Abstract
Objective
To explore the specificity of impaired praxis and postural knowledge to autism by examining three samples of children, including those with autism spectrum disorder (ASD), attention-deficit hyperactivity disorder (ADHD), and typically developing (TD) children.
Method
Twenty-four children with ASD, 24 children with ADHD, and 24 TD children, ages 8–13, completed measures assessing basic motor control (the Physical and Neurological Exam for Subtle Signs; PANESS), praxis (performance of skilled gestures to command, with imitation, and tool use) and the ability to recognize correct hand postures necessary to perform these skilled gestures (the Postural Knowledge Test; PKT).
Results
Children with ASD performed significantly worse than TD children on all three assessments. In contrast, children with ADHD performed significantly worse than TD controls on PANESS but not on the praxis examination or PKT. Furthermore, children with ASD performed significantly worse than children with ADHD on both the praxis examination and PKT, but not on the PANESS.
Conclusions
Whereas both children with ADHD and children with ASD show impairments in basic motor control, impairments in performance and recognition of skilled motor gestures, consistent with dyspraxia, appear to be specific to autism. The findings suggest that impaired formation of perceptual-motor action models necessary to development of skilled gestures and other goal directed behavior is specific to autism; whereas, impaired basic motor control may be a more generalized finding.
Keywords: imitation, motor learning, procedural learning, premotor cortex, inferior parietal lobe
Introduction
Abundant evidence from case reports and case-control studies has historically linked autism spectrum disorder (ASD) with impaired motor function, including unusual or clumsy gait, impairments in coordination, balance, tone, and posture, and abnormal performance of skilled gestures (Gidley Larson & Mostofsky, 2006). While the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision (DSM-IV-TR) specifies social, communicative, and repetitive behaviors as core features of autism, difficulties with motor control have been recognized since early descriptions of the disorder (Kanner, 1943; Ritvo & Provence, 1953). In recent decades, numerous studies have described impairments in motor function (DeMyer et al., 1972; Dewey, Cantell, & Crawford, 2007; Dowell, Mahone, & Mostofsky, 2009; Dziuk et al., 2007; Haas et al., 1996; Hallett et al., 1993; Jansiewicz et al., 2006; Jones & Prior, 1985; S.H. Mostofsky et al., 2006; Rapin, 1991; Vilensky, Damasio, & Maurer, 1981). These prominent findings reveal a large preponderance of individuals, yet not all children with ASD, have motor difficulties. Due to this heterogeneity, specification of motor impairments may lead to identification of clinically relevant endophenotypes.
Alternative approaches to unravel the neurobiology of ASD are critical to identifying subtypes and improving outcomes and will likely depend on the development of systematic, inexpensive, reliable methods to quantify outputs of biologically relevant neurobehavioral systems which reflect dimensions of impaired function in children with ASD. One such approach may be detailed examination of motor signs. Development of motor function parallels development of more complex social behavior, with skill-based learning necessary to both processes. Furthermore, motor signs are more clearly observable and quantifiable than are processes involved in more complex social interaction or communication. A better understanding of the neurobiology of ASD is vital for identifying treatments that improve outcomes. As such, ASD-associated anomalies in systems critical to motor control can help inform more broadly about neural substrates for behavioral control. Motor signs can therefore serve as biomarkers, important for guiding early diagnosis and treatment. Furthermore, the neural underpinnings of ASD may be teased out more effectively by examining motor signs, for which the neurological basis, anatomical and functional, is well mapped out.
One of the most consistent motor signs in autism is that children with ASD show difficulties with performance of skilled motor gestures to command, with imitation, and with tool use on praxis examination (Dewey et al., 2007; Dowell et al., 2009; Dziuk et al., 2007; S.H. Mostofsky et al., 2006; Rogers, Bennetto, McEvoy, & Pennington, 1996), observations consistent with “developmental dyspraxia” (Steinman, Mostofsky, & Denckla, 2010). Findings from these studies reveal that impaired performance on praxis examination correlated with measures of the core social and communicative features of autism (Dowell et al., 2009; Dziuk et al., 2007), suggesting that common mechanisms may contribute to impaired development of motor skills and social/communicative skills in autism. Furthermore, the association of praxis and social skill impairment remains even after accounting for deficits in basic motor controls (Dowell et al., 2009; Dziuk et al., 2007).
