Abstract
Previous research suggests that associative memory declines in normal aging and is severely affected by Alzheimer’s disease (AD); however, it is unclear whether and how this deficit can be minimized. The present study investigated whether emotional arousal enhances associative memory in healthy younger and older adults and patients with probable AD. We examined the effect of arousal on memory for item-location associations. Arousal enhanced memory for item location similarly across the three groups, while valence had no effect in any groups. Overall, our results suggest that arousal has beneficial effects on associative memory in healthy older adults and AD patients, as previously observed in younger adults.
Keywords: Memory binding, arousal, normal aging, Alzheimer’s disease
Memory binding refers to a person’s ability to remember various features of an object, a person, or an event together as a coherent whole. This is an essential component of episodic memory in everyday life; for instance, it is important to remember face-name associations to establish good relationships. Older adults may, in particular, experience difficulty remembering where they placed their personal belongings, such as car keys and eyeglasses, as a result of impaired recall of associations between items and locations. A number of laboratory studies have indeed demonstrated that older adults have a memory binding deficit compared to their younger counterparts (e.g., Chalfonte & Johnson, 1996; Kessels, Hobbel, & Postma, 2007; Mitchell, Johnson, Raye, Mather, & D’Esposito, 2000; Naveh-Benjamin, 2000; Pezdek, 1983).
People with Alzheimer’s disease (AD) face further challenges in remembering associative details. Associative learning deficits are an early sign of AD and associative learning tasks are used to help detect the disease (Blackwell et al., 2004; Fowler, Saling, Conway, Semple, & Louis, 2002; Lindeboom, Schmand, Tulner, Walstra, & Jonker, 2002; O’Connell et al., 2004). Moreover, associative learning tests can help differentiate AD from other types of dementia, such as vascular dementia and frontotemporal lobar degeneration (Lindeboom et al., 2002), and help predict who will develop Alzheimer’s disease (Fowler et al., 2002). AD patients have difficulty remembering various types of associative features, including item-location pairs commonly tested in the Paired Associative Learning Subtest of the Cambridge Neuropsychological Test Automated Battery (e.g., Swainson et al., 2001), item–color pairs (Parra et al., 2009), and interactive picture pairs, such as a monkey holding an umbrella (Lindeboom et al., 2002).
Despite the prevalence of memory binding impairments among older and AD individuals, little research has been conducted on whether and how this deficit can be minimized. Previous studies with younger adults, however, have demonstrated that emotional content can facilitate associative memory (Doerksen & Shimamura, 2001; Hadley & MacKay, 2006; Kensinger & Corkin, 2003; MacKay & Ahmetzanov, 2005). For instance, for younger adults, arousal elicited by target pictures predicts better memory for associations between items and their intrinsic features (picture-location combinations), a relationship that held up for both positive and negative pictures (Mather, Gorlick, & Nesmith, 2009; Mather & Nesmith 2008; Mather & Sutherland, 2009). However, Mather et al. (2009) found that the presence of an arousing item, regardless of its valence, had no effect on associative memory between that item and another item in the same scene. Hence, it seems that focused attention on an emotionally salient target enhances associative memory for within-item features that were the focus of attention, but yields no memory enhancement for between-item associations, as the other item was not the focus of attention. According to the arousal-biased competition theory (Mather & Sutherland, in press), an emotionally salient stimuli has high priority and therefore often wins the competition for attentional resources. This arousal-induced attentional focus on the target item results in deeper encoding of its associative information, which leads to memory enhancement. In this paper, we will call associative memory for within-item features that are focal to one’s attention ‘within-item memory binding.’ In contrast, we refer to associative memory for between-item features that are not central to one’s attention as ‘between-item memory binding’ (see Mather, 2007). Given the theoretical reasons to think that the effects of arousal should only be seen in within-item memory but not in between-item memory binding, the present study focused on investigating the effect of arousal on within-item associative memory.
Does emotional arousal enhance within-item memory binding in older adults, as previously observed in younger adults? The previous research investigating this question reveals a mixed picture. Kensinger, O’Brien, Swanberg, Garoff-Eaton, and Schacter (2007) examined whether emotional content improves younger and older adults’ reality-monitoring by having participants see or imagine neutral, positive, and negative items during the study phase and later testing them on whether the items were seen, imagined, or new. In Experiment 1, they found that younger adults showed enhanced memory for the source of negative items compared with positive and neutral items, whereas older adults did not show significant differences in reality monitoring for emotional and neutral items. In Experiment 2, with a different “imagined” task, both age groups showed reality-monitoring enhancement only for negative items. Similarly, another study demonstrated that older adults performed as well as younger adults in making associations between an item, its perceptually neutral information and its conceptually emotional information (e.g., the Horizon by Mazda was red and was rated as dangerous), whereas they showed impairments in making associations between an item, its perceptually neutral information and its conceptually neutral information (e.g., the Horizon by Mazda was a red luxury car; May, Rahhal, Berry, & Leighton, 2005). In a study examining memory for picture locations, Nashiro and Mather (in press) found that, whereas younger adults remembered the locations of emotional pictures better than the locations of neutral pictures, older adults did not show this arousal-enhanced memory binding. However, given evidence that the ability to detect emotionally arousing stimuli is relatively stable with normal aging (Knight et al., 2007; Leclerc & Kensinger, 2008; Mather & Knight, 2006) and that the effects of emotional arousal on memory remain similar in normal aging (Denburg, Buchanan, Tranel, & Adolphs, 2003; Kensinger, Brierley, Medford, Growdon, & Corkin, 2002), it seems possible that salience of emotional stimuli also facilitates older adults’ memory binding, even if it does so less than for younger adults. Thus, one goal of the current study was to see if we could find evidence of arousal-enhanced memory binding in older adults, and if so, if it would be similar for positive and negative arousing stimuli or only appear for negative stimuli as in Kensinger et al.’s (2007) study.
