellular matrix30) before the onset of proteinuria induced a preservation of GBM/extracellular matrix integrity, which in turn led to substantial disease protection.
Similarly, our findings demonstrate that with injection of AFSCs before the onset of proteinuria, levels of these two MMPs were significantly decreased within the first days after injection, resulting in a preservation of glomerular and interstitial structures, possibly including amelioration of the splitting of the GBM in treated mice, as confirmed by transmission electron microscopy analysis, because MMPs play a fundamental role in GBM turnover in Alport syndrome.29
In addition to findings suggesting that AFSCs can modulate TGFβ/BMP signaling, we found evidence that systemic injection of AFSCs may also provide protection by modulating immune function, in particular modulating macrophage recruitment and phenotype (M1 versus M2). Macrophages, together with the cytokine microenvironment, play a critical role in determining the balance between progressive fibrosis and resolution of inflammatory injury. High levels of INF-γ, TNFα, and M-CSF31 induce proinflammatory macrophages (M1), whereas elevated levels of IL-10 or IL-4 activate anti-inflammatory macrophages (M2c) that promote tissue remodeling.32–35 Mice that were treated with AFSC demonstrated a significant decrease in the number of M1 macrophages, as well as a decrease in levels of TNFα, CCL2, CXCL2, and M-CSF, which are highly expressed during chronic inflammation and in the presence of M1-type macrophages.35 We also observed an increase in levels of IL1-RII, typical of an M2 macrophage phenotype, indicating a possible switch toward M2 macrophages 5 days after injection in treated mice. A month after injection, macrophages were limited to the interstitial space and expression of IL1-RI, typical of M1,35 and CXCL2 and were decreased in our treated mice whereas expression of IL1-RII was still upregulated. Co-culture between AFSCs and lipopolysaccharide-stimulated macrophages indicated that our in vivo results were a direct effect of AFSCs because molecules such as RANTES (shown to be essential in macrophage recruitment in Alport syndrome36), IL-6, and TNFα were all downregulated in the presence of AFSCs. In addition, AFSCs were able to deactivate macrophages by blocking the expression of receptors such as CD80 and CD86, which, when normally expressed, promote a strong Th1 response and exert antiproliferative and cytotoxic activities.34
The positive effect of AFSCs was not limited to the interstitium, where inflammation and fibrosis were reduced; they also influenced podocyte preservation. Podocytes play a key role in the progression of Alport syndrome because they are the cell type responsible for synthesizing the GBM37 and podocyte loss is associated with the development of glomerular sclerosis.38 Injection of AFSCs in Alport syndrome mice preserved a normal number of podocytes in glomerular cross-sections compared with noninjected siblings.
Our in vitro co-culture experiments demonstrated that glomerular cells, predominantly podocytes, activated by angiotensin II, expressed high levels of ANGTR1, which is an important component of angiotensin II signaling. Excessive angiotensin II signaling induces podocyte damage and consequent detachment from the GBM.38 Addition of AFSCs to the co-culture system led to decreased ANGTR1 expression, similar to that induced by losartan, an angiotensin II antagonist. We speculate, on the basis of these experiments, that AFSCs have the ability to interfere with angiotensin II signaling to podocytes (by reducing expression of ANGTR1), thus protecting these cells from angiotensin-induced damage, resulting in a delay of podocyte detachment from the GBM.
An interesting study reported by Hayashi et al.39 showed that transient high doses of an angiotensin II receptor-blocker can effectively induce sustained regression of glomerular sclerosis even 6 months after cessation of treatment. We speculate that a single injection of AFSCs transiently interferes with angiotensin II signaling by decreasing ANGTR1 expression, leading to renoprotection over the intermediate term, in addition to effects on cytokine modulation and prevention of fibrosis.
