Abstract
Ultrasound (US) is used to enhance and target delivery of drugs and genes to cancer tissues. The present study further examines the role of acoustic cavitation in US-induced permeabilization of cell membranes and subsequent drug or gene uptake by the cell. Rat colon cancer cells were exposed to ultrasound at various static pressures to examine the hypothesis that oscillating bubbles, also known as cavitating bubbles, permeabilize cells. Increasing pressure suppresses bubble cavitation activity; thus if applied pressure were to reduce drug uptake, cell permeabilization would be strongly linked to bubble cavitation activity. Cells were exposed to 476 kHz pulsed ultrasound at average intensities of 2.75 W/cm2 and 5.5 W/cm2 at various pressures and times in an isothermal chamber. Cell fractions with reversible membrane damage (calcein uptake) and irreversible damage (propidium iodide uptake) were analyzed by flow cytometry. Pressurization to 3 atm nearly eliminated the biological effect of US in promoting calcein uptake. Data also showed a linear increase in membrane permeability based upon increased time and intensity. This research shows that US-mediated cell membrane permeability is likely linked to cavitation bubble activity.
Keywords: bubble cavitation, microbubbles, calcein, targeted drug delivery, flow cytometry, hydrostatic pressure
Introduction
Ultrasound currently finds significant application in diagnostic and therapeutic medicine. Diagnostic ultrasound, typically employing frequencies greater than 1 MHz, takes advantage of the ability of ultrasound to penetrate soft tissue and reflect from interfaces, thus allowing imaging of internal body structures. In physical therapy applications, ultrasound is used to warm targeted tissues, and typically employs frequencies around 1 MHz.
A recent development in therapeutic ultrasound is its application to deliver drugs and genes to a variety of tissues (Husseini et al. 2008; Mitragotri 2005; Muller et al. 2007; Pitt et al. 2004). Many drugs and genes have been delivered to specific tissues and locations such as tumors (Frenkel 2008; Nelson et al. 2002; Reddy 2005), cardiac tissue (Chen et al. 2003; Mayer et al. 2008; Muller et al. 2007), the brain (Hynynen 2008; Kinoshita et al. 2006; Meairs et al. 2007) and occluded vessels (Francis 2001). Ultrasound is associated with permeabilization of cell membranes, but the exact mechanism by which this occurs remains under investigation (Prentice et al. 2005; Schlicher et al. 2006; Tachibana et al. 1999). Because ultrasound is composed of oscillating pressure waves, it causes direct mechanical effects on tissue. It also acts physically on bubbles, causing them to oscillate in size—a phenomenon called cavitation. It has been hypothesized that intense cavitation, resulting from sufficiently intense ultrasonication, creates an increase in cell membrane permeability (Guzman et al. 2001; Prentice et al. 2005; Schlicher et al. 2006).
There are several mechanisms whereby ultrasonication and cavitation may be involved in drug delivery. Even in the absence of gas bubble cavitation, the oscillating pressure waves produce a slight oscillatory motion of fluid. This action will increase molecular transport by increasing local molecular motion. Another phenomenon called acoustic streaming occurs when the momentum of the sound wave is transferred to an absorbing fluid (like water), thus generating convective fluid flow in the direction of the propagating ultrasonic waves. Although these phenomena will enhance the transport of drug to a cell surface, it is doubtful that these low energy motions can change cell membrane permeability.
A more pronounced effect of ultrasonication is mediated by cavitation when gas bubbles are present. There are two general categories of cavitation: stable and collapse cavitation, also known as non-inertial and inertial cavitation. Stable cavitation is the cyclic volumetric oscillation of microbubbles without a violent collapse event. This stable oscillation phenomenon induces convective flow around the bubble called microstreaming which causes two important effects. First, genes or drugs in solution are convected at high velocities. Second, the rapid movement of fluid and changes in pressure associated with bubble oscillation generate high shear stresses adjacent to the bubble that may create stress on nearby cell membranes (Van Wamel et al. 2004). Under carefully controlled conditions, stable cavitation was found to be sufficiently energetic to permeabilize cell membranes (Nyborg 2001; Rooney 1970)
Inertial cavitation is much more violent than stable cavitation, and it occurs when the oscillations in bubble radius become so large that the inertia of the imploding wall compresses the internal gas to extremely high temperatures and pressures, which in turn forms free radicals and releases energy in the form of heat and powerful shockwaves (Brennen 1995). In addition, it has been shown that an asymmetric collapse may occur near cell surfaces, thus causing a high-speed jet of extracellular solution to be injected into the cell or tissue (Prentice et al. 2005). It is hypothesized that through sheer stresses and shockwaves associated with inertial cavitation events, cell membranes are permeabilized, therefore causing an increase in passive drug uptake (Schlicher et al. 2006).