Does emotional arousal mitigate within-item memory-binding deficits in patients with AD? To minimize these patients’ memory deficits, it may be possible to use their remaining skills. Some studies suggest that the ability to process emotional information is relatively preserved in AD patients compared with their general cognitive ability (Bucks & Radford, 2004; Koff, Zaitchik, Montepare, & Albert, 1999) and compared with normal controls (Lavenu, Pasquier, Lebert, Petit, & Van der Linden, 1999; Luzzi, Piccirilli, & Provinciali, 2007; Roudier et al., 1998). Furthermore, there is some evidence that their ability to recall emotional items is better than their ability to recall non-emotional items (Kazui, Mori, Hashimoto, & Hirono, 2003; Moayeri, Cahill, Jin, & Potkin, 2000; Satler et al. 2007). However, these findings are challenged by other studies demonstrating that emotional content has no effect on AD patients’ memory (Abrisqueta-Gomez, Bueno, Oliveira, & Bertolucci, 2002; Budson et al., 2006). The discrepancy seems to come from the fact that stimuli used in these experiments vary in intensity; prior studies demonstrating emotion-enhanced memory in AD patients used stimuli with high emotional intensity, such as natural disasters and the 9/11 terrorist attack, whereas those showing inconsistent results used stimuli with relatively low emotional intensity (for a review, see Kensinger, 2006). Taken together, these results suggest that AD patients may benefit from emotional salience of highly arousing stimuli in order to enhance within-item memory binding.
To summarize, evidence from previous studies indicates that older adults have memory binding deficits relative to their younger counterparts, and that associative memory and learning are even more severely affected by AD. Previous research has also suggested that emotional arousal enhances younger adults’ within-item memory binding, but it is unclear whether the same effect applies to healthy older adults and patients with AD. Since in previous studies, AD patients demonstrated emotional memory enhancement only for materials with high intensity, we selected stimuli pre-rated high in arousal (higher than 5 on a scale of 1 to 9, with 1 being not at all arousing and 9 being extremely arousing). This allowed us to examine the effect of a high level of arousal on item memory and memory binding.
Experiment 1
Method
Participants
We recruited 18 younger adults (Mage=20.72 years, 3 males, 15 females, age range 18–25 years, Meducation=14.61 years), 18 older adults over 60 years old who had not been diagnosed with dementia or other cognitive disorders from various retirement communities (Mage=72.67 years, 6 males, 12 females, age range 62–83 years, Meducation=15.03 years), and 18 patients with a clinical diagnosis of probable Alzheimer’s disease from Alzheimer’s Association early stage support groups in the Lafayette, Mountain View, Santa Cruz, and Rancho Mirage offices in California (Mage=72.44 years, 11 males, 7 females, age range 55–86 years, Meducation=16.22 years). Neuropsychological assessments were conducted by their respective physicians, neurologists, psychiatrists, and neuropsychologists. The neuropsychological tests used for the diagnosis of AD varied by clinician and included the Dementia Rating Scale (Mattis, 1988), Neurobehavioral Cognitive Status Examination (Mueller, Kiernan, & Langston, 2001), Wechsler Memory Scale III Logical Memory (Wechsler, 1997), and Delis-Kaplan Executive Function Scale (Delis, Kramer, &, Kaplan, 2001). To confirm the diagnoses of the disease, we asked participants to provide their diagnostic records.
In our experiment, we administered the Consortium To Establish A Registry Of Alzheimer’s Disease (CERAD) word list memory test (Welsh et al., 1994) to all participants except for one healthy older adult who had time constraints. In this test, participants learned a list of 10 words and were later asked to recall and recognize them. In the standard CERAD, recall and recognition tests are given across three-time periods. In the current study, due to the time constraints, we administered the tests once immediately after the learning phase; thus, the average scores would have been slightly lower if we had used the standard procedures. There were significant group differences in the proportion of words recalled (Myoung=.57, Range=.30–.80; Mold=.46, Range=.10–1.00; MAD=.21, Range=.00–.50), F(2,50)=18.13, MSE=.04, p<.001, and in corrected recognition scores (hits- false alarm rates) for words (Myoung=.97, Range=.80–1.00; Mold=.84, Range=.40–1.00; MAD=.53, Range=.00–.90), F(2,50)=29.66, MSE=.03, p<.001. Post hoc test revealed that younger and older controls preformed significantly better than the AD group on both tests, but there were no significant differences between the younger and healthy older groups.
Due to the different gender ratio among the three participant groups, we included gender as a covariate in all analyses. There were no significant effects of gender in any of the analyses; hence, we will not further discuss gender. Participants received monetary compensation, and the experiments were conducted either in the lab, at participants’ homes or at senior centers, using a laptop.
Materials
We used 64 photographs from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 1999) and from other sources. The photographs consisted of matched pairs of neutral and arousing pictures that were similar in appearance, complexity, content and focus of interest (for examples see Mather & Nesmith, 2008). We used IAPS ratings and pre-ratings by 10 undergraduates for picture selection and categorization (neutral, arousing-positive, and arousing-negative). We intermixed the arousing and non-arousing photographs and made two sets of 32 photographs (Set A, Set B) each of which contained an equal number of arousing and non-arousing photographs and had only one version from any matched picture pair. For example, Set A contained a neutral version from photograph pair 1, and therefore did not have the arousing version from that pair. Participants were randomly assigned to view Set A or Set B. Of the 32 photographs seen by each participant, 16 were neutral, and 16 were arousing images. Half of the arousing items were positive, and half were negative.
We also used 32 abstract shapes as stimuli, each of which was presented with a photograph. Picture-shape pairs were displayed with our initial purpose of examining memory for pairs as a test of between-item memory binding. However, the results showed that AD and older groups’ pair memory performance was at floor level. Thus, we will not discuss this further. We used PsyScope software (Cohen, MacWhinney, Flatt, & Provost, 1993) to present the stimuli and record the participants’ responses. The screen was divided into 3 × 3 grids, the outer eight cells of which were used to present the images. On each slide, one picture and one shape simultaneously appeared in two of the eight cells. One image (either a picture or a shape) was always located higher than the other. Each stimulus type appeared in each location equally frequently.
Procedure
Encoding was incidental; participants were instructed to view the items on the screen as if they were watching a slide show and were not informed about the upcoming memory tests. There were six blocks, each with 16 picture-shape pairs shown one pair at a time. Participants viewed all 32 picture-shape pairs in the assigned set across the first two blocks, which were then repeated in the second two blocks and the third two blocks in the same order. In order to keep the participants engaged, we asked them to do various encoding tasks. In three of the six blocks, each picture-shape pair was presented in two of the eight outer cells for 5000 ms. Immediately after the two images disappeared, either a blue or red dot was randomly presented in one of the eight outer cells of the screen with the question “is the dot blue or red?” in the center of the screen. The participants were asked to indicate their answers by pressing one of the keys labeled with a blue sticker or a red sticker. In the other three blocks, the following question appeared in the center of the screen during stimulus presentations: “is the picture higher (H) or lower (L) than the shape?” The participants responded by pressing one of the keys marked “H” or “L”, at which point the next pair was presented. The two questions alternated between blocks, and the order of which question came first was randomized (i.e., half of the participants saw the first 16 picture-shape pairs in Blocks 1, 3, and 5 with the blue/red question and saw the second 16 picture-shape pairs in Blocks 2, 4, 6 with the high/low question. The other half saw the first 16 pairs in Blocks 1, 3, and 5 with the high/low question and saw the second 16 pairs in Blocks 2, 4, 6 with the blue/red question). The types of questions participants received during encoding had no significant effects on their memory performance.
