Abstract
Methylselenol has been implicated as an active anticancer selenium (Se) metabolite. However, its in vivo efficacy against prostate cancer (PCa) has yet to be established. Here, we evaluated the growth inhibitory effects of two presumed methylselenol precursors methylseleninic acid (MSeA) and Se-methylselenocysteine (MSeC) in comparison with selenomethionine (SeMet) and selenite in DU145 and PC-3 human PCa xenografts in athymic nude mice. Each Se was given by a daily single oral dose regimen starting the day after the subcutaneous inoculation of cancer cells. We analyzed serum, liver and tumor Se content to confirm supplementation status and apoptosis indices and tumor microvessel density for association with antitumor efficacy. Furthermore, we analyzed lymphocyte DNA integrity to detect genotoxic effect of Se treatments. The data show that MSeA and MSeC exerted a dose-dependent inhibition of DU145 xenograft growth and both were more potent than SeMet and selenite, in spite of less tumor Se retention than in the SeMet-treated mice. Selenite treatment increased DNA single-strand breaks in peripheral lymphocytes, whereas the other Se forms did not. Terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) and cleaved caspase-3 indices (apoptosis) from MSeC-treated tumors were higher than tumors from control mice or MSeA-treated mice, whereas the microvessel density index was lower in tumors from MSeA-treated mice. In the PC-3 xenograft model, only MSeA was growth inhibitory at a dose of 3 mg/kg body wt. In summary, our data demonstrated superior in vivo growth inhibitory efficacy of MSeA over SeMet and selenite, against two human PCa xenograft models without the genotoxic property of selenite.
Introduction
Prostate cancer (PCa) is the most commonly diagnosed cancer in elderly American men and is the second leading cause of male cancer death (1). Treatment options for advanced PCa including hormone ablation therapy, radiation and surgery do not offer cure but delay the inevitable recurrence of the lethal hormone-refractory disease (2). Chemotherapy using available anticancer drugs, with the exception of the taxane drug docetaxel, for late stage PCa offers no survival benefit. All these treatments are costly and have significant side effects including impotence and incontinence that negatively affect the quality of life of the patients.
As an attractive alternative to treatment, cancer chemoprevention holds great promise to reduce the risk of PCa by blocking, delaying and reversing the carcinogenic processes. A landmark human clinical trial, led by the late Dr Larry Clark, with a daily oral supplement of selenium (Se)-enriched yeast has shown a significant cancer risk reduction for key internal organ sites in USA subjects, while slightly enhancing their risk of non-melanoma skin cancers (3). The prostate stood out as the most responsive organ site for the chemopreventive effect of Se. The ongoing Se-vitamin E cancer trial (SELECT) seeks to validate the efficacy of Se in the form of L-selenomethionine (SeMet, a major Se form identified in selenized yeast) alone or in combination with vitamin E (all-rac-α-tocopheryl acetate) to prevent PCa in a cohort of some 32 400 men (4). The enrollment of subjects was closed in June 2004. The results are eagerly anticipated, but a decade away. If a positive efficacy of SeMet or its combination with vitamin E is confirmed in the SELECT study, the public health impact of using that form of Se is self-evident. This will provide an outstanding impetus for further clinical trials to identify even more effective Se agents for maximizing the preventive benefits. However, should the results be negative, the quest for effective Se agents will take on great significance and urgency.
Earlier work in chemically induced mammary carcinogenesis models has suggested that the Se metabolite methylselenol may be the active species for cancer chemoprevention (5–8). Cell culture studies by us and others, using leukemia, mammary and PCa cell lines as well as vascular endothelial cells, have shown that methylselenol and its precursors such as Se-methylselenocysteine (MSeC), methylselenocyanate and methylseleninic acid (MSeA) induce G1 cell cycle arrest, caspase-mediated apoptosis and inhibit angiogenic switch mechanisms (8–14). These Se compounds lack the DNA single-strand break (SSB)-inducing activity (genotoxicity) of the inorganic selenite or selenide (5,8,9,14–16). SeMet is usually not effective for these activities at similar dose levels. These in vitro studies support the hypothesis that methylselenol precursor compounds would be a superior choice of Se for PCa chemoprevention over SeMet or selenite. However, little, if any, work has been done to rigorously validate their in vivo antitumor efficacy and to evaluate their potential for genotoxic damage to normal cells in preclinical human PCa models. Such studies are necessary and essential before any human translational studies are planned for these ‘second-generation’ Se agents.
Here, we report experiments comparing the inhibitory efficacy of the synthetic MSeA and the naturally occurring MSeC with SeMet and selenite against DU145 and PC-3 hormone-independent human PCa xenograft growth in nude mice. We chose SeMet because it is the Se form currently being evaluated in the SELECT study (4) and because SeMet and Se-yeast are widely marketed as dietary supplement in USA. On the other hand, sodium selenite has been the most often used reference Se compound in nutritional and cancer chemoprevention studies (5), and cell culture studies have shown it to be genotoxic (9,14–17). We therefore measured DNA strand breaks in the peripheral lymphocytes of the mice treated by the four Se compounds to test their systemic genotoxicity in normal cells in vivo. We determined the effects of these Se compounds on serum, liver and tumor Se content to confirm supplementation status. We also measured selected cancer cell apoptosis and angiogenesis indices to explore possible association of these cellular pathways with the in vivo anticancer activity.