We have hypothesized that a stem cell population may interrupt angiotensin II signaling, delaying the development of podocytopenia and suggesting for the first time a new mechanism to delay progression of a CKD, in addition to inhibition of fibrosis and inflammatory cell infiltrates. It is important to emphasize that CKD is not reversed by a single treatment with AFSCs and that treated animals do eventually succumb to ESRD, albeit later than untreated Alport syndrome mice. Further investigations are needed to determine whether multiple injections of AFSCs can induce a more potent protective effect.
Concise Methods
Isolation, Expansion, and Characterization of Mouse AFSCs
Samples of mouse amniotic fluid from pregnant C57BL/6 females (12–15 days of gestation) were extracted from each embryo and plated individually after three washes in PBS. The cells were then selected for c-kit expression and cultured as previously described.9 Characterization of the clonal mouse cells, including their expression of pluripotent markers and differentiation capability, was tested according to protocols outlined by Atala and colleagues.9
Animal Model and AFSC Injections
Transgenic Alport mice (B6.Cg-Col4α5tm1Yseg/J, stock #006283) on C57BL/6 background were purchased from the Jackson Laboratory. On the basis of our experience, mice homozygous for the Colα5 (IV) allele begin to succumb to the disease at 11 weeks of age, with a mean survival of 23.5 weeks. Alport colonies were maintained according to a homozygous/hemizygous breeding scheme. The animals were divided into three groups according to their treatment status: (1) wild-type C57BL/6 mice (n=15), (2) Col4a5−/− mice (n=25), and (3) Col4a5−/− mice + mouse AFSC, injected at 1.5 months of age (n=25).
On the basis of the type of experiment, injected mice and controls were killed 24 hours, 5 days, 1 month, and 2.5 months after injection. In a parallel experiment, 11 injected Alport syndrome mice and 15 noninjected Alport syndrome mice were left to reach the humane endpoint so that we could evaluate lifespan of both groups.
Immediately before injection, AFSCs were labeled with the cell surface marker CM-DiI (Invitrogen) following the manufacturer’s instructions, and 1×106 cells suspended in 0.1 mL of PBS were injected into the left ventricle using a 29.5-gauge needle under isoflurane anesthesia. All animal experiments were performed in adherence to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and with local Institutional Animal Care and Use Committee approval.
FACS
To evaluate the fate of the AFSCs in Col4a5−/− Alport mice after intracardiac infusion, 12 mice were injected with 1×106 stem cells prelabeled with Qdot immediately before the procedure (Invitrogen). The animals were killed at 24 hours (n=3), 5 days (n=3), 1 month (n=3), and 2.5 months (n=3) after treatment, and four major organs (the heart, kidney, liver, and lung) were processed for FACS analysis. A noninjected littermate served as the negative control throughout the analysis for each time point. In a separate experiment, Qdot-labeled AFSC were serially diluted and FACS results were analyzed to establish the minimum detectable signal (1:100,000 minimum detectable threshold).
Briefly, the heart tissue was minced and digested for 2 hours at 37°C with collagenase type II (Worthington) (0.5 mg/ml) and BSA (1.2 mg/ml) prepared in ADS buffer (116 mM NaCl, 20 mM Hepes, 1.0 mM NaH2PO4, 5 mM KCl, 0.8 mM Mg2SO4, 5.5 mM glucose, pH, 7.4). Then, the samples were centrifuged for 5 minutes at 500 rpm; the pellet was resuspended in PBS and subsequently filtered with 70- and 40-mm nylon mesh filters. Kidneys were harvested and minced, then digested with 16 U/ml Dispase (Worthington) as described by Held et al.40
To isolate the lung cells, the chest cavity was opened and the lungs were exposed. Dispase (20 U/ml; Worthington) was infused into the lungs with a 21-gauge angiocatheter inserted into the trachea. The lungs were then placed in a 50-ml tube with 1-ml Dispase inside and incubated at room temperature for 45 minutes on a shaker. The lungs were transferred onto a clean Petri dish and 0.2 mL DNAse I (Invitrogen) was added. After 10 minutes’ incubation, the lysate was filtered through a series of filters (100 μm to 40 μm) to obtain single cell suspension. Liver tissues were similarly minced and digested with 5 mg collagenase, 5 mg pronase (Worthington), and 1 mg DNase I prepared in 10 ml PBS for 2 hours at 37°C with shaking. The lysate was then passed through 70-μm nylon mesh filters twice and washed in PBS. Before organ harvest, all mice were exsanguinated with PBS through the left ventricle until all organs were pale or white. All cell suspensions were fixed in 1% paraformaldehyde before FACS analysis. Data acquisition and analysis of all organs by flow cytometry were performed with Vantage BD FACSDiva 5.0.1 flow cytometry system and BD FACSDiva5.1.3 software.
