Abstract
Intraflagellar transport (IFT) complexes A and B build and maintain primary cilia. In the mouse, kidney-specific or hypomorphic mutant alleles of IFT complex B genes cause polycystic kidneys, but the influence of IFT complex A proteins on renal development is not well understood. In the present study, we found that HoxB7-Cre–driven deletion of the complex A gene Ift140 from collecting ducts disrupted, but did not completely prevent, cilia assembly. Mutant kidneys developed collecting duct cysts by postnatal day 5, with rapid cystic expansion and renal dysfunction by day 15 and little remaining parenchymal tissue by day 20. In contrast to many models of polycystic kidney disease, precystic Ift140-deleted collecting ducts showed normal centrosomal positioning and no misorientation of the mitotic spindle axis, suggesting that disruption of oriented cell division is not a prerequisite to cyst formation in these kidneys. Precystic collecting ducts had an increased mitotic index, suggesting that cell proliferation may drive cyst expansion even with normal orientation of the mitotic spindle. In addition, we observed significant increases in expression of canonical Wnt pathway genes and mediators of Hedgehog and tissue fibrosis in highly cystic, but not precystic, kidneys. Taken together, these studies indicate that loss of Ift140 causes pronounced renal cystic disease and suggest that abnormalities in several different pathways may influence cyst progression.
Genesis of primary cilia—specialized microtubular-based sensory structures that project from most cells—requires intraflagellar transport (IFT), a bidirectional process that builds, maintains, and disassembles these organelles. IFT also supports diverse signaling roles played by primary cilia that influence development, differentiation, and cell cycle regulation.1–3 In the kidney, primary cilia play vital roles in promoting tubular development and maintaining normal renal morphology and function. Mutations that produce structural or functional defects in renal cell primary cilia cause abnormal proliferation of tubular epithelia, increased fluid secretion, and polycystic kidney disease.4–6 Discerning processes controlling IFT-mediated ciliary assembly and function is essential for understanding the pathogenic mechanisms underlying cystic renal diseases and other ciliopathies.
The IFT system consists of two large protein complexes, IFT complexes A and B, that are transported by kinesin-2 and cytoplasmic dynein-2.7–10 IFT complex B comprises at least 13 proteins11 and is required for ciliary assembly.12–16 In the mouse, strong alleles of IFT complex B genes typically produce midgestational lethality14,15 before renal development. Hypomorphic mutations in Ift88 or kidney-specific deletion of Ift20, two IFT complex B genes, cause renal cyst formation.13,16 Similarly, strong alleles of the kinesin-2 or the dynein-2 IFT motors produce midgestational lethality,15,17,18 whereas kidney-specific deletion of kinesin-2 results in cystic disease.19
In contrast, very little is known about what, if any, role is played by IFT complex A proteins on mammalian renal development and renal physiology. In invertebrate organisms, mutation or RNA interference depletion of individual IFT complex A proteins produces cilia that are shortened and often dilated and accumulate ciliary proteins.12,20–26 Similarly, RNA interference knockdown of complex A proteins in mammalian cells generated shortened cilia that accumulated IFT-B proteins.27 In mice, null alleles of individual IFT complex A proteins, including IFT139,28 IFT122,29,30 and IFT121,31 produced defects in skeletal, craniofacial, and nervous systems that caused embryonic or neonatal death. Because of the early lethality of IFT complex A mutants, there is no information as to whether IFT complex A defects would disrupt renal development or function in mice. In Ift122 zebrafish morphants, pronephric cysts were observed32; in contrast, Ift140 morphants showed no apparent ciliary or sensory neuron defects.33 Missense mutations in IFT-A proteins have been described in patients with cranioectodermal dysplasia/Sensenbrenner’s syndrome,31,32,34,35 a ciliopathy associated with extensive craniofacial, skeletal, heart, liver, and ectodermal abnormalities. Some cranioectodermal dysplasia patients exhibit renal disease characterized by extensive glomerular sclerosis, renal cysts, interstitial fibrosis with focal inflammatory cell infiltration, scattered tubular atrophy, and chronic renal failure.31,32,36 Nonetheless, our understanding of how IFT complex A proteins influence renal development and cystic disease is extremely limited, and the present studies addressed this question by characterizing IFT140 function in mouse kidney.
Results
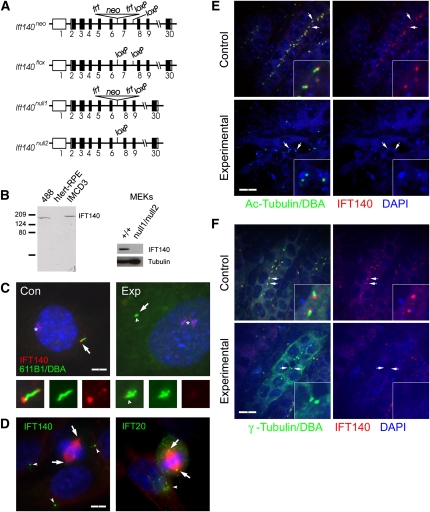
To understand the role of IFT140 in cystic kidney disease, we used Knockout Mouse Project (KOMP) embryonic stem (ES) cells37,38 to create flox and null1 alleles (Figure 1A). Animals homozygous for Ift140flox are viable with no detectable phenotypes, whereas animals homozygous for Ift140null1 die at midgestation and will be described in a separate publication. In this work, we used HoxB7-Cre to delete Ift140 in the collecting ducts. Control animals have the genotype HoxB7-Cre, Ift140flox/+, and experimental animals are HoxB7-Cre, Ift140flox/null1. The flox allele is converted to the null2 allele by Cre, and therefore, experimental animals are Ift140null2/null1 in the collecting ducts. An antibody generated against mouse IFT140 (Figure 1B) does not detect any IFT140 in extracts made from cell lines derived from experimental collecting ducts (Figure 1B), indicating that the Ift140null2/null1 combination produces a null or strong hypomorphic phenotype. During interphase, IFT140 localizes prominently to the ciliary base and tip and also is found along the ciliary shaft (Figure 1, C and D). Ift140null2/null1 cells assembled at most very short cilia that did not stain with the IFT140 antibody (Figure 1C). Other IFT proteins including IFT20 (Figure 1D) localize to the spindle pole during mitosis.39–42 In contrast, IFT140 does not seem to be associated with the spindle pole bodies during mitosis (Figure 1D). In control postnatal (p)5 kidneys, IFT140 labels the base of the cilium (Figure 1E) just adjacent to the centrosome (Figure 1F). Staining of experimental kidneys indicates that, at most, very short cilia remain at p5 and no IFT140 staining is observed. These results indicate that the conversion of the Ift140flox allele to the Ift140null2 allele is efficient and that IFT140 is required for ciliary assembly.
Figure 1.
