Abstract
More than a decade ago, cardiovascular disease (CVD) was recognized as a major cause of death in children with advanced CKD. This observation has sparked the publication of multiple studies assessing cardiovascular risk, mechanisms of disease, and early markers of CVD in this population. Similar to adults, children with CKD have an extremely high prevalence of traditional and uremia-related CVD risk factors. Early markers of cardiomyopathy, such as left ventricular hypertrophy and dysfunction, and early markers of atherosclerosis, such as increased carotid artery intima-media thickness, carotid arterial wall stiffness, and coronary artery calcification, are frequently present in these children, especially those on maintenance dialysis. As a population without preexisting symptomatic cardiac disease, children with CKD potentially receive significant benefit from aggressive attempts to prevent and treat CVD. Early CKD, before needing dialysis, is the optimal time to both identify modifiable risk factors and intervene in an effort to avert future CVD. Slowing the progression of CKD, avoiding long-term dialysis and, if possible, conducting preemptive transplantation may represent the best strategies to decrease the risk of premature cardiac disease and death in children with CKD.
Current registry data from the National Center for Health Statistics indicate that overall mortality rates in the general US pediatric population were 0.31 per 1000 population for children aged 1–19 years in 2008.1 In contrast, these rates were 35.6 (dialysis) and 3.5 (transplant) per 1000 patient-years at risk for children aged 0–19 years with ESRD according to 2006–2008 data from the US Renal Data System (USRDS).2 These large discrepancies in mortality rates exist despite the widespread availability of state-of-the-art renal replacement therapy and substantial advances in the care of children with CKD over the last 3 decades. Even more disturbing is the fact that for dialyzed children, all-cause mortality rates have not changed significantly since the 1980s, with the highest rates reported in children on maintenance dialysis who have never received a kidney transplant (Table 1). Furthermore, young adults developing ESRD during childhood have a significantly diminished life expectancy. Upon reaching adulthood, dialysis patients live 40–50 years less, and transplant patients live approximately 20–25 years less, compared with an age- and- race-matched US population.2
Table 1.
Annual mortality rates per 1000 patient-years at risk, period prevalent patients aged 0–19 years adjusted by sex, race, ethnicity, primary diagnosis, and patient vintage
| 1980–1989 | 1990–1999 | 2000–2008 | |
|---|---|---|---|
| Hemodialysis | 64 | 61 | 59 |
| Peritoneal dialysis | 83 | 75 | 80 |
| Dialysis/never transplanted | 126 | 151 | 134 |
| Transplants | 33 | 16 | 9 |
Data are from the USRDS (2011).2
In 1998, the National Kidney Foundation Task Force on Cardiovascular Disease declared an epidemic of cardiac disease in ESRD patients.3 Cardiovascular disease (CVD) mortality was especially high in young adults (aged 25–34 years) receiving maintenance dialysis, who died at a rate >100 times higher than the comparably aged general population. Although the task force focused only on adults, it raised important questions regarding pediatric patients with ESRD. Do they have cardiac disease? Do they die from cardiac disease? If so, how significant is the problem? This review summarizes the most current literature describing the epidemiology of CVD in children with CKD.
Cardiovascular Mortality Is the Leading Cause of Death in Children with CKD
After the task force publication, Parekh et al.4 used the USRDS database to evaluate the risk of cardiac death in children and young adults (aged 0–30 years) in 2002. Of 1380 deaths recorded between 1990 and 1996, 311 (23%) were due to cardiac causes. These data are in sharp contrast to the general pediatric population, in which CVD mortality is very low and accounts for <3% of all deaths.1
Subsequent reports from international registries confirm that CVD is the leading cause of death in both children with ESRD and in adults with childhood onset of CKD. The Australia and New Zealand Dialysis and Transplant,5 Dutch national cohort study,6 and a large German study7 reported that 40%–50% of all deaths are from cardiovascular or cerebrovascular causes.
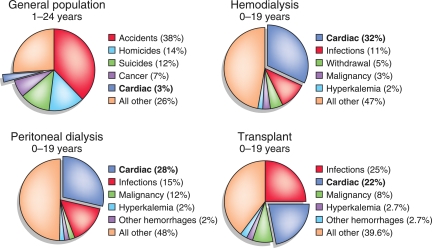
More current USRDS data reconfirmed CVD as one of the major causes of death in children with CKD (Figure 1). Cardiovascular death rates are similar in children on peritoneal dialysis and hemodialysis, whereas transplant recipients have a relatively lower risk of cardiac death.2 The most recent USRDS analysis of long-term survival in 18,911 patients who received a first kidney transplant during childhood (at <21 years old, 1983–2006) showed that the majority of deaths were from cardiovascular causes. Most of these deaths occurred in patients who had graft failure (45%), rather than in those with a functioning graft (25%).8 Furthermore, the hazard ratio was 7.8 for patients with cardiovascular mortality associated with dialysis after graft failure relative to those patients with a functioning graft. In agreement with prior studies, this recent report also noted decreased cardiovascular mortality in transplant recipients in the most recent era. However, even in these patients, the mortality rates are still significantly higher (approximately 10 times) than in the general pediatric population.
Figure 1.
