Abstract
The objective of the present study is to elucidate the feasibility of surgical maneuvers under the side-viewing endoscope during skull base tumor removal. The study focused on 51 patients who underwent tumor removal with the assistance of a side-viewing endoscope. The side-viewing endoscope enabled visualization and removal of residual tumors obscured by the skull base bone, cranial nerves, and other vital structures after a microscopic procedure. If the surgical field is surrounded by the dura or skull base tissue, not only curettage of a tumor but also semisharp dissection and bipolar coagulation are shown to be feasible. In the subarachnoid space, however, the primary feasible surgical maneuver was suctioning of the tumor. The extent of skull base resection could be reduced in 25 cases and additional tumor removal became possible in 47 cases. Application of the side-viewing endoscope enabled removal of the tumor compartment, the exposure of which has conventionally required an extensive skull base resection. This technique is a promising option for the treatment of skull base tumors.
Keywords: Side-viewing endoscope, skull base tumor, microsurgical maneuver, malleable instrument
The scope of endoscope-assisted microsurgery for treating lesions in the neurosurgical field is gradually widening. Surgical maneuvers under an endoscope are generally performed under a straight or slightly curved view of the endoscope with regular microsurgical instruments.1,2,3,4,5 A significant characteristic of the endoscope is, however, the feasibility of looking around corners with side-viewing lenses to circumvent certain obstacles. If sophisticated surgical maneuvers are feasible via such views, the surgical strategy for skull base lesions may be altered, providing alternatives in cases where an extended skull base resection would otherwise be mandatory. Therefore, we have performed microsurgical maneuvers using a side-viewing endoscope with currently available malleable instruments. Surgical situations were roughly categorized into four groups, and the surgical maneuvers that were feasible in each situation were elucidated.
METHODS
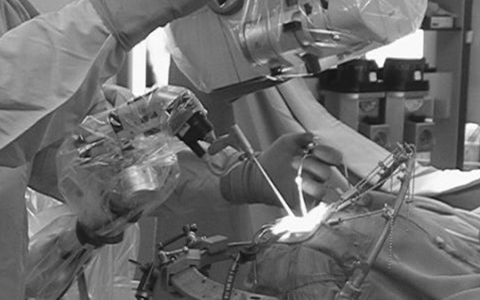
Fifty-four procedures on fifty-one skull base tumors of various types were performed between January 2003 and September 2008 whereby a major portion of the tumor was removed under the microscopic view and the residual compartment was obscured by the skull base tissue and required the use of the side-viewing endoscope (Table 1). Surgeries that were predominantly performed under a straight-view endoscope and in which the side-viewing endoscope was used only for observation were excluded. Each case of clival chordoma, dermoid in Meckel's cave, and schwannoma in the carotid canal was operated upon twice. Bayonet-shaped rigid endoscopes with a diameter of 2.7 or 4.0 mm and a viewing direction of 30 or 70 degrees were used (Aesculap Co., Tuttlingen, Germany; Olympus Optical Co., Tokyo, Japan). In the early cases, the endoscope was fixed on a holder equipped with a special platform enabling tiny adjustment of the endoscope position within the surgical field (Unitrak and Neuropilot; Aesculap Co.). Since May 2005, an integrated endoscope-holding system (EndoArm; Olympus Optical Co.) was introduced. The insertion of the endoscope and surgical instruments was monitored each time under the microscope. Basically, a bimanual maneuver was performed under the side-viewing endoscope. The endoscopic picture was displayed either on a separate monitor, which was set in front of the surgeon, or within the microscopic ocular using the picture-in-picture system. The positional relationship of the surgical field, endoscope, and microscope is shown in Fig. 1.
Table 1.