In adult populations, ideomotor praxis is characterized by impaired performance of skilled gestures and difficulty recognizing skilled gestures performed by others (Heilman & Gonzalez Rothi, 2003; Steinman et al., 2010). Accordingly, we recently examined this praxis recognition (so-called “postural knowledge”) in autism. Children with ASD were found to be impaired in their ability to recognize correct praxis gestures in others, as assessed by a Postural Knowledge Test (PKT) (Dowell et al., 2009). These findings parallel those of social dysfunction in autism, where children with ASD show impaired performance of social skills and deficits in social awareness (i.e. difficulty understanding others' social gestures). Understanding impairments that contribute to developmental dyspraxia and exploring whether or not these difficulties are specific to autism may therefore provide a window into impaired development of children with ASD. If these factors are distinctive to ASD, it may help pinpoint anomalies that contribute to hallmark deficits in social interaction and communication.
In this study, we explored the specificity of impaired basic motor control, postural knowledge, and praxis performance to autism by examining three samples of children, including children with ASD, children with attention-deficit hyperactivity disorder (ADHD), and TD children. Children with ADHD also exhibit deficits in motor control (Cole, Mostofsky, Larson, Denckla, & Mahone, 2008; MacNeil et al., 2011; S. H. Mostofsky, Newschaffer, & Denckla, 2003), but it is unclear if they also demonstrate impaired praxis. Findings from one previous study suggest children with ASD, but not those with ADHD, show impaired execution of skilled gestures on praxis examination (Dewey et al., 2007); however, postural knowledge was not assessed in children with ADHD, in that study or any others. We hypothesized that while both children with ASD and children with ADHD would exhibit impairments in basic motor control as compared with TD children, only children with ASD would demonstrate impaired praxis and postural knowledge.
Methods
Participants
Seventy-two children ages 8.02 to 13.00 years participated in the study: 24 children with ASD (mean age = 9.69, SD = 1.59; 5 girls; 1 left-handed, 2 mixed-handed, 21 right-handed), 24 children with ADHD (mean age = 9.73, SD = 1.30; 5 girls; 3 left-handed, 21 right-handed), and 24 TD children (mean age = 10.33, SD = 1.40; 5 girls; 3 left-handed, 2 mixed-handed, 19 right-handed). Within the group of children with ASD, subjects were either diagnosed with high-functioning autism (HFA; N = 12) or Asperger's syndrome (Asp; N = 12). Evidence from previous studies supports that children from each group, HFA and Asp, perform similarly on measures of basic motor skills, praxis, and postural knowledge (Dowell et al., 2009; Jansiewicz et al., 2006; S.H. Mostofsky et al., 2006). Additionally, preliminary analyses from the current data set supported the notion that children with Asp and children with HFA performed similarly on the PANESS, praxis examination, and PKT (see the Results section).
This study is a follow-up study to several from our laboratory: Mostofsky, 2006; Dziuk, 2007; Dowell, 2009. Fifty percent of the current group of children with ASD and 70% of the TD children were used in the Dowell, 2009 study. Therefore, in the current study, there is an additional group of interest, children with ADHD, and we have reanalyzed data from our most recent publication. Although there is some degree of overlap between subjects with Dowell, 2009, there is 0% subject overlap, ASD or TD, with our earlier studies, Mostofsky, 2006 or Dziuk, 2007.
Participants were recruited from a variety of sources including local schools, pediatricians' offices, outpatient clinics at the Kennedy Krieger Institute, advertisements posted in local community centers, local Autism Society of America chapters, local chapters of Children and Adults with Attention-Deficit Hyperactivity Disorder, and by word of mouth.
Children with autism met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria (Association, 1994). The Autism Diagnostic Interview—Revised (ADI-R) (Lord, Rutter, & Le Couteur, 1994), the Autism Diagnostic Observation Schedule-G, Module 3 (ADOS-G) (Lord et al., 2000), and clinical judgment were used to establish ASD diagnoses. All participants had to meet diagnosis criteria on the basis of clinical judgment of the examiner and meet diagnosis criteria on the ADOS-G, the ADI-R, or both.