Leading causes of death in general pediatric population and in children on renal replacement therapy. Data are presented as percentages. Data for dialysis and transplant patients are from the USRDS (2011).2 Data for general pediatric population are from Mathews et al. (2011).1
In older adults with ESRD, coronary artery disease (CAD) and cardiomyopathy-associated congestive heart failure are the leading causes of CVD mortality. The most common causes of ESRD in adults are diabetes and hypertension, two conditions having a strong association with other cardiovascular risk factors. Conversely, children have neither diabetes nor symptomatic atherosclerosis at the time of CKD diagnosis, possibly explaining why the causes of cardiac death in children are different from those in adults (Table 2). In looking more closely at cardiovascular deaths in children with CKD, cardiac arrest is the most common cause, followed by arrhythmia, cardiomyopathy, and cerebrovascular disease, with myocardial infarction rarely reported.2
Table 2.
Causes of cardiac death in children 0–19 years of age with CKD
| Dialysis | Transplant |
|---|---|
| Cardiac arrest/arrhythmias | Cardiac arrest/arrhythmias |
| Cerebrovascular disease | Cerebrovascular disease |
| Congestive heart failure/pulmonary edema | Acute myocardial infarction |
| Cardiomyopathy | Cardiomyopathy |
| Acute myocardial infarction | |
| Pericarditis |
Data are from the USRDS (2011).2
The prevalence of cardiac arrest in the youngest age group (0–4 years) is 5–10 times higher than in other pediatric age groups, perhaps reflecting the difficulty of ascertaining the true cause of sudden death in young children. These data may be confounded by comorbid conditions such as congenital disorders, which are not included in the USRDS database. As with mortality, a USRDS analysis of cardiac morbidity in children on maintenance dialysis confirms that cardiac disease in children is different from adults. A total of 1454 Medicare incident pediatric dialysis patients (aged 0–19 years) were identified from 1991 to 1996.9 Among them, 452 patients (31.2%) developed a cardiac-related event. Arrhythmia was most common (19.6%), followed by valvular disease (11.7%), cardiomyopathy (9.6%), and cardiac arrest (3%).
Children with CKD Are the Pediatric Population with the Highest Cardiovascular Risk
Although symptomatic CAD is rarely noted in children with CKD, atherosclerosis is already evident in children with advanced kidney disease. One small study from Turkey reported histopathological findings of internal iliac artery samples obtained at the time of kidney transplantation in 12 children.10 The authors showed that 5 of 12 arteries had evidence of atherosclerosis or arteriosclerotic lesions, including fibrous or fibroelastic intimal thickening, disruption of the internal elastic lamella, and atheromatous plaques. This evidence of early and accelerated atherosclerosis and arteriosclerosis likely explains why young adults with childhood onset of CKD are at increased risk for symptomatic CAD.
Not surprisingly, the American Heart Association’s guidelines for cardiovascular risk reduction in high-risk pediatric patients stratified pediatric CKD patients in the highest risk category for the development of CVD.11 This group includes those with pathologic and/or clinical evidence for manifestations of coronary disease before age 30 years. In addition to CKD, this highest risk category includes the following four other conditions: homozygous familial hypercholesterolemia, type 1 diabetes mellitus, heart transplantation, and Kawasaki disease with coronary aneurysms. However, even among these conditions, there is a wide variability in cardiovascular risk. For example, studies in young adults with childhood-onset type 1 diabetes—a condition that, like CKD, is not associated with primary heart disease—show that CVD-related death is very rare during the first 3 decades of life. Specifically, two large studies from Europe12,13 reported acute metabolic complications as the leading cause of death before age 30 years, whereas CVD was predominant after age 30 years. Another recent analysis of short-term mortality (mean follow-up 15.8 years) of young patients with childhood onset of type 1 diabetes from Italy reported no cardiac causes of deaths in the entire cohort.14
Prevalence of Cardiovascular Risk Factors in Children with CKD Is Similar to That in Adults with CKD
Children with CKD are at very high risk for the development of accelerated atherosclerosis, arteriosclerosis, and premature CVD during young adulthood due to a unique combination of traditional and uremia-related risk factors (Table 3).
Table 3.