Materials and methods
Se compounds
Sodium selenite, methaneseleninic acid (same as MSeA, >95%, white powder) and L-SeMet were purchased from Sigma Chemical Company (St Louis, MO). Se-MSeC was purchased from LKT Laboratories (St Paul, MN). Stock solutions of each compound were prepared in water and filter sterilized, and then stored in 1 ml aliquots in a −70°C freezer. A fresh vial was thawed for each day’s use. Starting the day after cancer cell inoculation, Se was given by a daily single oral dose delivered to the back of the tongue, 7 days a week, in a volume of 1 μl/g body wt (e.g. for a mouse weighing 30 g, 30 μl were delivered via a disposable plastic tip from a 100 μl Pipetman®). The mice ingested the small volume easily with minimal loss and without physical irritation to the esophagus that might have resulted from intragastric gavages. The tight range of the measured tissue Se content within each group (see results) indicated good consistency of this manner of Se delivery. The dosing frequency simulated that used in the Clark study (3) and the SELECT study (4).
Nude mice xenograft models
DU145 and PC-3 cells were obtained from the American Type Culture Collection, Manassas, VA. The DU145 cells were grown in minimum essential Eagle's medium supplemented with 10% fetal bovine serum without antibiotics. The PC-3 cells were grown in F-12K medium with 10% fetal bovine serum without antibiotics. Cells were expanded and used for inoculation within three to –four passages after thawing from liquid nitrogen storage.
The animal use protocol was approved by the Institutional Animal Care and Use Committee of the University of Minnesota, and carried out at the Hormel Institute's animal facility. Male Balb/c athymic nude mice were purchased from NxGen BioSciences (San Diego, CA), at 4–5 weeks of age. They were housed in a specific-pathogen free room with free access to water and a commercial rodent chow (Harlan Teklad, Madison, WI). Animals were maintained in clean high efficiency particulate air-filter top covered cages. After 2 weeks of quarantine, each mouse was inoculated with a single subcutaneous injection of DU145 or PC-3 cells. The detailed procedure was as follows.
On the day of tumor cell inoculation, DU145 or PC-3 cells were harvested by trypsinization and counted. Cells were concentrated by centrifugation 200g for 5 min. The cells were resuspended in serum-free medium and centrifuged again to wash once. The pelleted cells were resuspended in serum-free medium and the cell suspension was chilled in an ice bath. An equal volume of thawed Matrigel (BD Biosciences, New Bedford, MA) was added to the cells and mixed well. The cell suspension was kept on ice to prevent gelling. Each animal was given a subcutaneous injection on the left flank of 100 μl containing 2 × 106 cells for DU145 cells or 1 × 106 cells for PC-3 cells. Fewer PC-3 cells were used than DU145 cells because PC-3 cell line is more aggressive than DU145. Body weight was measured twice per week. Tumor size was measured using a vernier caliper in the two longest dimensions. Tumor volume was calculated with the following formula 0.5D1(D2)2, where D1 is the long measure and D2 is the short dimension. The experiments were terminated when the xenograft tumor size in the control group reached a group average calculated volume of ∼1000 mm3. Necropsy procedure was as follows.
Necropsy.
At 24 h after the last Se dose, mice were anesthetized by the inhalation of isoflurane and bled by cardiac puncture for serum preparation to determine the Se content or for lymphocyte preparation (see below). The mice were killed by cervical dislocation. The xenograft tumor was carefully dissected and weighed. All small tumors (<200 mg) and a piece of larger tumors were fixed in 10% neutral formalin and processed for hematoxylin and eosin staining and other immunohistochemistry staining. The rest of the tumor tissue was snap frozen in liquid nitrogen and stored at −70°C until analyzed. The liver was weighed and a portion was frozen until further analyses. In addition to liver, kidney, testes and prostate were fixed in 10% neutral formalin, and processed for hematoxylin and eosin staining for evaluation of possible toxic effects of the Se treatments.
Lymphocyte isolation from peripheral blood for COMET assay
Fresh whole blood (900 μl) from each mouse was added to an Eppendorf tube containing 100 μl of 3.8% sodium citrate and gently mixed. The blood was diluted with 1 ml of physiological saline solution and carefully layered over 3 ml of lymphocyte separation medium (Mediatech, Herndon, VA) in a sterile 15 ml centrifuge tube, creating a sharp blood-lymphocyte separation medium interface. After centrifugation at 400g at room temperature for 15–30 min, the top layer of clear plasma was removed to within 2–3 mm above the lymphocyte layer (buffy coat). The lymphocyte layer plus about half of the lymphocyte separation medium layer below it were transferred to a new centrifuge tube. An equal volume of phosphate-buffered saline was added and mixed. After centrifugation for 10 min at room temperature at 260g, the cells were recovered as above and washed again with phosphate-buffered saline. The recovered cells were resuspended at 1 × 107 cells/ml in 10% (vol/vol) dimethylsulfoxide, 40% (vol/vol) medium, 50% (vol/vol) fetal calf serum and then were aliquoted 2 × 106 cells per freezing vial. Cells were frozen at −70°C with −1°C per minute freezing rate and finally transferred into a liquid nitrogen tank for long-term storage.
COMET assay for DNA strand breaks
The principle of this assay is based upon the differential migration of intact DNA versus DNA with strand breaks in agarose gel under alkaline unwinding condition (single and double breaks) or under neutral pH condition (double-strand breaks only) (18). The assay was carried out essentially as described elsewhere (17) with the exception for ‘neutral’ electrophoresis; the buffer was 300 mM sodium acetate, 100 mM Tris, pH 9.0.
Se analyses
Se content in serum, liver and tumor xenograft was determined by using acid wet digestion and analysis by hydride generation, atomic absorption spectrometry at Grand Forks Human Nutrition Center.
Apoptosis indices
Apoptosis was detected by a TUNEL kit according to the manufacturer’s instruction (Calbiochem, Darmstadt, Germany). Immunostaining for cleaved caspase-3 was performed with an antibody from Cell Signaling Technology (Beverly, MA) after 5 μ sections had been processed and heat treated with citrate buffer in a microwave oven for antigen retrieval. For quantitation, each slide was scanned to get an overall impression of the staining patterns and 10 representative ×200 power photomicrographs were taken with a digital camera, avoiding gross necrotic areas. The positively stained cancer epithelial cells within each photomicrograph were counted. The counting of total cancer cells was aided with the ImagePro+ image processing program. The TUNEL and c-caspase-3 indices were based on the counting of ∼7000 total cells per tumor slide.
