Abstract
Internal carotid artery (ICA) injury is a dramatic complication of endonasal skull base approaches with massive bleeding. This study aims to design an animal model of ICA injury during endonasal skull base surgery. Eight sheep underwent ICA isolation followed by arterial pressure monitoring and placement of a rapid infuser. The Sinus Model Otorhino Neuro Trainer (Pro Delphus, Pernambuco, Brazil) nasal model was then modified. A novel posterior sphenoid wall was created, allowing the artery to be placed within and fixed to the model in a watertight fashion. A diamond-tipped bur allowed surgical exposure of the carotid artery. A standardized injury was created endoscopically. The “two-surgeon technique” allowed local packing measures to be performed. Outcome measures were mean arterial pressure (MAP) following injury, resuscitation fluid volume, survival time, and total blood loss. Mean preinjury weight was 51.8 ± 4.59 kg. All baseline hematologic parameters fell within normal limits. The mean preinjury and postinjury MAP was 65.7 ± 9.3 mm Hg versus 39.1 ± 6.9 mm Hg, respectively. The mean survival time was 50.25 ± 17.89 minutes, with mean resuscitation fluid volume of 10.89 ± 2.40 L and mean blood loss of 4943 ± 1089 mL. This model replicates the endoscopic surgical field of an ICA injury, with the potential to train endoscopic skull base teams in the skills require to manage an ICA injury.
Keywords: Endonasal surgery, carotid, packing, hemostasis
Skull base surgery has undergone a dramatic change in the last decade with the advent of improved technological developments and surgical instrumentation and an improved understanding of the endonasal skull base anatomy. This had led to the introduction of the expanded fully endoscopic endonasal skull base approaches.1 Endonasal surgical techniques have several advantages to their more tradition open approaches including the avoidance of external skin incisions, minimal sacrifice of intervening structures, improved visualization, reduced postoperative pain, and shorter hospital admission times.2 These advantageous have led to endonasal approaches rapidly becoming the standard of care for pituitary and other skull base tumors by both otolaryngologists and neurosurgeons worldwide.3
Endonasal skull base surgery was first introduced in 1961, and since then surgeons have considered internal carotid artery (ICA) injury the most dramatic complication of skull base surgery. ICA injury creates an immediately challenging surgical field, which may result in death of the patient. Although ICA injury during endoscopic sinus surgery is a relatively rare event, its frequency in endonasal skull base surgery is more significant. Ciric et al sent a questionnaire to 3172 neurosurgeons regarding the complications of transsphenoidal pituitary surgery. Results demonstrated that 52% of surgeons who had performed more than 500 endonasal pituitary approaches had experienced an ICA injury.4 More advanced surgical approaches have a higher incidence of ICA rupture. Four separate consecutive series of extended endonasal resections show an incidence of ICA injury of between 4% and 9%.5,6,7,8 These data demonstrate that increasing expertise and experience in endonasal skull base surgery and the increasingly challenging surgical pathologies encountered mean that it is likely that all specialist endonasal skull base surgeons will need to manage an ICA injury at some stage.
A review of the literature demonstrates that there is a lack of information with regards to the appropriate techniques and protocols in managing an ICA injury during endonasal surgery. Some authors advise that a hypotensive state should be avoided to maintain collateral cerebral perfusion9,10; however, others advise that a controlled hypotensive technique should be implemented,11 with the addition of carotid compression on those that fail.10,11 Weidenbecher et al advise immediate bilateral common carotid compression to maintain a surgical view.12 There are also conflicting reports regarding the best intervention for achieving hemostasis control. Although nasal packing is the most frequent technique employed, there are also case reports describing the use of bipolar diathermy, the muscle patch, and the use of a thrombin/gelatin matrix.9,12,13 An angiographic review of ICA injuries treated with nasal packing showed that 8 of the 12 patients had complete occlusion of the carotid, with a further patient suffering from occlusion of the middle cerebral and basilar artery. Another 4 of the 12 patients suffered from carotid stenosis. The authors concluded that “overpacking” contributes to the morbidity and mortality of the patient.14 Once the hemorrhage is controlled, many patients are transferred for immediate endovascular stenting or embolization; however, it is unclear which patients are suitable for this. Delayed complications include secondary hemorrhage, pseudoaneurysm formation, and carotid-cavernous fistula; however, the incidence of these complications remains unknown. Laws suggests that virtually all ICA injuries repaired by indirect measure will develop a pseudoaneurysm requiring endovascular embolization.15
To allow for further prospective scientific investigation of the management options and complications of an ICA injury, an animal model is needed. This model needs to be a large-animal model that recreates the hemodynamic similarities with the patient, creating a high-flow and high-pressure style injury. It needs to maintain the challenging anatomic constraints of the human nasal vestibule and nasal cavity. Additionally, the model needs to replicate the variable boney exposure that may be encountered during an unexpected vascular injury. Currently, there is no such model that can reproducibly recreate this challenging surgical scenario. The aim of this study is to design an animal model of ICA injury during endonasal skull base surgery.