HoxB7-Cre efficiently deletes the Ift140flox allele. (A) Diagram of targeting vector. Exons are displayed as boxes, whereas the coding region is shaded in black. frt, FlpE recombinase sites; loxP, Cre recombinase sites; neo, β-galactosidase-neomycin resistance gene fusion. (B) Affinity-purified anti-MmIFT140 detects a single band in protein extracts from the mouse cell lines 488 and IMCD3 but nothing in the human cell line hTert-RPE. This band is observed in extracts from mouse epithelial kidney (MEK) cells derived from a control kidney (+/+) but not from an experimental Ift140null2/null1 kidney. The MEK Western was also probed with a tubulin antibody (B-5-1-2) as a loading control. (C) In interphase control primary kidney cells, IFT140 (red) is found most strongly at the base (arrow) of the cilium (green, 6-11B-1) and is also found along the ciliary shaft and at the tip. In experimental cells (Ift140null2/null1), the cilia are very short or not present, and no IFT140 is associated with the remaining centrioles (arrow) or shortened cilium (arrowhead). These cells were also labeled with Dolichos biflorus agglutinin (DBA) in green to mark collecting duct cells. Note that DBA staining is far weaker than the 6-11B-1 staining. Asterisk marks an unidentified structure labeled by the IFT140 antibody in both experimental and control cells. Scale bar, 5 μm for both images. Bottom panels are three-time enlargements of the ciliary region shown as merged images and green (6-11B-1) and red (IFT140) channels separately. (D) In mitotic IMCD3 cells, IFT140 (green) is found in puncta in the cytoplasm but is not associated with the spindle pole bodies (arrows). Arrowheads mark cilia stained by IFT140 in neighboring cells. In contrast, IFT20 (green) is found at the spindle pole bodies (arrows) and in the cytoplasm. The arrowhead marks IFT20 staining the Golgi complex in a neighboring cell. In both images, red is anti–α-tubulin. Scale bar, 5 μm for both images. (E) Kidneys from control and experimental p5 animals were stained for cilia (green, 6-11B-1), collecting ducts (green, DBA), IFT140 (red), and nuclei (blue, 4',6-diamidino-2-phenylindole [DAPI]). Note that the DBA staining is weaker than the 6-11B-1 staining and does project in these images but was visible to identify collecting ducts. Arrows mark centrosomes in collecting ducts. Insets are three-time enlargements of cilia/centrosomes at arrows. Size bar, 10 μm for all images. Images are maximum projections of 13 confocal Z-images taken 0.25 μm apart. (F) Kidneys from control and experimental p5 animals were stained for centrosomes (green, γ-tubulin), collecting ducts (green, DBA), IFT140 (red), and nuclei (blue, DAPI). Note that the DBA staining is weaker than the γ-tubulin staining and does project in these images, but it was visible to identify collecting ducts. Arrows mark centrosomes in collecting ducts. Insets are three-time enlargements of centrosomes at arrows. Size bar, 10 μm for all images. Images are maximum projections of 13 confocal Z-images taken 0.25 μm apart.
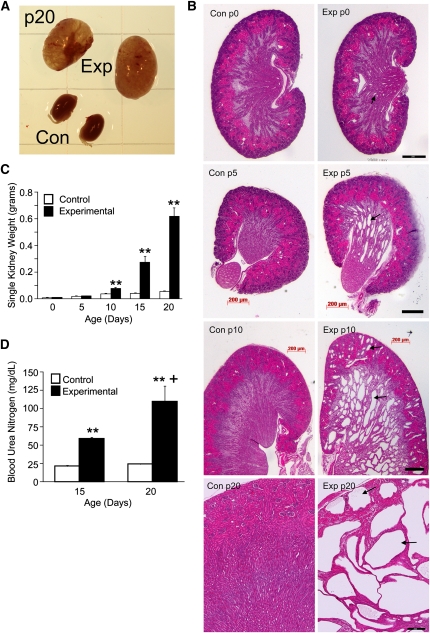
HoxB7-Cre expression begins with mesonephric duct development 6–9 days before birth43 before formation of the ureteric bud, the progenitor of adult collecting ducts. Collecting duct deletion of Ift140 led to pronounced postnatal renal cyst formation (Figure 2, A and B). At p0, there are modest medullary collecting duct dilations but no renal cysts. By p5, extensive medullary cysts are evident, with minimal cortical cysts. By p10, cysts are present in medullary and cortical regions, and by p20, extensive cysts are found throughout the kidney, with little remaining parenchymal tissue (Figure 2B). Kidney weights increased progressively (Figure 2C), and blood urea nitrogen levels were elevated in mutants at p15 and p20 (Figure 2D), consistent with renal failure during the third week of life.
Figure 2.
Deletion of Ift140 in mouse collecting ducts causes renal cysts and renal failure. (A) Gross morphology of experimental kidneys (top pair) and control kidneys at p20. (B) Hematoxylin/eosin (H&E) -stained sections of control and mutant kidneys at p0, p5, p10, and p20. Experimental kidneys at p0 are normal except for some minor dilation of the medullary collecting ducts (arrows) but become cystic (arrows) with age. Scale bars, 200 μm for both images in a pair. (C) Mean ± SEM individual kidney weights of control (open bars) and experimental (filled bars) animals at 5-day intervals between p0 and p20. **P<0.01 versus age-matched control (5–12 animals per time point). (D) BUN levels (mean ± SEM) in control and experimental animals at p15 and p20. n=3 animals at p15, and n=4 animals at p20 for each genotype. **P<0.01 comparing control and experimental animals at each time point. +P<0.05 comparing experimental animals between p15 and p20.
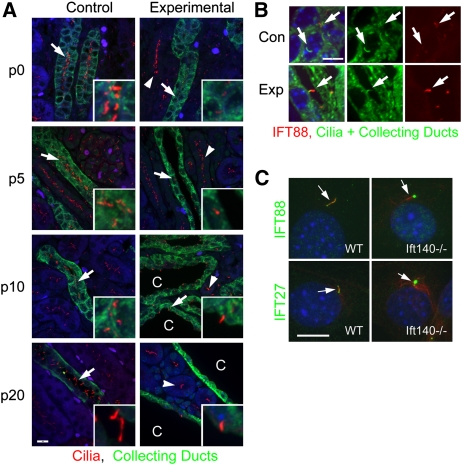
Control collecting ducts were highly ciliated at birth, whereas experimental p0 collecting ducts had no or very short cilia (Figure 3A). There was no ciliary loss in noncollecting duct cells (Figure 3A), supporting the specificity of HoxB7-Cre for the collecting duct system.43 Although there may be a progressive loss of these shortened cilia over time, many collecting duct cells still carried stumpy cilia at p10 and p20 (Figure 3A). These shortened cilia show increased staining with IFT88 compared with controls (Figure 3B). Similarly, Ift140-deleted cultured cells showed increased ciliary staining with IFT88 and IFT27 antibodies (Figure 3C).
Figure 3.