Microvessel counts
As a marker of angiogenesis, the microvessels were stained with CD34 rat monoclonal antibody (MEC 14.7) (Abcam plc, Cambridge, UK). A pathologist (Z.W.) evaluated the staining patterns and scored three most vascularized fields of each tumor slide to produce a maximal vessel density count for each tumor.
Statistical analyses
Analysis of variance was performed on data sets with multiple groups. Statistical significance was set at P = 0.05. Comparison of each treatment group mean with the control group was done by Dunnett's test. Other appropriate post hoc comparisons of multiple group means were also carried out when necessary. GraphPad Prism Software (San Diego, CA) was used for statistics.
Results
Experiment 1. The inhibitory efficacy of MSeA and MSeC against DU145 xenograft growth
Three groups of 10 mice each were used. One day after cancer cell inoculation, mice in the control group were given a daily oral dose of water. Mice in groups 2 and 3 were each given a daily oral dose of 4 mg Se/kg body wt as MSeA and MSeC, respectively. We chose this dose based on the information in our earlier study with rats in which 2 mg Se as MSeC/kg was given orally (19) and from the work by Cao et al. (20) with mice in which 200 μg of Se as MSeC was given to a nude mouse weighing ∼30 g (∼6.7 mg/kg).
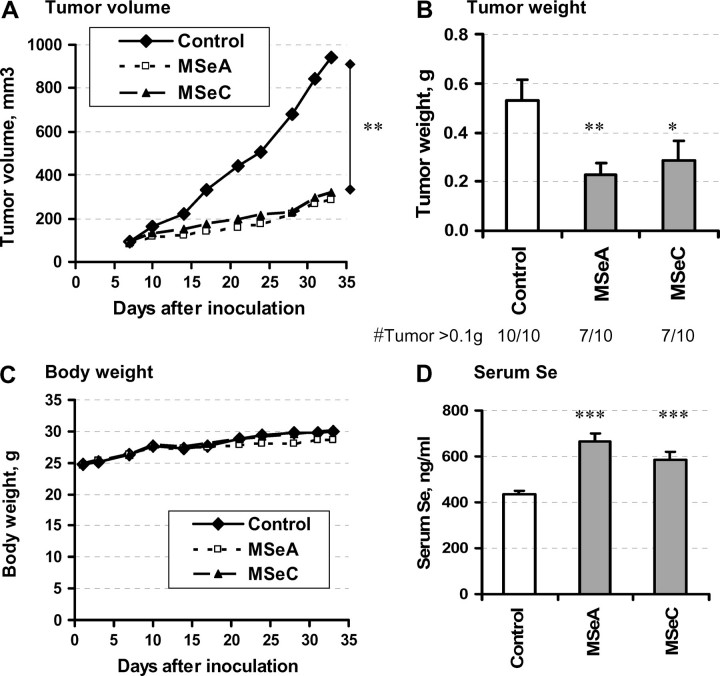
As shown in Figure 1A, the growth kinetics (i.e. calculated tumor volume as a function of time) for both Se-treated groups were decreased in comparison with the control mice. At the end of the experiment (35 days after inoculation), dissected tumors from MSeA-treated mice weighed significantly less than those of the control mice (Figure 1B, a 57% decrease for MSeA, P = 0.0055). MSeC treatment was as effective as MSeA, producing a 46% decrease (P = 0.0265). With 0.1 g tumor weight as an arbitrary cutoff threshold, 7 of 10 mice in MSeA and MSeC groups, respectively, had a significant tumor load compared with all 10 mice in the control group.
Fig. 1.
Effects of a daily single oral dose of MSeA and MSeC on the growth of DU145 PCa subcutaneous xenografts in athymic nude mice. Dosage for MSeA and MSeC was 4 mg Se/kg body wt. (A) Mean tumor volume as a function of time after inoculation. (B) Final tumor weight after necropsy, Mean ± SE, n = 10 mice. (C) Mean body weight of mice during the course of experiment. (D) Serum Se content at 24 h after the last Se dose, mean ± SE, n = 10 mice. Statistical significance from control group: *P < 0.05 and **P < 0.01. Experiment 1.
The treatment with MSeC did not affect the body weight of the mice (Figure 1C). The same dosage of MSeA resulted in a <6% suppression of the average body weight starting ∼3 weeks since the treatment was initiated, indicating possible mild adverse side effects (Figure 1C). However, histological examination by an experienced pathologist (J.D.L.) of the liver, kidney, testis and prostate sections of mice from either MSeA- or MSeC-treated group did not find evident pathologic alteration compared with control untreated animals (data not shown). The MSeA and MSeC treatment led to the expected increase in serum Se level, being 43% (P = 0.00007) and 35% (P = 0.0004) higher, respectively, than the control mice when the blood was taken 1 day after the last Se dose (Figure 1D). The change for MSeA-treated mice corresponded to elevating from the control value of 5.6–7.9 μM, an increment of 2.3 μM. The change in MSeC-treated mice to 7.5 μM amounted to an increment of 1.9 μM.
A low level (∼0.7%) of apoptosis by TUNEL assay was detected in the non-necrotic tumor epithelial cells in the control group (Figure 2A). MSeC-treated tumors contained a higher level of apoptosis detected by TUNEL (Figure 2A) or by cleaved caspase-3 (Figure 2B) than the control tumors, whereas MSeA-treated tumors did not show significant elevation of apoptosis. As a biomarker of angiogenesis, the microvessel counts in the most vascularized area of tumors were lower in the MSeA-treated tumors than in the control tumors (P = 0.0073), whereas MSeC treatment did not affect this parameter (Figure 2C). Taken together, the apoptosis and angiogenesis indices of the tumors after 35 days of treatment suggest that although the two methylselenol compounds were about equally active for suppressing DU145 xenograft growth, MSeA appeared to affect angiogenesis more than apoptosis, whereas MSeC appeared to induce apoptosis more than inhibiting angiogenesis.