Common risk factors for CVD in children with CKD
| Risk Factors | CKD (%)a | Dialysis (%) | Transplant (%) | References |
|---|---|---|---|---|
| Traditional | ||||
| hypertension | 47–54 | 52–75 | 63–81 | 15,21,25,31,54–58 |
| dyslipidemia | 45 | 33–87 | 55–84 | 19,21 |
| obesity | 15 | 8-11 | 12–22 | 20,21,25,59 |
| hyperglycemia | 4 | 11 | 22 | 20,21 |
| Uremia related | ||||
| anemia | 38–48 | 40–67 | 32–64 | 22,23,25,57,60–62 |
| hyperparathyroidism | 21 | 72 | 57,63 | |
| increased calcium-phosphorus product | 53–55 | 25,62 | ||
| increased C-reactive protein | 76 | 64 | ||
| hypoalbuminemia | 47 | 40–60 | 62,57 |
Traditional Risk Factors
Recent evidence highlights the high prevalence of traditional cardiovascular risk factors in children with even early stages of CKD. The Chronic Kidney Disease in Children (CKiD) study, an observational cohort of 586 children (aged 1–16 years) with stage 2–4 CKD, provides contemporary data on cardiovascular risk factors in this population. On enrollment, hypertension was present in 54% of participants.15 Despite the use of antihypertensive medication, 48% of these children were found to have inadequately controlled BP. Equally concerning, analysis of ambulatory BP monitoring data16 showed a high frequency of masked hypertension (38%), a rate four to five times higher than reported in unselected patients referred to pediatric hypertension clinics (7%–11%).17,18
In terms of other traditional risk factors, dyslipidemia was found in 45% and obesity in 15% of the CKiD cohort.19,20 As mentioned earlier, although diabetes is very rare cause of CKD in children, hyperinsulinemia and insulin resistance are present in 9% and 19%, respectively, of the CKiD population.20 Importantly, almost one-half of patients in the CKiD cohort have a combination of traditional risk factors. Even nonobese patients have a high prevalence of multiple traditional cardiovascular risks, with nearly one-quarter having two or three cardiovascular risk factors. Overweight or obese study participants have very high prevalence of multiple risk factors, similar to rates in severely obese (body mass index >40 kg/m2) children without kidney disease. This pattern differentiates the population of children with CKD from healthy children, in whom the coexistence of multiple cardiovascular risk factors is extremely infrequent, and is restricted to those who are obese (metabolic syndrome). The prevalence of these traditional risk factors increases as CKD progresses, and is highest in children on maintenance dialysis (Table 3). Although successful kidney transplantation leads to elimination of many uremia-related risk factors for atherosclerotic CVD (see below), transplant recipients remain at high risk for CVD from these traditional risk factors (Table 3). For example, a recent multicenter study determined that 38% of kidney transplant recipients had at least three traditional cardiovascular risk factors.21
Uremia-Related Risk Factors
The high prevalence of traditional risk factors may account for the accelerated CAD and premature cardiac death noted in young adults with a history of childhood-onset CKD. However, these risk factors do not fully explain the high rates of cardiac death in children aged 0–19 years on maintenance dialysis or in those receiving a kidney transplant. To better understand the increased risk of cardiac disease in these children, investigators have focused on uremia-related risk factors.
As in adults, abnormal mineral metabolism is common in children with CKD, a finding that becomes more frequent as kidney function decreases (Table 3). Despite the widespread use of erythropoiesis-stimulating agents and iron therapy, anemia is poorly controlled, especially in children with advanced CKD22 or in those on maintenance dialysis.23 Anemia in these children has been linked to overall mortality, but not to cardiac-related death.24 Inflammation is also likely a contributing risk factor in children receiving chronic dialysis (Table 3), although no study has examined the role of inflammation in cardiac death in these young patients.
As with traditional risk factors, there is frequently a coexistence of CKD-related risk factors in children. A cross-sectional analysis of all pediatric maintenance hemodialysis patients (aged 0.7–18 years, n=656) using data from the Centers for Medicare and Medicaid Services ESRD Clinical Performance Measures Project25 shows that 38% have anemia, 63% have serum phosphorus >5.5 mg/dl, and 55% have calcium-phosphorus product ≥55 mg2/dl.2
Despite the data presented above, no study has directly linked traditional and/or uremia-related risk factors with CVD mortality. However, the remarkable decrease in mortality after kidney transplant, compared with continued dialysis, clearly indicates that the uremic milieu is a major cause of cardiac death in children with CKD (Table 1).
Intermediate Cardiovascular Outcomes
Recent research has focused on identifying the presence of early cardiovascular abnormalities in children with CKD. Left ventricular (LV) abnormalities such as LV hypertrophy (LVH) and LV dysfunction, damage to the large arteries such as stiffness and increased intima-medial thickness (IMT) of the carotids, and coronary calcifications are now accepted as early markers of cardiomyopathy and atherosclerosis. These markers are strong, independent predictors of cardiac morbidity and mortality, both in the general population and in adults with CKD. Furthermore, over the last decade, these abnormalities have also been noted in children and young adults with CKD (Table 4).
Table 4.
Common early cardiac and vascular abnormalitiess in children with CKD
LVH is the Most Common Cardiac Abnormality in Children with CKD
Although most pediatric studies evaluating LV structure are cross-sectional and include few patients, they consistently show that LVH develops even when CKD is mild and progresses as kidney function deteriorates.26,27 Larger, more recent, multicenter studies in children with stage 2–4 CKD, on dialysis, and post-transplant have confirmed these previous investigations. The baseline CKiD data16 demonstrate an overall 17% prevalence of LVH. LVH is more frequent in children with sustained (both casual and ambulatory) (34%) and masked (20%) systolic or diastolic hypertension compared with children with normal casual and ambulatory BP (8%). At initiation of maintenance dialysis, 69%–82% of pediatric patients have evidence of LVH.28,29 LVH persists (40%–85%) during long-term dialysis28,30 with both concentric and eccentric geometric patterns of LVH present in these patients. Data from the International Pediatric Peritoneal Dialysis Network registry31 on 507 patients report the overall prevalence of LVH to be 48%. A Midwest Pediatric Nephrology Consortium study,21 utilizing data from six centers, demonstrated the prevalence of LVH to be 40% in children 1-year post-transplant. Most of these studies show the persistence of cardiac hypertrophy,32–34 but some report improvement post-transplant.35