Several methods have been used to study the role of cavitation in biological systems. Using sonoluminescence as an indicator of cavitation, Cochran measured flashes of light that were believed to be emitted upon the collapse of microbubbles (Cochran et al. 2001). Other evidence of the presence of cavitation has been to “listen” to acoustic signatures present in the fluid during sonication by using an acoustic detector (Ciaravino et al. 1981; Hill 1971; Husseini et al. 2007; Husseini et al. 2005; Tang et al. 2001; Tezel et al. 2002).
Cavitation phenomena can be reduced by pressurizing the bubbles (Brennen 1995; Ciaravino et al. 1981; Delius 1997; Richardson et al. 2007). Higher static pressure reduces the initial bubble radius, and increases the internal gas density. Therefore if a phenomenon associated with ultrasound decreases as static pressure increases, then the phenomenon is most probably caused by cavitation, not by fluid oscillation (independent of bubbles) or acoustic streaming. The unique aspect of this study is the use of a pressurized chamber to investigate the effect of ultrasound and cavitation on cellular uptake of a model drug. This study investigated the hypothesis that cavitation is involved in permeabilizing cell membranes, and not simply fluid oscillation or acoustic streaming. Specifically, we show that the application of static pressure correlates with a reduction in membrane permeability.
Materials and Methods
Reagents
Calcein (622.5 g/mol) (MP Biomedicals, Inc., Aurora, OH) was dissolved into 1X Ca2+/Mg2+-free Dulbecco’s phosphate-buffered saline (DPBS) to create a stock solution of 0.1 mM, stored at 4 C and sheltered from light. Fifty μl of stock solution were then added to 0.5 mL of cell suspension. Propidium iodide (PI, Sigma, St. Louis, MO) was added to the cell suspension approximately ten minutes prior to flow cytometry so that the final concentration was 10 μM.
Cell Culture
DHD/K12 TRb rat colon cancer cells (#90062901, European Cell Culture Collection) were cultured at 37 C, 5% CO2 in RPMI 1640 medium (Gibco BRL, Gaithersburg, MD) and supplemented with 20% cosmic calf serum (Hyclone, Logan, UT). The cells were harvested during their exponential phase of growth and suspended at a concentration of 1×106 cells/ml in medium containing 10 μM calcein. These cells were selected because they are responsive to ultrasonic-activated chemotherapy in vivo in a rat model (Nelson et al. 2002).
Apparatus
The chamber used for sonicating the cells was an aluminum box of ~2 L volume lined with about 1 L of acoustically absorbing rubber on the bottom and sides. This rubber had a corrugated surface (to scatter any non-absorbed sound). Placed on one wall was a 476 kHz ultrasonic transducer (model H-104, Sonic Concepts, Woodinville, WA), which has an active diameter of 64 mm, a radius of curvature of 62.64 mm, a focal length of 51.74 mm, and a center frequency of 476 kHz. Mapping of the interior of the box with a needle hydrophone (ONDA Corp., Sunnyvale, CA) showed that there were no standing waves inside the box when insonified at 476 kHz.
An XYZ stage on top of the box positioned a pressure tube containing the sample at the focal point of the transducer (Fig. 1). Within this tube of cellulose butyrate, a hollow rod held a small polyethylene pipette bulb filled with the cell suspension. A hole in the hollow rod connected the gas in the pressure tube with the gas above the cell suspension. Pressure was applied to the interior of the tube (and to the sample) from a compressed air source and regulator at 0, 1, 2, and 3 atmosphere (gauge pressure). The local pressure at Brigham Young University is 86 kPa, so the experimental pressures were 86, 187, 288 and 389 kPa absolute pressure.