Fig. 2.
Comparison of effects of MSeA and MSeC on apoptosis and angiogenesis indices. (A) TUNEL and (B) cleaved caspase-3 as biomarkers of cancer epithelial cell apoptosis. Mean ± SE, n = 7–10. Statistical significance from control group: *P < 0.05. (C) Microvessel counts as a biomarker of tumor angiogenesis, Mean ± SE, n = 7–10. Statistical significance from control group: **P <0.01. Experiment 1.
Experiment 2. Growth inhibition efficacy of four Se compounds and their effects on lymphocyte DNA integrity
The positive inhibitory efficacy of both methylselenol precursor compounds and the incongruent biomarker profiles of MSeA- versus MSeC-treated tumors in Experiment 1 prompted us to compare their efficacy with that of sodium selenite as well as SeMet. Based on the slight body weight suppression by 4 mg Se as MSeA/kg in Experiment 1, we chose a dosage of 3 mg Se/kg body wt for the efficacy comparison of all four Se forms. We also included 1 mg/kg of MSeA and MSeC to determine their respective dose–response pattern. The experiment was terminated at 49 days after cancer cell inoculation. Necropsy was the same as for Experiment 1, except the blood was used for lymphocyte preparation for COMET assay of DNA damage.
Tumor and body weights.
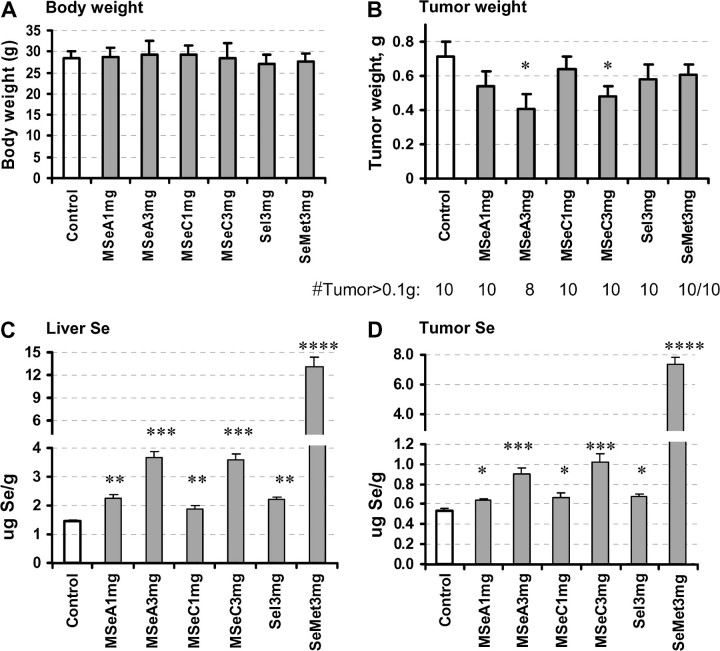
None of the four Se compounds at 3 mg/kg dose negatively affect the body weight of the mice, indicating a good tolerance to this dosage without adverse side effects (Figure 3A). Treatment with MSeA and MSeC resulted in a significant inhibition of the xenograft tumor growth at this dosage, suppressing the final tumor weight by 46% (group 3 versus 1, P = 0.025) and 33% (group 5 versus 1, P = 0.040), respectively (Figure 3B). Even though statistically the tumor weight was not decreased by MSeA at the 1 mg/kg dose (P = 0.170), a dose-dependent trend across the two MSeA doses was statistically significant (P = 0.019 for linear trend). Selenite and SeMet at the dosage of 3 mg Se/kg body wt did not lead to a statistically significant inhibition of xenograft tumor growth, although numerically the final tumor weight of the selenite and SeMet groups were 80% (group 6 versus 1, P = 0.286) and 85% (group 7 versus 1, P = 0.322) of the control group, respectively (Figure 3B).
Fig. 3.
The effects of a daily single oral dose of MSeA and MSeC in comparison with sodium selenite (Sel) and SeMet on the growth of DU145 PCa subcutaneous xenografts in athymic nude mice and their liver and tumor Se contents. (A) Body weight of mice at termination of experiment (49 days). Mean ± SD. Not statistical significant. (B) Tumor weight after necropsy. Mean ± SE, n = 10. (C) Liver Se content. Mean ± SE, n = 10. (D) Xenograft tumor Se content. Mean ± SE, n = 8–10. Statistical significance from control group: *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001. Experiment 2.
Tissue Se content.
Because the liver is the major organ for Se metabolism, we examined the Se content of liver as well as the DU145 xenograft tumor to confirm that the tumor inhibitory efficacy was associated with an increased retention of Se. Both MSeA and MSeC treatment led to a dose-dependent increase of liver Se content with similar efficacy (Figure 3C). Selenite (3 mg Se/kg selenite) was one-third as efficient as these methylselenol precursors. In contrast, SeMet treatment led to 9.1-fold more liver Se than the control mice (Figure 3C). The tumor Se content (Figure 3D) displayed identical patterns for each Se compound as in the liver, albeit the tumor Se content of each group appeared to be two to four times lower than the corresponding liver value. The data indicated that for each of the two methylselenol compounds, the DU145 xenograft growth inhibitory efficacy was proportional to the Se accumulated in the tumor and liver tissue. SeMet led to the greatest accumulation of Se in both the tumors and livers of the treated mice in comparison with the two methylselenol compounds and selenite, but was the least effective to inhibit tumor growth in vivo.
Apoptosis and angiogenesis indices.