Blood Collection, Creatinine, Proteinuria, and BUN Measurements
Blood samples (30 μL) from all experimental groups (n=16 per each experimental group) were collected into plasma separation tubes with lithium heparin. Serum creatinine and BUN levels were measured by colorimetric assay (BioAssay Systems Cat # DICT-500, and Cat # DIUR-500). Urine samples (n=10) were collected overnight from individual mice using metabolic cages (Harvard Apparatus #PY8 72-9061) before treatment (t=0) and at each experimental time point at which the animals were killed (1 month, 2.5 months). The urine albumin-to-creatinine ratio was determined using an ELISA for albuminuria (Immunology Consultants Laboratory # E90AL), and quantitative colorimetric assay kit for urine creatinine was performed following manufacturers’ protocols.
Histology, Real-Time PCR, and Western Blotting
Histology
Kidneys were harvested and fixed in formalin. Paraffin-embedded sections, 5 μm thick, were stained with periodic acid-Schiff reagent (Sigma-Aldrich) to evaluate kidney morphology, following standard protocols. Jones staining was performed on a Ventana NexES automated special stainer using a Jones hematoxylin and eosin staining kit from Ventana (catalog # 860-019) to evaluate basement membrane deposition (Pathology Laboratory, Children’s Hospital Los Angeles).
Immunohistochemistry
Thin deparaffinized kidney sections (5 μm) were blocked in 3% BSA and immunostained against macrophage/L1 protein/calprotectin Ab-1 (Thermo Scientific) at 1:50 dilution, S100A4 (Abcam) at 1:100 dilution with an overnight incubation at 4°C, and WT-1 (Santa Cruz) at 1:50 dilution and with an incubation time of 1 hour at 37°C. Biotinylated secondary antibodies (Vector Laboratories) were used at 1:100 dilutions. Slides were developed with DAB substrate (Vector Laboratories), followed by hematoxylin and eosin stains and mounted with Aqua Mount (Lerner Laboratories). A Leica DM100 microscope was used for imaging. Paraffin-embedded sections were also immunostained for fluorescence microscopy with antibody against the α1 chain of collagen type IV (Life Span Bioscience) at 1:50 dilution for 1 hour at room temperature, collagen I (Abcam) at 1:250 dilution, CD80 (Abcam) at 1:50 dilution, macrophage/L1 protein/calprotectin Ab-1 (Thermo Scientific) at 1:50 dilution at 4°C overnight, and VEGF (Abcam) at 1:200 dilution for 1 hour at room temperature, followed by FITC-conjugated secondary antibody at 1:100 dilution. Sections were counterstained with 4′,6-diamidino-2-phenylindole (Vector Laboratories). Frozen sections were stained with immunofluorescence CD150 (Abcam) at 1:25 dilution at 37°C for 1 hour, platelet/endothelial cell adhesion molecule-1 (PECAM-1) (BD Biosciences) at 1:200 dilution for 1 hour at room temperature. All α chains (Shingei Medical Research; H12 [1], H22 [2], H31 [3], RH42 [4], H53 [5], B66 [6]) were incubated at room temperature for 1 hour at 1:50 dilution. A Leica DM RA fluorescent microscope was used in conjunction with Open Lab 3.1.5 software to image the staining. To assess possible renal differentiation of AFSC into podocytes, we performed WT-1 staining as described, looking for double staining in conjugation with CM-DiI in the glomerular space.