Summary of 51 Surgical Cases and Surgical Maneuvers under the Side-Viewing Endoscope
| Pathology and Surgical Maneuver | Category of the Endoscopic Procedure | No. of Cases |
|---|---|---|
| Schwannoma (vestibular) | ||
| Fundus inspection and additional tumor removal | 2 | 12 |
| Schwannoma (jugular foramen) | ||
| Additional removal of the intraforaminal portion | 2 | 3 |
| Pituitary adenoma/Rathke's cyst/craniopharyngioma | ||
| Tumor removal along the cavernous sinus and diaphragma sellae | 1 | 15 |
| Meningioma (tuberculum sellae) | ||
| Additional tumor removal from the optic canal and tuberculum sellae | 2 | 4 |
| Chordoma (clivus) | ||
| Tumor removal through the transsphenoidal/transoral/transbasal route | 1 | 4 |
| Meningioma (petroclival)/adenoid cystic carcinoma (CP angle) | ||
| Tumor removal from Meckel's cave | 2 | 2 |
| Dermoid/epidermoid cyst/amyloidoma | ||
| Tumor removal behind the cranial nerves/cerebral peduncle | 4 | 7 |
| Schwannoma (extracranial compartment) | ||
| Tumor removal beyond the bony hindrance of the skull base bone | 3 | 4 |
| Total | 51 |
Figure 1.
Photo demonstrating intraoperative setting of the endoscope (EndoArm; Olympus Optical Co., Tokyo, Japan). The endoscope is fixed between the microscope and the patient so that the movement of the endoscope and surgical instruments can be monitored under the microscope.
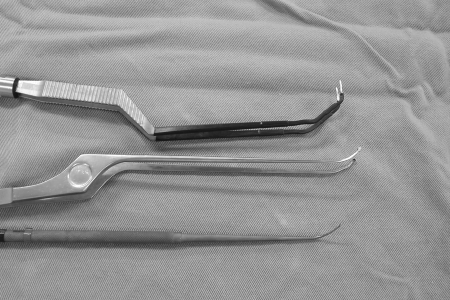
The malleable dissector and bipolar and tumor forceps used were manufactured by Fujita Medical Instruments Co. (Tokyo, Japan). A conventional sucker, which can be bent, was also used. The basic configuration of these instruments was the same as that of conventional ones, but a certain length of the tip was composed of silver for the bipolar forceps, titanium alloy for the dissectors, and stainless steel for tumor forceps so that the tip could be bent in the required direction (Fig. 2).
Figure 2.
Photo of the malleable bipolar, tumor forceps, and dissector developed for the surgical maneuver under the side-viewing endoscope. The length of the tip can be bent arbitrarily for desired angle and direction.
The situations in which surgical maneuvers under the side-viewing endoscope were effective were rather limited. We roughly categorized them into four groups according to the type of surgical maneuver that was feasible, which was mainly determined by the vulnerability of the surrounding structures. These groups were as follows: (1) removal of intra- and parasellar tumor, (2) removal of tumor extending into the neural foramina, (3) removal of tumor extending into the extracranial space beyond the skull base bone, and (4) removal of tumor in the subarachnoid space.
RESULTS
Category 1
During intra- and parasellar tumor surgery, the side-viewing endoscope enabled visualization of the medial wall of the cavernous sinus laterally and that of the diaphragma sellae upward. With the 70-degree endoscope fixed at one corner of the dural opening, the contralateral corner was clearly visualized. Although the boundary of the surgical field was composed of rather vulnerable structures, such as the inner wall of the cavernous sinus laterally and the arachnoid membrane superiorly, clear visualization of the size and location of the residual tumor enabled safe curettage and dissection. Tearing off of the tumor or sharp cutting, however, risked injuring the membranes and should generally be avoided.
Category 2
During the removal of tumor extending into the neural foramina, the residual tumor in the depth of the foramen and behind the cranial nerves could be well visualized. Additional bone resection was circumvented, and retraction of the vital structures such as cranial nerves could be avoided. As the margin of the tumor and the cranial nerve were clearly displayed, the dissector could be inserted precisely between them and its movement controlled without exerting excessive force on the cranial nerve. As the area was surrounded with the skull base bone and the dura, relatively rough surgical maneuvers such as semisharp dissection with a dissector were possible. If the cranial nerve was not in close proximity, coagulation with the malleable bipolar forceps was also feasible.