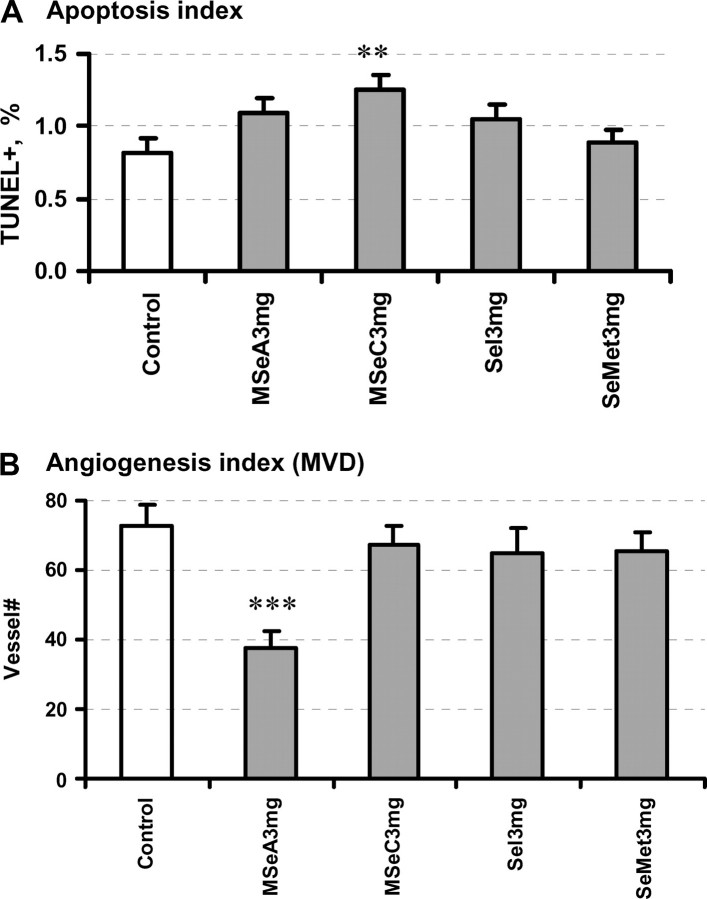
MSeC-treated tumors at the 3 mg/kg dose displayed statistically elevated incidence of apoptosis (P = 0.009) (Figure 4A), whereas MSeA-treated tumors approached statistical significance (P = 0.065). The microvessel counts in the most vascularized area of each tumor were decreased by MSeA treatment (P = 0.0002) (Figure 4B). The vessel count was not decreased by MSeC, selenite or SeMet at 3 mg/kg (P > 0.4). These data therefore were consistent with the differential impacts of the two methylselenol compounds on DU145 cancer xenograft cell apoptosis and angiogenesis observed in Experiment 1.
Fig. 4.
Evaluation of (A) TUNEL index for DU145 xenograft cancer epithelial apoptosis and (B) microvessel counts as a biomarker of angiogenesis. Se dose level was 3 mg Se/kg body wt. Mean ± SE, n = 8–10. Statistical significance from control group: **P <0.01 and ***P < 0.001. Experiment 2.
DNA strand breaks in peripheral lymphocytes.
Figure 5A shows representative images of the electrophoretic patterns of lymphocyte nuclei from a control mouse (left panel) and those of a selenite-treated mouse (right panel) under alkaline unwinding pH, a condition that detects both DNA SSBs and double-strand breaks. Quantitation of the incidence of nuclei with moderate to extensive fantails (Figure 5B) showed a doubling of cells with DNA strand breaks in the selenite-treated mice in comparison with the control mice (P = 0.00091). Neither MSeA nor MSeC, at the effective tumor inhibitory doses, increased DNA strand breaks in the peripheral lymphocytes, nor did SeMet (Figure 5B). There was no detectable increase by any of the four Se compounds of the DNA strand breaks in the lymphocytes under neutral pH, a condition that detects only DNA double-strand breaks (Figure 5C). These results indicated that daily oral selenite treatment increased the in vivo incidence of DNA SSBs in the peripheral lymphocytes, but the two methylselenol compounds did not induce this kind of in vivo genotoxicity.
Fig. 5.
Assessment of DNA integrity by single cell electrophoresis assay (COMET) of the peripheral lymphocytes of athymic nude mice after 48 days of daily oral treatment with MSeA, MSeC, selenite and SeMet. (A) Representative images of lymphocyte nuclei from a control mouse (left panel) and from a selenite-treated mouse (right panel). The fantails indicate DNA strand breakage. (B) Proportion of nuclei with moderate-to-extensive DNA strand breaks under alkaline electrophoresis condition (measuring both SSB and double-strand breaks). A total of 100 nuclei were scored for each mouse. (C) Proportion of nuclei with moderate-to-extensive DNA strand breaks under neutral electrophoresis condition (measuring double-strand breaks). A total of 200 nuclei were scored for each mouse. Mean ± SE, n = 10 mice. Statistical significance from control group: ***P < 0.001. Experiment 2.
Experiment 3. Comparison of efficacy of four Se compounds against PC-3 xenograft
To determine the general applicability of the findings of superior tumor growth inhibitory activity of the two methylselenol compounds and potential differences in the cellular and molecular targets by MSeA versus MSeC, we evaluated the in vivo growth inhibitory efficacy of the four Se compounds against PC-3 xenograft growth. Each group contained 16 mice to increase the statistical power of detecting a treatment effect. We chose only the 3 mg/kg dose for each Se compound. The experiment was terminated at 45 days after PC-3 cancer cell inoculation.