Real-Time PCR
Kidneys were minced into small pieces and the RNA extracted using Qiagen RNeasy kit according to the manufacturer’s instructions. Quantitative real-time PCR for TNFα, CCL2, CXCL2, M-CSF, and IL1-RII was carried out using a Roche Light Cycler 480 and Light Cycler TaqMan Master Mix. Real-time PCR conditions were as follows: 90°C for 10 minutes, 60°C for 10 seconds, and 72°C for 1 second with the analysis of the fluorescent emission at 72°C. Thirty-five cycles were performed for each experiment. To assess whether the rare AFSC present in the glomeruli were able to produce new Col4a5, and the expression of the α chains (Col4α1, Col4α2, Col4α3, Col4α4, Col4α6), we performed real-time PCR for these genes at all different time points as previously described.
Western Blot Analysis
Total protein from kidneys was collected and stored at −80°C in a radioimmunoprecipitation assay buffer supplemented with protease and phosphatase inhibitors (Santa Cruz) until use. Proteins were transferred onto a polyvinylidene fluoride 0.45-μm membrane (Millipore) and probed with antibodies to ID2 at 1:1000 dilution (Abcam), BMP-7 at 1:500 dilution (Abcam), pSMAD2 at 1:1000 dilution (Cell Signaling), and pSMAD1/5 at 1:500 dilution (Cell Signaling). Peroxide-conjugation secondary antibodies were applied (Sigma-Aldrich) in concentrations as follows: 1:10,000 for anti-mouse and 1:20,000 for anti-rabbit. Antigens were detected using the ECL Western blotting detection reagents (Amersham Biosciences/GE Healthcare), impressed on Biomax Light Film (GE Healthcare). Data from four independent experiments were quantified by densitometry (all measurements were normalized against their corresponding housekeeping gene, β-actin). Western blotting for α chains (α2, α3, α5) was performed by following the protocols described by Sugimoto et al.7 Kidneys were minced and homogenized in PBS with complete protease inhibitors, centrifuged, and resuspended in PBS with protease inhibitors and were homogenized for the second time. Tissue homogenates were centrifuged and pellets were resuspended in 1M NaCl containing DNAse I (25 mg/ml, Invitrogen). The samples were vortexed and incubated at room temperature for 10 minutes. After centrifugation, pellets were resuspended in 2% sodium deoxycholate with protease inhibitors, vortexed, and centrifuged again. Pellets were digested in collagenase I (1 unit/ml, Worthington) overnight to solubilize the NC1 domains of type IV collagen. Under reducing conditions, protein extracts were separated on 4%–20% Tris-Glycin gel (Invitrogen) and transferred onto a polyvinylidene fluoride 0.45-μm membrane. Blotted membranes were blocked with 3% BSA containing 50 mM Tris-HCl buffer (pH, 7.5) and 150 mM NaCl for over 8 hours, washed three times with 0.1% Tween-Tris buffer, and then treated with primary type IV collagen antibodies for 2 hours diluted 1:100 in 1% BSA containing 50 mM Tris-HCl buffer (pH, 7.5) and 150 mM NaCl (H22, H31, M54) (Shigei Medical Research Institute, Japan). Horseradish peroxidase–conjugated secondary antibody was applied thereafter and the blots were developed as described earlier.
In Vivo Stimulation of Macrophages and In Vitro Co-Culture with AFSCs
In vivo macrophages were stimulated in C57BL/6J mice (n=5) by injecting 1 mL of a 4% sterile thioglycollate solution (Brewer thioglycollate medium, DIFCO) into the peritoneal cavity under isoflurane. These mice (n=5) were killed with CO2 administration after 4 days, and macrophages were harvested by lavage of the peritoneal cavity.41 Cellular culture was established for 24 hours for the following groups: (1) nonactivated macrophages, (2) lipopolysaccharide (30 ng/ml, Sigma-Aldrich)-activated macrophages, (3) activated macrophages plus AFSC in 10:1 ratio, (4) AFSC alone, and (5) AFSC stimulated with lipopolysaccharide. Supernatant was collected and analyzed for multiple cytokine array (Proteome Profiler Array Kit, c# ARY006), as suggested from the protocol (R&D Systems). RNA was collected and real-time PCR for CD80, Cd86, MCR1, IL1-RI, IL1-RII, and YM1 was performed as described.