As in adults, hypertension is the main cause of cardiac hypertrophy in children before ESRD or post-transplant. Elevated parathyroid hormone (PTH) might contribute to progression of LVH in children with stage 2–4 CKD36 and hypertension associated with volume overload has been linked to the development of LVH in the dialysis population.29
Children on Maintenance Dialysis Are at Highest Risk for LV Dysfunction
Tissue Doppler imaging demonstrates impaired LV filling and compliance early in the progression of pediatric CKD.37 The prevalence of diastolic dysfunction increases in patients undergoing maintenance dialysis.37 The functional consequence of these changes has been suggested by a recent study linking diastolic dysfunction to altered maximal aerobic capacity.38
Several reports using echocardiography document subtle alterations in LV wall mechanics, especially in children on maintenance dialysis. These alterations include decreased shortening at the myocardial mid-wall,39,40 depressed contractile reserve during stress/exercise,26,41 and acute reductions in global and segmental myocardial blood flow with a concomitant increase in myocardial stunning.42
Arterial Abnormalities Develop during Early CKD
As with LV structure and function, vascular changes such as an increase in carotid IMT (cIMT) and arterial stiffness (pulse wave velocity) begin at predialysis stages of CKD33,43 and are worst in children on maintenance dialysis33,43 (Table 4). There are some reports suggesting improvements in these arterial abnormalities post-transplant.44,45 In children with stage 2–4 CKD, increased cIMT is associated with hypertension and dyslipidemia. Unlike in predialysis CKD, abnormal mineral metabolism (high phosphorus, calcium-phosphorus product, and PTH) is the major predictor of vascular changes in children on dialysis. A recent study suggests that both low and high levels of 1,25-dihydroxyvitamin D are associated with high cIMT.46 These apparently contradictory relationships may be due to both the effects of vitamin D on calcium-phosphorus homeostasis and its proinflammatory properties.44
Coronary Artery Calcification Is Frequent in Children on Maintenance Dialysis
An early autopsy study of pediatric patients with ESRD who died between 1960 and 1983 showed a high prevalence of vascular calcinosis.47 In this study, coronary artery calcification (CAC) was present in 28%. Peak calcium-phosphorus product, peak serum P, and cumulative dose of calcitriol were significantly associated with the severity of the calcinosis. Despite significant advances in the care of children with ESRD since the autopsy study, the frequency of CAC has not changed much (Table 4). As in the above study, higher time-integrated serum phosphorus, calcium-phosphate product, PTH, and the amount of cumulative calcium-containing oral phosphate binders predict the presence of CAC in recent reports. As in adults, cardiac valve calcification is also described in children on maintenance dialysis.48 However, in a study of 40 young adults (mean age 23.6 years) who developed ESRD at mean age of 11.5 years, cIMT is similar to healthy controls and only 4 (10%) patients have evidence of coronary calcification.45 The authors noticed this relatively low rate of cardiac calcification compared with other studies might be explained by a significantly lower amount of prescribed calcium-containing phosphate binders and vitamin D preparations in their patients.
In the decade since the recognition of CVD as a major cause of morbidity and mortality in children with CKD, there has been important progress in our understanding of the mechanisms leading to the development of early cardiac and vascular abnormalities in these patients. Yet the vast majority of data come from observational (registries) or small, cross-sectional studies. There have been no high-quality studies evaluating the association of early markers/intermediate outcomes of CVD during childhood and cardiac morbidity and mortality later in life. Nor have there been any interventional studies on controlling modifiable cardiovascular risks in children with CKD.
Despite these gaps in the literature, existing data on cardiovascular risks, early cardiac and vascular abnormalities, and CVD morbidity and mortality all point to dialysis vintage as a major factor associated with poor outcomes in children with ESRD.2,8 Therefore, primary among all management strategies in childhood CKD/ESRD is the avoidance of long-term dialysis, ideally with preemptive transplantation when feasible. Although cardiovascular risk remains high relative to the general population, successful transplantation can significantly improve uremia-related risk factors and, most importantly, increase life expectancy by age 20–30 years compared with long-term dialysis.
For those children who are unwilling or unable to receive a kidney transplant, several strategies should be used to reduce the cardiovascular risks associated with maintenance dialysis. Aggressive monitoring and management of hypertension, dyslipidemia, mineral metabolism, anemia, nutrition, and inflammation cannot be overemphasized. Unfortunately, the prescription of adequate dialysis, as measured by Kt/V, will not necessarily decrease the risk associated with these CKD-associated complications. Pediatric data from the Centers for Medicare and Medicaid Services End-Stage Renal Disease Clinical Performance Measures (CPM) Project25,49 indicate that 89% of patients receiving hemodialysis and 87% of patients receiving peritoneal dialysis achieved the recommended modality-specific Kt/V. Yet, one-third of children had significant anemia, almost one-half had low serum albumin, and two-thirds had uncontrolled hypertension and a high calcium-phosphorus product.
These studies, supported by epidemiologic data on cardiac death in peritoneal dialysis and hemodialysis patients, clearly indicate that current dialysis practice guidelines based primarily on Kt/V will not decrease CVD morbidity and mortality in children with ESRD. Unfortunately, unlike in adults,50 there have been no randomized studies in pediatric patients examining the role of alternative dialysis strategies (quotidian hemodialysis) to improve cardiac outcomes. However, small, single-center studies have shown clinically important improvements in cardiac hypertrophy and function when children receive dialysis more frequently than the traditional, thrice-weekly in-center schedule.51,52 Considering the potential for a longer and possibly more productive life, the benefits of more frequent and longer dialysis treatment might therefore be more far reaching in children than in adults.53
Disclosures
None.
