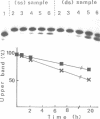
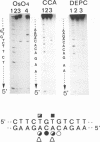
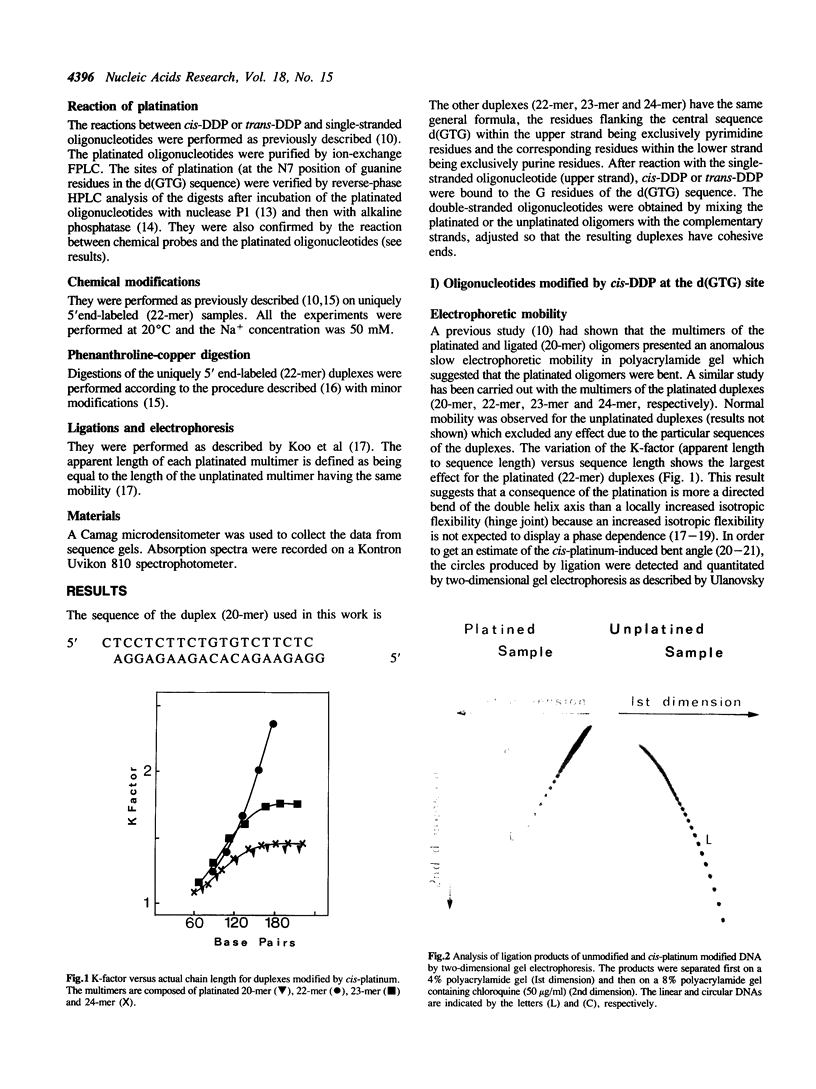
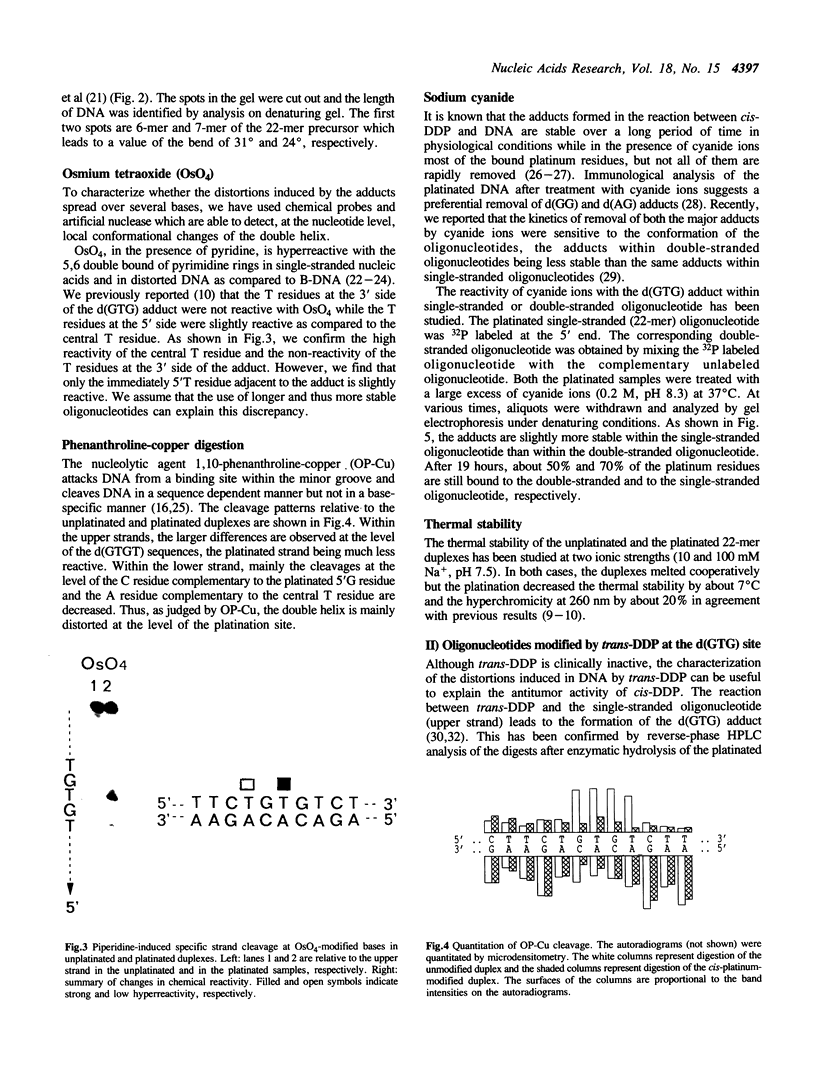
Abstract
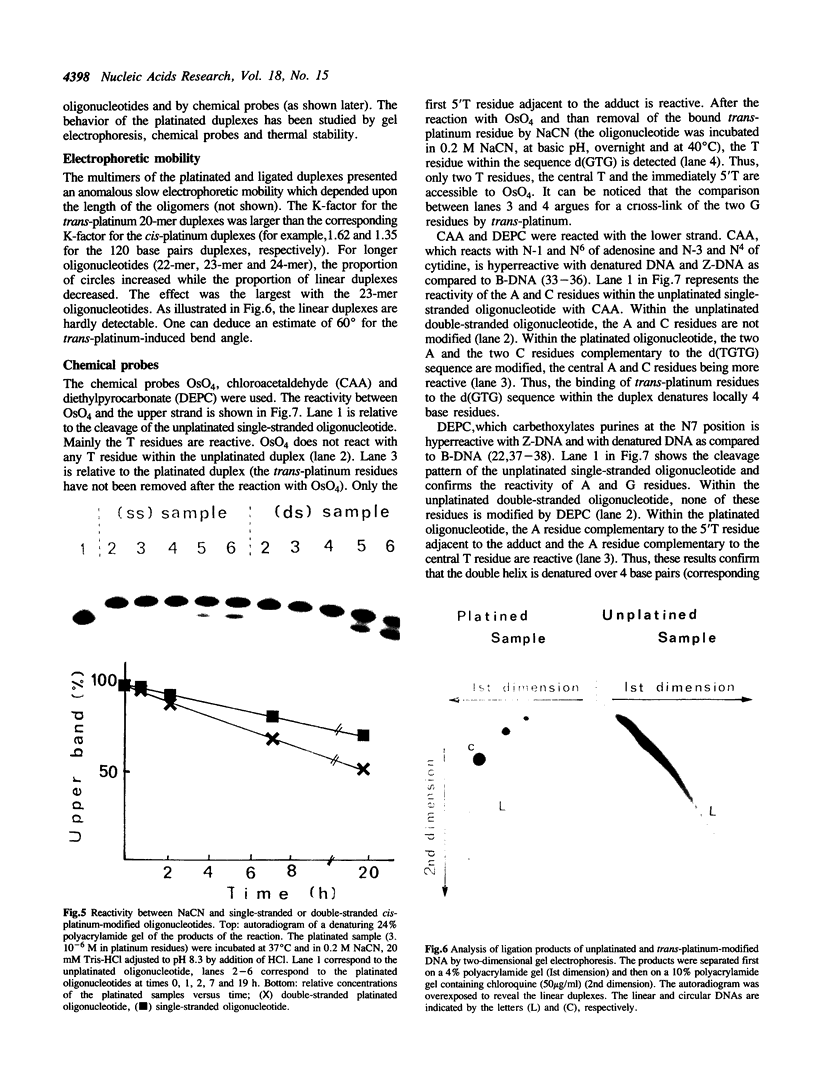
Conformational changes induced in double-stranded oligonucleotides by the binding of trans- or cis-diamminedichloro platinum(II) to the d(GTG) sequence have been characterized by means of melting temperatures, electrophoretic migrations in non-denaturing polyacrylamide gels, reactivities with the artificial nuclease Phenanthroline-copper and with chemical probes. The cis-platinum adduct behaves more as a centre of directed bend than as a hinge joint, the induced bend angle being of the order of 25-30 degrees. The double helix is locally denatured over 2 base pairs (corresponding to the platinated 5'G residue and the central T residue) and is distorted over 4-5 base pairs. The trans-platinum adduct behaves also more as a centre of directed bend than as a hinge joint, the induced bend angle being of the order of 60 degrees. The double helix is locally denatured over 4 base pairs (corresponding to the immediately 5'T residue adjacent to the adduct and to the three base residues of the adduct). Both the cis- and trans-platinum adducts decrease the thermal stability of the double helix.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W., Gonias S. L., Kam S. K., Wu K. C., Lippard S. J. Binding of the antitumor drug platinum uracil blue to closed and nicked circular duplex DNAs. Biochemistry. 1978 Mar 21;17(6):1060–1068. doi: 10.1021/bi00599a019. [DOI] [PubMed] [Google Scholar]
- Brouwer J., van de Putte P., Fichtinger-Schepman A. M., Reedijk J. Base-pair substitution hotspots in GAG and GCG nucleotide sequences in Escherichia coli K-12 induced by cis-diamminedichloroplatinum (II). Proc Natl Acad Sci U S A. 1981 Nov;78(11):7010–7014. doi: 10.1073/pnas.78.11.7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnouf D., Duane M., Fuchs R. P. Spectrum of cisplatin-induced mutations in Escherichia coli. