Abstract
We sought to determine the risk of tumor incisional recurrence in patients receiving surgery and postoperative radiation therapy for locally advanced sinonasal malignancies. Medical records for 70 patients newly diagnosed with nonmetastatic American Joint Committee on Cancer stage II to stage IV sinonasal malignancies between 1991 and 2003 were retrospectively reviewed. Patient demographics and tumor variables were recorded. All patients underwent upfront surgical resection with postoperative three-dimensional conformal proton beam radiotherapy. Recurrence and survival-related outcomes were recorded. Two patients with squamous cell carcinoma had pathologically confirmed tumor recurrence at the incision site. The actuarial risk of incisional recurrence for the entire group at 1 year was 3%. One of the two patients had a maxillary sinus tumor and developed isolated skin recurrence along the transfacial incision. The other patient with an ethmoid sinus tumor developed isolated dural recurrence along the craniotomy incision. Both patients underwent multiple courses of salvage surgery and radiation therapy. One was successfully salvaged locally but developed distant metastases and the other died of local recurrence. Tumor seeding following transfacial and craniotomy surgery can occur, especially for squamous cell carcinoma. Sound oncological surgical technique, even when utilizing these difficult surgical approaches, is important to minimize incisional recurrence.
Keywords: Incisional recurrence, wound metastasis, tumor implantation, anterior skull base surgery, transfacial surgery
Tumor seeding is a serious and potentially avoidable complication after oncological surgery. Consequently, surgical techniques that minimize tumor manipulation and ensure en bloc resection are routinely employed by head and neck surgeons. When incisional recurrence does occur, however, it generally results in poor prognosis.1
Wound seeding has been widely reported following open surgical excision of a variety of tumors including carcinomas of the endometrium,2 cervix,3 lung,4 colon,5 ovary,6 and gallbladder.7 Specific to head and neck surgery, tumor seeding leading to local recurrence has been seen after incisional and excisional biopsies of neck masses and after tumor spillage during parotid surgery and can even occur in the abdominal wall after percutaneous gastrostomy tube placement in patients with tumors of the upper aerodigestive tract.8,9,10
Surgery of the anterior skull base has the additional challenges of limited exposure as well as several nearby vital neurovascular structures that prevent wide oncological resection. In an effort to optimize results, advancements have been made through the addition of endoscopic assistance and image guidance systems. In addition, improvements in the field of radiation oncology have allowed for successful outcomes even in some cases where complete oncological resection is impossible.
This study evaluates the risk of tumor seeding in patients with locally advanced sinonasal malignancies undergoing upfront surgical resection as part of their treatment.
METHODS
Study Cohort
Between October 1991 and November 2003, 70 patients with newly diagnosed nonmetastatic American Joint Committee on Cancer stage II to stage IV sinonasal malignancies were treated with adjuvant postoperative proton beam with or without photon radiation therapy at the Massachusetts General Hospital. The study was approved by the Institutional Review Board. All available pathology slides were reviewed at our institutions before treatment. Each patient was seen jointly by radiation oncologists and otolaryngologists, and the decision was made jointly for the patient to undergo surgery followed by radiation therapy (not all surgeries were performed at Massachusetts General Hospital, but all radiation therapy and follow-up care were provided there). The median follow-up for the entire group was 65 months. The median follow-up of all living patients was 92 months.
Tumor and Treatment Characteristics
The age range was 17 to 82 years with a median of 50 years. There were 36 (51%) male and 34 (49%) female patients. None of the patients received previous irradiation to the primary site. The histological types were squamous cell carcinoma in 29, tumors with neuroendocrine differentiation in 15, adenoid cystic carcinoma in 11, adenocarcinoma in 3, and sarcoma in 12 patients. All patients underwent upfront surgical resection. The surgical approach was transfacial alone in 43 (61%), craniofacial in 23 (33%), and craniotomy in 4 (6%) patients. The median time between surgery and the initiation of radiation was 1.7 months.
All patients were treated with postoperative proton beam with or without photon radiation therapy as curative intent. Proton beam therapy was delivered at the Harvard Cyclotron Laboratory or Northeast Proton Therapy Center at the Massachusetts General Hospital using 160 MeV and 230 MeV beams, respectively. The median prescribed dose to gross tumor volume or high-risk clinical target volume was 69 Gy (range 59.4 to 77.8). The median proportion of protons was 58% (range 23 to 100%). Seventy-four percent of the patients received twice-daily radiation.
For the entire group of patients, none received concurrent chemotherapy. Sixty-seven percent of the patients with neuroendocrine tumors received induction chemotherapy that consisted of cisplatin and etoposide.