Glomeruli Isolation and In Vitro Co-Culture with AFSC
C57BL/6J mice (n=5) were infused with 15 ml of 90% DMEM and 10% embryonic stem cell qualified FBS under pentobarbital anesthesia. Kidneys were extracted, minced, and digested in collagenase I (Gibco), 1 mg/ml collagenase A, and 100 U/ml deoxyribonuclease I (Sigma-Aldrich) in PBS at 37°C for 30 minutes, then filtered twice through a 100-μm cell strainer and once more through a 70-μm cell strainer. The solution was centrifuged at 1500 rpm for 5 minutes and the cells plated. The glomeruli were cultured in 88.8% DMEM, 10% embryonic stem cell qualified FBS, 1% antibiotics, and 0.2% puromycin.42,43 After 3 days, cells began to disperse from the glomeruli. Cells were used within four or five passages. Cellular culture was evaluated for WT-1 expression to identify podocytes, PECAM-1 for endothelial cells, and α smooth muscle actin (at 1:25 dilution at 37°C for 1 hour [Abcam]) for mesangial cells using immunofluorescence as previously described. Cellular culture was established for 4 hours for the following groups: (1) glomerular cells, (2) glomerular cells in the presence of angiotensin II (Sigma-Aldrich) at concentration of 10−5 M twice at a 2-hour interval, (3) glomerular cells in the presence of angiotensin II at concentration of 10−5 M twice at a 2-hour interval plus 100 μM of losartan (Sigma-Aldrich), (4) glomerular cells in the presence of angiotensin II at concentration of 10−5 M twice at a 2-hour interval plus AFSC in 10:1 ratio, (5) AFSC only, and (6) AFSC in the presence of angiotensin II at concentration of 10−5 M twice at a 2-hour interval. The cells were then collected and teal-time PCR for ANGTR1 was performed as previously described.
Evaluation of Glomerular Sclerosis, Collagen I, S100A4-Positive Cells, and Count of WT-1
The degree of glomerular sclerosis was determined in histologic sections (n=10 mice per experimental group) stained with periodic acid-Schiff reagent. The sclerotic index was examined in 50 randomly selected glomeruli. Glomerular sclerosis was assessed using a three-level scale: normal-mild (for normal to minimally sclerotic glomeruli with less than 33% fibrosis), moderate (for intermediate glomeruli with 33%–66% fibrosis), and severe (for glomeruli with over 66% fibrosis).44 The number of WT-1–positive cells was determined in 50 randomly selected glomeruli per mouse (n=10 per experimental group). The fraction of S100A4-positive cells was evaluated in four different interstitial areas per cross-section per mouse (n=10 per experimental group). Collagen I was evaluated in four different interstitial areas per cross-section per mouse (n=10 per experimental group), and its deposition was assessed using TissueQuest 3.0.1.0131 software (Tissue Gnostics). All counts were performed in a blinded fashion.
TGFβ/BMP and Epithelial–Tomesenchymal Transition (Cellular Activation, Proliferation, and Differentiation) PCR Arrays
mRNA from wild-type (n=3) and Col4a5−/−-noninjected (n=3) and Col4a5−/−-injected mice (n=3) was obtained as previously described for the real-time PCR. Briefly, RT2 First Strand Kit (SABiosciences) was used to convert mRNA to cDNA. This cDNA was then added to the SAbiosciences RT2 SYBR Green qPCR Master Mix. Each sample was used to perform quantitative gene expression analysis on specific arrays c# PAMM-035F and c# PAMM-090F. All steps were done according to the manufacturer’s protocol for the Roche Light Cycler 480. The online tool (http://www.sabiosciences.com/pcrarraydataanalysis.php) offered by the manufacturer was used to analyze the data, including significant values and fold changes. Fold changes for the gene expression were investigated between injected Col4a5−/− mice and their noninjected siblings.