Acknowledgments
This manuscript is supported by research grants (DK076957 and DK090070) from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Mathews TJ, Miniño AM, Osterman MJ, Strobino DM, Guyer B: Annual summary of vital statistics: 2008. Pediatrics 127: 146–157, 2011 [DOI] [PubMed] [Google Scholar]
- 2.US Renal Data System : USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2011 [Google Scholar]
- 3.Levey AS, Beto JA, Coronado BE, Eknoyan G, Foley RN, Kasiske BL, Klag MJ, Mailloux LU, Manske CL, Meyer KB, Parfrey PS, Pfeffer MA, Wenger NK, Wilson PW, Wright JT Jr: Controlling the epidemic of cardiovascular disease in chronic renal disease: What do we know? What do we need to learn? Where do we go from here? National Kidney Foundation Task Force on Cardiovascular Disease. Am J Kidney Dis 32: 853–906, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Parekh RS, Carroll CE, Wolfe RA, Port FK: Cardiovascular mortality in children and young adults with end-stage kidney disease. J Pediatr 141: 191–197, 2002 [DOI] [PubMed] [Google Scholar]
- 5.McDonald SP, Craig JC. Australian and New Zealand Paediatric Nephrology Association: Long-term survival of children with end-stage renal disease. N Engl J Med 350: 2654–2662, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Groothoff JW, Gruppen MP, Offringa M, Hutten J, Lilien MR, Van De Kar NJ, Wolff ED, Davin JC, Heymans HS: Mortality and causes of death of end-stage renal disease in children: A Dutch cohort study. Kidney Int 61: 621–629, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Oh J, Wunsch R, Turzer M, Bahner M, Raggi P, Querfeld U, Mehls O, Schaefer F: Advanced coronary and carotid arteriopathy in young adults with childhood-onset chronic renal failure. Circulation 106: 100–105, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Foster BJ, Dahhou M, Zhang X, Platt RW, Hanley JA: Change in mortality risk over time in young kidney transplant recipients. Am J Transplant 11: 2432–2442, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Chavers BM, Li S, Collins AJ, Herzog CA: Cardiovascular disease in pediatric chronic dialysis patients. Kidney Int 62: 648–653, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Nayir A, Bilge I, Kiliçaslan I, Ander H, Emre S, Sirin A: Arterial changes in paediatric haemodialysis patients undergoing renal transplantation. Nephrol Dial Transplant 16: 2041–2047, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Kavey RE, Allada V, Daniels SR, Hayman LL, McCrindle BW, Newburger JW, Parekh RS, Steinberger J. American Heart Association Expert Panel on Population and Prevention Science American Heart Association Council on Cardiovascular Disease in the Young American Heart Association Council on Epidemiology and Prevention American Heart Association Council on Nutrition, Physical Activity and Metabolism American Heart Association Council on High Blood Pressure Research American Heart Association Council on Cardiovascular Nursing American Heart Association Council on the Kidney in Heart Disease Interdisciplinary Working Group on Quality of Care and Outcomes Research: Cardiovascular risk reduction in high-risk pediatric patients: A scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research: Endorsed by the American Academy of Pediatrics. Circulation 114: 2710–2738, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Laing SP, Swerdlow AJ, Slater SD, Botha JL, Burden AC, Waugh NR, Smith AW, Hill RD, Bingley PJ, Patterson CC, Qiao Z, Keen H: The British Diabetic Association Cohort Study, II: Cause-specific mortality in patients with insulin-treated diabetes mellitus. Diabet Med 16: 466–471, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Skrivarhaug T, Bangstad HJ, Stene LC, Sandvik L, Hanssen KF, Joner G: Long-term mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in Norway. Diabetologia 49: 298–305, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Bruno G, Cerutti F, Merletti F, Novelli G, Panero F, Zucco C, Cavallo-Perin P. Piedmont Study Group for Diabetes Epidemiology: Short-term mortality risk in children and young adults with type 1 diabetes: The population-based Registry of the Province of Turin, Italy. Nutr Metab Cardiovasc Dis 19: 340–344, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Flynn JT, Mitsnefes M, Pierce C, Cole SR, Parekh RS, Furth SL, Warady BA. Chronic Kidney Disease in Children Study Group: Blood pressure in children with chronic kidney disease: A report from the Chronic Kidney Disease in Children study. Hypertension 52: 631–637, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitsnefes M, Flynn J, Cohn S, Samuels J, Blydt-Hansen T, Saland J, Kimball T, Furth S, Warady B. CKiD Study Group: Masked hypertension associates with left ventricular hypertrophy in children with CKD. J Am Soc Nephrol 21: 137–144, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lurbe E, Torro I, Alvarez V, Nawrot T, Paya R, Redon J, Staessen JA: Prevalence, persistence, and clinical significance of masked hypertension in youth. Hypertension 45: 493–498, 2005 [DOI] [PubMed] [Google Scholar]