Immediately after the encoding phase, participants completed recall and location memory tests. In the recall test, participants were asked to describe as many pictures as they could remember in as much detail as possible. The location memory test assessed within-item memory binding, or how well participants remembered combinations of pictures and an intrinsic feature of the picture (its location). The test was a two-alternative forced choice in which each of the 32 pictures was presented in two different locations with number labels (one in its original location and another in a new location). Participants were asked to indicate in which location they believed the picture had appeared in during the encoding trials by pressing one of the keys marked 1 through 8. A pair memory test was given last to examine how well participants remembered picture-shape combinations. As mentioned earlier, these pair memory results will not be presented here since both groups’ performance was near floor level.
Results
Item memory
During free recall, the experimenter documented participants’ descriptions of pictures. Two coders later evaluated the accuracy of the descriptions, coding participants’ descriptions with numbers that corresponded with each of the pictures. Inter-rater reliability was .96; the coders discussed discrepancies until mutual agreement was reached. One point was given for each accurately described picture, and the total points were calculated for each participant. The proportion of pictures recalled of each type was computed.
A 3 (group: younger, older and AD) × 2 (arousal type: arousing and non-arousing) repeated-measures ANOVA revealed a main effect of group (Myoung=.29, SE=.02; Mold=.23, SE=.02; MAD=.06, SE=.02), F(2,51)=28.27, MSE=.02, p<.001, ηp2=.53. Tukey’s post hoc analysis showed that younger and older adults recalled a significantly greater proportion of pictures than did the AD group (p<.001 for both comparisons), whereas younger and older adults did not differ significantly (p=.22). There was a main effect of arousal, indicating that arousing pictures were more likely to be recalled than non-arousing pictures (Marousing=.29, SE=.02; Mnon-arousing =.09, SE=.01), F(51,1)=86.83, MSE=.01, p<.001, ηp2=.63. We also found an interaction between group and arousal, F(52,2)=14.16, MSE=.01, p<.001, ηp2=.36 (see Table 1 for all means and standard errors). Separate analyses for each group indicated that all groups recalled significantly more arousing than non-arousing pictures; however, the effect of arousal was greater in younger adults, F(1,17)=53.02, MSE=0.01, p<.001, ηp2=.78, and healthy older adults, F(1,17)=33.70, MSE=0.02, p<.001, ηp2=.67, than in AD patients, F(1,17)=5.64, MSE=0.01, p<.05, ηp2=.25.
Table 1.
Item and Location Memory Accuracy for Arousing (Negative and Positive) and Non-arousing Items. For Experiment 1, proportion of total items of that type that were recalled is reported; for Experiment 2, corrected recognition (hits – false alarms) is reported. Location memory is the proportion of responses that were correct.
| Experiment 1 | Experiment 2 | ||||||
|---|---|---|---|---|---|---|---|
| Recall | Younger | Older | AD | Recognition | Younger | Older | AD |
| Arousing | .42 (.04) | .38 (.04) | .08 (.04) | Arousing | .88 (.05) | .79 (.05) | .53 (.05) |
| negative | .41 (.04) | .33 (.04) | .06 (.04) | negative | .84 (.05) | .78 (.05) | .60 (.06) |
| positive | .43 (.04) | .42 (.04) | .10 (.04) | positive | .92 (.05) | .79 (.05) | .46 (.06) |
| Non-arousing | .15 (.02) | .09 (.02) | .04 (.02) | Non-arousing | .81 (.05) | .70 (.05) | .54 (.05) |
|
| |||||||
| Location | Younger | Older | AD | Location | Younger | Older | AD |
| Arousing | .89 (.04) | .85 (.04) | .63 (.04) | Arousing | .86 (.03) | .82 (.03) | .64 (.04) |
| negative | .89 (.04) | .89 (.04) | .66 (.04) | negative | .85 (.05) | .81 (.05) | .68 (.05) |
| positive | .90 (.05) | .84 (.05) | .59 (.05) | positive | .87 (.04) | .83 (.04) | .60 (.05) |
| Non-arousing | .86 (.04) | .81 (.04) | .59 (.04) | Non-arousing | .87 (.04) | .70 (.04) | .59 (.04) |
Note: Standard errors are in parentheses.
To examine the effect of valence, we conducted a 3 (group) × 2 (valence: positively vs. negatively arousing) repeated-measures ANOVA. We found a main effect of valence (Mnegative =.26, SE=.03; Mpositive =.32, SE=.02), F(1,51)=5.84, MSE=.01, p<.05, ηp2=.10, indicating that participants across the groups recalled more positive than negative pictures. There was no significant interaction between group and valence (see Table 1).
Location memory (within-item memory binding)
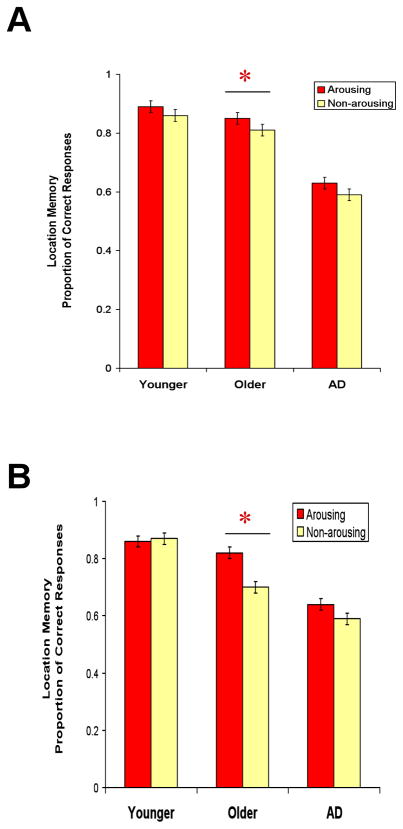
We used a 3 (group) × 2 (arousal type) repeated-measures ANOVA to examine the proportion of location forced-choice responses that consisted of correct responses. There was a main effect of group (Myoung=.88, SE=.04; Mold=.83, SE=.04; MAD=.61, SE=.04), F(2,51)=15.17, MSE=.05, p<.001, ηp2=.37. Tukey’s post hoc analysis showed that younger and older adult controls performed significantly better than the AD group (p<.001 for both comparisons), whereas younger and older adults did not differ significantly (p=.67). There was a main effect of arousal indicating that arousal enhanced location memory (Marousing=.79, SE=.02; Mnon-arousing =.76, SE=.02), F(2,51)=5.28, MSE=.01, p<.05, ηp2=.09, but no interaction between group and arousal, F(2,51)=.08, MSE=.01, p=.92, ηp2=.003 (see Table 1).