Effects of Ift140 deletion on cilia. (A) Kidneys from control and experimental animals were stained for cilia (red, 6-11B-1), collecting ducts (green, DBA), and nuclei (blue, DAPI) at the day of birth (p0), and postnatal days 5, 10, and 20. Arrows mark collecting ducts, and arrowheads mark ciliated nephrons near collecting ducts; cysts and dilated ducts are marked with a C. Size bar, 10 μm. Insets are four-time enlargements of areas marked by the arrow. Images are maximum projections of 16 confocal Z-images taken 0.5 μm apart. (B) IFT88 accumulates in mutant cilia. Kidneys from p5 control and experimental animals were stained for IFT88 (red), acetylated tubulin (green, cilia), DBA (green, collecting ducts), and nuclei (blue, DAPI). Size bar, 5 μm for all images in C. Images are maximum projections of 26 confocal Z-images taken 0.2 μm apart. (C) Mouse embryonic fibroblasts derived from wild-type (WT) and Ift140null2/null2 (−/−) animals were stained for acetylated tubulin (red) to mark cilia (arrows) and IFT88 (green) or IFT27 (green) along with DAPI (blue). Note that Ift140 experimental cells accumulate IFT88 and IFT27 in the cilia. Size bar, 10 μm for all images.
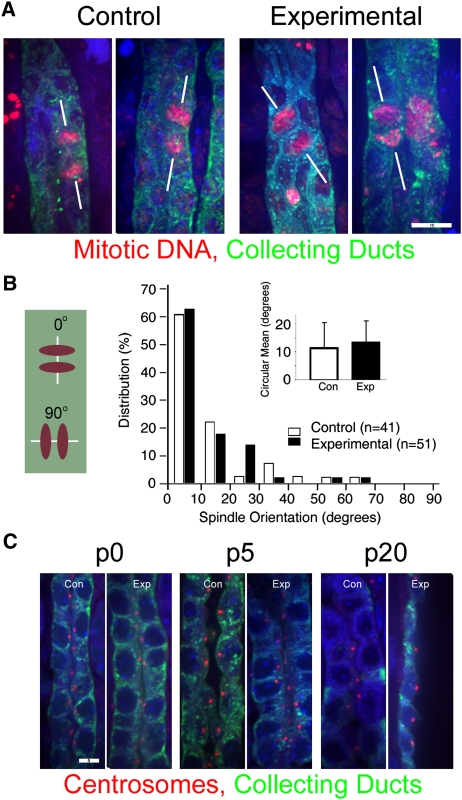
During normal tubular development, the mitotic spindle of dividing cells orients in parallel to the longitudinal axis of the nephron.44 In many different polycystic kidney disease (PKD) models,16,45,46 randomization of the mitotic spindle axis may be a critical part of the cystogenic program. At p5, many experimental collecting ducts were already dilated; these ducts were excluded from analysis to focus on events occurring before the duct lost its normal architecture. In control collecting ducts, mitotic spindles aligned with the long axis of the tubule. In contrast to what we observed in Ift20-deleted kidneys,16 loss of Ift140 did not alter mitotic spindle orientation in precystic collecting ducts (Figure 4, A and B). Whereas deletion of Ift20 from the collecting ducts caused mislocalization of the centrosome from the center of the apical end of the cell,16 deletion of Ift140 did not produce this phenotype, because the centrosomes remained apically positioned even in the highly cystic p20 ducts (Figure 4C).
Figure 4.
Deletion of Ift140 does not lead to mitotic spindle misorientation or centrosomal abnormalities in collecting duct cells. (A) In normal p5 kidney tubules, mitotic spindles (red, phospho-histone H3) typically orient parallel to the long axis of the collecting duct (green, DBA; A, left and B, open bars). The absence of IFT140 does not alter this relationship in p5 collecting ducts (A, right and B, filled bars). Size bar, 10 μm for all four images. Images are maximum projections of 15 confocal Z-images taken 0.5 μm apart. (B) Mitotic spindle orientation quantitation. Mitotic collecting duct cells were photographed, and the angle between the long axis of the tubule and the spindle was measured as depicted in the schematic diagram in left; angles were grouped into 10° bins. Inset bar graph shows circular mean mitotic spindle orientation and the 95% confidence interval about the mean in collecting ducts of control (open bars, n=41) and experimental (filled bars, n=51) kidneys. (P=0.479, NS, Kolmogorov–Smirov test). (C) Centrosome phenotypes. In normal kidneys, centrosomes (red, GTU-88) are normally found at the center of apical surface of the cell. This location is not altered when Ift140 is deleted. Scale bar, 5 μm for all images in C. Images are maximum projections of three confocal Z-images taken 0.5 μm apart.
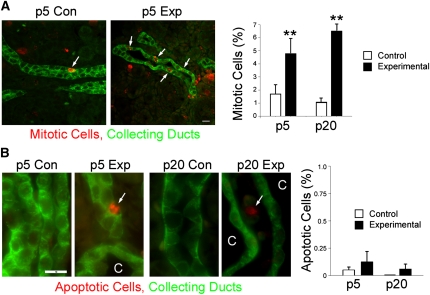
Increased cell proliferation is a hallmark of cystic kidney diseases.5 Mouse kidneys undergo substantial postnatal development, and consequently, both experimental and control kidneys have relatively high levels of mitotic cells at p5; however, the percentage of mitotic cells is significantly higher when Ift140 is deleted (Figure 5A). Postnatal development normally completes within the first 3 weeks of life and the rate of proliferation declines, but in Ift140-deleted collecting ducts, proliferation remains high (Figure 5A). Apoptosis is normally very low in healthy kidneys but is elevated in cystic kidneys.47 Similar to published reports, we found very low numbers of cleaved caspase-3–positive cells in the collecting ducts of control animals at either p5 or p20, and this finding was not significantly altered when Ift140 was deleted. At p20, apoptosis was observed in noncollecting duct cells, probably a result of the expanding cysts impinging on their neighbors (Figure 5B).
Figure 5.
Proliferation and apoptosis in Ift140-defective collecting ducts. (A) p5 kidneys labeled with phospho-histone H3 (red) and DBA (green) to identify mitotic collecting duct cells (arrows). Size bar, 10 μm. Mitotic (phospho-histone H3-positive) cells were counted in >1000 cells from cortical collecting ducts or cortical cysts in p5 and p20 Ift140 control (open bars) and experimental (filled bars) kidneys. Bars depict mean percent ± SEM; n=4–5 animals of each genotype and age. **P<0.01 comparing control and experimental animals. (B) p5 and p20 kidneys labeled with cleaved caspase-3 (red) and DBA (green) to identify apoptotic collecting duct cells (arrow). At p20, most apoptotic cells (arrows) were outside of the cysts (C). Images are maximum projections of three wide-field images taken 2 μm apart. Size bar, 10 μm for all images in B. Apoptotic (cleaved caspase-3–positive) cells were counted in 1000 cells from cortical collecting ducts or cortical cysts in p5 and p20 Ift140 control (open bars) and experimental (filled bars) kidneys. Bars depict mean percent ± SEM; n=4–5 animals of each genotype and age. The differences were NS (P was not <0.05).