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3758–3762. doi: 10.1073/pnas.84.11.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman A., Jennerwein M. M., Nagel D. L. Characterization of bifunctional adducts produced in DNA by trans-diamminedichloroplatinum(II). Chem Biol Interact. 1988;67(1-2):71–80. doi: 10.1016/0009-2797(88)90087-7. [DOI] [PubMed] [Google Scholar]
- Eastman A. Reevaluation of interaction of cis-dichloro(ethylenediamine)platinum(II) with DNA. Biochemistry. 1986 Jul 1;25(13):3912–3915. doi: 10.1021/bi00361a026. [DOI] [PubMed] [Google Scholar]
- Eastman A. The formation, isolation and characterization of DNA adducts produced by anticancer platinum complexes. Pharmacol Ther. 1987;34(2):155–166. doi: 10.1016/0163-7258(87)90009-x. [DOI] [PubMed] [Google Scholar]
- Fichtinger-Schepman A. M., van der Veer J. L., den Hartog J. H., Lohman P. H., Reedijk J. Adducts of the antitumor drug cis-diamminedichloroplatinum(II) with DNA: formation, identification, and quantitation. Biochemistry. 1985 Jan 29;24(3):707–713. doi: 10.1021/bi00324a025. [DOI] [PubMed] [Google Scholar]
- Herr W. Diethyl pyrocarbonate: a chemical probe for secondary structure in negatively supercoiled DNA. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8009–8013. doi: 10.1073/pnas.82.23.8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston B. H., Rich A. Chemical probes of DNA conformation: detection of Z-DNA at nucleotide resolution. Cell. 1985 Oct;42(3):713–724. doi: 10.1016/0092-8674(85)90268-5. [DOI] [PubMed] [Google Scholar]
- Kohwi-Shigematsu T., Manes T., Kohwi Y. Unusual conformational effect exerted by Z-DNA upon its neighboring sequences. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2223–2227. doi: 10.1073/pnas.84.8.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H. S., Wu H. M., Crothers D. M. DNA bending at adenine . thymine tracts. Nature. 1986 Apr 10;320(6062):501–506. doi: 10.1038/320501a0. [DOI] [PubMed] [Google Scholar]
- Lepre C. A., Chassot L., Costello C. E., Lippard S. J. Synthesis and characterization of trans-[Pt(NH3)2Cl2] adducts of d(CCTCGAGTCTCC).d(GGAGACTCGAGG). Biochemistry. 1990 Jan 23;29(3):811–823. doi: 10.1021/bi00455a031. [DOI] [PubMed] [Google Scholar]
