Abstract
We report the pathology and outcome of secondary skull base tumors in patients previously treated with external beam radiation for retinoblastoma (Rb). Rb patients are at increased risk of second head and neck primary malignancies due to early radiation exposure during treatment and loss of RB1 protein in genetic carriers. An institutional database was reviewed for patients with retinoblastoma who had previously received radiation therapy and subsequently developed skull base tumors. Seventeen patients met the selection criteria. The median age of Rb diagnosis was 12 months. Thirteen cases underwent enucleation in addition to radiation therapy as part of initial Rb treatment. A median of 19 years elapsed between the diagnosis of Rb and diagnosis of skull base malignancy. The most common tumors were osteogenic sarcoma (39%) and leiomyosarcoma (22%). Eleven (71%) patients received postoperative chemotherapy, and 7 (41%) received postoperative radiotherapy. Three (24%) patients underwent salvage surgery for recurrent disease. Five-year survival was 68%, and 10-year survival was 51% by Kaplan-Meier analysis. Secondary malignancy in Rb patients is a well-defined event. The use of surgery with appropriate adjuvant therapy was associated with a 51% 10-year survival in this study population.
Keywords: Skull base neoplasms, retinoblastoma, neoplasms, second primary, radiotherapy
Retinoblastoma (Rb) is a cancer arising from the retina that primarily affects the pediatric population, with 90% of cases usually diagnosed before age 5.1 The disease is caused by mutations in the retinoblastoma (Rb1) gene, a tumor suppressor gene and the first tumor suppressor gene to be cloned. Rb affects ~1 in 23,000 live births and occurs clinically in two forms. When the mutation is in every cell of the body (“genetic form”), the disease is often bilateral (85% of cases), and these children are at lifelong risk for the development of second cancers. When the mutation is only in the eye, patients are not at risk for germ line–related second cancers.2
More than 100 years ago, it was demonstrated that radiation alone could be curative for Rb, and in the past, radiation was employed to avoid surgical removal of the eye.2 However, the risk of second nonocular cancers is further increased, and the latency period decreased, when patients are treated with radiation.2 A dose–response relationship has been demonstrated for development of secondary sarcomas of the head and neck after radiation.3 The incidence of these malignancies is further increased if children are radiated during the first year of life.4 Although older data suggested that the most common secondary malignancy, regardless of radiation status, was osteosarcoma,5 more recent data demonstrated that soft tissue sarcomas are slightly more frequent than bone sarcomas in irradiated hereditary Rb patients as they survive to older ages. Among hereditary Rb patients previously reported from New York, radiotherapy increased the cumulative risk of developing a second malignancy by ~38% at 50 years after treatment for Rb.6
At our institution, we have treated several Rb patients with secondary skull base malignancies who have previously received radiation as part of their initial Rb treatment. The purpose of this study was to report the outcomes following management of these high-risk patients.
METHODS
Following institutional review board approval, a retrospective review of a database of skull base tumors at Memorial Sloan Kettering Cancer Center was performed. The database spans from 1974 to present. Patients were included if they had a history of Rb whose initial treatment included radiation therapy. Kaplan-Meier curves were calculated using SPSS (Chicago, IL), and log-rank (Mantel-Cox) statistics were used to determine significance.
RESULTS
Overall, 17 patients were identified: 13 males and 4 females. The median age of Rb diagnosis was 12 months (range 1 to 29 months). Sixteen patients (94%) presented with bilateral tumors. Thirteen patients underwent enucleation as part of initial treatment, including two who underwent bilateral enucleation. All patients received external radiation to a median dose of 4400 cGy (range 3500 to 7000 cGy).
Subsequent malignancy occurred at a mean of 23 years after radiation treatment (median 19 years, range 6 to 65 years). The mean age of development of secondary malignancy was 24 years old. In total, 18 skull base tumors were resected in this cohort, including one patient who developed two different tumors 10 years apart (Fig. 1).
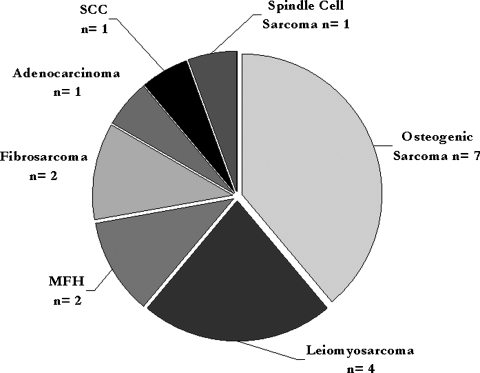
Figure 1.