Statistical Analysis
Overall survival was measured from the end of radiotherapy until death, censoring patients who were alive at last follow-up. Disease-free survival was measured from the end of radiotherapy until death or tumor recurrence. Local control, regional control, and freedom from distant metastasis were measured from the end of radiotherapy until local, regional, or distant recurrence, respectively. Freedom from incisional recurrence was measured from the date of initial surgery until the date of incisional recurrence. The Kaplan-Meier product-limit method was used to estimate all survival and tumor control probabilities.
RESULTS
At a median follow-up of 65 months for the entire group, the local control rate at 5 years was 86%. The disease-free and overall survival rates at 5 years were 55% and 59%, respectively.
Two patients with squamous cell carcinoma had pathologically confirmed tumor recurrence at the incision site 7.6 and 4.6 months, respectively, after surgery. Both had undergone complete resection by craniotomy and transfacial approaches, respectively. The actuarial risk of incisional recurrence at 1 year was 3% for the entire group. For patients with squamous cell carcinoma, the actuarial risk of developing incisional recurrence at 1 year was 7%.
Patient One
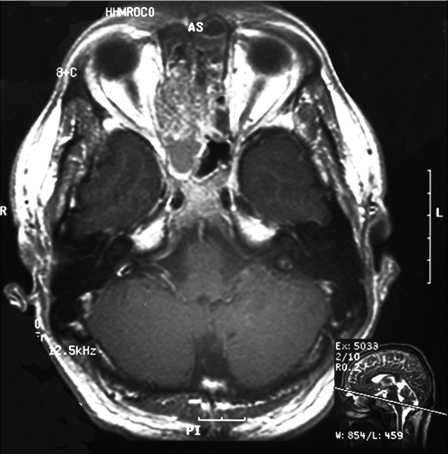
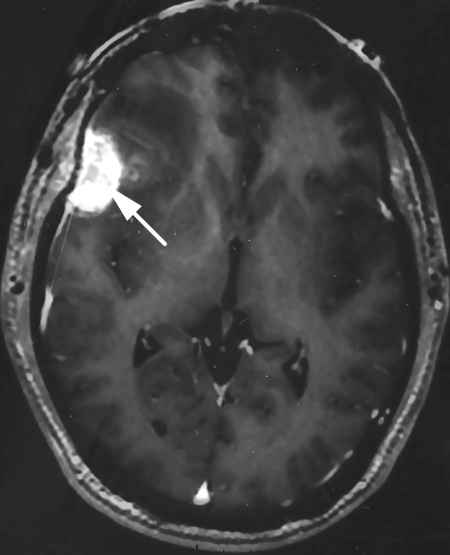
A 37-year-old man presented initially with a 3-month history of headaches and facial pressure and was ultimately found to have T4N0M0 disease with a mass centered in the right ethmoid sinus that was proven by biopsy to be nonkeratinizing squamous cell carcinoma. A preoperative magnetic resonance imaging (MRI) showed an extensive tumor of the right ethmoid sinuses and nasal cavity with involvement of the cribriform plate and extension into the anterior cranial fossa without infiltration into the orbit soft tissues (Fig. 1). After multidisciplinary evaluation, he underwent bifrontal craniotomy and endoscopic resection at an outside institution. Postoperatively, he received a full course of proton beam therapy to a total of 66.6 Gy in 1.8 Gy per fraction. Three months posttreatment on his planned follow-up, he was noted on MRI to have a large, multilobulated, enhancing mass along the dural incision of the bifrontal craniotomy scar, representing a leptomeningeal metastasis (Fig. 2). He underwent gross total resection of this mass. Postoperative MRI at 2 weeks revealed gross tumor recurrence along the dura within as well as immediately next to the surgical bed. He received postoperative reirradiation with fractionated proton beam therapy to the surgical bed followed and proton beam radiosurgery boost to the gross disease. Sixteen months following the completion of this treatment, he was again noted to have another small focus of intracranial metastasis that was treated with proton beam radiosurgery with good results.
Figure 1.
Non–contrast-enhanced T1-weighted magnetic resonance imaging of the paranasal sinuses demonstrates a lesion epicentered in the right ethmoid sinuses with mass effect on the posterior right orbit. There was significant extension through the cribriform plate into the anterior cranial fossa.
Figure 2.
Contrast-enhanced T1-weighted magnetic resonance imaging demonstrates an enhancing mass involving the dura immediately beneath the prior bifrontal craniotomy incision (white arrow).