- 18.McNiece KL, Gupta-Malhotra M, Samuels J, Bell C, Garcia K, Poffenbarger T, Sorof JM, Portman RJ. National High Blood Pressure Education Program Working Group: Left ventricular hypertrophy in hypertensive adolescents: Analysis of risk by 2004 National High Blood Pressure Education Program Working Group staging criteria. Hypertension 50: 392–395, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saland JM, Pierce CB, Mitsnefes MM, Flynn JT, Goebel J, Kupferman JC, Warady BA, Furth SL. CKiD Investigators: Dyslipidemia in children with chronic kidney disease. Kidney Int 78: 1154–1163, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson AC, Schneider MF, Cox C, Greenbaum LA, Saland J, White CT, Furth S, Warady BA, Mitsnefes MM: Prevalence and correlates of multiple cardiovascular risk factors in children with chronic kidney disease. Clin J Am Soc Nephrol 6: 2759–2765, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson AC, Greenbaum LA, Barletta GM, Chand D, Lin JJ, Patel HP, Mitsnefes M: High prevalence of the metabolic syndrome and associated left ventricular hypertrophy in pediatric renal transplant recipients. Pediatr Transplant 14: 52–60, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Furth SL, Abraham AG, Jerry-Fluker J, Schwartz GJ, Benfield M, Kaskel F, Wong C, Mak RH, Moxey-Mims M, Warady BA: Metabolic abnormalities, cardiovascular disease risk factors, and GFR decline in children with chronic kidney disease. Clin J Am Soc Nephrol 6: 2132–2140, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atkinson MA, Martz K, Warady BA, Neu AM: Risk for anemia in pediatric chronic kidney disease patients: A report of NAPRTCS. Pediatr Nephrol 25: 1699–1706, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Warady BA, Ho M: Morbidity and mortality in children with anemia at initiation of dialysis. Pediatr Nephrol 18: 1055–1062, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Chavers BM, Solid CA, Sinaiko A, Daniels FX, Chen SC, Collins AJ, Frankenfield DL, Herzog CA: Diagnosis of cardiac disease in pediatric end-stage renal disease. Nephrol Dial Transplant 26: 1640–1645, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitsnefes MM, Kimball TR, Witt SA, Glascock BJ, Khoury PR, Daniels SR: Left ventricular mass and systolic performance in pediatric patients with chronic renal failure. Circulation 107: 864–868, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Matteucci MC, Wühl E, Picca S, Mastrostefano A, Rinelli G, Romano C, Rizzoni G, Mehls O, de Simone G, Schaefer F. ESCAPE Trial Group: Left ventricular geometry in children with mild to moderate chronic renal insufficiency. J Am Soc Nephrol 17: 218–226, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Mitsnefes MM, Daniels SR, Schwartz SM, Khoury P, Strife CF: Changes in left ventricular mass in children and adolescents during chronic dialysis. Pediatr Nephrol 16: 318–323, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Ulinski T, Genty J, Viau C, Tillous-Borde I, Deschênes G: Reduction of left ventricular hypertrophy in children undergoing hemodialysis. Pediatr Nephrol 21: 1171–1178, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Mitsnefes MM, Barletta GM, Dresner IG, Chand DH, Geary D, Lin JJ, Patel H: Severe cardiac hypertrophy and long-term dialysis: The Midwest Pediatric Nephrology Consortium study. Pediatr Nephrol 21: 1167–1170, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Bakkaloglu SA, Borzych D, Soo Ha I, Serdaroglu E, Büscher R, Salas P, Patel H, Drozdz D, Vondrak K, Watanabe A, Villagra J, Yavascan O, Valenzuela M, Gipson D, Ng KH, Warady BA, Schaefer F. International Pediatric Peritoneal Dialysis Network: Cardiac geometry in children receiving chronic peritoneal dialysis: Findings from the International Pediatric Peritoneal Dialysis Network (IPPN) registry. Clin J Am Soc Nephrol 6: 1926–1933, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bullington N, Kartel J, Khoury P, Mitsnefes M: Left ventricular hypertrophy in pediatric kidney transplant recipients: Long-term follow-up study. Pediatr Transplant 10: 811–815, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Mitsnefes MM, Schwartz SM, Daniels SR, Kimball TR, Khoury P, Strife CF: Changes in left ventricular mass index in children and adolescents after renal transplantation. Pediatr Transplant 5: 279–284, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Kitzmueller E, Vécsei A, Pichler J, Böhm M, Müller T, Vargha R, Csaicsich D, Aufricht C: Changes of blood pressure and left ventricular mass in pediatric renal transplantation. Pediatr Nephrol 19: 1385–1389, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Becker-Cohen R, Nir A, Ben-Shalom E, Rinat C, Feinstein S, Farber B, Frishberg Y: Improved left ventricular mass index in children after renal transplantation. Pediatr Nephrol 23: 1545–1550, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Mitsnefes MM, Kimball TR, Kartal J, Witt SA, Glascock BJ, Khoury PR, Daniels SR: Progression of left ventricular hypertrophy in children with early chronic kidney disease: 2-year follow-up study. J Pediatr 149: 671–675, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Mitsnefes MM, Kimball TR, Border WL, Witt SA, Glascock BJ, Khoury PR, Daniels SR: Impaired left ventricular diastolic function in children with chronic renal failure. Kidney Int 65: 1461–1466, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Weaver DJ, Jr, Kimball TR, Knilans T, Mays W, Knecht SK, Gerdes YM, Witt S, Glascock BJ, Kartal J, Khoury P, Mitsnefes MM: Decreased maximal