Figure 1.
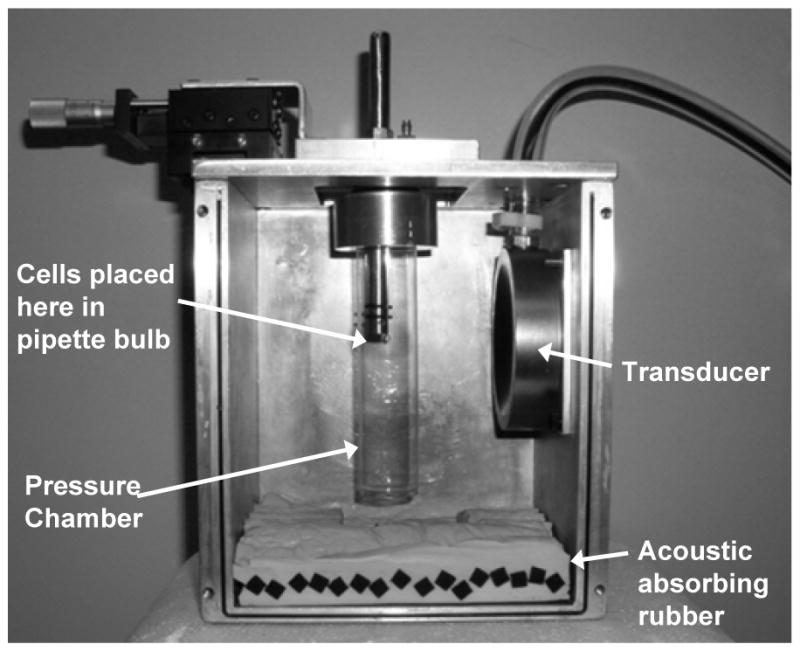
Photograph of the pressurized chamber and transducer box. During the experiments absorbing rubber was placed on all sides of the box except the top. There was about 2 cm of air space between the water surface and the aluminum when the samples were exposed to ultrasound.
The wall thicknesses of the pressure tube and PE sample holder were 0.46 and 0.42 mm respectively. Overpressurization to 3 atm was calculated to expand the tube radius by 0.6%, and although it may have shifted the center of the focal volume by 1%, any changes in cell permeabilization were estimated to be less than the noise level of experimental error in this system. The calculated peak rarefaction pressure within the PE sample holder was not corrected for any attenuation of the thin films of cellulose butyrate and PE.
The water in the chamber external to the pressure tube was recirculated through a heater at 37°C and then through a 0.2-μm filter to remove particulate and gas bubbles that might serve as nuclei for cavitation bubbles.
Electronics
A sine wave at 476 kHz was produced by a signal generator (Hewlett Packard, model 33120A). A 1:10 duty cycle was produced with a100 cycle burst at a frequency of 476 Hz and was monitored on a digital oscilloscope. This signal was amplified (ENI Model 240L, Rochester, NY) and then sent through a matching network to the ultrasonic transducer. The 10% duty cycle was selected because it was a good compromise between a very low duty cycle (say 1% that would require long experimental times) and continuous insonation that would heat up the sample. The intensity values reported herein are spatial peak temporal averages (Ispta) unless otherwise noted.
The intensity of US at the focal point was determined in separate experiments using a pendulum radiation meter consisting of a 1.5-mm-diameter stainless steel ball suspended by 2 Kevlar fibers in an inverted V-configuration (Chen et al. 2004; Dunn et al. 1977). The deflection of the steel ball was measured with a micrometer (0.005 mm precision) as follows. The pendulum assembly was mounted on an XYZ micrometer stage, and the steel ball located at the acoustic focal point and illuminated by a laser. When the transducer was activated the ball was deflected backward, and then the horizontal micrometer of the stage was adjusted to bring the ball back to the focal point illuminated by the laser. The difference in micrometer readings gave the deflection from which the acoustic intensity was calculated.