Same as in Experiment 2, daily single oral treatment with all four Se compounds at 3 mg/kg dose did not negatively affect the body weight of the mice (Figure 6A). Treatment with MSeA resulted in a 34% decrease of the PC-3 xenograft growth (group 2 versus 1, P = 0.049) (Figure 6B). MSeC, selenite and SeMet did not lead to a significant inhibition of PC-3 xenograft tumor growth (P > 0.5). The number of mice that had to be euthanized due to tumor burden before the planned termination of experiment was as follows: 2/16 in control group, none (0/16) in MSeA group, 3/16 in MSeC group, 3/16 in selenite group and 4/16 in SeMet group. In comparison with the DU145 xenografts, the PC-3 xenografts showed a much wider size range within each group (Figure 6C). When the tumors within each group were sorted by their final weight and plotted by rank order, a clear separation of the curve of the MSeA group from that of the control or the other groups was seen in the large (fast growing) tumors (#1–8). Apoptosis and angiogenesis indices were not performed in this study due to the wide range of xenograft growth within each group complicating sample choices and interpretation. Since tumor weight was the most reliable measurement, we believe the results (Figure 6B) support MSeA as the most active among the four Se forms tested against PC-3 PCa xenograft.
Fig. 6.

The effects of a daily single oral dose of MSeA, MSeC, sodium selenite and SeMet on the growth of PC-3 PCa subcutaneous xenografts in athymic nude mice. (A) Body weight of mice before termination of experiment. Mean ± SD, n = 12–16. (B) Tumor weight after necropsy. Mean ± SE, n = 16. Statistical significance from control group: *P < 0.05 (one sided). Euthanasia # reflects the number of tumor-bearing mice requiring euthanasia due to heavy tumor burden to comply with institutional animal care and use committee regulation before the preset termination time. (C) Rank-order plot of individual PC-3 tumors from the five groups from heaviest to lightest. Experiment 3.
Discussion
Cancer prevention clinical translation research with Se has had a rich history (3,21,22), culminating to the ongoing SELECT study for PCa prevention (4), a lung cancer prevention study led by the Eastern Cooperative Oncology Group (23), and other large trials elsewhere (24). However, on the key issue of the selection of the forms of Se for such trials, the research did not follow the usual drug/agent development paradigm of identifying the active compounds in test tube- and cell culture-based studies, establishing their efficacy in preclinical animal models and then progressing to human clinical trials. Rigorous debates on what forms of Se to use preceded the difficult decision that had to be made for the SELECT study (4) and the discussions and arguments can be assured to continue into the future for additional human trials. To this end, the data from the present study allow us to make several significant points.
First, the current work is, to our knowledge, the first comprehensive study to demonstrate the in vivo growth inhibition of two aggressive androgen-independent human PCa xenografts by MSeA and to compare with MSeC, SeMet and selenite. The daily single oral Se dosing regimen used was designed to mimic the frequency used in the Clark study (3), the SELECT study (4) and the lung cancer prevention trial (23). The DU145 xenograft growth suppression data for MSeA and MSeC in two separate experiments suggest an overall dose-dependent response for each methylselenol precursor. MSeA was comparable with MSeC in this model (Figures 1B and 3B), perhaps with a slight advantage over the latter. The dose-response patterns of liver and tumor Se accumulation after MSeA and MSeC treatment (Figure 3C and D) and their corresponding DU145 xenograft inhibitory effects suggest the dependence on enriching these Se forms and/or their metabolites to support their anticancer efficacy. Worthy of note is the superior efficacy of these second-generation Se compounds over selenite in the DU145 model was consistent with similar findings of better chemopreventive activity of MSeC and MSeA than selenite against chemically induced mammary carcinogenesis in the rats (5–8,25).
These data further indicate that MSeA at 3 mg Se/kg body wt was safe and effective against both DU145 (Figure 3B) and PC-3 xenografts (Figure 6B) with little adverse effects on the body weight of the mice (Figures 3A and 6A) and without evident histological alteration in liver, kidney, testes and prostate organs. The efficacy of MSeC, however, was lower against the PC-3 xenograft in comparison with the DU145 xenograft (Figure 6B versus Figure 3B). Since MSeC metabolism by the host mice could be presumed to remain the same whether they were inoculated with DU145 or PC-3 cells, the observed difference in efficacy against these two PCa xenografts most probably arose from differences in the xenografts themselves, especially their differences in cell proliferation, survival and angiogenesis signaling pathways. Knowledge of the accumulated Se through speciation technology and characterization of the in vivo differences of signaling pathways in the two types of xenografts in the future may provide the needed insights to understand the difference in efficacy.
The second point is that our data provide the first of its kind in vivo evidence of a systemic genotoxicity to peripheral lymphocytes as a result of selenite oral dosing (Figure 5B). This finding confirms a long-held belief predicted by cell culture studies that selenite can be genotoxic (14–16,26). Neither methylselenol precursor induced DNA SSBs at the effective dose for xenograft tumor suppression. Therefore, selenite may not be recommendable for chronic chemoprevention use due to the potential for genotoxicity-induced mutagenesis in normal cells, which may in turn, paradoxically, increase cancer risk. However, it should be pointed out that the dose of selenite used in the present study was two orders of magnitude higher than that required for nutritional selenite supplementation to correct Se deficiency in the mouse (e.g. 0.1 p.p.m. in diet or a daily intake of 0.01–0.02 mg Se/kg body wt), an application not expected to cause genotoxicity.
The third point concerns the differences in tissue Se patterns after treatment with methylselenol precursor compounds versus SeMet. The mice given SeMet accumulated 3.6-fold more liver Se than those given the same dose of either MSeA or MSeC (Figure 3C). In the DU145 xenograft tumors, SeMet resulted in 8.1-fold more Se accumulation than did MSeA and 7.2-fold more than did MSeC (Figure 3D). Our data are consistent with earlier reports of a greater accumulation of Se in major organs and mammary tumors of rodents fed SeMet in the diets than those fed selenite or methylated Se in the diets (5,7,27). Mechanistically, the greater accumulation of Se in tissues in the SeMet-treated mice is most probably due to non-specific incorporation of SeMet into proteins in the place of Met. While the health impact of this Se accumulation in the mice was not apparent in our study, massive tissue Se accumulation in people with chronic consumption of SeMet may pose a risk of selenosis during episodes of tissue breakdown.