Category 3
During the removal of the tumor extending into the extracranial space, the side-viewing endoscope was inserted into the skull base defect created by the lesion itself, and additional incision in the cervical region was not necessary. Beside curettage and dissection, coagulation and evaporation of the tumor with high-frequency cauterization was feasible as the structures surrounding the tumor were extracranial soft tissues that lacked vulnerable components. An occasional limitation during this procedure was the inability to clearly distinguish the tumor from the surrounding soft tissue.
Category 4
During the tumor removal in the subarachnoid space, a wide subarachnoid area could be inspected with regular-sized craniotomy. The tumor compartment's full depth and that behind the neural tissue could be sucked out with the bent suction tube. The present range of surgical instruments, however, did not allow performance of precise coagulation of tiny vessels under the side-viewing endoscope even with the use of the malleable bipolar forceps. Thus, this type of surgery is mainly suggested for soft, less vascularized tumors such as dermoid and epidermoid tumors.
There were two cases of visual acuity impairment after removal of the tuberculum sellae meningioma, both of which exhibited gradual recovery during the postoperative course. Mechanical stress on the optic nerve generated by inadvertent movement of the surgical instrument under the side-viewing endoscope was considered to be a major cause of this. Continuous irrigation is considered to be mandatory when using the endoscope close to vital structures, as heat may otherwise become an additional cause of injury.6 Although we irrigated the endoscope tip frequently during the surgery, the possibility of heat injury in these cases cannot be completely excluded. No other morbidity or mortality relating to the endoscopic procedure was encountered.
The extent of skull base resection was reduced in 25 cases of categories 2 and 3, as the residual tumor could be managed without additional bone removal. The tumor removal rate was increased in 47 cases of categories 1, 2, and 4, as the residual tumor compartment that was difficult to expose even with additional skull base bone resection could be visualized and removed under the side-viewing endoscope.
ILLUSTRATIVE CASE
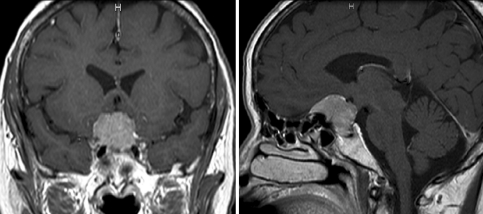
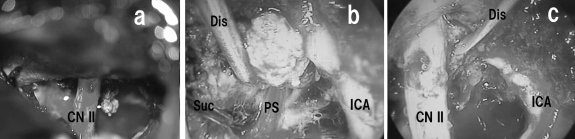
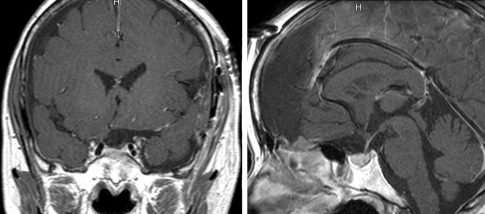
A 66-year-old woman was diagnosed with a meningioma at the tuberculum sellae by magnetic resonance imaging (MRI) performed during a brain check-up (Fig. 3). The preoperative neurological examination results were normal. The patient underwent tumor extirpation under the right subfrontal approach. The major suprasellar compartment was removed under the microscopic view (Fig. 4a) and the residual compartment, which was obscured by the anterior skull base bone and optic nerve, was removed under the endoscope without additional removal of the anterior clinoid process or opening of the optic canal. The tumor attached to the diaphragma sellae could be clearly visualized with the endoscope with a 70-degree viewing direction (EndoArm) and was dissected safely from the pituitary stalk (Fig. 4b). As the endoscope was firmly fixed, bimanual maneuver was feasible. The tumor extending into the optic canal could also be removed under endoscopic control with the malleable dissector, of which ~15 mm of the tip was bent at a right angle (Fig. 4c). Finally, the tumor attachment at the tuberculum sellae was coagulated with the malleable bipolar. The postoperative MRI showed total removal of the tumor (Fig. 5). The patient developed no complications besides deterioration of her right visual acuity, which then gradually recovered.