Transmission Electron Microscopy Procedure
Fresh kidneys (n=3 per experimental group) underwent primary fixation with 2% glutaraldehyde in sodium phosphate buffer. They were then post-fixed in 1% osmium tetroxide for 1 hour and dehydrated in 50%, 70%, 90%, 95%, 100%, ethanol and propylene oxide for 10 minutes each. Samples were further infiltrated with epoxy resin mixture (Eponate 12 resin). Ultra-thin sections were collected on copper grids, and sections were stained using 10% uranyl acetate in 50% methanol and modified Sato lead stain. A Morgagni 268 electron microscope was used for picture acquisition (Pathology Laboratory, Children’s Hospital Los Angeles).
Statistical Analyses
Statistical analyses were performed using SigmaPlot 11 data analysis software (Systat Software Inc). Statistical differences between groups were determined using ANOVA and an unpaired t test for data sets that passed the Shapiro-Wilk normality test or a Mann-Whitney rank-sum test for data that failed the Shapiro-Wilk normality test. Kaplan-Meier curves were used for the survival study, and the log-rank (Mantel-Cox) test was used to determine statistical significance. A P value of 0.05 was considered to represent a statistically significant difference. Data are shown as mean ± SEM.
Acknowledgments
This study was supported by the Alport Foundation and by the California Institute for Regenerative Medicine (CIRM).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011030243/-/DCSupplemental.
References
- 1.Bonventre JV: Pathophysiology of acute kidney injury: roles of potential inhibitors of inflammation. Contrib Nephrol 156: 39–46, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Humphreys BD, Bonventre JV: Mesenchymal stem cells in acute kidney injury. Annu Rev Med 59: 311–325, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Herrera MB, Bussolati B, Bruno S, Morando L, Mauriello-Romanazzi G, Sanavio F, Stamenkovic I, Biancone L, Camussi G: Exogenous mesenchymal stem cells localize to the kidney by means of CD44 following acute tubular injury. Kidney Int 72: 430–441, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Hudson BG, Reeders ST, Tryggvason K: Type IV collagen: Structure, gene organization, and role in human diseases. Molecular basis of Goodpasture and Alport syndromes and diffuse leiomyomatosis. J Biol Chem 268: 26033–26036, 1993 [PubMed] [Google Scholar]
- 5.Noël LH: Renal pathology and ultrastructural findings in Alport's syndrome. Ren Fail 22: 751–758, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Prodromidi EI, Poulsom R, Jeffery R, Roufosse CA, Pollard PJ, Pusey CD, Cook HT: Bone marrow-derived cells contribute to podocyte regeneration and amelioration of renal disease in a mouse model of Alport syndrome. Stem Cells 24: 2448–2455, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Sugimoto H, Mundel TM, Sund M, Xie L, Cosgrove D, Kalluri R: Bone-marrow-derived stem cells repair basement membrane collagen defects and reverse genetic kidney disease. Proc Natl Acad Sci USA 103: 7321–7326, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LeBleu V, Sugimoto H, Mundel TM, Gerami-Naini B, Finan E, Miller CA, Gattone VH, 2nd, Lu L, Shield CF, 3rd, Folkman J, Kalluri R: Stem cell therapies benefit Alport syndrome. J Am Soc Nephrol 20: 2359–2370, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Coppi P, Bartsch G, Jr, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, Furth ME, Soker S, Atala A: Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 25: 100–106, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Carraro G, Perin L, Sedrakyan S, Giuliani S, Tiozzo C, Lee J, Turcatel G, De Langhe SP, Driscoll B, Bellusci S, Minoo P, Atala A, De Filippo RE, Warburton D: Human amniotic fluid stem cells can integrate and differentiate into epithelial lung lineages. Stem Cells 26: 2902–2911, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Da Sacco