Children with ADHD met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria. Diagnosis of ADHD was determined by the Diagnostic Interview for Children and Adolescents-IV (DICA-IV) (Reich, Welner, & Herjanic, 1997). Parents completed the Conners' Parent Rating Scales-Revised (CPRS-R) (Conners, 1997) and the ADHD Rating Scale-IV, home and school versions (ADHDRS) (DuPaul, Power, Anastopoulos, & Reid, 1998). Inclusion in ADHD was made based on the following criteria: (1) DSM-IV-TR diagnosis of ADHD based on positive scores on at least one of the parent rating scales (i.e., T-score of 65 or higher on scale L (DSM-IV: inattentive) or M (DSM-IV: hyperactive-impulsive) on the CPRS-R Long Form or children receiving scores of 2 or 3 on at least 6/9 items on the Inattentive or Hyperactivity/Impulsivity scales of the ADHDRS); and (2) confirmation of ADHD diagnosis by DICA-IV psychiatric interview. Two subtypes of ADHD were included: combined (ADHD-C: n = 16, 3 girls) and inattentive (ADHD-I: n = 8, 2 girls). Preliminary analyses were conducted to assess performance differences between the ADHD subtypes. Data supported that children with combined-type and inattentive-type ADHD performed similarly on the PANESS, praxis examination, and PKT (see the Results section). As such, we have included both subtypes in our group of ADHD children, but have also included the mean motor scores for each ADHD subtype. Children were excluded if they met criteria for diagnosis of autism spectrum disorder, conduct, mood, generalized anxiety, separation anxiety or obsessive-compulsive disorders on DICA-IV. Diagnoses of oppositional defiant disorder (ODD) and simple phobias were allowed. While findings from previous studies suggest that ADHD associated with conduct disorder may be a distinct subtype, this is not the case for ADHD associated with ODD (Biederman, Faraone, & Lapey, 1992; Faraone et al., 1995).
Children were excluded from all three groups if there was a prior documented history of a definitive neurological disorder (including seizures, tumors, traumatic brain injury, stroke, or lesions), presence of a severe chronic medical disorder, visual impairment, history of substance abuse or dependence, or presence of childhood schizophrenia or psychosis. Potential participants were screened and excluded appropriately after gathering information during a phone screening. If there was a history of known etiology for autism (e.g., fragile × syndrome, tuberous sclerosis, phenylketonuria, or congenital rubella) or a history of documented prenatal or perinatal insult then children were excluded from the ASD group.
Participants were not eligible for the group of typically developing children if, based on the DICA-IV, they had a history of a developmental or psychiatric disorder. They were also excluded if they had an immediate family member with autism or another pervasive developmental disorder.
Parents of children taking stimulants were asked to withhold medication the day prior to and the day of each study visit. Medications other than stimulants were taken as prescribed. Children in the control group were not taking any psychotropic medications.
The Johns Hopkins Medicine Institutional Review Board approved this study. Written consent was obtained from a parent or legal guardian and assent was obtained from every child.
Procedures
The three diagnostic groups of children were matched based on age, gender, race, socioeconomic status (SES) (Hollingshead, 1975), handedness, and Perceptual Reasoning Index (PRI). The SES of each participant's family was assessed using information from the medical history to calculate a Hollingshead four-factor index of social status value. Handedness was confirmed on the Edinburgh Inventory (Oldfield, 1971) and the PANESS.
To assess intellectual functioning, each participant was administered the Wechsler Intelligence Scale for Children (4th ed.; WISC-IV) (Wechsler, 2003). Research indicates that it is more appropriate to use a task-specific measure of intelligence than a general measure of intelligence when assessing children with ASD (Mottron, 2004). Therefore, instead of using the Full Scale IQ (FSIQ) from the WISC-IV, the current study used the Perceptual Reasoning Index (PRI) to assess the intellectual functioning of each child, because the tasks in this study were perceptually based, nonverbal tasks.
Basic motor control examination
PANESS, a standardized childhood assessment, was used to assess basic motor control (Denckla, 1985). The PANESS evaluates gait, balance, coordination, and aim and the presence of subtle neurological signs, such as overflow movements, abnormal posturing, and dysrhythmia, during these tasks. Similar to the Dowell et al., 2009 study, a measure of “total timed repetitive movements” (TTRM) was used as a relevant measure of the contribution of basic motor control during the production of skilled gestures during the praxis examination. It is the cumulative time (seconds) it takes to complete 20 hand pats (fixing the heel of the hand to the lap and patting the front of the hand to the lap), 20 finger taps (tapping the pointer finger and thumb together), and 20 foot taps (fixing the heel of the foot on the floor and tapping the front of the foot to the floor) on each side. These times are believed to be an appropriate measure of basic motor control because limb movements are necessary to perform skilled gestures. Additionally, the frontal and frontal-subcortical contributions necessary to perform these limb movements are integrated into the performance of skilled gestures (Dowell et al., 2009). We also used a total score from the PANESS, calculated as the total gaits and stations score (gait and balance movements, including walking on the heels, toes, sides of feet, and tandem) plus the total timed score (repetitive, simple flexion/extension movements and patterned movements), as a measure of overall motor control which incorporates the overall performance and the presence of neurological subtle signs during each movement.