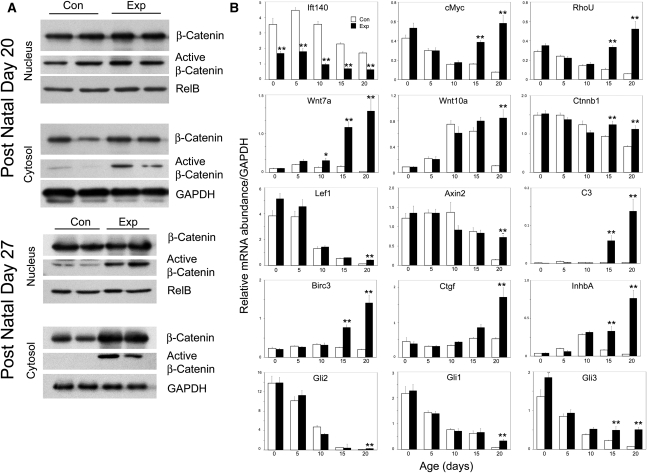
Abnormalities in diverse signaling pathways have been implicated in renal cyst initiation or expansion.5 For example, canonical Wnt is up-regulated in many cystic kidney models and human autosomal dominant PKD.48,49 Activation of canonical Wnt signaling promotes increased nuclear accumulation of dephospho-β-catenin and transcriptional activation of Wnt-responsive target genes.49 Deletion of Ift140 increases activated dephospho-β-catenin levels in both nucleus and cytoplasm (Figure 6A). To understand the relationship between cyst development and Wnt signaling, we performed a time course analysis, examining expression of genes associated with canonical Wnt signaling (Figure 6B). We also measured Ift140 mRNA, which was significantly reduced but not absent at all time points. This finding was expected, because mRNA was isolated from whole kidney; however, Ift140 was deleted only in collecting ducts. Expression of Axin2 and Lef1 declined over postnatal development in both groups, with rate of decline greater in controls; therefore, both genes were significantly higher in p20 mutants. Postnatal β-catenin (Ctnnb1) expression gradually declined in control but not in mutant kidneys, resulting in significantly elevated Ctnnb1 mRNA in mutant kidneys at p15 and p20. c-Myc and RhoU mRNAs showed a similar pattern of expression, declining significantly in both experimental and control kidneys until p10, continuing to decrease in controls at p15 and p20, and significantly rising in mutants at the latter two time points. Wnt10a expression is low in control and experimental kidneys at p0, increases in both groups up until p10, and then declines in controls over the next 10 days while continuing to increase in mutant kidneys. Wnt7a mRNA expression is very low at p0 in control and experimental kidneys, modestly increases in both groups at p5, declines significantly in controls by p20, and rises >50-fold by p20.
Figure 6.
Altered canonical Wnt signaling and selected gene expression in collecting duct Ift140-deleted kidneys. (A) Western blot analysis of β-catenin. Control (Con) and experimental (Exp) kidneys were fractionated into cytosol and nuclear fractions and analyzed by Western blots with antibodies to β-catenin and dephosphorylated (active) β-catenin. RelB and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are loading controls. Each lane is a different animal. (B) Quantitative PCR analysis of 12 genes in experimental (filled bars) and control (open bars) kidneys at selected postnatal times. Bars depict mean ± SEM of 5–11 individual mouse kidneys in each treatment and age group. Gene expression data are normalized to GAPDH expression. **P<0.01, *P<0.05; Tukey HSD post hoc test. Raw data and statistical analysis of temporal changes of gene expression are included in Supplemental Tables 1–3.
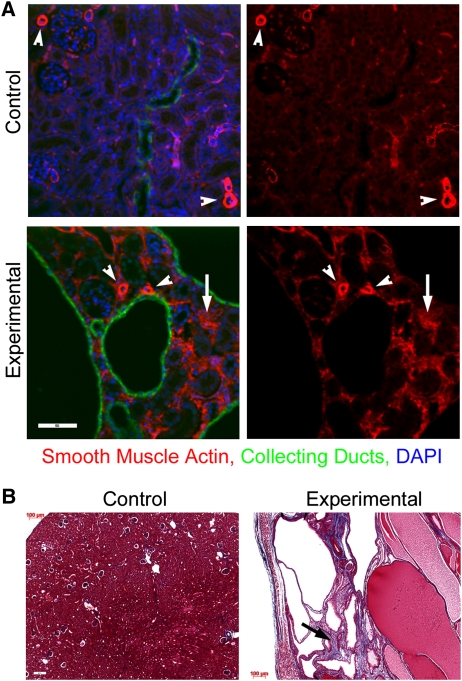
To understand how deletion of Ift140 affects other signaling processes, selected genes from additional candidate pathways implicated in kidney development and cystic disease were examined. Although Hedgehog signaling has not been implicated in cystic disease, it plays essential roles in kidney development and is highly interconnected with cilia.50,51 Expression of three Hedgehog signaling effectors Gli1, Gli2, and Gli3 steadily declines in postnatal control and experimental kidneys, although decreasing somewhat more slowly in experimental kidneys. Hippo signaling has recently been implicated in cystic disease,52 and expression of the Hippo-regulated gene Birc3 is elevated late in cystic disease development in Ift140-deleted kidneys. The fibrosis-associated genes Ctgf and InhbA are downstream targets of Hippo signaling, and their expression in Ift140-deleted kidneys is similarly elevated late in disease. Consistent with increased expression of fibrosis genes, Ift140-deleted cystic kidneys were fibrotic, showing increased interstitial cell smooth muscle actin staining (Figure 7A) and increased collagen deposition (Figure 7B). Activation of innate immunity has been described in cystic kidneys.53 Expression of complement C3, which plays pivotal roles in innate immunity, is normally expressed at a very low level but is induced >100-fold late in cystic expansion in Ift140-deleted kidneys (Figure 6B).
Figure 7.
Fibrosis accompanies the development of cystic disease. (A) Interstitial cells in experimental p20 animals show increased staining with smooth muscle actin (arrow) compared with controls. Arrowheads depict vascular structures in both genotypes. Scale bar, 50 μm. (B) Trichrome blue staining reveals increased collagen deposition (arrow) in experimental kidneys at p20. Scale bar, 100 μm.