The effect of valence on location memory was analyzed with a 3 (group) × 2 (positively vs. negatively arousing) repeated-measures ANOVA. There was no main effect of valence and no interaction between group and valence (see Table 1). The AD group appeared to benefit more from negative than positive context but the difference was not significant (p=.25).
Discussion
All groups recalled a greater number of arousing and non-arousing items. However, the effect of arousal on recall was smaller in the patient group than control groups, suggesting that the benefit of emotional content on item memory is present but does diminish with the disease. Moreover, participants across groups demonstrated similar memory enhancement for the locations of arousing items than the locations of non-arousing items.
In contrast with this finding of consistent arousal-enhanced location memory across age groups, in a previous study, we found that healthy older adults showed no arousal-based enhancement in within-item memory binding (Nashiro & Mather, in press). The encoding procedures in the two studies were identical except that we repeated stimuli twice in the current experiment rather than once in the previous study. Thus, one possible explanation for why the current study found more evidence for arousal-enhanced memory binding than the previous study was that, while reducing encoding load by repeating items more frequently, repetition increased the effect of arousal on location memory for older adults. Thus, in the second experiment we tried increasing repetition further to see if we could replicate the current results.
One limitation of Experiment 1 was the unbalanced gender ratio in our sample; we had fewer males than females in the younger and older groups. Our initial analyses indicated that gender had no effect on item memory and memory binding. However, given previous findings on gender differences in emotional memory at both behavioral and neural levels (e.g., Canli, Desmond, Zhao, & Gabrieli, 2002), it is important to further clarify that our results were not specific to any gender. In the next experiment, we attempted to have a more balanced gender ratio within and across groups.
Experiment 2
In Experiment 2, we were interested in replicating and extending the results from Experiment 1. As repetition seemed to increase the effect of arousal for older adults, we increased the number of repetitions from two to three. In addition, in order to further reduce cognitive load during encoding, we presented fewer items (16 pictures instead of 32). With these changes, we aimed to determine 1) whether the memory advantage for arousing stimuli observed in recall in Experiment 1 would replicate for recognition memory, and 2) whether the enhanced location memory for arousing pictures would replicate.
Method
Participants
We recruited 24 younger adults (Mage=19.17 years, 7 males, 17 females, age range 18–21 years, Meducation=12.88 years), 24 older adults over 60 who had not been diagnosed with dementia or other cognitive disorders from various retirement communities (Mage=74.89 years, 7 males, 17 females, age range 65–89 years, Meducation=13.18 years), and 18 patients with a clinical diagnosis of probable Alzheimer’s disease from Alzheimer’s Association early stage support groups in the Lafayette, Mountain View, and Santa Cruz offices in California (Mage=73.50 years, 8 males, 10 females, age range 58–90 years, Meducation=16.83 years). Neuropsychological assessments were conducted by their respective physicians, neurologists, psychiatrists, and neuropsychologists. To confirm the diagnoses of the disease, we asked participants to provide their diagnostic records. All AD participants were diagnosed with mild probable Alzheimer’s disease within two years prior to participating in this study. No patient had a history of stroke, head injury, or other neurological illness.
The CERAD was conducted in the same manner as in Experiment 1. One healthy older adult and one AD patient did not complete the tests due to time constraints. Compared with the AD group, younger and older controls had significantly higher scores on the word recall test (Myoung=.48, Range=.20–.80; Mold=.45, Range=.20–.80; MAD=.16, Range=.00–.50), F(2,61)=25.56, MSE=.02, p<.001, and on the recognition test (hits-false alarm rates), (Myoung=.89, Range=.70–1.00; Mold=.86, Range=.50–1.00; MAD=.45, Range=.00–.90), F(2,61)=31.28, MSE=.04, p<.001. There was no difference between the younger and older control groups on either test.
Due to the different gender ratio and levels of education in the two participant groups, we included gender and education as covariates in all analyses. There were no significant effects of gender and education in any of the analyses; hence, we will not discuss them further. Participants received monetary compensation, and the experiments were conducted either in the lab, at participants’ homes or at senior centers, using a laptop.
Materials
We used the same 64 photographs as in Experiment 1, which were divided into Set A and Set B. Participants were randomly assigned to view either set. Half of the assigned set was used as study materials, and the other half as lures in the recognition test. Which half of the set was presented during the study phase was randomized, and each set contained an equal number of neutral and arousing photographs. In order to keep the procedure consistent with that in Experiment 1, we presented the 32 shapes used in Experiment 1 together with the photographs.
Procedure
The procedure was the same as that in Experiment 1 except for the following modifications. There were four blocks of trials; in each of these participants viewed the same set of 16 photograph-shape pairs one at a time. Various encoding tasks were given to the participants to keep them engaged. The encoding task in Block 1 and 4 were the same as the dot color identification task used in Experiment 1 (participants indicated whether the dot was blue or red between trials). The task in Block 2 was identical to the other encoding task described in Experiment 1 in which participants indicated whether the photograph was higher or lower than the shape. In Block 3, participants were asked: “is the photograph bigger (B) or smaller (S) than the shape?” Participants answered by pressing one of the keys marked “B” or “S”, at which point the next pair was presented.
Immediately after the encoding phase, participants completed recognition and location memory tests. In the recognition test, we randomly presented 16 studied and 16 non-studied photographs and asked participants to indicate whether they had seen the pictures in the study session by pressing one of the keys marked “YES” or “NO”. The location memory test was the same as that in Experiment 1. A pair memory test was given last, but the results will not be discussed here, as again, performance was near floor on this test.