METHODS
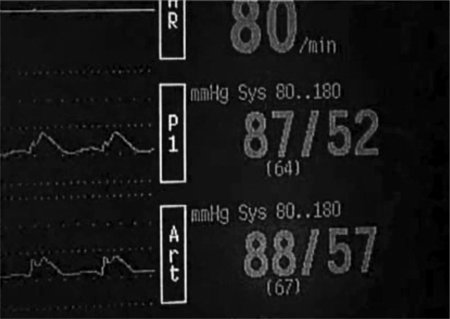
All sheep were weighed and underwent coagulation profiling and a full blood examination prior to general anesthesia. Animals were fed a standard diet and observed for 3 days prior to surgery to ensure a good state of health. All sheep were fasted 12 to 18 hour before surgery with free access to water. Induction of general anesthesia was performed via injection with sodium thiopentone (19 mg/kg body weight) into the left jugular vein. Endotracheal intubation then followed with anesthesia maintained by inhalation of 1.5 to 2.0% isoflurane, to a depth that allowed spontaneous ventilation. The sheep were positioned on their backs, and a midline neck incision was performed from the thyroid cartilage to the base of the neck, extending down to the superficial layer of the deep cervical fascia. The fascia was incised, and dissection continued to the anterior tracheal wall. The visceral fascia was then dissected from the lateral tracheal walls to reveal the right carotid sheath. The sheath was then incised and the carotid artery dissected free for a length of 15 cm from the angle of the mandible to the base of the neck. The left carotid artery was also identified as described above. Both carotid arteries were cannulated at the level of the mandible to allow for continuous invasive arterial pressure monitoring bilaterally. The left internal jugular vein was identified by dissection posterior to the left sternocleidomastoid muscle and then cannulated with a rapid infusion catheter exchange set (Arrow International Inc., Reading, PA) to allow for rapid fluid resuscitation (Fig. 1).
Figure 1.
Continuous bilateral invasive carotid arterial pressure monitoring ensuring no compression of vessel on entry/exit through model.
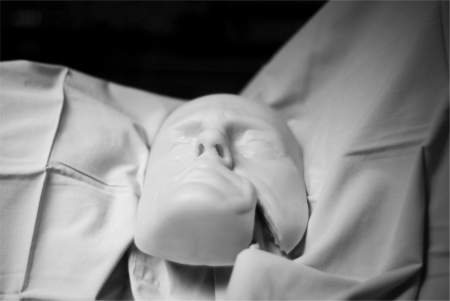
The Sinus Model Otorhino Neuro Trainer (SIMONT, Pro Delphus, Pernambuco, Brazil) was chosen to simulate the endoscopic environment so that the carotid injuries could be managed with the anatomic limitations and confines seen in the human nasal vestibule, nasal cavity, and sphenoid sinus (Fig. 1). This model is a life-sized, anatomically accurate reconstruction of the nasal cavity and paranasal sinuses that uses a novel material called “surgical neoderma,” which recreates the colors, consistency, and elasticity of nasal mucosa and paranasal sinus boney architecture. Additionally, it allows the use of routine sinus and skull base surgical instrumentation and provides the realistic tissue resistances encountered during endonasal surgery. In this model, bilateral large sphenoidotomies and partial middle turbinectomies were performed, as is routinely performed during advanced skull base surgery. To place the artery in the sphenoid sinus, the model was modified and refashioned with the removal of the posterior wall of the sphenoid sinus. A plastic backing (0.5 mm thick) was then constructed, with a narrow channel to allow the placement of the freely dissected carotid artery. A novel detachable posterior sphenoid sinus wall was recreated. This was passed behind the left ICA and, along with the plastic backing, subsequently fixed by four fasteners to the posterior aspect of the SIMONT model, allowing a watertight seal. Absence of carotid compression on entry and exit of the carotid artery was confirmed visually and by observing no change in the mean arterial pressure (MAP between the left and right carotid arteries (Fig. 2). The model was then fixed to the operating table and onto the neck of the sheep to prevent displacement during intervention (Fig. 3).