Discussion
It has now well appreciated that ciliary defects lead to cystic disease and that IFT is critical for building cilia and maintaining the proper signaling environment within these organelles. However, the role of IFT complex A proteins in cystic disease was not previously known, because all of the mouse IFT mutants with cystic phenotypes were in IFT complex B proteins or molecular motors. Analysis of complex A mutants in nonvertebrates suggested that complex A proteins may not be as important to ciliary assembly as complex B proteins.12,20–26 Whereas complex B defects typically cause a complete lack of ciliary assembly, complex A defects often present with shortened cilia containing abnormal accumulations of protein. These observations suggested that complex B is more important for anterograde transport of materials from the cell body to the cilium tip, whereas complex A is more important for retrograde transport of materials from ciliary tip to cell body. This binary model overlooks the complexity of the IFT system. Complex A and B proteins are trafficked in both directions, likely as part of a large train, and retrograde transport requires prior anterograde transport to deliver IFT particles to the tip of the cilium before retrograde transport can actually occur.54 Moreover, two recent studies implicate complex A proteins in anterograde trafficking of materials into cilia. Mutations in Drosophila Ift140 produced shortened chordotonal cilia with reduced amounts of a TRPV calcium channel.23 Similarly, RNA interference-induced depletion of IFT-A proteins, including IFT140, blocked G protein–coupled receptor trafficking into mammalian primary cilia.27 Taken together, these findings suggest that IFT140 and other complex A proteins play additional roles in ciliary function in addition to mediating retrograde IFT.
Our finding that the loss of IFT140 did not affect mitotic spindle orientation was unexpected, because the presence of aberrant mitotic spindle orientation in a number of cystic models suggested that it may be a driving force for the expansion of tubules into cysts.45 However, the presence of normally oriented mitotic spindles in Ift140-deleted collecting ducts indicates that randomization of spindle orientation is not a prerequisite for cystic disease. This result is similar to the work of Nishio et al.55 that found normal spindle orientation in Pkd1 and Pkd2 mutant kidneys before cyst formation. The difference in the ability of Ift20 and Ift140 mutant cells to orient their spindles may relate to our observation that, unlike IFT20 and other complex B proteins, IFT140 does not localize to the mitotic spindle pole. Recent work indicated that IFT88 localizes to the spindle pole and is important in the formation of astral microtubules needed to orient the mitotic spindle within cells.42 If astral microtubule formation is a general function of IFT complex B proteins, it may explain why Ift20-deleted cells have misoriented spindles. The absence of IFT140 at the spindle pole suggests that this function is not likely a function for this particular protein and possibly all of complex A. Another difference between IFT20 and IFT140 that may contribute to the difference in oriented cell division is the observation that Ift140 mutant cells still assemble a short ciliary remnant, whereas no ciliary remnants are seen on Ift20 mutant cells. In Ift20 mutant cells, centrosome position varied widely, localizing anywhere on the apical surface of cells early in cyst development and anywhere in the cell in advanced cysts. In contrast, centrosome position was maintained at the center of the apical end of Ift140 mutant cells even in advanced cystic disease. It is possible that the short cilium assembled on the Ift140 mutant cells is sufficient to properly position the centrosome. If the interphase centrosome position determines the division plane, then a short cilium may be sufficient to maintain proper orientation of cell division.
How then do kidney cysts form in the face of normally oriented mitoses? Increased epithelial proliferation and apoptosis have been proposed to contribute to cystic expansion.5,6,56 Enhanced proliferation in Ift140-deleted collecting ducts must certainly play a role in the cystic expansion of these kidneys. Enhanced apoptosis is a characteristic in many PKD models47; however, its role in cyst expansion has not been resolved. For example, apoptosis has been posited to promote cyst cavitation, trigger proliferation, or serve as a line of defense against neoplastic transformation of damaged cells.47 However, increased apoptosis after the loss of Pax2 reduced cystogenesis in pck mice.57 There was no significant increase in apoptosis in Ift140-deleted collecting ducts either early or late in the disease, suggesting that apoptosis itself is not driving cytogenesis. However, it is possible that enhanced proliferation in the absence of enhanced apoptosis is responsible for the rapid cystic expansion observed in Ift140-deleted collecting ducts.
A large unanswered question in ciliary biology and cystic disease research is what is the function of the primary cilium in maintaining tubule architecture and preventing cyst formation? It is generally agreed that primary cilia are sensory organelles that integrate extracellular signals and regulate signal transduction to control cell physiology. To understand the pathways influenced by the cilium, we interrogated a number of signal transduction pathways that had been previously identified as being altered in cystic disease. Canonical Wnt/β-catenin signaling is known to play a major role in kidney development and cystic disease,49 and it also influences expression of cMyc, a master regulator of proliferation.58 Hedgehog signaling is critical in kidney development,50 and in vertebrates, it is organized around the primary cilium.51 Hippo signaling regulates organ size and has recently been proposed to be altered in cystic disease.52,59 Fibrosis is a common feature of PKDs60 and intriguingly, may be linked to Hippo signaling and activation of innate immunity pathways that are upregulated in cystic disease in both mouse and humans.53,61 Although several genes showed modest elevations early in development of cystic disease, the largest changes came late in the development of the disease and in some cases, were quite dramatic. It is likely that elevation of proproliferative genes occurs within the rapidly dividing collecting duct epithelium, whereas fibrosis and innate immunity genes may be upregulated in noncollecting cells in response to cyst formation. The observation that most genes are upregulated after initiation of cyst formation suggests a model in which ciliary dysfunction does not initiate the pathways leading to cystic disease but rather, serves to restrain these pathways to prevent disorganized cell division. This idea is consistent with the observation that deletion of ciliary genes after postnatal development is completed does not result in cystic disease unless kidney damage occurs.62,63
Concise Methods
Mouse Breeding
Ift140-targeted ES cell line EPD0073_5_F01 was obtained from the National Institutes of Health-supported KOMP Repository (www.komp.org)37 and injected into C57Bl/6J albino blastocysts to generate chimeric animals. Chimeric mice were mated to C57Bl/6J albino mice (B6[Cg]-Tyrc-2J/J, Jax 000058) or C57Bl/6J mice (Jax 000664), and all the animals used in this study were C57Bl/6 congenics. Embryonic ages were determined by timed mating, with the day of the plug designated embryonic day 0.5. The original allele derived from the KOMP cells was designated the Ift140neo allele. As described in Figure 1A, this allele was converted to the Ift140null1 allele by deleting exon 7 by the action of Cre recombinase expressed in the germline (C57Bl/6 Prm-Cre)64 or to the Ift140flox allele by the action of FlpE expressed in the germline (C57Bl/6 Flp1).65 HoxB7-Cre43 was used to delete exon 7 in kidney collecting ducts, creating Ift140null2 in these specific cells. Mice with kidney collecting duct deletion of IFT140 were generated by crossing HoxB7-Cre, Ift140null1/+ males to Ift140 flox/flox females. HoxB7-Cre, Ift140null1/flox offspring mice were used as experimental animals, and HoxB7-Cre, Ift140+/flox mice were used as controls. All mouse work was carried out at the University of Massachusetts Medical School and was approved by the University of Massachusetts Medical School Institutional Animal Care and Use Committee.