Results
Item Memory
Corrected recognition scores were calculated (hits - false alarm rates). A 3 (group: younger, older and AD) × 2 (arousal type: arousing and non-arousing) repeated-measures ANOVA revealed a main effect of group (Myoung=0.85, SE=0.04; Mold=0.74, SE=0.04; MAD=0.53, SE=.05), F(2,63)=12.91, MSE=0.08, p<.001, ηp2=.29. Tukey’s post hoc analysis showed that younger and older adults recognized a significantly greater proportion of pictures than did the AD group (p<.001 and p=.004, respectively), whereas younger and older adults did not differ significantly (p=.18). There was a main effect of arousal (Marousing=0.73, SE=0.03; Mnon-arousing=0.68, SE=0.03), F(2,63)=4.21, MSE=0.02, p<.05, ηp2=.06, but no interaction between group and arousal (p=.28). A 3 (group) × 2 (valence: positively vs. negatively arousing) repeated-measures ANOVA revealed that there was no main effect of valence but a significant interaction between group and valence, F(2,63)=3.60, MSE=.03, p<.05, ηp2=.10 (see Table 1). Further analyses suggested that the AD group had better recognition memory for negative than positive pictures, F(1,17)=4.21, MSE=.04, p=.06, ηp2=.20, although the difference was only marginally significant. Neither younger nor older healthy controls showed a significant difference between memory for negative and positive pictures.
In addition, the hit and false alarm rates were separately examined. A 3 (group: younger, older and AD) × 2 (arousal type: arousing and non-arousing) repeated-measures ANOVA of hit rates revealed no significant findings. The same analysis for false alarm rates found a main effect of group, F(2,63)=19.42, MSE=.03, p<.001, ηp2=.38 (see Table 2 for all means and standard errors). Tukey’s post hoc analysis suggested that AD patients made more false alarms than did the younger and older groups (p<.001 for both comparisons), whereas younger and older adults did not differ significantly (p=.507). A 3 (group) × 2 (valence: positively vs. negatively arousing) repeated-measures ANOVA of hit rates revealed a main effect of group, F(2,63)=3.41, MSE=.06, p<.05, ηp2=.10, suggesting that younger adults obtained significantly more hits than did AD patients. The same analysis for false alarm rates found a main effect of group, F(2,63)=14.58, MSE=.04, p<.001, ηp2=.32, suggesting that AD patients made significantly more false alarms than did the two other groups. Overall, we did not find the effects of emotion and valence on false alarm rates in any groups (c.f., Gallo, Foster, Wong, & Bennett, 2010, Kapucu, Rotello, Ready, & Seidl, 2008; Thomas & Hasher, 2006). However, this lack of effects of valence on false alarms may have been due to very low false alarm rates in the younger and older groups.
Table 2.
Item Hit and False Alarm (FA) Rates for Arousing (Negative and Positive) and Non-arousing Items in Experiment 2
| Hits | Younger | Older | AD |
|---|---|---|---|
| Arousing | .89 (.03) | .82 (.03) | .75 (.04) |
| negative | .85 (.04) | .82 (.04) | .78 (.05) |
| positive | .92 (.04) | .82 (.04) | .72 (.05) |
| Non-arousing | .82 (.04) | .76 (.04) | .78 (.05) |
|
| |||
| FAs | Younger | Older | AD |
|
| |||
| Arousing | .01 (.03) | .04 (.03) | .22 (.03) |
| negative | .01 (.03) | .04 (.03) | .18 (.04) |
| positive | .00 (.03) | .03 (.03) | .26 (.04) |
| Non-arousing | .01 (.03) | .06 (.03) | .24 (.03) |
Note: Standard errors are in parentheses.
Location Memory
Location memory accuracy was calculated by the proportion of correct responses of the total responses. A 3 (group) × 2 (arousal type) repeated-measure ANOVA revealed a main effect of group (Myoung=0.86, SE=0.03, Mold=0.76, SE=0.03, MAD=0.62, SE=0.04), F (2,63)=15.11, MSE=0.04, p<.001, ηp2=.32. Tukey’s post hoc analysis showed that younger and older adults performed significantly better than the AD group (p<.001 and p=.005, respectively), whereas younger and older adults did not quite differ significantly (p=.05). There was a main effect of arousal (Marousing=0.77, SE=0.02; Mnon-arousing=0.72, SE=0.02), F (2,63)=5.60, MSE=0.02, p<.05, ηp2=.08, but no significant interaction between group and arousal. The results were consistent with those in Experiment 1, indicating an overall arousal-based enchantment of location memory that did not significantly differ between the groups.
The effect of valence on location memory was analyzed with a 3 (group) × 2 (positively vs. negatively arousing) repeated-measures ANOVA. There was no main effect of valence and no interaction between group and valence (see Table 1).
Discussion
Arousal enhanced item memory for healthy younger and older adults, but the effect was diminished for AD patients. The results of location memory were also similar to those in Experiment 1. We found a main effect of arousal but no group by arousal interaction; suggesting that, in general, participants had better memory for the locations of arousing than non-arousing pictures. Valence had little influence on location memory in all groups.
General Discussion
The current study examined the effect of arousal on item memory and within-item memory binding in healthy younger and older adults and AD patients. A previous study we conducted using similar methods (Nashiro & Mather, in press) revealed that, whereas younger adults showed arousal-enhanced location memory, older adults did not. Given mixed findings in previous research, the current study further probed the effects of arousal on older adults’ location memory to see if there are any benefits of emotional arousal for location memory binding when using easier memory tasks than in Nashiro and Mather’s (in press) study.
The results from two experiments revealed that healthy older adults and AD patients remembered a greater number of arousing than non-arousing items, indicating that emotional arousal enhances item memory in normal aging and in AD, although the item memory enhancement effect was smaller in the patient group. Importantly, participants across groups had better memory for the locations of arousing than non-arousing pictures, whereas valence had little influence on location memory. The results demonstrate that the effects of emotional salience on within-item memory binding previously found in younger adults (Mather et al., 2009; Mather & Nesmith 2008; Mather & Sutherland, 2009) are similarly present in older adults with and without AD.
Task difficulty may play a role in determining the size of the arousal effect for older adults with and without AD. In Nashiro and Mather’s (in press) study, healthy older participants viewed 32 pictures twice and showed no arousal enhancement in location memory. In Experiment 1 of the current study, we presented the same number of items three times, and older participants showed better location memory for arousing than non-arousing pictures. In Experiment 2 of the present study, we reduced encoding load further by increasing the number of presentations to four and reducing the number of pictures to 16. In Experiment 1 and 2, older adults showed an effect of arousal on location memory, with a larger effect in Experiment 2 (Ms = .85 vs. .81 for arousing vs. non-arousing in Exp. 1 and Ms = .82 vs. .70 in Exp. 2). One possible interpretation of these results is that combining the benefits of emotional salience and adequate exposure to items in the present study led to location memory enhancement in healthy older adults. Since older adults have age-related memory binding deficits to begin with, they may need more exposure to stimuli than younger adults to benefit from arousing content to enhance within-item feature binding. In the case of AD patients, it is unclear whether more exposure to items would benefit them since they showed similar effects of arousal on location memory in Experiments 1 and 2 (Ms = .63 vs. .59 for arousing vs. non-arousing in Exp. 1 and Ms = .64 vs. .59 in Exp. 2).