S, Sedrakyan S, Boldrin F, Giuliani S, Parnigotto P, Habibian R, Warburton D, De Filippo RE, Perin L: Human amniotic fluid as a potential new source of organ specific precursor cells for future regenerative medicine applications. J Urol 183: 1193–1200, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perin L, Giuliani S, Sedrakyan S, DA Sacco S, De Filippo RE: Stem cell and regenerative science applications in the development of bioengineering of renal tissue. Pediatr Res 63: 467–471, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Perin L, Sedrakyan S, Giuliani S, Da Sacco S, Carraro G, Shiri L, Lemley KV, Rosol M, Wu S, Atala A, Warburton D, De Filippo RE: Protective effect of human amniotic fluid stem cells in acute tubular necrosis. PLoS One 5:e9357, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nörgaard JO: Cellular outgrowth from isolated glomeruli. Origin and characterization. Lab Invest 48: 526–542, 1983 [PubMed] [Google Scholar]
- 15.Spear GS: The pathology of the kidney in the Alport syndrome. Birth Defects Orig Artic Ser 10: 109–113, 1974 [PubMed] [Google Scholar]
- 16.Andrews KL, Mudd JL, Li C, Miner JH: Quantitative trait loci influence renal disease progression in a mouse model of Alport syndrome. Am J Pathol 160: 721–730, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katayama K, Kawano M, Naito I, Ishikawa H, Sado Y, Asakawa N, Murata T, Oosugi K, Kiyohara M, Ishikawa E, Ito M, Nomura S: Irradiation prolongs survival of Alport mice. J Am Soc Nephrol 19: 1692–1700, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gross O, Borza DB, Anders HJ, Licht C, Weber M, Segerer S, Torra R, Gubler MC, Heidet L, Harvey S, Cosgrove D, Lees G, Kashtan C, Gregory M, Savige J, Ding J, Thorner P, Abrahamson DR, Antignac C, Tryggvason K, Hudson B, Miner JH: Stem cell therapy for Alport syndrome: The hope beyond the hype. Nephrol Dial Transplant 24: 731–734, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bielesz B, Sirin Y, Si H, Niranjan T, Gruenwald A, Ahn S, Kato H, Pullman J, Gessler M, Haase VH, Susztak K: Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J Clin Invest 120:4040–4054, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Hir M, Hegyi I, Cueni-Loffing D, Loffing J, Kaissling B: Characterization of renal interstitial fibroblast-specific protein 1/S100A4-positive cells in healthy and inflamed rodent kidneys. Histochem Cell Biol 123: 335–346, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Hauser PV, De Fazio R, Bruno S, Sdei S, Grange C, Bussolati B, Benedetto C, Camussi G: Stem cells derived from human amniotic fluid contribute to acute kidney injury recovery. Am J Pathol 177: 2011–2021, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villanueva S, Ewertz E, Carrión F, Tapia A, Vergara C, Céspedes C, Sáez PJ, Luz P, Irarrázabal C, Carreño JE, Figueroa F, Vio CP: Mesenchymal stem cell injection ameliorates chronic renal failure in a rat model. Clin Sci (Lond) 121: 489–499, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Ninichuk V, Gross O, Segerer S, Hoffmann R, Radomska E, Buchstaller A, Huss R, Akis N, Schlöndorff D, Anders HJ: Multipotent mesenchymal stem cells reduce interstitial fibrosis but do not delay progression of chronic kidney disease in collagen4A3-deficient mice. Kidney Int 70: 121–129, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Sison K, Eremina V, Baelde H, Min W, Hirashima M, Fantus IG, Quaggin SE: Glomerular structure and function require paracrine, not autocrine, VEGF-VEGFR-2 signaling. J Am Soc Nephrol 21: 1691–1701, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doi K, Noiri E, Fujita T: Role of vascular endothelial growth factor in kidney disease. Curr Vasc Pharmacol 8: 122–128, 2010. [Review] [DOI] [PubMed] [Google Scholar]