- Lilley D. M., Palecek E. The supercoil-stabilised cruciform of ColE1 is hyper-reactive to osmium tetroxide. EMBO J. 1984 May;3(5):1187–1192. doi: 10.1002/j.1460-2075.1984.tb01949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley D. M. Structural perturbation in supercoiled DNA: hypersensitivity to modification by a single-strand-selective chemical reagent conferred by inverted repeat sequences. Nucleic Acids Res. 1983 May 25;11(10):3097–3112. doi: 10.1093/nar/11.10.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippard S. J., Hoeschele J. D. Binding of cis- and trans-dichlorodiammineplatinum(II) to the nucleosome core. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6091–6095. doi: 10.1073/pnas.76.12.6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrot L., Leng M. Chemical probes of the conformation of DNA modified by cis-diamminedichloroplatinum(II). Biochemistry. 1989 Feb 21;28(4):1454–1461. doi: 10.1021/bi00430a005. [DOI] [PubMed] [Google Scholar]
- Mazeau K., Vovelle F., Rahmouni A., Leng M., Ptak M. Structure of the intrastrand cis-[Pt(NH3)2(d(GpCpG))] adduct in a dodecanucleotide duplex: II. A molecular mechanics modeling study. Anticancer Drug Des. 1989 Jun;4(1):63–78. [PubMed] [Google Scholar]
- McLean M. J., Larson J. E., Wohlrab F., Wells R. D. Reaction conditions affect the specificity of bromoacetaldehyde as a probe for DNA cruciforms and B-Z junctions. Nucleic Acids Res. 1987 Sep 11;15(17):6917–6935. doi: 10.1093/nar/15.17.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palecek E., Boublíková P., Galazka G., Klysik J. Inhibition of restriction endonuclease cleavage due to site-specific chemical modification of the B-Z junction in supercoiled DNA. Gen Physiol Biophys. 1987 Aug;6(4):327–341. [PubMed] [Google Scholar]
- Ptak M., Rahmouni A., Mazeau K., Thuong N. T., Leng M. Structure of the intrastrand cis-[Pt(NH3)2(d(GpCpG))] adduct in a dodecanucleotide duplex: I. A 1H and 31P n.m.r. study. Anticancer Drug Des. 1989 Jun;4(1):53–61. [PubMed] [Google Scholar]