Figure 3.
Preoperative coronal (left) and sagittal (right) magnetic resonance images of the patient demonstrating a meningioma at the tuberculum sellae. Note the steep angle of the anterior wall of the sella turcica.
Figure 4.
Intraoperative photographs after removal of the tumor under the microscope (a) during the dissection of the tumor from the pituitary stalk (b) and from the optic nerve (c) under the side-viewing endoscope. Note the bimanual maneuver under the endoscope in Fig. 4b. CN II, optic nerve; Dis, dissector; ICA, internal carotid artery; PS, pituitary stalk; Suc, suction tube.
Figure 5.
Postoperative coronal (left) and sagittal (right) magnetic resonance imaging demonstrates gross total removal of the tumor.
DISCUSSION
The use of endoscopes in neurosurgery has increased gradually in the last decades. This has been supported by technological advancements of the endoscope itself as well as that of its supporting equipment such as monitors and holding systems. The term endoscope-assisted microsurgery, introduced by Perneczky and Fries, includes any microsurgical maneuver accomplished with endoscopic assistance.7 Compared with endoscopic sinus surgeries performed in a single-hand fashion by an otorhinolaryngologist, the concept encompasses procedures involving fixing of the endoscope to a special holder to make bimanual maneuvers feasible. The development of a special platform and integrated holding system has contributed much to the advancement of this concept. The latter in particular enables endoscope movement and fixation in a gravity-free manner, so that frequent insertion, fixation, and removal of the endoscope is now far less stressful to the surgeon, which has resulted in enhanced safety.8
Thanks to the side-viewing capability of the endoscope, one can look around corners that are blind in the microscopic view without additional bone resection or retraction of surrounding vital structures. This enables the recognition and management of lesions that remain behind obstacles after a microscopic procedure.9 There are already several reports about the use of the side-viewing endoscope during skull base surgery. The maneuvers in these reports are, however, mostly limited to observation or simple curettage of the tumor.1,6,10,11,12 Bimanual surgical maneuvers similar to those performed under the surgical microscope, such as dissection, holding, and coagulation of a tumor, are desired to fully exploit the potential of the side-viewing endoscope. To perform a sophisticated surgical maneuver under an angled view of the side-viewing endoscope, however, the availability of a set of instruments suited for this purpose is essential. The ideal version of such instruments should have the capability to be bent in any direction, while maintaining maneuverability. A form of robotic forceps would be the ultimate answer.13 The development of such forceps is, however, still in progress, and it will be some time before they become available in a clinical setting. We therefore considered the malleable form of conventional instruments as a tentative substitute. As demonstrated, malleable dissector and tumor and bipolar forceps enabled rough dissection and holding of the tumor, and even hemostasis under the side-viewing endoscope. We actually observed an increased tumor removal rate as the complete range of malleable instruments required gradually became available. This was especially evident in the case of recurrent tumors, in which various surgical maneuvers became possible in the second surgery owing to the development of malleable instruments. During the time course, the endoscopic surgical maneuvers shifted from simple single-hand curettage to bimanual maneuvers similar to those under a microscope. However, there are still several issues to be resolved for this type of surgery to become widely accepted. With the present instruments, precise hemostasis, which is mandatory during maneuvers in the subarachnoid space, is not feasible. The lack of cutting instruments is another important problem to be solved. As malleable scissors are structurally not possible to manufacture, another method of cutting has to be invented.