Genotyping was carried out with the following primers: 140komp2 TCAGCCCTCTATGCCACTCT, 140komp3 CTTCCCTATGCCTTCAGCAG, and 140komp6 TGGTTTGTCCAAACTCATCAA. The expected products sizes are 140komp3+140komp2: WT=190 bp, neo=269 bp, null1=0, flox=269 bp, and null2=0; 140komp2+140komp6: WT=0, neo=1099 bp, null1=328 bp, flox=0, and null2=0.
Serum Chemistry
Blood urea nitrogen was analyzed by a Roche Cobra Integra 400 Plus at the Comparative Pathology Laboratory, Baylor College of Medicine (Houston, TX).
Cell Culture
IMCD3 (ATCC) and 48866 cells were grown in DMEM (4.5 g/L glucose)/F12 with penicillin and streptomycin and supplemented with 5% or 10% FBS, respectively. hTert-RPE cells (Clontech, Mountain View, CA) were grown in DMEM (1.0 g/L glucose)/F12 with penicillin and streptomycin and supplemented with 10% FBS.
Primary kidney cells were generated by dispersing kidneys in 0.25% Trypsin and 2.21 mM EDTA and plating in DMEM (4.5g/L glucose)/F12 with penicillin and streptomycin supplemented with 10% FBS. Stable lines were selected in the same medium supplemented with 150 mM NaCl and 150 mM urea.66 Primary mouse embryonic fibroblasts were generated by dispersing embryos in 0.25% Trypsin and 2.21 mM EDTA and then plating in 90% DMEM (high glucose) and 10% FBS with penicillin and streptomycin.
Immunofluorescence Microscopy
Cells for immunofluorescence microscopy were grown, fixed, and stained as described previously.40 Mouse tissues were fixed overnight at 4°C with 4% paraformaldehyde (EM Sciences, Hatfield PA) in PBS, embedded in paraffin, and processed as described in ref. 16.
Primary antibodies used included antitubulins (6-11B-1, GTU-88, B-5-1-2; Sigma, St. Louis, MO), antiphospho-histone H3Ser10 (Upstate, Lake Placid, NY), anti–β-catenin (Cell Signaling, Danvers, MA), antiactive β-catenin (clone 8E7; Upstate), anti–glyceraldehyde-3-phosphate dehydrogenase (clone 14C10; Cell Signaling), anti-RelB (N-17; Santa Cruz Biotechnology, Santa Cruz, CA), antismooth muscle actin (clone 1A4; Sigma-Aldrich), and anticleaved caspase-3 (Millipore, Billerica, MA). Anti-MmIFT140 was made by expressing the last 356 residues of the mouse protein in bacteria as a maltose binding protein fusion and injecting into rabbits. Antibodies were affinity-purified against the same fragment expressed as a glutathione S-transferase fusion. FITC-conjugated Dolichos biflorus agglutinin (Sigma) was added with the secondary antibodies. AlexaFluor-labeled secondary antibodies (Invitrogen Molecular Probes, Carlsbad, CA) were used to detect the primary antibodies.
Wide-field images were acquired by an Orca ER camera (Hamamatsu, Bridgewater, NJ) on a Zeiss Axiovert 200M microscope equipped with a Zeiss 100× plan–Apochromat 1.4 numerical aperture objective. Images were captured by Openlab (Improvision, Waltham, MA) and adjusted for contrast in Adobe Photoshop. For comparisons made between images, the photos were taken with identical conditions and manipulated equally. Confocal images were acquired by a Nikon TE-2000E2 inverted microscope equipped with a Solamere Technology-modified Yokogawa CSU10 spinning disk confocal scan head. Z-stacks were acquired at 0.5-μm intervals and converted to single planes by maximum projection with MetaMorph software. Bright-field images were acquired using a Zeiss Axioskop 2 Plus equipped with an Axiocam HRC color digital camera and Axiovision 4.0 acquisition software.
Cell Fractionation and Protein Analysis
Kidney cytoplasmic and nuclear extracts were prepared using the CelLytic Nuclear Extraction Kit (Sigma) protocol. For Western blot analysis, proteins were separated by SDS-PAGE and electrotransferred to Immobilon P (Millipore, Bedford, MA). After transfer, blots were blocked with Tris-buffered saline with 0.05% Tween 20 (TBST) containing 5% dry milk and then incubated with primary antibodies in the same solution.
mRNA Analysis
Individual kidneys were frozen at −80°C in RNAlater (Qiagen Inc., Valencia, CA). RNA isolation, cDNA synthesis, PCR primer design, and quantitative real-time PCR were performed as described previously16 using an ABI Prism 7500 (Applied Biosystems, Foster City, CA). PCR primers are listed in Supplemental Table 1. All quantitative PCR reactions were performed in duplicate, and melting curves verified that a single product was amplified. Standard curves were generated by 10-fold serial dilutions of a pool of mutant p20 mouse kidney cDNA, and for each gene, the threshold cycle was related to log cDNA dilution by linear regression analysis. Gene expression data were normalized to glyceraldehyde-3-phosphate dehydrogenase expression.
Statistical Analyses
To normalize the variance, kidney weight and BUN data were logarithmically transformed, and mitotic index data (calculated by counting the number of phosphohistone-staining cells present in at least 1000 collecting duct cells) were square root transformed; then, data were analyzed by one-way ANOVA and compared using the Tukey compromise posthoc test (SuperANOVA; Abacus Concepts, Berkeley, CA). Gene expression data were normalized by transformation to natural logarithms and analyzed by two-factor factorial ANOVA (PASW version 18; IBM-SPSS Inc., Chicago, IL), examining the effect of kidney genotype and animal age; in the presence of a significant age × genotype interaction, pairwise posthoc comparisons were made using Tukey’s Honestly Significant Difference test. Differences between groups were considered statistically significant if P<0.05. Circular mean mitotic spindle orientation, circular standard deviation, and 95% confidence intervals were calculated as previously described16 using the von Mises distribution followed by Kolmogorov–Smirov two-sample testing to ascertain whether there was a statistically significant difference in the distribution of mitotic spindle orientation angles in control versus experimental mice. Computations were performed using the PMag 4.2a (Hounslow) and PASW Version 18.0 statistical software packages. Accession number for mouse IFT140 is NM_134126.
Disclosures
None.
Acknowledgments
We thank William Monis, Dr. Brian Keady, and members of the Harvard Center for PKD research for critical comments, Drs. Stephen Jones (University of Massachusetts Medical School Transgenic Mouse Core) and Paul Furcinitti (University of Massachusetts Medical School Digital Imaging Core) for assistance, Dr. Paul Odgren for use of his bright-field microscope, and Bethany Walker for making the IFT140 antibody.
J.A.J. is a member of the Harvard Center for PKD Research (P50 DK074030). Studies were supported by National Institutes of Health Grant GM060992 (to G.J.P.) and the Order of the Eagles (G.J.P.). Core resources supported by Diabetes Endocrinology Research Center Grant DK32520 were used.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011080829/-/DCSupplemental.