Why would arousal enhance within-item memory binding? The arousal-biased competition theory (Mather & Sutherland, in press) proposes that an emotional item has high priority and captures attention when there is no other competing high priority information. This arousal-induced attention enhances within-item feature binding, leading to deeper encoding and thus better retention of bound information (see Mather & Sutherland, in press, for further discussion of this issue). Previous research has shown that the ability to detect emotionally arousing stimuli is relatively stable with normal aging (Knight et al., 2007; Leclerc & Kensinger, 2008; Mather & Knight, 2006). Prior evidence also suggests that AD patients have normal physiological responses to emotional stimuli (Hoefer et al., 2008) and the ability to process emotional information as well as do healthy controls (Lavenu et al., 1999; Luzzi et al. 2007; Roudier et al., 1998). Furthermore, the effects of emotional arousal on memory remain similar in normal aging (Denburg et al., 2003; Kensinger et al., 2002) and are relatively well preserved in AD (Kazui, Mori, Hashimoto, & Hirono, 2003; Moayeri, Cahill, Jin, & Potkin, 2000; Satler et al. 2007). Preserved arousal enhancement of memory in both groups seem to compensate for their memory binding impairment when memory tasks are relatively easy, as evidenced by our finding that participants across groups had better location memory for arousing than non-arousing pictures. Presumably, arousing components of items attracted their attention, which strengthened perceptual binding and hence enhanced memory for within-item features.
Our results were in line with previous studies suggesting that emotional information embedded in target items enhanced older adults’ source memory (e.g., May, Rahhal, Berry, & Leighton, 2005; Rahhal, May, & Hasher, 2002). However, there is also counter-evidence suggesting that neither younger nor older adults showed benefits of emotional content on source memory (Davidson, McFarland, & Glisky, 2006). One possible explanation for this discrepancy is differences in levels of task difficulty across studies. As described earlier, our previous and current results together suggest that healthy older adults benefit more from emotional content when tasks are relatively easy. Thus, it is possible that studies suggesting no effect of emotion on source memory used tasks that are cognitively challenging for older adults. However, the fact that Davidson et al. (2006) failed to replicate previous findings on beneficial effects of emotion on source memory by using similar methods as those in previous studies (Doerksen & Shimamura, 2001; Kensinger & Corkin, 2003) warrants further investigation into discrepancies across studies.
One limitation of the current study was younger adults’ possible ceiling effects in location memory, indicating that the tasks were too easy for them, which may have reduced any potential arousal effects. Future studies should use more appropriate levels of task difficulty for each group to avoid this issue. Another limitation of the current study was the small number of stimuli. Although the low number of stimuli was intentionally selected, it might have been limited our ability to observe larger effects of emotion on memory performance. Despite these limitations, the current study provides important information about the benefits of emotional arousal on older adults’ and AD patients’ memory binding. These findings may lead to strategies to help reduce memory-binding impairments previously observed in both groups.
Figure 1.
(A) In Experiment 1, healthy older adults showed better location memory for arousing than non-arousing pictures, but the AD group did not show this advantage. (B) Experiment 2 replicated the finding of Experiment 1.
Acknowledgments
Funding
This work was supported by grants from the National Institute on Aging (R01AG025340, K02 AG032309 and 5T32AG000037).
References
- Abrisqueta-Gomez J, Bueno OF, Oliveira MG, Bertolucci PH. Recognition memory for emotional pictures in Alzheimer’s patients. Acta Neurologica Scandinavica. 2002;105:51–4. doi: 10.1034/j.1600-0404.2002.00035.x. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal volume in men: a magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Basso M, Yang J, Warren L, MacAvoy MG, Varma P, Bronen RA, van Dyck CH. Volumetry of amygdala and hippocampus and memory performance in Alzheimer’s disease. Psychiatry Research: Neuroimaging. 2006;146:251–261. doi: 10.1016/j.pscychresns.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Blackwell AD, Sahakian BJ, Vesey R, Semple JM, Robbins TW, Hodges JR. Detecting dementia: Novel neuropsychological markers of preclinical Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders. 2004;17:42–48. doi: 10.1159/000074081. [DOI] [PubMed] [Google Scholar]
- Bucks RS, Radford SA. Emotion processing in Alzheimer’s disease. Aging and Mental Health. 2004;8:222–232. doi: 10.1080/13607860410001669750. [DOI] [PubMed] [Google Scholar]
- Budson AE, Todman RW, Chong H, Adams EH, Kensinger EA, Krangel TS, Wright CI. False recognition of emotional word lists in aging and Alzheimer disease. Cognitive and Behavioral Neurology. 2006;19:71–78. doi: 10.1097/01.wnn.0000213905.49525.d0. [DOI] [PubMed] [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Gabrieli JDE. Sex differences in the neural basis of emotional memories. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10789–10794. doi: 10.1073/pnas.162356599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfonte BL, Johnson MK. Feature memory and binding in young and older adults. Memory & Cognition. 1996;24:403–416. doi: 10.3758/bf03200930. [DOI] [PubMed] [Google Scholar]
- Chee MWL, Goh JOS, Venkatraman V, Tan JC, Gutchess A, Sutton B, Park D. Age-related changes in object processing and contextual binding revealed using fMR adaptation. Journal of Cognitive Neuroscience. 2006;18:495–507. doi: 10.1162/jocn.2006.18.4.495. [DOI] [PubMed] [Google Scholar]
- Cohen J, MacWhinney B, Flatt M, Provost J. Psy-Scope: An interactive graphic system for designing and controlling experiments in the psychology laboratory using Macintosh computers. Behavior Research Methods, Instruments, & Computers. 1993;25:257–271. [Google Scholar]