Insonation of Cells
The polyethylene bulb was filled with the suspension of cells in calcein and then attached to the hollow rod. The pressurizeable tube was filled with degassed water and sealed to the lid of the box with a sealing ring. Once the tube and sample were pressurized to the desired static pressure, the lid was placed on the Al box and the sample positioned at the focal point with the XYZ micrometer stage. Ultrasound was applied for various times and intensities, after which the static pressure was released and the sample recovered.
Flow Cytometry
After insonation, cells were washed three times in 1X DPBS Ca2+/Mg2+ free. PI was added to 0.5 mL of cell solution at a concentration of 10 μM. Cells were analyzed in a flow cytometer (Epics XL, Beckman Coulter, Fullerton, CA) for forward and side scatter, cell fractions with reversible (calcein uptake), and irreversible membrane damage (PI uptake).
Results
Acoustic intensity versus exposure time
A minimal, and often negligible, amount of calcein uptake occurred between temporal average intensities of 0.125 W/cm2 and 1.17 W/cm2 (data not shown). However, at temporal average intensities of 2.75 W/cm2 and 5.5 W/cm2 there were much higher levels of uptake that were more easily detected; therefore these intensities were selected for subsequent experiments.
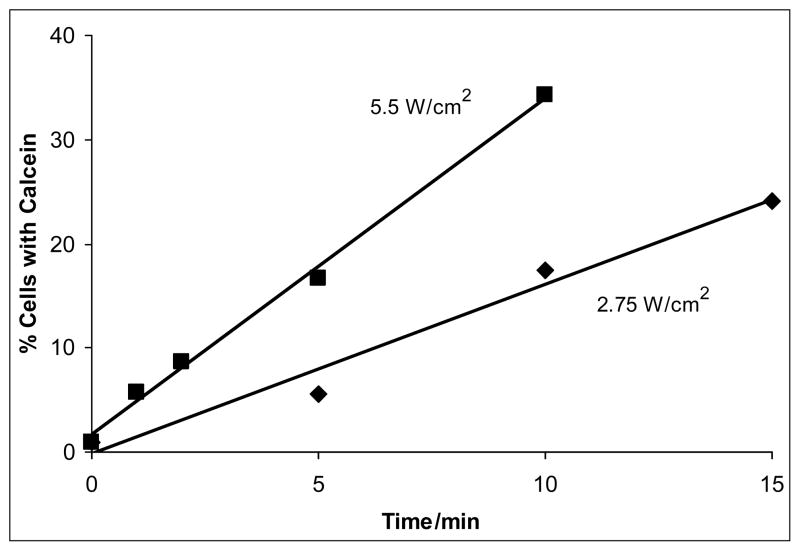
As measured by calcein uptake into viable cells, the amount of molecular uptake was directly proportional to the time and intensity of insonation (see Fig. 2). For example, at ten minutes of ultrasonic exposure, the average percentage of cells with calcein uptake was 17.5 % at 2.75 W/cm2, while the average uptake was 34.3 % at 5.5 W/cm2. Similarly, after 5 min of exposure, the average uptake at 2.75 W/cm2 and 5.5 W/cm2 were 5.6 % and 16.7 % respectively . The data showed that increasing either time or intensity increased the calcein permeability of cells.
Figure 2.
Average (n=3) calcein uptake as a function of time and average intensity at atmospheric pressure (86 kPa). At 5.5 W/cm2 (Ispta), there were consistently higher amounts of drug uptake than at 2.75 W/cm2 (Ispta). The lines are a least square fit of the data.