Pie chart showing histological distribution of secondary skull base malignancies. MFH, malignant fibrous histiocytoma; SCC, squamous cell carcinoma.
The most common histological diagnosis was osteogenic sarcoma, seen in 7 (39%) of cases. The next most common histology were four cases of leiomyosarcoma (22%), two cases each of malignant fibrous histiocytoma and fibrosarcoma (11%), and one case each of adenocarcinoma, squamous cell carcinoma, and spindle cell sarcoma (6%; Fig. 2).
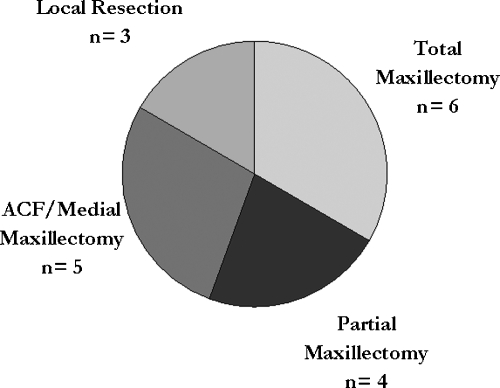
Figure 2.
Pie chart showing types of surgical resection for secondary skull base malignancies. ACF, anterior cranial fossa.
Surgical management included total maxillectomy in six cases (33%), anterior craniofacial approach/medial maxillectomy in five cases (27%), partial maxillectomy in four cases (22%), and local resection in three cases (17%). All local resection group patients had tumors that invaded the superior or lateral orbit into the anterior skull base. Five cases (27%), three in the total maxillectomy and two in the partial maxillectomy groups, underwent orbital exenteration. A rectus free flap was utilized in 10 (56%) cases for reconstruction.
Adjuvant treatment included chemotherapy and additional radiotherapy. Eleven (71%) cases received postoperative chemotherapy. Methotrexate and doxorubicin were the most commonly used chemotherapeutic agents. Postoperative radiotherapy was given in 7 (41%) cases (range 5040 to 6080 cGy). In addition, three cases underwent salvage surgery for recurrent disease, two of whom were successfully salvaged. Only two cases did not receive any adjuvant treatment.
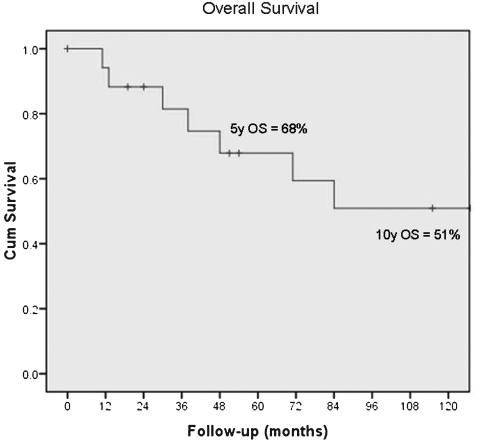
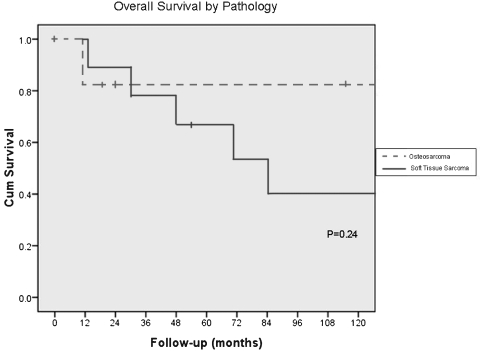
At the time of review, all patients showed no evidence of disease or had died of disease. The 5-year survival was 68% and the 10-year survival was 51% by Kaplan-Meier analysis (Fig. 3). Median survival was 165 months after surgery. Those patients who died of disease survived a median of 46 months (range 13 to 165 months) after surgery. Four patients died of local disease and three patients died of distant disease. Cause of death in one patient could not be determined. The type of sarcoma, osteosarcoma versus soft tissue sarcoma, was not associated with a difference in survival (log-rank, p = 0.24; Fig. 4).
Figure 3.
Kaplan-Meier survival curve showing overall survival (OS) of 68% at 5 years and 51% at 10 years.
Figure 4.
Kaplan-Meier survival curve showing overall survival by pathology (p = 0.24).
DISCUSSION
As noted by Kleinerman et al, hereditary Rb patients who receive radiation therapy are at significant risk for development of secondary malignancies, especially sarcomas.7 In this series, 76% of all cancers presenting before age 25 were sarcomas, and 48% of cancers after age 25 were sarcomas. In nonirradiated hereditary Rb patients, 33% of all cancers were sarcomas under the age of 25 versus only 8% for those older than 25. Approximately equal numbers of bone and soft tissue sarcomas were noted. These data suggest that radiation therapy in hereditary Rb increases the risk of sarcoma development.