- Cuénod CA, Denys A, Michot JL, Jehenson P, Forette F, Kaplan D, Boller F. Amygdala atrophy in Alzheimer’s disease. An in vivo magnetic resonance imaging study. Archives of Neurology. 1993;50:941–945. doi: 10.1001/archneur.1993.00540090046009. [DOI] [PubMed] [Google Scholar]
- Davidson PSR, McFarland CR, Glisky EL. Effects of emotion on item and source memory in young and older adults. Cognitive, Affective & Behavioral Neuroscience. 2006;6:306–322. doi: 10.3758/cabn.6.4.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: insights from relational and item-based learning. Journal of Neurophysiology. 2002;88:982–90. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E. The Delis-Kaplan Executive Function Scale. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Denburg NL, Buchanan TW, Tranel D, Adolphs R. Evidence for preserved emotional memory in normal older persons. Emotion. 2003;3:239–253. doi: 10.1037/1528-3542.3.3.239. [DOI] [PubMed] [Google Scholar]
- Doerksen S, Shimamura AP. Source memory enhancement for emotional words. Emotion. 2001;1:5–11. doi: 10.1037/1528-3542.1.1.5. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Bunsey M. On the binding of associations in memory: Clues from studies on the role of the hippocampal region in paired-associate learning. Current Directions in Psychological Science. 1995;4:19–23. [Google Scholar]
- Fowler KS, Saling MM, Conway EL, Semple JM, Louis WJ. Paired associate performance in the early detection of DAT. Journal of the International Neuropsychological Society. 2002;8:58–71. [PubMed] [Google Scholar]
- Gallo DA, Foster KT, Wong JT, Bennett DA. False recollection of emotional pictures in Alzheimer’s disease. Neuropsychologia. 2010;48:3614–3618. doi: 10.1016/j.neuropsychologia.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh JO, Chee MW, Tan JC, Venkatraman V, Hebrank A, Leshikar ED, Park DC. Age and culture modulate object processing and object-scene binding in the ventral visual area. Cognitive Affective & Behavioral Neuroscience. 2007;7:44–52. doi: 10.3758/cabn.7.1.44. [DOI] [PubMed] [Google Scholar]
- Hadley CB, Mackay DG. Does emotion help or hinder immediate memory? Arousal versus priority-binding mechanisms. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2006;32:79–88. doi: 10.1037/0278-7393.32.1.79. [DOI] [PubMed] [Google Scholar]
- Hoefer M, Allison SC, Schauer GF, Neuhaus JM, Hall J, Dang JN, Rosen HJ. Fear conditioning in frontotemporal lobar degeneration and Alzheimer’s disease. Brain: A Journal of Neurology. 2007;131:1646–1657. doi: 10.1093/brain/awn082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K, Yamaguchi K, Watanabe M, Kainuma E, Hikake N, Saitoh S, Kimura R. Crucial role of thalami and basal ganglia in emotional memory and cognition: Association with the recognition of Niigata Ken Chuetsu earthquake 2004. Psychogeriatrics. 2007;7:58–63. [Google Scholar]
- Kapucu A, Rotello CM, Ready RE, Seidl KN. Response bias in “remembering” emotional stimuli: A new perspective on age differences. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34:703–711. doi: 10.1037/0278-7393.34.3.703. [DOI] [PubMed] [Google Scholar]
- Kazui H, Mori E, Hashimoto M, Hirono N. Enhancement of declarative memory by emotional arousal and visual memory function in Alzheimer’s disease. The Journal of Neuropsychiatry and Clinical Neurosciences. 2003;15:221–226. doi: 10.1176/jnp.15.2.221. [DOI] [PubMed] [Google Scholar]
- Kensinger EA. Remembering emotional information: Effects of aging and Alzheimer’s disease. In: Welsh EM, editor. Fronteirs in Alzheimer’s disease research. Hauppauge, NY: Nova Science; 2006. pp. 213–226. [Google Scholar]
- Kensinger EA, Brierley B, Medford N, Growdon JH, Corkin S. Effects of normal aging and Alzheimer’s disease on emotional memory. Emotion. 2002;2:118–134. doi: 10.1037/1528-3542.2.2.118. [DOI] [PubMed] [Google Scholar]
- Kensinger E, Corkin S. Memory enhancement for emotional words: Are emotional words more vividly remembered than neutral words? Memory & Cognition. 2003;31:1169–1180. doi: 10.3758/bf03195800. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, O’Brien JL, Swanberg K, Garoff-Eaton RJ, Schacter DL. The effects of emotional content on reality-monitoring performance in young and older adults. Psychology And Aging. 2007;22:752–764. doi: 10.1037/0882-7974.22.4.752. [DOI] [PubMed] [Google Scholar]
- Kessels RPC, Hobbel D, Postma A. Aging, context memory and binding: A comparison of “what, where and when” in young and older adults. International Journal of Neuroscience. 2007;117:795–810. doi: 10.1080/00207450600910218. [DOI] [PubMed] [Google Scholar]
- Knight M, Seymour TL, Gaunt JT, Baker C, Nesmith K, Mather M. Aging and goal-directed emotional attention: Distraction reverses emotional biases. Emotion. 2007;7:705–714. doi: 10.1037/1528-3542.7.4.705. [DOI] [PubMed] [Google Scholar]
- Koff E, Zaitchik D, Montepare J, Albert MS. Emotion processing in the visual and auditory domains by patients with Alzheimer’s disease. Journal of the International Neuropsychological Society. 1999;5:32–40. doi: 10.1017/s1355617799511053. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. The international affective picture system (IAPS): Technical manual and affective ratings. Gainesville, FL: University of Florida, The Center for Research in Psychophysiology; 1999. [Google Scholar]
- Lavenu I, Pasquier F, Lebert F, Petit H, Van der Linden M. Perception of emotion in frontotemporal dementia and in Alzheimer’s disease. Alzheimer Disease & Associated Disorders. 1999;13:96–101. doi: 10.1097/00002093-199904000-00007. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA. Effects of age on detection of emotional information. Psychology and Aging. 2008;23:209–215. doi: 10.1037/0882-7974.23.1.209. [DOI] [PubMed] [Google Scholar]
- Lindeboom J, Schmand B, Tulner L, Walstra G, Jonker C. Visual association test to detect early dementia of the Alzheimer type. Journal of Neurology, Neurosurgery, and Psychiatry. 2002;73:126–133. doi: 10.1136/jnnp.73.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzi S, Piccirilli M, Provinciali L. Perception of emotions on happy/sad chimeric faces in Alzheimer disease: relationship with cognitive functions. Alzheimer Disease & Associated Disorders. 2007;21:130–135. doi: 10.1097/WAD.0b013e318064f445. [DOI] [PubMed] [Google Scholar]