Forty-three months after the diagnosis of his first leptomeningeal disease, he was diagnosed with biopsy-proven multiple-lung metastasis. There was no evidence of locoregional disease at last follow-up. He was undergoing palliative chemotherapy at last follow-up.
Patient Two
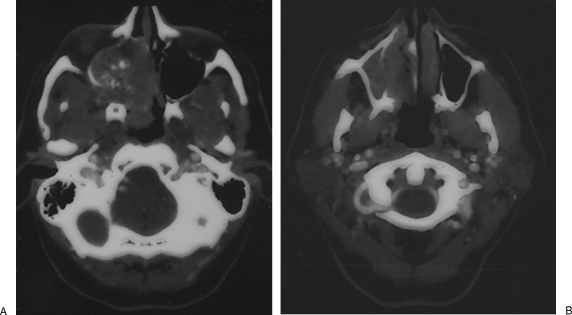
A 59-year-old man with a history of T4aN0M0 squamous cell carcinoma of the right maxillary sinus initially presented with a 5-year complaint of progressive right nasal blockage. Examination at that time showed a large tumor involving the right nasal cavity; computed tomography was performed, which showed a large tumor of the right maxillary sinus with extension through the posterior wall to involve the pterygopalatine fossa and infratemporal fossa (Fig. 3A). The right ethmoid air cells and orbit were involved, and no clear plane could be visualized between the tumor and the anterior skull base. Of note, there was no erosion of the anterior wall of the maxilla or involvement of the premaxillary soft tissues (Fig. 3B).
Figure 3.
Axial computed tomography scan with contrast demonstrates a large tumor of the right maxillary sinus with extension through the posterior wall to involve the pterygopalatine fossa and infratemporal fossa (A). There was no erosion of the anterior wall of the maxilla and no involvement of the premaxillary soft tissues (B).
After multidisciplinary evaluation, he underwent a right radical maxillectomy, with right orbital exenteration with pathology revealing a 6-cm squamous cell carcinoma associated with an inverted papilloma. The tumor was present at the medial posterior margin and close to the superior and lateral margins without any extension to the anterior margin by imaging and operative findings.
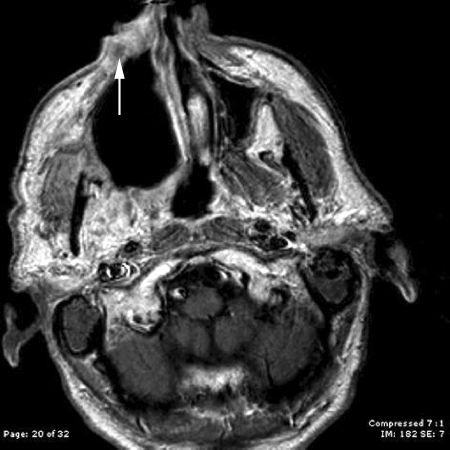
Postoperatively, he underwent proton beam radiation therapy to a total dose of 70 Gy. Seven weeks after the completion of radiation, he was noted to have a 1-cm superficial skin nodule along the inferior portion of his right lateral rhinotomy incision scar that was proven by biopsy to be invasive squamous cell carcinoma (Fig. 4). He underwent a wide local excision of this lesion with clear margins as well as a nasolabial rotational flap reconstruction, followed by postoperative reirradiation. He subsequently developed regional recurrence in his submental lymph nodes requiring neck dissection and a course of postoperative chemoradiation therapy. He was later found to have inoperable skull base recurrence as well as lip and neck dermal metastases. He died from recurrent disease at 5 years after initial surgery.
Figure 4.
Axial contrast-enhanced T1-weighted magnetic resonance imaging scan demonstrates the soft tissue nodule along the lateral rhinotomy incision line (white arrow).