The fourth point is the seemingly different profiles of cell apoptosis (TUNEL, cleaved caspase-3) and angiogenesis (microvessel density) indices in the MSeA-treated versus MSeC-treated DU145 tumors (Figures 2 and 5), in spite of their comparable tumor growth suppression efficacy (Figures 1B and 4B). Much work is needed to substantiate these observations in future experiments with greater attention to these dynamic processes during the acute and early stage of Se exposure to support or refute the hypothesis that MSeA and MSeC may affect different in vivo molecular targets and cellular pathways. Their difference in efficacy against PC-3 xenograft (Figure 6B) as discussed above favors such a speculation. Consistent with in vivo proapoptotic activity of MSeC or metabolites against advanced PCa cells in our study, dietary feeding of MSeC to rat in a mammary carcinogenesis model induced 3–4-fold increase of TUNEL positive apoptosis in both small and large intraductal proliferative lesions, whereas an antiproliferative activity measured by bromodeoxyuridine incorporation was only observed in the small intraductal proliferatives but not in the large intraductal proliferatives (28). In line with the anti-angiogenic actions of MSeA, our cell culture studies have shown that MSeA, but not selenite, inhibits the expression of vascular endothelial growth factor in cancer epithelial cells and matrix metalloproteinase-2 in vascular endothelial cells (10). These molecules should be examined in future experiments to determine their in vivo involvement as antiangiogenic targets of MSeA.
In addition to these major points, it is noteworthy of a couple of recent PCa xenograft studies concerning Se. In a study with androgen-dependent LNCaP subcutaneous xenograft, a daily single dose of 100 μg Se as MSeC per mouse by intraperitoneal injection was observed to decrease xenograft growth (29). Assuming an average body weight of 30 g per mouse, this dosage would be equivalent to ∼3.3 mg Se/kg, which was within the dose range of our study. Another study using an orthotopic intraprostatic inoculation of PC-3 cells did not show a growth inhibitory effect of MSeC or SeMet when provided through the drinking water at 3 p.p.m. Se (30). This provided an estimated daily Se intake of 0.5 mg/kg body wt by assuming 5 ml of daily water consumption per mouse, which would be lower than the dosages used in our work. That study, however, revealed an inhibitory efficacy of sodium selenate (30). Since selenate is known to be much less effective than selenite for most cultured cells, the orthotopic inoculation of PC-3 cells might have altered how these cells responded to this form of Se in vivo. The organ-specific environment should also be taken into consideration in future studies to assess the merit of different Se compounds for PCa chemoprevention.
In summary, our data support a dose-dependent inhibition of human DU145 PCa xenograft growth in vivo by MSeA and MSeC and a significant inhibition of PC-3 xenograft growth by MSeA, without adverse effect on the body weight of the host mice or observable histological alterations in several organs. SeMet and selenite at the same dosage were not growth inhibitory against either xenograft model. Furthermore, MSeA and MSeC at the well-tolerated dose of 3 mg/kg did not induce DNA SSBs in peripheral lymphocytes, whereas selenite induced this systemic genotoxicity in vivo. The observed differences in the cancer cell apoptosis and angiogenesis indices in the MSeA- versus MSeC-treated DU145 xenografts and the lack of in vivo efficacy of MSeC against PC-3 xenograft raised a possibility of differential targeting actions of MSeA versus MSeC to mediate the respective anticancer efficacy, in addition to the actions of their presumed common methylselenol metabolite. This hypothesis requires further investigation.
Funding
Hormel Foundation; National Cancer Institute (CA95642, CA126880); Department of Defense Prostate Cancer Research Program (DAMD17-02-1-0007).
Acknowledgments
We thank Hormel Institute Animal Facility personnel for their help with excellent animal care and health monitoring. We thank Dr Yan Zhao and Dr Lei Wang for technical assistance. We also thank Andria Hansen for secretarial support.
Conflict of Interest Statement: None declared.