References
- 1.Berbari NF, O’Connor AK, Haycraft CJ, Yoder BK: The primary cilium as a complex signaling center. Curr Biol 19: R526–R535, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pedersen LB, Rosenbaum JL: Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol 85: 23–61, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Satir P, Christensen ST: Structure and function of mammalian cilia. Histochem Cell Biol 129: 687–693, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pazour GJ: Intraflagellar transport and cilia-dependent renal disease: The ciliary hypothesis of polycystic kidney disease. J Am Soc Nephrol 15: 2528–2536, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Gallagher AR, Germino GG, Somlo S: Molecular advances in autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis 17: 118–130, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris PC, Torres VE: Polycystic kidney disease. Annu Rev Med 60: 321–337, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL: Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol 141: 993–1008, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scholey JM: Intraflagellar transport motors in cilia: Moving along the cell’s antenna. J Cell Biol 180: 23–29, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole DG, Snell WJ: SnapShot: Intraflagellar transport. Cell 137: 784–784.e1, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Ou G, Koga M, Blacque OE, Murayama T, Ohshima Y, Schafer JC, Li C, Yoder BK, Leroux MR, Scholey JM: Sensory ciliogenesis in Caenorhabditis elegans: Assignment of IFT components into distinct modules based on transport and phenotypic profiles. Mol Biol Cell 18: 1554–1569, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Follit JA, Xu F, Keady BT, Pazour GJ: Characterization of mouse IFT complex B. Cell Motil Cytoskeleton 66: 457–468, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perkins LA, Hedgecock EM, Thomson JN, Culotti JG: Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol 117: 456–487, 1986 [DOI] [PubMed] [Google Scholar]
- 13.Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG: Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol 151: 709–718, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murcia NS, Richards WG, Yoder BK, Mucenski ML, Dunlap JR, Woychik RP: The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left-right axis determination. Development 127: 2347–2355, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV: Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426: 83–87, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Jonassen JA, San Agustin J, Follit JA, Pazour GJ: Deletion of IFT20 in the mouse kidney causes misorientation of the mitotic spindle and cystic kidney disease. J Cell Biol 183: 377–384, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marszalek JR, Ruiz-Lozano P, Roberts E, Chien KR, Goldstein LSB: Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc Natl Acad Sci USA 96: 5043–5048, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N: Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 95: 829–837, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, Igarashi P: Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci USA 100: 5286–5291, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Absalon S, Blisnick T, Kohl L, Toutirais G, Doré G, Julkowska D, Tavenet A, Bastin P: Intraflagellar transport and functional analysis of genes required for flagellum formation in trypanosomes. Mol Biol Cell 19: 929–944, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin H, Rosenbaum JL, Barr MM: An autosomal recessive polycystic kidney disease gene homolog is involved in intraflagellar transport in C. elegans ciliated sensory neurons. Curr Biol 11: 457–461, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Absalon S, Blisnick T, Bonhivers M, Kohl L, Cayet N, Toutirais G, Buisson J, Robinson D, Bastin P: Flagellum elongation is required for correct structure, orientation and function of the flagellar pocket in Trypanosoma brucei. J Cell Sci 121: 3704–3716, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Lee E, Sivan-Loukianova E, Eberl DF, Kernan MJ: An IFT-A protein is required to delimit functionally distinct zones in mechanosensory cilia. Curr Biol 18: 1899–1906, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blacque OE, Li C, Inglis PN, Esmail MA, Ou G, Mah AK, Baillie DL, Scholey JM, Leroux MR: The WD repeat-containing protein IFTA-1 is required for retrograde intraflagellar transport. Mol Biol Cell 17: 5053–5062, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iomini C, Babaev-Khaimov V, Sassaroli M, Piperno G: Protein particles in Chlamydomonas flagella undergo a transport cycle consisting of four phases. J Cell Biol 153: 13–24, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iomini C, Li L, Esparza JM, Dutcher SK: Retrograde intraflagellar transport mutants identify complex A proteins with multiple genetic interactions in Chlamydomonas reinhardtii. Genetics 183: 885–896, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukhopadhyay S, Wen X, Chih B, Nelson CD, Lane WS, Scales SJ, Jackson PK: TULP3 bridges the IFT-A complex and membrane phosphoinositides to promote trafficking of G protein-coupled receptors into primary cilia. Genes Dev 24: 2180–2193, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tran PV, Haycraft CJ, Besschetnova TY, Turbe-Doan A, Stottmann RW, Herron BJ, Chesebro AL, Qiu H, Scherz PJ, Shah JV, Yoder BK, Beier DR: THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia. Nat Genet 40: 403–410, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cortellino S, Wang C, Wang B, Bassi MR, Caretti E, Champeval D, Calmont A, Jarnik M, Burch J, Zaret KS, Larue L, Bellacosa A: Defective ciliogenesis, embryonic lethality and severe impairment of the Sonic Hedgehog pathway caused by inactivation of the mouse complex A intraflagellar transport gene Ift122/Wdr10, partially overlapping with the DNA repair gene Med1/Mbd4. Dev Biol 325: 225–237, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin J, Lin Y, Norman RX, Ko HW, Eggenschwiler JT: Intraflagellar transport protein 122 antagonizes Sonic Hedgehog signaling and controls ciliary localization of pathway components. Proc Natl Acad Sci USA 108: 1456–1461, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mill P, Lockhart PJ, Fitzpatrick E, Mountford HS, Hall EA, Reijns MA, Keighren M, Bahlo M, Bromhead CJ, Budd P, Aftimos S, Delatycki MB, Savarirayan R, Jackson IJ, Amor DJ: Human and mouse mutations in WDR35 cause short-rib polydactyly syndromes due to abnormal ciliogenesis. Am J Hum Genet 88: 508–515, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walczak-Sztulpa J, Eggenschwiler J, Osborn D, Brown DA, Emma F, Klingenberg C, Hennekam RC, Torre G, Garshasbi M, Tzschach A, Szczepanska M, Krawczynski M, Zachwieja J, Zwolinska D, Beales PL, Ropers HH, Latos-Bielenska A, Kuss AW: Cranioectodermal Dysplasia, Sensenbrenner syndrome, is a ciliopathy caused by mutations in the IFT122 gene. Am J Hum Genet 86: 949–956, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsujikawa M, Malicki J: Intraflagellar transport genes are essential for differentiation and survival of vertebrate sensory neurons. Neuron 42: 703–716, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Gilissen C, Arts HH, Hoischen A, Spruijt L, Mans DA, Arts P, van Lier B, Steehouwer