The age at the time of radiation delivery is also important. Abramson and Frank showed that when patients are irradiated at less than a year of age, they are at significantly higher risk of developing secondary malignancies in comparison to patients irradiated after 1 year.4 Although external beam radiation therapy has served as an effective therapy for Rb for many years, there is a general trend away from using external beam therapy in favor of brachytherapy or chemotherapy.2 As more experience is gained with these other treatment modalities, an eye function-preserving approach can be employed, which does not carry the same elevated risk of secondary development of malignancy.
The types of secondary malignancies reported in this series are consistent with reported literature for Rb patients. Both bone and soft tissue sarcomas have previously been observed in hereditary Rb patients, with soft tissue sarcomas being slightly more frequent as patients age.8 Among soft tissue sarcomas, the three most common types reported in order are leiomyosarcoma, fibrosarcoma, and malignant fibrous histiocytoma.7 Our data reflect a similar pattern.
A few limitations should be noted in our study. Because this is a study from review of the database of craniofacial surgery, patients were selected for inclusion only if they had surgery via an anterior craniofacial or other skull base approach. A selection bias remains in this study, as only patients with tumors amenable to surgical resection were identified for inclusion. We do not have the data on how many of our Rb patients developed secondary head and neck malignancies but did not undergo surgical treatment. Our outcome data reflect results from this limited set of surgical skull base patients. Furthermore, the limited data prevent determining the contribution of chemotherapy or radiation therapy to the overall survival outcome.
This single-institution, retrospective study reports that surgery with adjuvant therapy can provide benefit for Rb patients who develop secondary skull base malignancies. Although these challenging cases should each be managed under multidisciplinary consultation, our data suggest that there may be a benefit to surgery with adjuvant therapy.
NOTE
Presented at the North American Skull Base Society Meeting, New Orleans, Louisiana, 2009.
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of the National Institutes of Health, the National Cancer Institute, Division of Cancer Epidemiology and Genetics. The project described was supported by Award Number T32CA009685 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
References
- Hong W K, Holland J F, DeSancho M T, et al. Holland-Frei Cancer Medicine, 8th ed. Shelton, CT: People's Medical Publishing House; 2010. [Google Scholar]
- Abramson D H, Schefler A C. Update on retinoblastoma. Retina. 2004;24:828–848. doi: 10.1097/00006982-200412000-00002. [DOI] [PubMed] [Google Scholar]
- Wong F L, Boice J D, Jr, Abramson D H, et al. Cancer incidence after retinoblastoma. Radiation dose and sarcoma risk. JAMA. 1997;278:1262–1267. doi: 10.1001/jama.278.15.1262. [DOI] [PubMed] [Google Scholar]
- Abramson D H, Frank C M. Second nonocular tumors in survivors of bilateral retinoblastoma: a possible age effect on radiation-related risk. Ophthalmology. 1998;105:573–579. discussion 579–580. doi: 10.1016/S0161-6420(98)94006-4. [DOI] [PubMed] [Google Scholar]
- Abramson D H, Ellsworth R M, Kitchin F D, Tung G. Second nonocular tumors in retinoblastoma survivors. Are they radiation-induced? Ophthalmology. 1984;91:1351–1355. doi: 10.1016/s0161-6420(84)34127-6. [DOI] [PubMed] [Google Scholar]
- Kleinerman R A, Tucker M A, Tarone R E, et al. Risk of new cancers after radiotherapy in long-term survivors of retinoblastoma: an extended follow-up. J Clin Oncol. 2005;23:2272–2279. doi: 10.1200/JCO.2005.05.054. [DOI] [PubMed] [Google Scholar]
- Kleinerman R A, Tucker M A, Abramson D H, Seddon J M, Tarone R E, Fraumeni J F., Jr Risk of soft tissue sarcomas by individual subtype in survivors of hereditary retinoblastoma. J Natl Cancer Inst. 2007;99:24–31. doi: 10.1093/jnci/djk002. [DOI] [PubMed] [Google Scholar]
- Marees T, Moll A C, Imhof S M, de Boer M R, Ringens P J, Leeuwen F E van. Risk of second malignancies in survivors of retinoblastoma: more than 40 years of follow-up. J Natl Cancer Inst. 2008;100:1771–1779. doi: 10.1093/jnci/djn394. [DOI] [PubMed] [Google Scholar]