Figure 2.
Sinus Model Otorhino Neuro Trainer (Pro Delphus, Pernambuco, Brazil) model placed at neck of sheep, and fixed to operating table.
Figure 3.
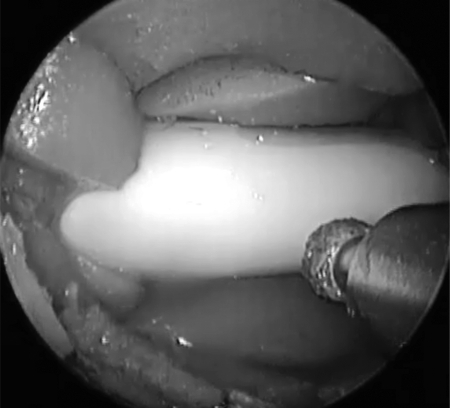
Diamond-tipped bur used to drill through plastic plate to reveal carotid artery.
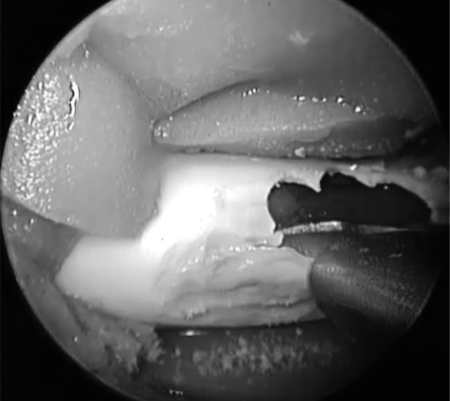
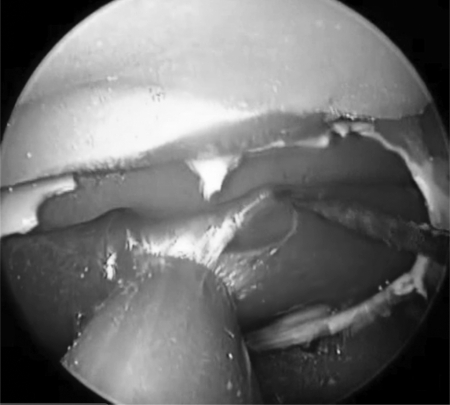
Using a 0-degree rigid endoscope, a 3-mm, 15-degree, diamond-tipped bur was then used to drill away the plastic plate, simulating the thin boney covering of the carotid siphon within the sphenoid sinus (Fig. 4). The Hajek punch was then used to expose the carotid artery, creating a boney window revealing the pulsatile carotid artery (Fig. 5). This allowed for the variable boney exposure that may be experienced during an unanticipated vascular event. An 11-blade scalpel was used to create an approximately 4-mm longitudinal incision through the anterior wall of the carotid artery. Immediately rapid bleeding occurred, obstructing the surgical view. To confirm a challenging and high-pressure injury, local packing was performed of the injury site only, ensuring that vascular flow was still maintained. This was confirmed by observing a pulse pressure on the invasive pressure monitor placed distal to the carotid injury site.
Figure 4.
Hajek punch used to remove thinned plastic plate to reveal carotid artery.
Figure 5.
Number 11 scalpel blade used to create the 4-mm carotid injury.
Simultaneous fluid resuscitation with warmed normal saline (Baxter, New South Wales, Australia) was commenced at 200 mL/min. Resuscitation was stopped once hemostasis was achieved and the MAP achieved its preinjury level. Aggressive simultaneous fluid resuscitation ensured a high-flow, high-pressure vascular injury model. A thermal blanket was used to ensure a constant temperature and prevent the adverse affects of hypothermia on the coagulation cascade. Specific outcome measures for this study were the preinjury and postinjury MAP despite rapid fluid resuscitation, the resuscitation fluid volume used, and survival time and total blood loss.
RESULTS
A total of eight sheep were used for validation of this animal model. The mean weight was 51.8 ± 4.59 kg. Baseline coagulation and hematologic parameters were similar for all animals with no significant difference between each animal. All parameters fell within standard means as set by the Institute of Medical and Veterinary Pathology, Adelaide, Australia. The mean preinjury MAP, pulse, and temperature were 65.7 ± 9.3 mm Hg, 100 ± 14.84 beats per minute, and 40.9 ± 0.64°C, respectively. The mean postinjury MAP (10 minutes postinjury) was 39.1 ± 6.9 mm Hg despite maximal resuscitation efforts at 200 mL/min. The mean resuscitation fluid used at time of exsanguination was 10.89 ± 2.40 L, with a mean total blood loss of 4943 ± mL. With the performance of local packing measures only, which did not obstruct vascular flow, hemostasis was not achieved and resulted in all animals exsanguinating with a mean survival time for each animal of 50.25 ± 17.89 minutes with local cottonoid packing only.