aerobic capacity in pediatric chronic kidney disease. J Am Soc Nephrol 19: 624–630, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weaver DJ, Jr, Kimball T, Witt SA, Glascock BJ, Khoury PR, Kartal J, Mitsnefes MM: Subclinical systolic dysfunction in pediatric patients with chronic kidney disease. J Pediatr 153: 565–569, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Chinali M, de Simone G, Matteucci MC, Picca S, Mastrostefano A, Anarat A, Caliskan S, Jeck N, Neuhaus TJ, Peco-Antic A, Peruzzi L, Testa S, Mehls O, Wühl E, Schaefer F. ESCAPE Trial Group: Reduced systolic myocardial function in children with chronic renal insufficiency. J Am Soc Nephrol 18: 593–598, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Mese T, Guven B, Yilmazer MM, Serdaroglu E, Tavli V, Haydar A, Bak M: Contractility reserve in children undergoing dialysis by dobutamine stress echocardiography. Pediatr Cardiol 31: 937–943, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Hothi DK, Rees L, Marek J, Burton J, McIntyre CW: Pediatric myocardial stunning underscores the cardiac toxicity of conventional hemodialysis treatments. Clin J Am Soc Nephrol 4: 790–797, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Litwin M, Wühl E, Jourdan C, Trelewicz J, Niemirska A, Fahr K, Jobs K, Grenda R, Wawer ZT, Rajszys P, Tröger J, Mehls O, Schaefer F: Altered morphologic properties of large arteries in children with chronic renal failure and after renal transplantation. J Am Soc Nephrol 16: 1494–1500, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Litwin M, Wühl E, Jourdan C, Niemirska A, Schenk JP, Jobs K, Grenda R, Wawer ZT, Rajszys P, Mehls O, Schaefer F: Evolution of large-vessel arteriopathy in paediatric patients with chronic kidney disease. Nephrol Dial Transplant 23: 2552–2557, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Briese S, Wiesner S, Will JC, Lembcke A, Opgen-Rhein B, Nissel R, Wernecke KD, Andreae J, Haffner D, Querfeld U: Arterial and cardiac disease in young adults with childhood-onset end-stage renal disease-impact of calcium and vitamin D therapy. Nephrol Dial Transplant 21: 1906–1914, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Shroff RC, Donald AE, Hiorns MP, Watson A, Feather S, Milford D, Ellins EA, Storry C, Ridout D, Deanfield J, Rees L: Mineral metabolism and vascular damage in children on dialysis. J Am Soc Nephrol 18: 2996–3003, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Milliner DS, Zinsmeister AR, Lieberman E, Landing B: Soft tissue calcification in pediatric patients with end-stage renal disease. Kidney Int 38: 931–936, 1990 [DOI] [PubMed] [Google Scholar]
- 48.Dursun H, Küçükosmanoğlu O, Noyan A, Ozbarlas N, Büyükçelik M, Soran M, Bayazit AK, Anarat A: Mitral annular calcification and brown tumor of the rib in a child with chronic renal failure. Pediatr Nephrol 20: 673–675, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Fadrowski JJ, Frankenfield D, Amaral S, Brady T, Gorman GH, Warady B, Furth SL, Fivush B, Neu AM: Children on long-term dialysis in the United States: Findings from the 2005 ESRD clinical performance measures project. Am J Kidney Dis 50: 958–966, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, Gorodetskaya I, Greene T, James S, Larive B, Lindsay RM, Mehta RL, Miller B, Ornt DB, Rajagopalan S, Rastogi A, Rocco MV, Schiller B, Sergeyeva O, Schulman G, Ting GO, Unruh ML, Star RA, Kliger AS. FHN Trial Group: In-center hemodialysis six times per week versus three times per week. N Engl J Med 363: 2287–2300, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fischbach M, Terzic J, Laugel V, Dheu C, Menouer S, Helms P, Livolsi A: Daily on-line haemodiafiltration: A pilot trial in children. Nephrol Dial Transplant 19: 2360–2367, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Hoppe A, von Puttkamer C, Linke U, Kahler C, Booss M, Braunauer-Kolberg R, Hofmann K, Joachimsky P, Hirte I, Schley S, Utsch B, Thumfart J, Briese S, Gellermann J, Zimmering M, Querfeld U, Müller D: A hospital-based intermittent nocturnal hemodialysis program for children and adolescents. J Pediatrics 158: 95–99, 99.e1, 2011 [DOI] [PubMed] [Google Scholar]
- 53.Lacson E, Jr, Lazarus M: Dialysis time: Does it matter? A reappraisal of existing literature. Curr Opin Nephrol Hypertens 20: 189–194, 2011 [DOI] [PubMed] [Google Scholar]
- 54.Mitsnefes M, Ho PL, McEnery PT: Hypertension and progression of chronic renal insufficiency in children: A report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS). J Am Soc Nephrol 14: 2618–2622, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Mitsnefes M, Stablein D: Hypertension in pediatric patients on long-term dialysis: A report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS). Am J Kidney Dis 45: 309–315, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Tkaczyk M, Nowicki M, Bałasz-Chmielewska I, Boguszewska-Baçzkowska H, Drozdz D, Kołłataj B, Jarmoliński T, Jobs K, Kiliś-Pstrusińska K, Leszczyńska B, Makulska I, Runowski D, Stankiewicz R, Szczepańska M, Wierciński R, Grenda R, Kanik A, Pietrzyk JA, Roszkowska-Blaim M, Szprynger K, Zachwieja J, Zajaczkowska MM, Zoch-Zwierz W, Zwolińska D, Zurowska A: Hypertension in dialysed children: The prevalence and therapeutic approach in Poland–a nationwide survey. Nephrol Dial Transplant 21: 736–742, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Staples AO, Greenbaum LA, Smith JM, Gipson DS, Filler G, Warady BA, Martz K, Wong CS: Association between clinical risk factors and progression of chronic kidney disease in children. Clin J Am Soc Nephrol 5: 2172–2179, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kramer AM, van Stralen KJ, Jager KJ, Schaefer F, Verrina E, Seeman T, Lewis MA, Boehm M, Simonetti GD, Novljan G, Groothoff JW: Demographics of blood pressure and hypertension in children on renal replacement therapy in Europe. Kidney Int 80: 1092–1098, 2011 [DOI] [PubMed] [Google Scholar]