The kind of surgical maneuver that is feasible under the endoscope is also defined by the vulnerability of the surrounding structures to which force is exerted. We have roughly categorized the surgical situations into four groups according to the vulnerability of the surrounding structures and analyzed the groups separately. In the sellar region, the cavernous sinus medial wall and arachnoid membrane are prone to injury during rough dissection. Thus, surgical maneuvers should mainly be restricted to gentle curettage. During tumor removal from the neural foramina, however, relatively rough dissection is feasible as the surrounding structures are skull base bone or dura. This also applies for cases of tumors extending beyond the skull base into the extracranial space. As the surrounding structures are the extracranial soft tissue, one can dissect, coagulate, and sometimes even evaporate the tumor. In contrast, the accuracy of presently feasible surgical maneuvers in the subarachnoid space under the side-viewing endoscope is inferior to that performed under the surgical microscope. Until precise hemostasis and cutting become feasible, the primary procedure must be restricted to suctioning of a soft tumor with a sucker. When performing surgery under the side-viewing endoscope, it is important for the surgeon to be aware of the above-mentioned limitations.
We experienced two cases of postoperative visual acuity impairment in patients harboring meningioma at the tuberculum sellae. This can be attributed to the inadvertent movement of surgical instruments during the maneuver under the side-viewing endoscope. Hand-eye coordination under such circumstances is rather difficult to control, and the need to switch the surgeon's gaze from the microscopic ocular to the endoscope monitor is an additional factor that complicates the maneuver. There are already several microscopes available that are equipped with the picture-in-picture system, but the endoscopic image displayed in the microscopic ocular is generally not clear enough to rely upon. To overcome these problems, a systematic training system including the use of a simulator should be developed, and a microscope with a simultaneous display of both microscopic and endoscopic images with satisfactory quality is desired.
The usefulness of the endoscope in skull base surgery should be evaluated from two distinct perspectives: one is in reducing the surgical invasiveness of the conventional approach with the endoscope14 and the other is in accomplishing surgical procedures that have conventionally been almost impossible to execute with a microscope alone.15 Compared with the straight-view endoscope, the side-viewing endoscope is more promising in the latter point. In the cases analyzed in the present study, approach-related invasiveness could be reduced mainly in categories 2 and 3 by reducing the size of the craniotomy and avoiding additional bone removal around the neural foramina. Not only the reduction in skull base resection, but also the elimination of the retraction of vital structures, was a major advantage. In categories 1, 2, and 4, surgical procedures that are almost impossible to execute under a conventional surgical microscope became feasible, as the tumor compartment that is difficult to expose even with additional skull base bone resection could be visualized and removed under the side-viewing endoscope. These procedures included visualization and management of the residual tumor compartment along the cavernous sinus and diaphragma sellae, and behind the cranial nerves and brain parenchyma. As a result, the tumor removal rate may have increased. Although several difficulties are still to be overcome, the present results indicate that the technique has the possibility of providing a novel surgical strategy for the treatment of skull base lesions. It is hoped that this technique will become one of the most valuable options available during skull base surgery.
CONCLUSION
Application of the side-viewing endoscope during skull base surgery enabled the surgeon to observe lesions behind various skull base tissues, which would only be possible at the expense of extended skull base resection under a microscope. With special malleable instruments, lesions can also be treated under the endoscopic view. As the full range of malleable instruments has become available, the variety of feasible surgical maneuvers has increased. Hand-eye coordination under the side-viewing endoscope is, however, rather difficult to control. Therefore, the importance of systematic education and practice cannot be overemphasized. Although several issues are still to be resolved, this technique has the possibility of providing a novel surgical strategy for the treatment of skull base lesions.
ACKNOWLEDGMENT
This study was supported partly by a grant from the Japanese Foundation for Research and Promotion of Endoscopy.