M, van Reeuwijk J, Kant SG, Roepman R, Knoers NV, Veltman JA, Brunner HG: Exome sequencing identifies WDR35 variants involved in Sensenbrenner syndrome. Am J Hum Genet 87: 418–423, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arts HH, Bongers EM, Mans DA, van Beersum SE, Oud MM, Bolat E, Spruijt L, Cornelissen EA, Schuurs-Hoeijmakers JH, de Leeuw N, Cormier-Daire V, Brunner HG, Knoers NV, Roepman R: C14ORF179 encoding IFT43 is mutated in Sensenbrenner syndrome. J Med Genet 48: 390–395, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Zaffanello M, Diomedi-Camassei F, Melzi ML, Torre G, Callea F, Emma F: Sensenbrenner syndrome: A new member of the hepatorenal fibrocystic family. Am J Med Genet A 140: 2336–2340, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Austin CP, Battey JF, Bradley A, Bucan M, Capecchi M, Collins FS, Dove WF, Duyk G, Dymecki S, Eppig JT, Grieder FB, Heintz N, Hicks G, Insel TR, Joyner A, Koller BH, Lloyd KC, Magnuson T, Moore MW, Nagy A, Pollock JD, Roses AD, Sands AT, Seed B, Skarnes WC, Snoddy J, Soriano P, Stewart DJ, Stewart F, Stillman B, Varmus H, Varticovski L, Verma IM, Vogt TF, von Melchner H, Witkowski J, Woychik RP, Wurst W, Yancopoulos GD, Young SG, Zambrowicz B: The knockout mouse project. Nat Genet 36: 921–924, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skarnes WC, Rosen B, West AP, Koutsourakis M, Bushell W, Iyer V, Mujica AO, Thomas M, Harrow J, Cox T, Jackson D, Severin J, Biggs P, Fu J, Nefedov M, de Jong PJ, Stewart AF, Bradley A: A conditional knockout resource for the genome-wide study of mouse gene function. Nature 474: 337–342, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deane JA, Cole DG, Seeley ES, Diener DR, Rosenbaum JL: Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr Biol 11: 1586–1590, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Follit JA, Tuft RA, Fogarty KE, Pazour GJ: The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol Biol Cell 17: 3781–3792, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robert A, Margall-Ducos G, Guidotti JE, Brégerie O, Celati C, Bréchot C, Desdouets C: The intraflagellar transport component IFT88/polaris is a centrosomal protein regulating G1-S transition in non-ciliated cells. J Cell Sci 120: 628–637, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Delaval B, Bright A, Lawson ND, Doxsey S: The cilia protein IFT88 is required for spindle orientation in mitosis. Nat Cell Biol 13: 461–468, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu J, Carroll TJ, McMahon AP: Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development 129: 5301–5312, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Costantini F, Kopan R: Patterning a complex organ: Branching morphogenesis and nephron segmentation in kidney development. Dev Cell 18: 698–712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, Yaniv M, Pontoglio M: Defective planar cell polarity in polycystic kidney disease. Nat Genet 38: 21–23, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, Gessler M, Quaggin SE, Harrison R, Mount R, McNeill H: Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet 40: 1010–1015, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Goilav B: Apoptosis in polycystic kidney disease. Biochim Biophys Acta 1812: 1272–1280, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Saadi-Kheddouci S, Berrebi D, Romagnolo B, Cluzeaud F, Peuchmaur M, Kahn A, Vandewalle A, Perret C: Early development of polycystic kidney disease in transgenic mice expressing an activated mutant of the beta-catenin gene. Oncogene 20: 5972–5981, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Merkel CE, Karner CM, Carroll TJ: Molecular regulation of kidney development: is the answer blowing in the Wnt? Pediatr Nephrol 22: 1825–1838, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reidy KJ, Rosenblum ND: Cell and molecular biology of kidney development. Semin Nephrol 29: 321–337, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong SY, Reiter JF: The primary cilium at the crossroads of mammalian hedgehog signaling. Curr Top Dev Biol 85: 225–260, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Happé H, van der Wal AM, Leonhard WN, Kunnen SJ, Breuning MH, de Heer E, Peters DJ: Altered Hippo signalling in polycystic kidney disease. J Pathol 224: 133–142, 2011 [DOI] [PubMed] [Google Scholar]
- 53.Mrug M, Zhou J, Woo Y, Cui X, Szalai AJ, Novak J, Churchill GA, Guay-Woodford LM: Overexpression of innate immune response genes in a model of recessive polycystic kidney disease. Kidney Int 73: 63–76, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Pigino G, Geimer S, Lanzavecchia S, Paccagnini E, Cantele F, Diener DR, Rosenbaum JL, Lupetti P: Electron-tomographic analysis of intraflagellar transport particle trains in situ. J Cell Biol 187: 135–148, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nishio S, Tian X, Gallagher AR, Yu Z, Patel V, Igarashi P, Somlo S: Loss of oriented cell division does not initiate cyst formation. J Am Soc Nephrol 21: 295–302, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu B, He X, Li A, Qiu Q, Li C, Liang D, Zhao P, Ma J, Coffey RJ, Zhan Q, Wu G: Cystogenesis in ARPKD results from increased apoptosis in collecting duct epithelial cells of Pkhd1 mutant kidneys. Exp Cell Res 317: 173–187, 2011 [DOI] [PubMed] [Google Scholar]
- 57.Ostrom L, Tang MJ, Gruss P, Dressler GR: Reduced Pax2 gene dosage increases apoptosis and slows the progression of renal cystic disease. Dev Biol 219: 250–258, 2000 [DOI] [PubMed] [Google Scholar]
- 58.Soucek L, Evan GI: The ups and downs of Myc biology. Curr Opin Genet Dev 20: 91–95, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Habbig S, Bartram MP, Müller RU, Schwarz R, Andriopoulos N, Chen S, Sägmüller JG, Hoehne M, Burst V, Liebau MC, Reinhardt HC, Benzing T, Schermer B: NPHP4, a cilia-associated protein, negatively regulates the Hippo pathway. J Cell Biol 193: 633–642, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Norman J: Fibrosis and progression of autosomal dominant polycystic kidney disease (ADPKD). Biochim Biophys Acta 1812: 1327–1336, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou J, Ouyang X, Cui X, Schoeb TR, Smythies LE, Johnson MR, Guay-Woodford LM, Chapman AB, Mrug M: Renal CD14 expression correlates with the progression of cystic kidney disease. Kidney Int 78: 550–560, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davenport JR, Watts AJ, Roper VC, Croyle MJ, van Groen T, Wyss JM, Nagy TR, Kesterson RA, Yoder BK: Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr Biol 17: 1586–1594, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patel V, Li L, Cobo-Stark P, Shao X, Somlo S, Lin F, Igarashi P: Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum Mol Genet 17: 1578–1590, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’Gorman S, Dagenais NA, Qian M, Marchuk Y: Protamine-Cre recombinase transgenes efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proc Natl Acad Sci USA 94: 14602–14607, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Farley FW, Soriano P, Steffen LS, Dymecki SM: Widespread recombinase expression using FLPeR (flipper) mice. Genesis 28: 106–110, 2000 [PubMed] [Google Scholar]
- 66.Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB: Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol 12: R378–R380, 2002 [DOI] [PubMed] [Google Scholar]