DISCUSSION
Local recurrence along surgical incisions is a rare and potentially avoidable complication that can result after oncological surgery. When it does occur, however, it is considered a cutaneous metastasis and generally results in a poor prognosis.1
There are many reports in the literature of incisional recurrence following open surgical excision of intra-abdominal and pelvic malignancies, with the overall incidence rate of 5% or less.11 Some examples include cancers of the cervix (0.8%),12 colon (0.8 to 3.3%),13,14 and rectum (5.3%).15 There has been considerable debate with regards to the route of spread leading to these recurrences. Some believe that implantation of viable tumor cells that are dislodged at the time of surgery is the likely mechanism,16 although others have suggested that the mode of transmission is retrograde spread of tumor secondary to lymphatic obstruction.17
Since the advent of laparoscopic and endoscopic equipment and surgical techniques, there has been a push to maintain the effectiveness of a procedure while minimizing the morbidity of the surgical approach. It would make sense then, especially if one ascribes to the notion of tumor seeding at the time of the primary surgery, that tumor seeding at the portals of entry for the instruments could be a site of possible tumor recurrence. This idea has been supported by reports of tumor recurrence at trocar sites for thoracoscopic4 and laparoscopic5,7 surgery for tumors and is further enforced by the numerous reports of gastrostomy site seeding from tumors of the upper aerodigestive tract.8,9,10
Oncological surgery of the skull base brings with it additional challenges of limited exposure as well as several nearby vital neurovascular structures that preclude wide oncological margins, at times making en bloc resection impossible. Given that tumor fragmentation may occur intentionally or inadvertently during resection, it would be reasonable to assume that incisional recurrence may be more frequent following craniofacial surgery compared with open approaches in other body locations. This was not observed in our patient population, however. In our group, there were a total of two pathologically confirmed tumor recurrences along the surgical incision line out of a total of 70 patients undergoing up-front surgical management of their tumors. This 3% risk is similar to what is seen following surgery for cervical,12 colon,13,14 and rectal15 tumors. With regards to skull base malignancies, clival chordomas of the skull and cervical spine have been found to carry a risk of ~5% for incisional recurrence when managed with combined surgery and radiation.18,19
In the current study, both patients who developed incisional recurrence in our series had squamous cell carcinomas as their primary tumor. This is similar to what has been observed in other parts of the body where adenocarcinoma, squamous cell carcinoma, and undifferentiated carcinoma3,20 were the most frequently described tumors resulting in incisional recurrence. This suggests that, when possible, en bloc resection of these tumors is advisable and should be considered when choosing the surgical approach.
There are times, however, when en bloc resection of an anterior skull base tumor is not possible. In these instances, endoscopic assistance should be considered. When a true transnasal endoscopic approach is utilized to assist the neurosurgeon, the raw surface for potential tumor seeding would be reduced, minimizing the likelihood of developing incisional recurrence.
Our series of patients demonstrates that, despite the limitations of exposure in the anterior skull base, there was a similar risk for developing tumor recurrence along the surgical excision line compared with other body sites.2,3,4,5 Those patients at highest risk were those with squamous cell carcinoma as the pathology of their primary tumors. As a result, when contemplating surgery as part of the management for these types of sinonasal malignancies, surgical approaches that avoid tumor handling and fragmentation while minimizing raw surface edges of the surrounding soft tissue should be employed.
Management of incisional recurrence usually entails salvage surgery with or without reirradiation. If the nidus is affecting only the skin incision without significant depth of penetration, electron beam radiation can be utilized to avoid additional tissue damage to the prior treatment area. In our patient population, salvage surgery was performed with reirradiation, using protons and proton radiosurgery boost for the dural recurrence in an attempt to maximize local control while minimizing radiation damage to the underlying brain parenchyma. Despite these efforts, however, the prognosis in these patients is generally poor, and avoidance is the best strategy.
CONCLUSION
Tumor seeding following transfacial and craniotomy surgery can occur, especially for squamous cell carcinoma. Sound oncological surgical techniques, even in the setting of the challenging exposure of the anterior skull base, are important to minimize recurrence from tumor seeding.
NOTES
Presented in part at the 17th North America Skull Base Society, February 16 to 19, 2006, Phoenix, Arizona.