- Rice J. A., Crothers D. M., Pinto A. L., Lippard S. J. The major adduct of the antitumor drug cis-diamminedichloroplatinum(II) with DNA bends the duplex by approximately equal to 40 degrees toward the major groove. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4158–4161. doi: 10.1073/pnas.85.12.4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runkel L., Nordheim A. Conformational DNA transition in the in vitro torsionally strained chicken beta-globin 5' region. Nucleic Acids Res. 1986 Sep 25;14(18):7143–7158. doi: 10.1093/nar/14.18.7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A., Marrot L., Leng M. Conformation of DNA modified at a d(GG) or a d(AG) site by the antitumor drug cis-diamminedichloroplatinum(II). Biochemistry. 1989 Oct 3;28(20):7975–7979. doi: 10.1021/bi00446a001. [DOI] [PubMed] [Google Scholar]
- Shore D., Langowski J., Baldwin R. L. DNA flexibility studied by covalent closure of short fragments into circles. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4833–4837. doi: 10.1073/pnas.78.8.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulanovsky L., Bodner M., Trifonov E. N., Choder M. Curved DNA: design, synthesis, and circularization. Proc Natl Acad Sci U S A. 1986 Feb;83(4):862–866. doi: 10.1073/pnas.83.4.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt N., Marrot L., Rousseau N., Malfoy B., Leng M. Chloroacetaldehyde reacts with Z-DNA. J Mol Biol. 1988 Jun 20;201(4):773–776. doi: 10.1016/0022-2836(88)90474-3. [DOI] [PubMed] [Google Scholar]
- Yoon C., Kuwabara M. D., Law R., Wall R., Sigman D. S. Sequence-dependent variability of DNA structure. Influence of flanking sequences and fragment length on digestion by conformationally sensitive nucleases. J Biol Chem. 1988 Jun 15;263(17):8458–8463. [PubMed] [Google Scholar]
- de Boer J. G., Glickman B. W. Sequence specificity of mutation induced by the anti-tumor drug cisplatin in the CHO aprt gene. Carcinogenesis. 1989 Aug;10(8):1363–1367. doi: 10.1093/carcin/10.8.1363. [DOI] [PubMed] [Google Scholar]