DISCUSSION
Modern skull base surgery has undergone a paradigm shift in recent years from traditional external approaches to the expanded fully endoscopic endonasal skull base approach. Limited access surgery has several advantages; however, the surgeon needs to be aware of the potential for catastrophic vascular complications to occur. This article describes a reproducible animal model of a lethal endonasal carotid artery injury. Importantly, this model recreates the endonasal confines and limitations of the human nasal cavity and nasal vestibule, with hemodynamic similarities to the human patient. The pulsatile nature of this injury recreates the difficult surgical view encountered by the surgeon.
With appropriate safe surgical principles, most endoscopic sinus surgeons are unlikely to manage an ICA injury. However, ICA injury is a more likely event to the endoscopic skull base surgeon. All literature to date relies on retrospective studies and case reports to dictate the management options in such a catastrophic event. The surprised surgeon maybe ill equipped to deal with such a challenging surgical scenario. Surgeons rely on indiscriminate nasal packing in an attempt to achieve immediate hemorrhage control, often resulting in complete occlusion of the vessel, which contributes to the mortality and morbidity of the patient.14
The high-flow, high-pressure bleeding characteristics of an ICA injury creates an immediately challenging surgical scenario with massive blood loss that may prove fatal for the patient. The narrow nasal corridor means that even a little blood rapidly obstructs the surgeon's view, and the pulsatile nature of bleeding results in the endoscope tip rapidly becoming soiled with blood. These characteristics cause the surgical team to rapidly becoming disorientated and lose control of the surgical field. Frequently, a significant amount of experience, coordination, and teamwork is needed by both surgeons for the “two-surgeon team” to navigate through the bleeding and maintain a surgical view.9 It is these challenging surgical characteristics that may result in exsanguination of the patient, and indiscriminate nasal packing is often all surgeons are equipped to do in an attempt to achieve hemostasis.
Animal models have played an important role in surgical education and training and have been used in the medical field since 384 B.C.16 Remaining challenges in endoscopic skull base techniques include the ability to train a new generation of endonasal endoscopic skull base surgeons in a stepwise fashion including training in the potential for vascular injuries to occur.17 This reproducible model allows the surgical steps that a skull base team should undertake to be defined in this catastrophic scenario. Importantly, it provides the opportunity to train endonasal endoscopic surgical teams in the skills required to manage the surgical field in such a catastrophic arterial injury, in a stepwise fashion.
Kassam et al concluded that the most significant limitation of endoneurosurgical hemostasis is the inability to repair large arteries primarily.9 This model creates the opportunity for further research and development to be performed and allows the design and investigation of different treatment techniques that may be employed. It is important to recognize that not every vascular injury will have the same anatomic constraints, and this reproducible model allows scientific investigation into developing the surgical techniques required to manage both a minimally accessible injury and also a maximally exposed injury site. With animal recovery following carotid injury control, it allows investigation into both the short-term and long-term complications of these techniques.
CONCLUSION
The increasing frequency of extended endoscopic endonasal skull base approaches means that specialist endonasal skull base surgeons need to be familiar with the techniques required to manage an inadvertent carotid injury. This model is the first to replicate the challenging endoscopic surgical management of a high-flow/high-pressure vascular injury, with the potential to train future endoscopic skull base surgeons in the skills required to manage such an event. Additionally, it allows for the development of novel hemostatic techniques and surgical instrumentation.
NOTE
P.J.W. receives royalties for design of instruments from Medtronic ENT.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Sam Boase and Dr. Josh Jervis-Bardy for the technical assistance with this model.