Praxis examination
A version of the Florida Apraxia Battery (Gonzalez Rothi, Raymer, & Heilman, 1997), modified for children (S.H. Mostofsky et al., 2006), was used to assess ability to perform skilled gestures. Children had to perform skilled gestures in the response to three different standardized, verbal prompts: verbal command (gesture to command, GTC), imitation of the examiner performing the gesture (gesture to imitation, GTI), and actual tool use in response to the tool being placed on the table (gesture with tool use, GTU). Each subject's examination was video recorded and later scored independently by two raters, both blinded to diagnosis. There were 25 GTC items, 34 GTI items, and 17 GTU items that were each evaluated for the occurrence of errors (See Dowell, et al., 2009 for a detailed summary of errors). Inter-rater reliability of at least 80% was achieved for each subject. Average total errors were used to assess praxis performance. For comprehensive descriptions of the modified praxis examination, scoring methodology, and reliability data, reference Mostofsky et al. (2006) and Dziuk et al. (2007).
Postural knowledge examination
A modified version, adapted for children from Mozaz et al. (2002), of a PKT was used to assess recognition of skilled gestures in others. Children had to recognize and identify skilled gestures in three ways. First, when presented with a drawing of a person with a missing hand performing a gesture involving the use of a tool (12 transitive gestures, i.e. hammering), the participant had to select from three drawings of hand positions and point to the hand that best showed how the tool should be held. Second, the child was presented with a drawing of a person with a missing hand performing a gesture that does not involve a tool (12 intransitive gestures, i.e. waving goodbye) and again had to choose from three options of hand positions and point to the hand that best depicted how the gesture should be performed. In the third section (ten items) of the PKT, three drawings of hands holding a tool were presented to the child and they were asked to point to the drawing that best demonstrated how the tool should be held. The number of correct responses from each section was totaled to arrive at an overall measure of postural, or representational, knowledge which will be referred to as `PKT total correct'.
Statistical Analysis
Statistical analyses were completed with Statistical Package for the Social Sciences 17.0 (SPSS; Chicago, IL). Group demographics were compared using univariate analysis of variance (ANOVA) and Chi-square as appropriate. Group differences between children with Asp and children with HFA and children with ADHD-C and ADHD-I on the PANESS, praxis examination, and PKT were also compared using ANOVA. A three-way multivariate analysis of variance (MANOVA) was used to assess multivariate effect of diagnosis for the three assessments (total score and TTRM from the PANESS, average total errors on the praxis examination, and PKT total correct). Additionally, in order to rule out the possible effects of small group differences in age and PRI, the MANOVA was rerun with these factors as covariates and the significance of effects remained. Next, an ANOVA along with post hoc single-step Tukey-HSD multiple comparisons of these variables were completed to compare each diagnostic groups' mean performance on each assessment with the other two groups. A two-tailed significance level of 0.05 was used as the standard for significance in all analyses.
Results
Demographic Information
The three groups of children (ASD, ADHD, and TD), did not differ from each other significantly with respect to age (F(2, 69) = 1.48, p = .23, η2 = .04), PRI (F(2, 69) = 2.13, p = .13, η2 = .06), SES (F(2, 67) = 2.17, p = .12, η2 = .06), gender (X2(2, N = 72) = 0.00, p = 1.00), race (X2(6, N = 72) = 8.92, p = .18), or handedness (X2(4, N = 72) = 3.03, p = .55).
Comparison of Children within ASD Group: Asp and HFA
Children diagnosed with Asp and children diagnosed with HFA demonstrated similar performance on the PANESS (total score: F(1, 22) = 1.97, p = .18, η2 = .08; TTRM: F(1, 22) = 1.91, p = .18, η2 = .08), praxis examination (F(1, 22) = 1.16, p = .29, η2 = .05), and PKT (F(1, 22) = 1.34, p = .26, η2 = .06). These results support past literature (Dowell et al., 2009; Jansiewicz et al., 2006; S.H. Mostofsky et al., 2006) and validate the decision to collapse both groups, Asp and HFA, into a single ASD group.