References
- Charalampaki P, Kafadar A M, Grunert P, Ayyad A, Perneczky A. Vascular decompression of trigeminal and facial nerves in the posterior fossa under endoscope-assisted keyhole conditions. Skull Base. 2008;18:117–128. doi: 10.1055/s-2007-1003927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Divitiis E, Cappabianca P, Cavallo L M. Endoscopic transsphenoidal approach: adaptability of the procedure to different sellar lesions. Neurosurgery. 2002;51:699–705. discussion 705–707. doi: 10.1097/00006123-200209000-00016. [DOI] [PubMed] [Google Scholar]
- Gardner P A, Kassam A B, Thomas A, et al. Endoscopic endonasal resection of anterior cranial base meningiomas. Neurosurgery. 2008;63:36–52. discussion 52–54. doi: 10.1227/01.NEU.0000335069.30319.1E. [DOI] [PubMed] [Google Scholar]
- Jho H D, Carrau R L. Endoscopic endonasal transsphenoidal surgery: experience with 50 patients. J Neurosurg. 1997;87:44–51. doi: 10.3171/jns.1997.87.1.0044. [DOI] [PubMed] [Google Scholar]
- Menovsky T, Grotenhuis J A, de Vries J, Bartels R H. Endoscope-assisted supraorbital craniotomy for lesions of the interpeduncular fossa. Neurosurgery. 1999;44:106–110. discussion 110–112. doi: 10.1097/00006123-199901000-00062. [DOI] [PubMed] [Google Scholar]
- Schroeder H W, Oertel J, Gaab M R. Endoscope-assisted microsurgical resection of epidermoid tumors of the cerebellopontine angle. J Neurosurg. 2004;101:227–232. doi: 10.3171/jns.2004.101.2.0227. [DOI] [PubMed] [Google Scholar]
- Perneczky A, Fries G. Endoscope-assisted brain surgery: part 1—evolution, basic concept, and current technique. Neurosurgery. 1998;42:219–224. discussion 224–225. doi: 10.1097/00006123-199802000-00001. [DOI] [PubMed] [Google Scholar]
- Morita A, Okada Y, Kitano M, Hori T, Taneda M, Kirino T. Development of hybrid integrated endoscope-holder system for endoscopic microneurosurgery. Neurosurgery. 2004;55:926–931. discussion 931–932. doi: 10.1227/01.neu.0000139490.72543.27. [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Kato A, Taki T, et al. Endoscope assisted removal of jugular foramen schwannoma; report of 3 cases. Minim Invasive Neurosurg. 2005;48:365–368. doi: 10.1055/s-2005-915631. [DOI] [PubMed] [Google Scholar]
- Cappabianca P, Cavallo L M, de Divitiis E. Endoscopic endonasal transsphenoidal surgery. Neurosurgery. 2004;55:933–940. discussion 940–941. doi: 10.1227/01.neu.0000137330.02549.0d. [DOI] [PubMed] [Google Scholar]
- Hori T, Okada Y, Maruyama T, Chernov M, Attia W. Endoscope-controlled removal of intrameatal vestibular schwannomas. Minim Invasive Neurosurg. 2006;49:25–29. doi: 10.1055/s-2006-932125. [DOI] [PubMed] [Google Scholar]
- Tatagiba M, Matthies C, Samii M. Microendoscopy of the internal auditory canal in vestibular schwannoma surgery. Neurosurgery. 1996;38:737–740. [PubMed] [Google Scholar]
- Hongo K, Kobayashi S, Kakizawa Y, et al. NeuRobot: telecontrolled micromanipulator system for minimally invasive microneurosurgery-preliminary results. Neurosurgery. 2002;51:985–988. discussion 988. doi: 10.1097/00006123-200210000-00024. [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Perneczky A. Subtemporal keyhole approach to the suprasellar and petroclival region: microanatomic considerations and clinical application. Neurosurgery. 1997;41:592–601. doi: 10.1097/00006123-199709000-00017. [DOI] [PubMed] [Google Scholar]
- Morita A, Shin M, Sekhar L N, Kirino T. Endoscopic microneurosurgery: usefulness and cost-effectiveness in the consecutive experience of 210 patients. Neurosurgery. 2008;62(Suppl 2):607–613. doi: 10.1227/01.neu.0000316264.59596.c5. [DOI] [PubMed] [Google Scholar]