Glossary
Abbrevations
- MSeA
methylseleninic acid
- MSeC
Se-methylselenocysteine
- PCa
prostate cancer
- Se
selenium
- SELECT
selenium-vitamin E cancer trial
- SeMet
selenomethionine
- SSB
single-strand break
References
- 1.Jemal A, et al. Cancer statistics, 2006. CA Cancer J. Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Petrylak DP. The current role of chemotherapy in metastatic hormone-refractory prostate cancer. Urology. 2005;65:3–7. doi: 10.1016/j.urology.2005.03.053. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 3.Clark LC, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957–1963. [PubMed] [Google Scholar]
- 4.Lippman SM, et al. Designing the Selenium and Vitamin E Cancer Prevention Trial (SELECT) J. Natl Cancer Inst. 2005;97:94–102. doi: 10.1093/jnci/dji009. [DOI] [PubMed] [Google Scholar]
- 5.Ip C. Lessons from basic research in selenium and cancer prevention. J. Nutr. 1998;128:1845–1854. doi: 10.1093/jn/128.11.1845. [DOI] [PubMed] [Google Scholar]
- 6.Ip C, et al. Activity of methylated forms of selenium in cancer prevention. Cancer Res. 1990;50:1206–1211. [PubMed] [Google Scholar]
- 7.Ip C, et al. Chemical form of selenium, critical metabolites, and cancer prevention. Cancer Res. 1991;51:595–600. [PubMed] [Google Scholar]
- 8.Ip C, et al. In vitro and in vivo studies of methylseleninic acid: evidence that a monomethylated selenium metabolite is critical for cancer chemoprevention. Cancer Res. 2000;60:2882–2886. [PubMed] [Google Scholar]
- 9.Lu J, et al. Selenium and cancer chemoprevention: hypotheses integrating the actions of selenoproteins and selenium metabolites in epithelial and non-epithelial target cells. Antioxid. Redox Signal. 2005;7:1715–1727. doi: 10.1089/ars.2005.7.1715. [DOI] [PubMed] [Google Scholar]
- 10.Jiang C, et al. Monomethyl selenium–specific inhibition of MMP-2 and VEGF expression: implications for angiogenic switch regulation. Mol. Carcinog. 2000;29:236–250. doi: 10.1002/1098-2744(200012)29:4<236::aid-mc1006>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 11.Jiang C, et al. Caspases as key executors of methyl selenium-induced apoptosis (anoikis) of DU-145 prostate cancer cells. Cancer Res. 2001;61:3062–3070. [PubMed] [Google Scholar]
- 12.Jiang C, et al. Distinct effects of methylseleninic acid versus selenite on apoptosis, cell cycle, and protein kinase pathways in DU145 human prostate cancer cells. Mol. Cancer Ther. 2002;1:1059–1066. [PubMed] [Google Scholar]
- 13.Wang Z, et al. Antimitogenic and proapoptotic activities of methylseleninic acid in vascular endothelial cells and associated effects on PI3K-AKT, ERK, JNK and p38 MAPK signaling. Cancer Res. 2001;61:7171–7178. [PubMed] [Google Scholar]
- 14.Kim T, et al. Se-methylselenocysteine induces apoptosis through caspase activation in HL-60 cells. Carcinogenesis. 2001;22:559–565. doi: 10.1093/carcin/22.4.559. [DOI] [PubMed] [Google Scholar]
- 15.Lu J, et al. Dissociation of the genotoxic and growth inhibitory effects of selenium. Biochem. Pharmacol. 1995;50:213–219. doi: 10.1016/0006-2952(95)00119-k. [DOI] [PubMed] [Google Scholar]
- 16.Lu J, et al. Selenite induction of DNA strand breaks and apoptosis in mouse leukemic L1210 cells. Biochem. Pharmacol. 1994;47:1531–1535. doi: 10.1016/0006-2952(94)90528-2. [DOI] [PubMed] [Google Scholar]
- 17.Li GX, et al. Differential involvement of reactive oxygen species in apoptosis induced by two classes of selenium compounds in human prostate cancer cells. Int. J. Cancer. 2007;120:2034–2043. doi: 10.1002/ijc.22480. [DOI] [PubMed] [Google Scholar]
- 18.Singh NP. Microgels for estimation of DNA strand breaks, DNA protein crosslinks and apoptosis. Mutat. Res. 2000;455:111–127. doi: 10.1016/s0027-5107(00)00075-0. [DOI] [PubMed] [Google Scholar]
- 19.Jiang C, et al. Selenium-induced inhibition of angiogenesis in mammary cancer at chemopreventive levels of intake. Mol. Carcinog. 1999;26:213–225. doi: 10.1002/(sici)1098-2744(199912)26:4<213::aid-mc1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 20.Cao S, et al. Selective modulation of the therapeutic efficacy of anticancer drugs by selenium containing compounds against human tumor xenografts. Clin. Cancer Res. 2004;10:2561–2569. doi: 10.1158/1078-0432.ccr-03-0268. [DOI] [PubMed] [Google Scholar]
- 21.Yu SY, et al. Protective role of selenium against hepatitis B virus and primary liver cancer in Qidong. Biol. Trace Elem. Res. 1997;56:117–124. doi: 10.1007/BF02778987. [DOI] [PubMed] [Google Scholar]
- 22.Blot WJ, et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J. Natl Cancer Inst. 1993;85:1483–1492. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- 23.Karp DD. ECOG 5597: phase III chemoprevention trial of selenium supplementation in persons with resected stage I non-small-cell lung cancer. Clin. Adv. Hematol. Oncol. 2005;3:313–315. [Google Scholar]
- 24.Larsen EH, et al. Speciation and bioavailability of selenium in yeast-based intervention agents used in cancer chemoprevention studies. J. AOAC Int. 2004;87:225–232. [PubMed] [Google Scholar]
- 25.Ip C, et al. Comparative effect of inorganic and organic selenocyanate derivatives in mammary cancer chemoprevention. Carcinogenesis. 1994;15:187–192. doi: 10.1093/carcin/15.2.187. [DOI] [PubMed] [Google Scholar]
- 26.Garberg P, et al. Studies of the role of DNA fragmentation in selenium toxicity. Biochem. Pharmacol. 1988;37:3401–3406. doi: 10.1016/0006-2952(88)90688-0. [DOI] [PubMed] [Google Scholar]
- 27.Ip C, et al. Tissue selenium levels in selenium-supplemented rats and their relevance in mammary cancer protection. Carcinogenesis. 1989;10:921–925. doi: 10.1093/carcin/10.5.921. [DOI] [PubMed] [Google Scholar]
- 28.Ip C, et al. Methylselenocysteine modulates proliferation and apoptosis biomarkers in premalignant lesions of the rat mammary gland. Anticancer Res. 2001;21:863–867. [PubMed] [Google Scholar]
- 29.Lee SO, et al. Monomethylated selenium inhibits growth of LNCaP human prostate cancer xenograft accompanied by a decrease in the expression of androgen receptor and prostate-specific antigen (PSA) Prostate. 2006;66:1070–1075. doi: 10.1002/pros.20329. [DOI] [PubMed] [Google Scholar]
- 30.Corcoran NM, et al. Inorganic selenium retards progression of experimental hormone refractory prostate cancer. J. Urol. 2004;171:907–910. doi: 10.1097/01.ju.0000092859.16817.8e. [DOI] [PubMed] [Google Scholar]