Comparison of Children within ADHD Group: ADHD-C and ADHD-I
Children diagnosed with ADHD-C and ADHD-I performed similarly on the PANESS total score (F(1, 22) = 1.10, p = .31, η2 = .05; ADHD-C: M = 30.25, SD = 8.66; ADHD-I: M = 34.50, SD = 10.74), PANESS TTRM (F(1, 22) = 1.23, p = .28, η2 = .05; ADHD-C: M = 35.41, SD = 5.15; ADHD-I: M = 38.55, SD = 8.79), praxis examination (F(1, 22) = 0.96, p = .34, η2 = .04; ADHD-C: M = 21.44, SD = 11.28; ADHD-I: M = 17.25, SD = 5.84), and PKT (F(1, 22) = 0.03, p = .88, η2 = .001; ADHD-C: M = 29.06, SD = 2.52; ADHD-I: M = 28.88, SD = 3.14).
Comparison Analyses between Diagnostic Groups: ASD, ADHD, and TD
A three-way MANOVA revealed a significant multivariate effect of diagnosis for the performance measures from all three behavioral assessments: PANESS total; PANESS TTRM; praxis average total errors; and PKT total correct (Pillai's Trace: p < .001, η2p = .28). Significant effects of diagnosis were also observed with univariate tests for each dependent variable (PANESS total: F = 18.52, p < .001, η2p = .35; PANESS TTRM: F = 10.77, p < .001, η2p = .24; praxis average total errors: F = 18.66, p < .001, η2p = .35; and PKT total correct: F = 6.35, p = .003, η2p = .16).
Basic motor control examination
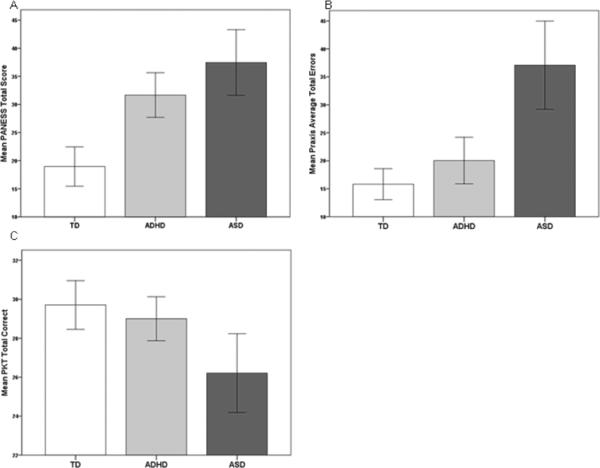
Post hoc analyses revealed at 95% confidence interval that children with ASD were significantly slower than TD children on PANESS TTRM (p < .001) and performed worse on the PANESS overall (p < .001). Children with ADHD also were significantly slower than TD children on PANESS TTRM (p = .003) and had a worse PANESS total score (p < .001). There were no significant differences between PANESS performance of children with ASD and children with ADHD, considering both the TTRM and total score (see Figure 1 and Table 1).
Figure 1.
Note. TD = typically developing; ADHD = attention deficithyper activity disorder; ASD = autism spectrum disorder. Error bars: 95% conficlnce interval. (A) Children with ASD and ADHD performed significantly worse than TD children on the PANESS (both p<0.001). (B) Children with ASD committed significantly more averege totle error on the praxis examination than TD and ADHD children (both p<0.001). (C) Children with ASD performed signicantly worse than TD and ADHD children on the PKT (p=0.003 and p=0.024, respectively.)
Table 1.
Group Differences
| TD (n = 24) |
ADHD (n = 24) |
ASD (n = 24) |
Significant Group Differences | |
|---|---|---|---|---|
| M(SD) | M(SD) | M(SD) | ||
| PANESS | ||||
| Total Score | 18.96 (8.31) | 31.67 (9.39) | 37.46 (13.82) | a***, b*** |
| Total Timed Repetitive | 30.43 (4.88) | 36.46 (6.57) | 38.32 (6.83) | a***, b** |
| Praxis Examination | ||||
| Total Average Errors | 15.81 (6.57) | 20.04 (9.87) | 37.08 (18.67) | a***, c*** |
| Postural Knowledge Test | ||||
| Total Correct | 29.71 (2.96) | 29.00 (2.67) | 26.21 (4.79) | a**, c* |
Note. TD = typically developing; ADHD = attention deficit hyperactivity disorder; ASD = autism spectrum disorder
ASD performed worse than TD
ADHD performed worse than TD
ASD performed worse than ADHD
p = 0.024
p = 0.003
p < 0.001
Praxis examination
Post hoc analyses showed at 95% confidence interval that children with ASD had significantly more average total errors during the praxis examination and therefore performed worse than TD children and children with ADHD (both comparisons p < .001). Children with ADHD and TD children did not exhibit significantly different levels of performance on the praxis examination (see Figure 1 and Table 1).