- 26.Hohenstein B, Colin M, Foellmer C, Amann KU, Brekken RA, Daniel C, Hugo CP: Autocrine VEGF-VEGF-R loop on podocytes during glomerulonephritis in humans. Nephrol Dial Transplant 25: 3170–3180, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Gouédard L, Chen YG, Thevenet L, Racine C, Borie S, Lamarre I, Josso N, Massague J, di Clemente N: Engagement of bone morphogenetic protein type IB receptor and Smad1 signaling by anti-Müllerian hormone and its type II receptor. J Biol Chem 275: 27973–27978, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Rider CC, Mulloy B: Bone morphogenetic protein and growth differentiation factor cytokine families and their protein antagonists. Biochem J 429: 1–12, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Zeisberg M, Khurana M, Rao VH, Cosgrove D, Rougier JP, Werner MC, Shield CF, 3rd, Werb Z, Kalluri R: Stage-specific action of matrix metalloproteinases influences progressive hereditary kidney disease. PLoS Med 3: e100, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jernigan S, Eddy A: Experimental insights into the mechanisms of tubulo-interstitial scarring. In: Mechanisms and clinical management of chronic renal failure, edited by El Nahas A, Harris K, Anderson S, Oxford, Oxford University Press, 2000, pp 104–145 [Google Scholar]
- 31.Chen Y, Wood KJ: Interleukin-23 and TH17 cells in transplantation immunity: Does 23+17 equal rejection? Transplantation 84:1071–1074, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M: The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25: 677–686, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Mosser DM: The many faces of macrophage activation. J Leukoc Biol 73: 209–212, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Cao Q, Zheng D, Wang YP, Harris DC: Macrophages and dendritic cells for treating kidney disease. Nephron, Exp Nephrol 117: e47–e52, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Ricardo SD, van Goor H, Eddy AA: Macrophage diversity in renal injury and repair. J Clin Invest 118: 3522–3530, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ninichuk V, Gross O, Reichel C, Khandoga A, Pawar RD, Ciubar R, Segerer S, Belemezova E, Radomska E, Luckow B, Perez de Lema G, Murphy PM, Gao JL, Henger A, Kretzler M, Horuk R, Weber M, Krombach F, Schlöndorff D, Anders HJ: Delayed chemokine receptor 1 blockade prolongs survival in collagen 4A3-deficient mice with Alport disease. J Am Soc Nephrol 16: 977–985, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Miner JH: Glomerular basement membrane composition and the filtration barrier. Pediatr Nephrol 26: 1413–1417, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffmann S, Podlich D, Hähnel B, Kriz W, Gretz N: Angiotensin II type 1 receptor overexpression in podocytes induces glomerulosclerosis in transgenic rats. J Am Soc Nephrol 15: 1475–1487, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Hayashi K, Sasamura H, Ishiguro K, Sakamaki Y, Azegami T, Itoh H: Regression of glomerulosclerosis in response to transient treatment with angiotensin II blockers is attenuated by blockade of matrix metalloproteinase-2. Kidney Int 78: 69–78, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Held PK, Al-Dhalimy M, Willenbring H, Akkari Y, Jiang S, Torimaru Y, Olson S, Fleming WH, Finegold M, Grompe M: In vivo genetic selection of renal proximal tubules. Mol Ther 13: 49–58, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Li YM, Baviello G, Vlassara H, Mitsuhashi T: Glycation products in aged thioglycollate medium enhance the elicitation of peritoneal macrophages. J Immunol Methods 201: 183–188, 1997 [DOI] [PubMed] [Google Scholar]
- 42.da Silva Meirelles L, Chagastelles PC, Nardi NB: Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci 119: 2204–2213, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C: A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lauronen J, Häyry P, Paavonen T: An image analysis-based method for quantification of chronic allograft damage index parameters. APMIS 114: 440–448, 2006 [DOI] [PubMed] [Google Scholar]