References
- Kassam A B, Gardner P, Snyderman C, Mintz A, Carrau R. Expanded endonasal approach: fully endoscopic, completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Neurosurg Focus. 2005;19:E6. [PubMed] [Google Scholar]
- Casler J D, Doolittle A M, Mair E A. Endoscopic surgery of the anterior skull base. Laryngoscope. 2005;115:16–24. doi: 10.1097/01.mlg.0000150681.68355.85. [DOI] [PubMed] [Google Scholar]
- Carrau R L, Kassam A B, Snyderman C H. Pituitary surgery. Otolaryngol Clin North Am. 2001;34:1143–1155. ix. doi: 10.1016/s0030-6665(05)70371-6. [DOI] [PubMed] [Google Scholar]
- Ciric I, Ragin A, Baumgartner C, Pierce D. Complications of transsphenoidal surgery: results of a national survey, review of the literature, and personal experience. Neurosurgery. 1997;40:225–236. discussion 236–237. doi: 10.1097/00006123-199702000-00001. [DOI] [PubMed] [Google Scholar]
- Gardner P A, Kassam A B, Snyderman C H, et al. Outcomes following endoscopic, expanded endonasal resection of suprasellar craniopharyngiomas: a case series. J Neurosurg. 2008;109:6–16. doi: 10.3171/JNS/2008/109/7/0006. [DOI] [PubMed] [Google Scholar]
- Stippler M, Gardner P A, Snyderman C H, Carrau R L, Prevedello D M, Kassam A B. Endoscopic endonasal approach for clival chordomas. Neurosurgery. 2009;64:268–277. discussion 277–278. doi: 10.1227/01.NEU.0000338071.01241.E2. [DOI] [PubMed] [Google Scholar]
- Frank G, Sciarretta V, Calbucci F, Farneti G, Mazzatenta D, Pasquini E. The endoscopic transnasal transsphenoidal approach for the treatment of cranial base chordomas and chondrosarcomas. Neurosurgery. 2006;59(Suppl 1):ONS50–ONS57. discussion ONS50–ONS57. doi: 10.1227/01.NEU.0000219914.17221.55. [DOI] [PubMed] [Google Scholar]
- Couldwell W T, Weiss M H, Rabb C, Liu J K, Apfelbaum R I, Fukushima T. Variations on the standard transsphenoidal approach to the sellar region, with emphasis on the extended approaches and parasellar approaches: surgical experience in 105 cases. Neurosurgery. 2004;55:539–547. discussion 547–550. doi: 10.1227/01.neu.0000134287.19377.a2. [DOI] [PubMed] [Google Scholar]
- Kassam A, Snyderman C H, Carrau R L, Gardner P, Mintz A. Endoneurosurgical hemostasis techniques: lessons learned from 400 cases. Neurosurg Focus. 2005;19:E7. [PubMed] [Google Scholar]
- Pepper J P, Wadhwa A K, Tsai F, Shibuya T, Wong B J. Cavernous carotid injury during functional endoscopic sinus surgery: case presentations and guidelines for optimal management. Am J Rhinol. 2007;21:105–109. doi: 10.2500/ajr.2007.21.2901. [DOI] [PubMed] [Google Scholar]
- Park A H, Stankiewicz J A, Chow J, Azar-Kia B. A protocol for management of a catastrophic complication of functional endoscopic sinus surgery: internal carotid artery injury. Am J Rhinol. 1998;12:153–158. doi: 10.2500/105065898781390154. [DOI] [PubMed] [Google Scholar]
- Weidenbecher M, Huk W J, Iro H. Internal carotid artery injury during functional endoscopic sinus surgery and its management. Eur Arch Otorhinolaryngol. 2005;262:640–645. doi: 10.1007/s00405-004-0888-8. [DOI] [PubMed] [Google Scholar]
- Cappabianca P, Esposito F, Esposito I, Cavallo L M, Leone C A. Use of a thrombin-gelatin haemostatic matrix in endoscopic endonasal extended approaches: technical note. Acta Neurochir (Wien) 2009;151:69–77. discussion 77. doi: 10.1007/s00701-008-0172-6. [DOI] [PubMed] [Google Scholar]
- Raymond J, Hardy J, Czepko R, Roy D. Arterial injuries in transsphenoidal surgery for pituitary adenoma; the role of angiography and endovascular treatment. AJNR Am J Neuroradiol. 1997;18:655–665. [PMC free article] [PubMed] [Google Scholar]
- Laws E R., Jr Vascular complications of transsphenoidal surgery. Pituitary. 1999;2:163–170. doi: 10.1023/a:1009951917649. [DOI] [PubMed] [Google Scholar]
- Cohen B J, Loew F M, editors. Laboratory Animal Medicine: Historical Perspectives in Laboratory Animal Medicine. Orlando, FL: Academic Press, Inc.; 1984. [Google Scholar]
- Mehta R P, Cueva R A, Brown J D, et al. What's new in skull base medicine and surgery? Skull Base Committee Report. Otolaryngol Head Neck Surg. 2006;135:620–630. doi: 10.1016/j.otohns.2006.04.018. [DOI] [PubMed] [Google Scholar]