Postural knowledge examination
Post hoc analyses revealed at 95% confidence interval that children with ASD performed significantly worse than TD children (p = .003) and children with ADHD (p = .024) on the PKT. Children with ADHD and TD children did not demonstrate significantly different performance levels on the PKT (see Figure 1 and Table 1).
Discussion
Consistent with our hypothesis and prior studies, children with ASD exhibited impairments in basic motor control, postural knowledge, and praxis performance (Dewey et al., 2007; Dowell et al., 2009; Dziuk et al., 2007; S.H. Mostofsky et al., 2006). Additionally, this study revealed that impairments in the recognition and performance of skilled gestures are specific to children with ASD, while impairments in basic motor control may be a more generalized finding, as it was also observed in children with ADHD.
The current study sought to clarify and expand upon previous findings that have contributed to understanding developmental dyspraxia in ASD. Our finding that children with ASD, but not those with ADHD, exhibit impaired performance of skilled gestures is consistent with findings from Dewey, et al., 2007. The current results also support and extend our prior findings (Dowell et al., 2009) revealing that children with ASD are not only impaired in performance of skilled gestures, but also in their ability to recognize these gestures in others. In this study we not only confirmed that children with ASD show impaired gesture awareness (postural knowledge), we also provide evidence of specificity: Children with ADHD's performance on the PKT was equivalent to that of TD children, and was significantly better than that of children with ASD.
Our findings of autism-specific impairment in praxis and postural knowledge may have their basis in anomalous patterns of action model formation, the process by which one learns a novel action and its associated sensory feedback, and related abnormalities in neural connectivity particular to autism (Dowell et al., 2009; Haswell, Izawa, Dowell, Mostofsky, & Shadmehr, 2009; Steinman et al., 2010). Procedural learning and resulting action model formation is essential not only to development of skilled gestures, but also to developing perceptual representation of those actions (Oztop, Kawato, & Arbib, 2006). Examination of processes underlying action model formation reveal that children with ASD show a distinctive pattern of motor learning, with a bias toward reliance on proprioceptive feedback from their own intrinsic body space, with relative discounting of visual feedback from the external world (Haswell et al., 2009). Furthermore, this bias toward proprioceptive feedback, mediated by closely connected anatomic regions within the primary sensorimotor cortex, was found to robustly predict impaired praxis (and imitation) in autism as well as the core social features of the disorder (Haswell et al., 2009). This further supports our conclusions drawn in Dowell et al., 2009, that the combined autism-associated deficits in praxis and postural knowledge, may be the consequence of down-regulation of inferior parietal-premotor connections necessary to visual-motor learning, including “mirror neuron” processes involving motor imitation (Hwang & Shadmehr, 2005; Scott, Sergio, & Kalaska, 1997). These same connections are also necessary to downstream processes for maintaining the stored perceptual-motor representations of these actions, as assessed on praxis examination (Dowell et al., 2009; Halsband & Lange, 2006; Heilman & Gonzalez Rothi, 2003; Steinman et al., 2010).
As in all investigations, there are limitations of the study design to consider. Our group of children with ASD was composed of high functioning children, meaning they were of at least average intelligence as assessed by IQ. This may limit the interpretation of the current findings across the entire autism spectrum. We were not able to include children with developmental coordination disorder (DCD) or DCD/ADHD. Including these diagnostic groups in future studies would help clarify differences among neurodevelopmental disorders. Another potential limitation of our study is that we did not specifically assess visual abilities. There was no evidence of visual perception impairment in any participants, but since parts of the tasks rely on vision, it is important to note that no vision assessments were completed. Our results would also be strengthened with increased numbers and a completely independent sample since our sample is not completely independent of our most recently published study, Dowell, 2009. Finally, improvements to the PKT could be made in future studies to make the gestures more child-appropriate since the current exam was developed for adults (Mozaz, Rothi, Anderson, Crucian, & Heilman, 2002) and increase the number of items to improve power.
The findings nevertheless, provide further evidence that autism is associated with impairments in both performance and understanding of skilled actions. The specificity of these impairments to children with ASD further suggests that anomalous formation of action models may contribute to patterns of motor and social skill impairment particular to autism. Furthermore, this specificity to ASD warrants further work examining genetic and imaging data, as this could help discriminate between meaningful, biologically relevant endophenotypes. Advancements in understanding developmental dyspraxia in autism could also have far-reaching therapeutic applications. Methods for altering patterns of skill learning in children with ASD, beginning at an early age, could not only lead to improved social interaction with more facile execution of communicative gestures and other social skills, it may also help advance children's ability to understand others' actions with resulting improvements in social cognition.