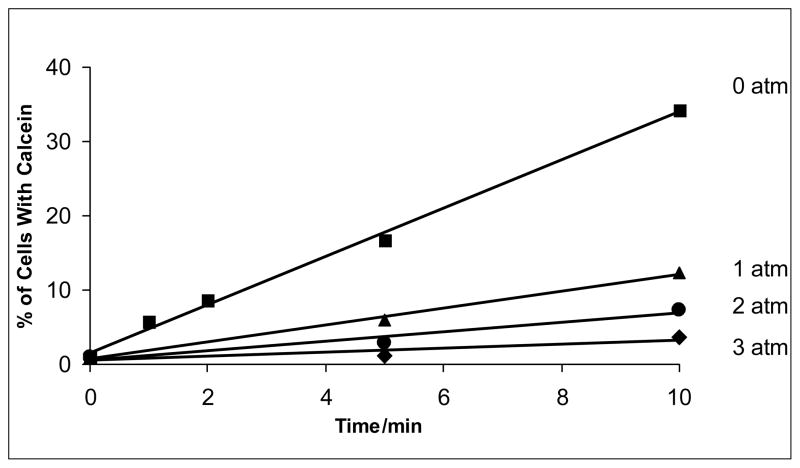
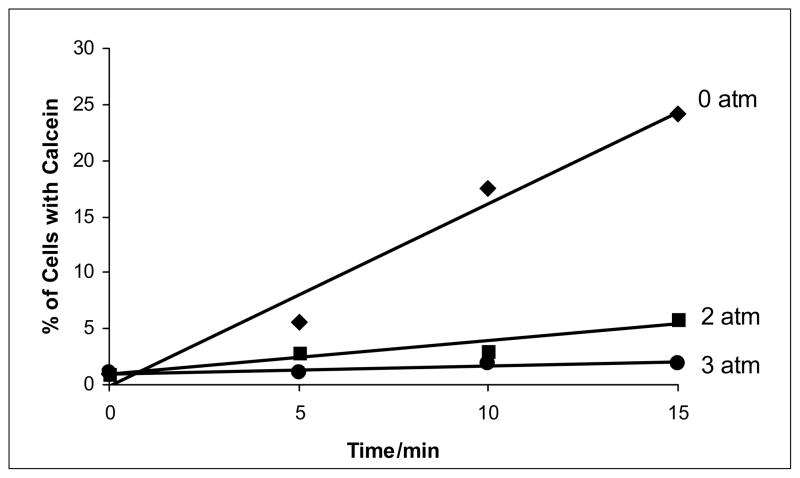
Additionally, experiments showed a similar linear relationship between calcein uptake and exposure time at different pressures (see Figs. 3 and 4). Most remarkably, pressurization of the samples during the insonation procedure drastically decreased the percent of cells with calcein uptake.
Figure 3.
Calcein uptake as a function of overpressure and ultrasound exposure time at an average intensity of 5.5 W/cm2 (Ispta).The lines are a least square fit of the data. The pressures (given in atmospheres of overpressure) correspond to absolute pressures of 86, 187, 288, and 289 kPa.
Figure 4.
Calcein uptake as a function of overpressure and exposure time to ultrasound at an average intensity of 2.75 W/cm2 (Ispta). The lines are a least square fit of the data. The pressures (given in atmospheres of overpressure) correspond to absolute pressures of 86, 187, 288, and 289 kPa.
In sham experiments in which the cells were pressurized but not exposed to ultrasound, no significant uptake in calcein was measured beyond the normal baseline amount. Therefore, overpressurizing the cells alone did not contribute to calcein uptake.
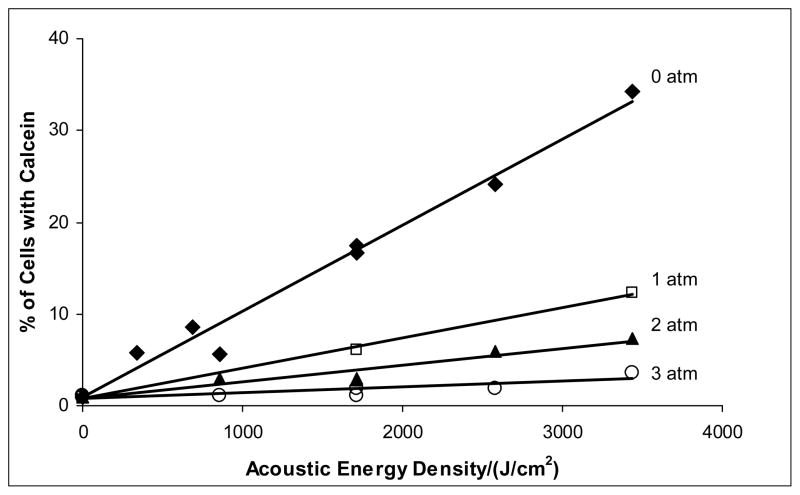
The total energy density delivered to the sample is the product of the average intensity (W/cm2) and the exposure time. The relationship between calcein uptake and total energy density is depicted in Figure 5. Acoustic energy density appears to be a fundamental parameter because data from both intensities correlate in a linear relationship at a given gauge pressure.