References
- Rosen T. Cutaneous metastases. Med Clin North Am. 1980;64:885–900. doi: 10.1016/s0025-7125(16)31572-3. [DOI] [PubMed] [Google Scholar]
- Joshi S C, Sharma D N, Khurana N, Mohanta P K, Bahadur A K. Endometrial carcinoma with recurrence in the incisional scar: a case report. Int J Gynecol Cancer. 2003;13:901–903. doi: 10.1111/j.1525-1438.2003.13376.x. [DOI] [PubMed] [Google Scholar]
- Srivastava K, Singh S, Srivastava M, Srivastava A N. Incisional skin metastasis of a squamous cell cervical carcinoma 3.5 years after radical treatment—a case report. Int J Gynecol Cancer. 2005;15:1183–1186. doi: 10.1111/j.1525-1438.2005.00173.x. [DOI] [PubMed] [Google Scholar]
- Chen T P, Liu H P, Lu H I, Hsieh M J, Liu Y H, Wu Y C. Incidence of incisional recurrence after thoracoscopy. Surg Endosc. 2004;18:540–542. doi: 10.1007/s00464-003-8215-9. [DOI] [PubMed] [Google Scholar]
- Reilly W T, Nelson H, Schroeder G, Wieand H S, Bolton J, O'Connell M J. Wound recurrence following conventional treatment of colorectal cancer. A rare but perhaps underestimated problem. Dis Colon Rectum. 1996;39:200–207. doi: 10.1007/BF02068076. [DOI] [PubMed] [Google Scholar]
- Rafii A, Ferron G, Lacroix-Triki M, Dalenc F, Gladieff L, Querleu D. Abdominal wall metastasis of ovarian carcinoma after low transverse abdominal incision: report of two cases and review of literature. Int J Gynecol Cancer. 2006;16(Suppl 1):334–337. doi: 10.1111/j.1525-1438.2006.00511.x. [DOI] [PubMed] [Google Scholar]
- Hu J B, Sun X N, Xu J, He C. Port site and distant metastases of gallbladder cancer after laparoscopic cholecystectomy diagnosed by positron emission tomography. World J Gastroenterol. 2008;14:6428–6431. doi: 10.3748/wjg.14.6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacke W, Hecker U, Woenckhaus C, Lerch M M. Percutaneous endoscopic gastrostomy site metastasis in a patient with esophageal cancer. Endoscopy. 2004;36:472. doi: 10.1055/s-2004-814379. [DOI] [PubMed] [Google Scholar]
- Kurdow R, Schniewind B, Delere Y, Boehle A S, Lüttges J, Doniec J M. Implantation metastasis of a hypopharyngeal carcinoma at the site of a percutaneous endoscopic gastrostomy. Endoscopy. 2003;35:462. doi: 10.1055/s-2003-38764. [DOI] [PubMed] [Google Scholar]
- Ananth S, Amin M. Implantation of oral squamous cell carcinoma at the site of a percutaneous endoscopic gastrostomy: a case report. Br J Oral Maxillofac Surg. 2002;40:125–130. doi: 10.1054/bjom.2001.0740. [DOI] [PubMed] [Google Scholar]
- Brady L W, O'Neill E A, Farber S H. Unusual sites of metastases. Semin Oncol. 1977;4:59–64. [PubMed] [Google Scholar]
- Bolli J A, Doering D L, Bosscher J R, et al. Squamous cell carcinoma antigen: clinical utility in squamous cell carcinoma of the uterine cervix. Gynecol Oncol. 1994;55:169–173. doi: 10.1006/gyno.1994.1272. [DOI] [PubMed] [Google Scholar]
- Hughes E S, McDermott F T, Polglase A L, Johnson W R. Tumor recurrence in the abdominal wall scar tissue after large-bowel cancer surgery. Dis Colon Rectum. 1983;26:571–572. doi: 10.1007/BF02552962. [DOI] [PubMed] [Google Scholar]
- Gunderson L L, Sosin H, Levitt S. Extrapelvic colon—areas of failure in a reoperation series: implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 1985;11:731–741. doi: 10.1016/0360-3016(85)90305-0. [DOI] [PubMed] [Google Scholar]
- Gunderson L L, Sosin H. Areas of failure found at reoperation (second or symptomatic look) following “curative surgery” for adenocarcinoma of the rectum. Clinicopathologic correlation and implications for adjuvant therapy. Cancer. 1974;34:1278–1292. doi: 10.1002/1097-0142(197410)34:4<1278::aid-cncr2820340440>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Kotwall C A, Kirkbride P, Zerafa A E, Murray D. Endometrial cancer and abdominal wound recurrence. Gynecol Oncol. 1994;53:357–360. doi: 10.1006/gyno.1994.1147. [DOI] [PubMed] [Google Scholar]
- Malfetano J H. Skin metastases from cervical cancer: a fatal event. Gynecol Oncol. 1986;24:177–182. doi: 10.1016/0090-8258(86)90025-9. [DOI] [PubMed] [Google Scholar]
- Fischbein N J, Kaplan M J, Holliday R A, Dillon W P. Recurrence of clival chordoma along the surgical pathway. AJNR Am J Neuroradiol. 2000;21:578–583. [PMC free article] [PubMed] [Google Scholar]
- Fagundes M A, Hug E B, Liebsch N J, Daly W, Efird J, Munzenrider J E. Radiation therapy for chordomas of the base of skull and cervical spine: patterns of failure and outcome after relapse. Int J Radiat Oncol Biol Phys. 1995;33:579–584. doi: 10.1016/0360-3016(95)02014-3. [DOI] [PubMed] [Google Scholar]
- Brownstein M H, Helwig E B. Patterns of cutaneous metastasis. Arch Dermatol. 1972;105:862–868. [PubMed] [Google Scholar]