Acknowledgements
This research was supported in part by grants from the National Institutes of Health, R01 NS048527 (NINDS), R01 MH078160 (NIMH), and R01 MH085328 (NIMH), and Autism Speaks, grant 2506.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/neu
References
- Association, A. P. Diagnostic and statistical manual of mental disorders. Fourth Edition-Revised Washington, DC: 1994. [Google Scholar]
- Biederman J, Faraone SV, Lapey KA. Comorbidity of diagnosis in attention-deficit disorder. In: Weiss G, editor. Attention-Deficit Hyperactivity Disorder. W.B. Saunders; Philadelphia: 1992. pp. 335–360. [Google Scholar]
- Cole WR, Mostofsky SH, Larson JC, Denckla MB, Mahone EM. Age-related changes in motor subtle signs among girls and boys with ADHD. Neurology. 2008;71(19):1514–1520. doi: 10.1212/01.wnl.0000334275.57734.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK. Conners' Rating Scales - Revised. Multi-Health Systems Inc.; North Tonawanda, New York: 1997. [Google Scholar]
- DeMyer M, Alpern G, Barton S, DeMyer W, Churchill D, Hingtgen J, et al. Imitation in autistic, early schizophrenic, and non-psychotic subnormal children. Journal of Autism and Childhood Schizophrenia. 1972;2:264–287. doi: 10.1007/BF01537618. [DOI] [PubMed] [Google Scholar]
- Denckla MB. Revised neurological examination for subtle signs. Psychopharmacology Bulletin. 1985;21:773–779. [PubMed] [Google Scholar]
- Dewey D, Cantell M, Crawford SG. Motor and gestural performance in children with autism spectrum disorders, developmental coordination disorder, and/or attention deficit hyperactivity disorder. Journal of the International Neuropsychological Society. 2007;13(2):246–256. doi: 10.1017/S1355617707070270. [DOI] [PubMed] [Google Scholar]
- Dowell LR, Mahone EM, Mostofsky SH. Associations of postural knowledge and basic motor skill with dyspraxia in autism: Implication for abnormalities in distributed connectivity and motor learning. Neuropsychology. 2009;23(5):563–570. doi: 10.1037/a0015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale-IV. Guilford Press; New York: 1998. [Google Scholar]
- Dziuk MA, Gidley Larson JC, Apostu A, Mahone EM, Denckla MB, Mostofsky SH. Dyspraxia in autism: association with motor, social, and communicative deficits. Developmental Medicine and Child Neurology. 2007;49(10):734–739. doi: 10.1111/j.1469-8749.2007.00734.x. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Chen WJ, Milberger S, Warburton R, Tsuang MT. Genetic Heterogeneity in Attention Deficit Hyperactivity Disorder (ADHD): Gender, Psychiatric Comorbidity, and Maternal ADHD. Journal of Abnormal Psychology. 1995;104(2):334–345. doi: 10.1037/0021-843X.104.2.334. [DOI] [PubMed] [Google Scholar]
- Gidley Larson JC, Mostofsky SH. Motor Deficits in Autism. In: Tuchman R, Rapin I, editors. Autism: A Neurological Disorder of Early Brain Development. Mac Keith Press for the International Review of Child Neurology Series; London: 2006. pp. 231–247. [Google Scholar]
- Gonzalez Rothi LJ, Raymer AM, Heilman KM. Limb praxis assessment. In: Rothi LJG, Heilman KM, editors. Apraxia: the cognitive neuropsychology of action. Psychology Press; Hove, UK: 1997. pp. 61–74. [Google Scholar]
- Haas R, Townsend J, Courchesne E, Lincoln A, Schriebman L, Yeung-Courchesne R. Neurologic abnormalities in infantile autism. Journal of Child Neurology. 1996;11(2):84–92. doi: 10.1177/088307389601100204. [DOI] [PubMed] [Google Scholar]
- Hallett M, Lebiedowska MK, Thomas SL, Stanhope SJ, Denckla MB, Rumsey J. Locomotion of autistic adults. Archives of Neurology. 1993;50:1304–1308. doi: 10.1001/archneur.1993.00540120019007. [DOI] [PubMed] [Google Scholar]
- Halsband U, Lange RK. Motor learning in man: a review of functional and clinical studies. Journal of Physiology - Paris. 2006;99(4–6):414–424. doi: 10.1016/j.jphysparis.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Haswell CC, Izawa J, Dowell LR, Mostofsky SH, Shadmehr R. Representation of internal models of action in the autistic brain. Nature Neuroscience. 2009;12(8):970–972. doi: 10.1038/nn.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman KM, Gonzalez Rothi LJ. Apraxia. In: Heilman KM, Valenstein E, editors. Clinical Neuropsychology. Fourth Edition Oxford University Press; New York: 2003. pp. 215–235. [Google Scholar]