Figure 5.
Calcein uptake as a function of acoustic energy density. There is a linear relationship between acoustic energy density and drug uptake at all overpressures. The lines are a least square fit of the data. The pressures (given in atmospheres of overpressure) correspond to absolute pressures of 86, 187, 288, and 289 kPa.
Cell death
Flow cytometry data of propidium iodide uptake (cell death but not cell rupture) showed that there was not a significant increase in dead cells above the usual amount found in the control, with the exception of samples exposed to 5.5 W/cm2 for 15 minutes. In other words, the cells that were permeabilized enough to uptake calcein, were able to re-establish membrane integrity after sonication at the specified energies except at the most severe treatment. At this energy density (4,950 J/cm2), more than half of the cells were lysed by the ultrasonication process. Therefore this data point was not included in the subsequent analysis, and higher energy densities were not investigated. Cells that received 3,300 J/cm2 or lower were permeabilized enough to uptake calcein and apparently were able to re-establish membrane integrity after insonation.
Increased pressure decreases uptake
Figures 3–5 show that the application of pressure above ambient decreases the cell membrane damage as assessed by calcein uptake. Again, the uptake increased linearly with time (Figs. 3 and 4) and acoustic energy density (Fig. 5), but the rate of uptake decreased as overpressure increased. For example, application of 3 atm overpressure decreased calcein uptake by about 90% for samples at all the times and intensities studied (Fig. 4).
Discussion
Our observations that cell membrane damage and calcein uptake correlates with the intensity, time, and total energy of ultrasonic exposure is not novel, but is confirmatory of the observations of Prausnitz et al. (Guzman et al. 2001; Guzman et al. 2001; Keyhani et al. 2001; Schlicher et al. 2006). However, our observation that model drug uptake is a strong function of ambient pressure is novel and aids in explaining the nature of the mechanism that causes membrane damage. We posit that the ultrasound itself does not have a direct effect on the cell membrane, because the ultrasonic acoustic vibrations themselves are not a function of the ambient pressure, except for a negligible change in acoustic wave velocity. However, the behavior of cavitating bubbles is strongly influenced by the ambient pressure; thus the ultrasound is apparently acting indirectly through the action of the cavitating bubbles, which are strongly responsive to the ambient pressure. Richardson et al. have also shown that increasing the ambient pressure during 80-kHz insonation reduced the background emission attributed to inertial cavitation (Richardson et al. 2007).
Because increasing the pressure was effective in decreasing calcein uptake, the hypothesis that bubble cavitation is the cause of increased membrane permeability is supported. However, the question remains as to whether stable or inertial cavitation (or both) is responsible for the cell membrane permeability. In the presence of a wide range of bubble sizes, the likelihood of inertial cavitation occurring can be generally estimated by a parameter called the “mechanical index”(MI), which is defined as the peak negative pressure (in MPa) divided by the square root of the ultrasonic frequency (in MHz). Inertial cavitation in water containing bubbles (at atmospheric pressure and all sizes) begins to occur above a threshold in mechanical index of about 0.2 to 0.5 (Apfel 1982; Barnett 1998; Bouakaz et al. 2005; Church 2005; Husseini et al. 2007; Husseini et al. 2005). Biological effects (again at atmospheric pressure) have been reported above an MI of 0.6 (Barnett 1998; Korosoglou et al. 2006; Miller et al. 1995). Church points out that the MI threshold defined above is a predictor of inertial cavitation activity for a single acoustic cycle event, and that with more cycles, there is a decrease in the value of the MI threshold required to produce inertial cavitation (Church 2005). His equations predict that the threshold MI required to produce inertial cavitation in water with 476 kHz US is 0.17 MPa. In our experiments at 5.5 W/cm2, the MI during the 100-cycle pulse was 0.6, and the peak rarefaction pressure was 0.41 MPa. Thus, it is possible that inertial cavitation is occurring in these experiments at ambient atmospheric pressure, and it is also possible that inertial cavitation is at least somewhat responsible for increasing membrane permeability.