- 59.Hanevold CD, Ho PL, Talley L, Mitsnefes MM: Obesity and renal transplant outcome: A report of the North American Pediatric Renal Transplant Cooperative Study. Pediatrics 115: 352–356, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Chavers BM, Roberts TL, Herzog CA, Collins AJ, St Peter WL: Prevalence of anemia in erythropoietin-treated pediatric as compared to adult chronic dialysis patients. Kidney Int 65: 266–273, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Mitsnefes MM, Subat-Dezulovic M, Khoury PR, Goebel J, Strife CF: Increasing incidence of post-kidney transplant anemia in children. Am J Transplant 5: 1713–1718, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Smith LB, Fadrowski JJ, Howe CJ, Fivush BA, Neu AM, Furth SL: Secondary hyperparathyroidism and anemia in children treated by hemodialysis. Am J Kidney Dis 55: 326–334, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bek K, Akman S, Bilge I, Topaloğlu R, Calişkan S, Peru H, Cengiz N, Söylemezoğlu O: Chronic kidney disease in children in Turkey. Pediatr Nephrol 24: 797–806, 2009 [DOI] [PubMed] [Google Scholar]
- 64.Goldstein SL, Currier H, Watters L, Hempe JM, Sheth RD, Silverstein D: Acute and chronic inflammation in pediatric patients receiving hemodialysis. J Pediatr 143: 653–657, 2003 [DOI] [PubMed] [Google Scholar]
- 65.Johnstone LM, Jones CL, Grigg LE, Wilkinson JL, Walker RG, Powell HR: Left ventricular abnormalities in children, adolescents and young adults with renal disease. Kidney Int 50: 998–1006, 1996 [DOI] [PubMed] [Google Scholar]
- 66.Mitsnefes MM, Kimball TR, Kartal J, Witt SA, Glascock BJ, Khoury PR, Daniels SR: Progression of left ventricular hypertrophy in children with early chronic kidney disease: 2-year follow-up study. J Pediatr 149: 671–675, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Sinha MD, Tibby SM, Rasmussen P, Rawlins D, Turner C, Dalton RN, Reid CJ, Rigden SP, Booth CJ, Simpson JM: Blood pressure control and left ventricular mass in children with chronic kidney disease. Clin J Am Soc Nephrol 6: 543–551, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mitsnefes MM, Kimball TR, Kartal J, Witt SA, Glascock BJ, Khoury PR, Daniels SR: Cardiac and vascular adaptation in pediatric patients with chronic kidney disease: Role of calcium-phosphorus metabolism. J Am Soc Nephrol 16: 2796–2803, 2005 [DOI] [PubMed] [Google Scholar]
- 69.Delucchi A, Dinamarca H, Gainza H, Whitttle C, Torrealba I, Iñiguez G: Carotid intima-media thickness as a cardiovascular risk marker in pediatric end-stage renal disease patients on dialysis and in renal transplantation. Transplant Proc 40: 3244–3246, 2008 [DOI] [PubMed] [Google Scholar]
- 70.Krmar RT, Balzano R, Jogestrand T, Cedazo-Minguez A, Englund MS, Berg UB: Prospective analysis of carotid arterial wall structure in pediatric renal transplants with ambulatory normotension and in treated hypertensive recipients. Pediatr Transplant 12: 412–419, 2008 [DOI] [PubMed] [Google Scholar]
- 71.Muscheites J, Meyer AA, Drueckler E, Wigger M, Fischer DC, Kundt G, Kienast W, Haffner D: Assessment of the cardiovascular system in pediatric chronic kidney disease: A pilot study. Pediatr Nephrol 23: 2233–2239, 2008 [DOI] [PubMed] [Google Scholar]
- 72.Ziolkowska H, Brzewski M, Roszkowska-Blaim M: Determinants of the intima-media thickness in children and adolescents with chronic kidney disease. Pediatr Nephrol 23: 805–811, 2008 [DOI] [PubMed] [Google Scholar]
- 73.Mitsnefes MM, Kimball TR, Witt SA, Glascock BJ, Khoury PR, Daniels SR: Abnormal carotid artery structure and function in children and adolescents with successful renal transplantation. Circulation 110: 97–101, 2004 [DOI] [PubMed] [Google Scholar]
- 74.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB: Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 342: 1478–1483, 2000 [DOI] [PubMed] [Google Scholar]
- 75.Ishitani MB, Milliner DS, Kim DY, Bohorquez HE, Heimbach JK, Sheedy PF 2nd, Morgenstern BZ, Gloor JM, Murphy JG, McBane RD, Bielak LF, Peyser PA, Stegall MD: Early subclinical coronary artery calcification in young adults who were pediatric kidney transplant recipients. Am J Transplant 5: 1689–1693, 2005 [DOI] [PubMed] [Google Scholar]
- 76.Civilibal M, Caliskan S, Adaletli I, Oflaz H, Sever L, Candan C, Canpolat N, Kasapcopur O, Kuruoglu S, Arisoy N: Coronary artery calcifications in children with end-stage renal disease. Pediatr Nephrol 21: 1426–1433, 2006 [DOI] [PubMed] [Google Scholar]
- 77.Civilibal M, Caliskan S, Kurugoglu S, Candan C, Canpolat N, Sever L, Kasapcopur O, Arisoy N: Progression of coronary calcification in pediatric chronic kidney disease stage 5. Pediatr Nephrol 24: 555–563, 2009 [DOI] [PubMed] [Google Scholar]