- Hollingshead AB. Four factor index of social status. Yale University, Department of Sociology; New Haven, CT: 1975. [Google Scholar]
- Hwang EJ, Shadmehr R. Internal models of limb dynamics and the encoding of limb state. Journal of Neural Engineering. 2005;2(3):S266–278. doi: 10.1088/1741-2560/2/3/S09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansiewicz E, Goldberg MC, Newschaffer CJ, Denckla MB, Landa RJ, Mostofsky SH. Motor signs distinguish children with high functioning autism and Asperger's syndrome from controls. Journal of Autism and Developmental Disorders. 2006;36(5):613–621. doi: 10.1007/s10803-006-0109-y. [DOI] [PubMed] [Google Scholar]
- Jones V, Prior M. Motor imitation ability and neurological signs in autistic children. Journal of Autism and Developmental Disorders. 1985;15:37–46. doi: 10.1007/BF01837897. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EHJ, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- MacNeil LK, Xavier P, Garvey MA, Gilbert DL, Ranta ME, Denckla MB, et al. Quantifying excessive mirror overflow in children with attention-deficit/hyperactivity disorder. Neurology. 2011;76(7):622–628. doi: 10.1212/WNL.0b013e31820c3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Dubey P, Jerath VK, Jansiewicz EM, Goldberg MC, Denckla MB. Developmental dyspraxia is not limited to imitation in children with autism spectrum disorders. Journal of the International Neuropsychological Society. 2006;12:314–326. doi: 10.1017/s1355617706060437. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Newschaffer CJ, Denckla MB. Overflow movements predict impaired response inhibition in children with ADHD. Perceptual and Motor Skills. 2003;97(3 Pt 2):1315–1331. doi: 10.2466/pms.2003.97.3f.1315. [DOI] [PubMed] [Google Scholar]
- Mottron L. Matching strategies in cognitive research with individuals with high-functioning autism: current practices, instrument biases, and recommendations. Journal of Autism and Developmental Disorders. 2004;34(1):19–27. doi: 10.1023/b:jadd.0000018070.88380.83. [DOI] [PubMed] [Google Scholar]
- Mozaz M, Rothi LJ, Anderson JM, Crucian GP, Heilman KM. Postural knowledge of transitive pantomimes and intransitive gestures. Journal of the International Neuropsychological Society. 2002;8(7):958–962. doi: 10.1017/s1355617702870114. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oztop E, Kawato M, Arbib M. Mirror neurons and imitation: a computationally guided review. Neural Networks. 2006;19(3):254–271. doi: 10.1016/j.neunet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Rapin I. Autistic children: Diagnosis and clinical features. Pediatrics. 1991;87:751–760. [PubMed] [Google Scholar]
- Reich W, Welner Z, Herjanic B. The Diagnostic Interview for Children and Adolescents-IV. Multi-Health Systems; North Tonawanda: 1997. [Google Scholar]
- Ritvo A, Provence S. Form perception and imitation in some autistic children. Psychoanalytic Study of the Child. 1953;8:155–161. [Google Scholar]
- Rogers S, Bennetto L, McEvoy R, Pennington B. Imitation and pantomime in high-functioning adolescents with autism spectrum disorders. Child Development. 1996;67:2060–2073. [PubMed] [Google Scholar]
- Scott SH, Sergio LE, Kalaska JF. Reaching movements with similar hand paths but different arm orientations. II. Activity of individual cells in dorsal premotor cortex and parietal area 5. Journal of Neurophysiology. 1997;78(5):2413–2426. doi: 10.1152/jn.1997.78.5.2413. [DOI] [PubMed] [Google Scholar]
- Steinman KJ, Mostofsky SH, Denckla MB. Toward a narrower, more pragmatic view of developmental dyspraxia. Journal of Child Neurology. 2010;25(1):71–81. doi: 10.1177/0883073809342591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilensky JA, Damasio AR, Maurer RG. Gait disturbance in patients with autistic behavior. Archives of Neurology. 1981;38:646–649. doi: 10.1001/archneur.1981.00510100074013. [DOI] [PubMed] [Google Scholar]
- Wechsler DL. Wechsler Intelligence Scale for Children. Fourth Edition The Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]