- MacKay DG, Ahmetzanov MV. Emotion, memory, and attention in the taboo Stroop paradigm: An experimental analogue of flashbulb memories. Psychological Science. 2005;16:25–32. doi: 10.1111/j.0956-7976.2005.00776.x. [DOI] [PubMed] [Google Scholar]
- Mather M. Emotional arousal and memory binging: An object-based framework. Perspectives on Psychological Science. 2007;2:33–52. doi: 10.1111/j.1745-6916.2007.00028.x. [DOI] [PubMed] [Google Scholar]
- Mather M, Gorlick MA, Nesmith K. The limits of arousal’s memory impairing effects on nearby information. American Journal of Psychology. 2009;122:349–370. [PMC free article] [PubMed] [Google Scholar]
- Mather M, Knight MR. Angry faces get noticed quickly: Threat detection is not impaired among older adults. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2006;61:54–57. doi: 10.1093/geronb/61.1.p54. [DOI] [PubMed] [Google Scholar]
- Mather M, Nesmith K. Arousal-enhanced location memory for pictures. Journal of Memory and Language. 2008;58:449–464. doi: 10.1016/j.jml.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Sutherland M. Disentangling the effects of arousal and valence on memory for intrinsic details. Emotion Review. 2009;1:118–119. doi: 10.1177/1754073908100435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Sutherland MR. Arousal-biased competition in perception and memory. Perspectives on Psychological Science. doi: 10.1177/1745691611400234. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale professional manual. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- Maunoury C, Michot JL, Caillet H, Parlato V, LeroyWillig A, Jehenson P, Boller F. Specificity of temporal amygdala atrophy in Alzheimer’s disease: Quantitative assessment with magnetic resonance imaging. Dementia. 1996;7:10–14. doi: 10.1159/000106846. [DOI] [PubMed] [Google Scholar]
- May CP, Rahhal T, Berry EM, Leighton EA. Aging, Source Memory, and Emotion. Psychology and Aging. 2005;20:571–578. doi: 10.1037/0882-7974.20.4.571. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK, Raye CL, D’Esposito M. fMRI evidence of age-related hippocampal dysfunction in feature binding in working memory. Cognitive Brain Research. 2000;10:197–206. doi: 10.1016/s0926-6410(00)00029-x. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK, Raye CL, Mather M, D’Esposito M. Aging and reflective processes of working memory: Binding and test load deficits. Psychology and Aging. 2000;15:527–541. doi: 10.1037//0882-7974.15.3.527. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Raye CL, Johnson MK, Greene EJ. An fMRI investigation of short-term source memory in young and older adults. NeuroImage. 2006;30:627–633. doi: 10.1016/j.neuroimage.2005.09.039. [DOI] [PubMed] [Google Scholar]
- Moayeri SE, Cahill L, Jin Y, Potkin SG. Relative sparing of emotionally influenced memory in Alzheimer’s disease. Neuroreport. 2000;11:653–655. doi: 10.1097/00001756-200003200-00001. [DOI] [PubMed] [Google Scholar]
- Mori E, Ikeda M, Hirono N, Kitagaki H, Imamura T, Shimomura T. Amygdalar volume and emotional memory in Alzheimer’s disease. American Journal of Psychiatry. 1999;156:216–22. doi: 10.1176/ajp.156.2.216. [DOI] [PubMed] [Google Scholar]
- Mueller J, Kiernan R, Langston JW. Manual for Cognistat. Fairfax, CA: The Northern California Neurobehavioral Group Inc; 2001. [Google Scholar]
- Nashiro K, Mather M. How arousal affects younger and older adults’ memory binding. Experimental Aging Research. doi: 10.1080/0361073X.2011.536746. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveh-Benjamin M. Adult age differences in memory performance: Tests of an associative deficit hypothesis. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26:1170–1187. doi: 10.1037//0278-7393.26.5.1170. [DOI] [PubMed] [Google Scholar]
- O’Connell H, Coen R, Kidd N, Warsi M, Chin AV, Lawlor BA. Early detection of Alzheimer’s disease (AD) using the CANTAB paired Associates Learning Test. International Journal of Geriatric Psychiatry. 2004;19:1207–8. doi: 10.1002/gps.1180. [DOI] [PubMed] [Google Scholar]
- Olson IR, Page K, Moore KS, Chatterjee A, Verfaellie M. Working memory for conjunctions relies on the medial temporal lobe. Journal of Neuroscience. 2006;26:4596–4601. doi: 10.1523/JNEUROSCI.1923-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra MA, Abrahams S, Fabi K, Logie R, Luzzi S, Della Sala Sergio. Short-term memory binding deficits in Alzheimer’s disease. Brain. 2009;132:1057–1066. doi: 10.1093/brain/awp036. [DOI] [PubMed] [Google Scholar]
- Pezdek K. Memory for items and their spatial locations by young and elderly adults. Developmental Psychology. 1983;19:895–900. [Google Scholar]
- Rahhal TA, May CP, Hasher L. Truth and character: Sources That Older Adults Can Remember. Psychological Science. 2002;13:101–105. doi: 10.1111/1467-9280.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cerebral Cortex. 2005;15:1679–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Roudier M, Marcie P, Grancher AS, Tzortzis C, Starkstein S, Boller F. Discrimination of facial identity and of emotions in Alzheimer’s disease. Journal of the Neurological Sciences. 1998;154:151–158. doi: 10.1016/s0022-510x(97)00222-0. [DOI] [PubMed] [Google Scholar]
- Satler C, Garrido LM, Sarmiento EP, Leme S, Conde C, Tomaz C. Emotional arousal enhances declarative memory in patients with Alzheimer’s disease. Acta Neurologica Scandinavica. 2007;116:355–60. doi: 10.1111/j.1600-0404.2007.00897.x. [DOI] [PubMed] [Google Scholar]
- Swainson R, Hodges JR, Galton CJ, Semple J, Michael A, Dunn BD, Sahakian BJ. Early detection and differential diagnosis of Alzheimer’s disease and depression with neuropsychological tasks. Dementia and Geriatric Cognitive Disorders. 2001;12:265–280. doi: 10.1159/000051269. [DOI] [PubMed] [Google Scholar]
- Thomas RC, Hasher L. The influence of emotional valence on age differences in early processing and memory. Psychology and Aging. 2006;21:821–825. doi: 10.1037/0882-7974.21.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale. 3. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Welsh KA, Butters N, Mohs RC, Beekly D, Edland S, Fillenbaum G, Heyman A. The consortium to establish a registry for Alzheimer’s disease (CERAD). Part V. A Normative Study Of The Neuropsychological Battery. Neurology. 1994;44:609–614. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]