Little theoretical work has been reported on how increasing static pressure will increase the threshold of inertial cavitation, but there are several experimental observations showing that increasing pressure suppresses the amount of inertial cavitation (Fry et al. 1951; Hill 1971; Morton et al. 1983; Richardson et al. 2007) and cell damage (Ciaravino et al. 1981; Delius 1997; Hill 1971; Morton et al. 1983).
In contrast with other studies that used contrast agents to provide bubbles, our study used no external sources of bubbles. However, micron sized gas bubbles are stable in aqueous solutions in the presence of surfactant molecules that surround and stabilize the bubble. The proteins and other biomolecules found in cell nutrient solutions are adequate surfactants to stabilize gas bubbles (Williams 1983). The solutions used herein were not degassed prior to resuspending the cells, and thus it is postulated that normal amounts of air dissolved in these solutions were nucleated into gas bubbles when the first few low-pressure cycles of ultrasound propagated through the cell suspension.
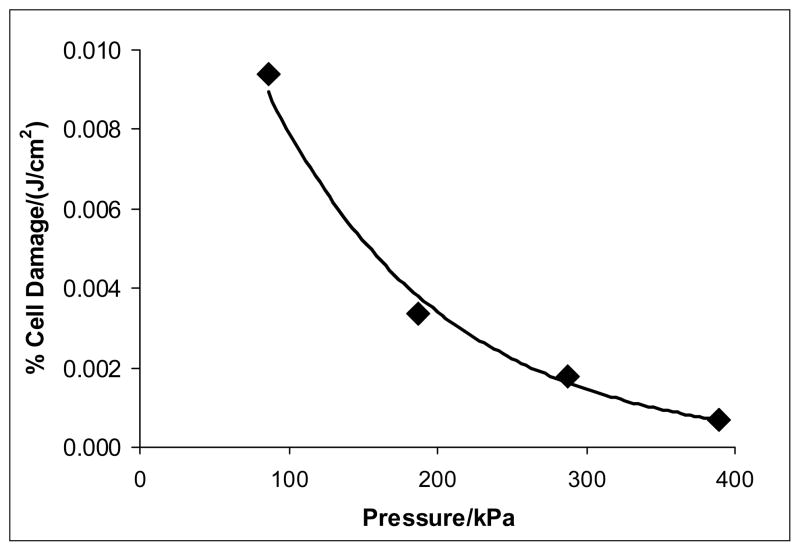
The slopes of the lines in Figures 3 and 4 represent the rate of cell permeabilization at various pressures. This linear increase with time is consistent with other observations, as is the observation that the cell permeabilization toward drugs is related to treatment time multiplied by acoustic intensity (Guzman et al. 2001). But perhaps even more significantly, the slopes of the lines in Figure 5 represent the percentage of cells permeabilized per increment of energy delivered at 476 kHz. Thus the slope of these lines represents the sensitivity of the cells to an increment of applied energy density, and it is apparent that this sensitivity to damage decreases as pressure increases, as is plotted in Figure 6. In fact, the rate of cell damage can be represented by a decaying exponential model of the following form:
where A = 0.0185 % damage/(J/cm2), k = 0.0085/kPa, and P is absolute pressure. This expression proposes that at 1 atm pressure (101 kPa), 100% of the cells would take up calcein if exposed to 27,760 J/cm2. This might possibly be achieved by long exposures at low intensities where cell death would be a minimum.
Figure 6.
Sensitivity of the cells to damage per increment of applied energy density. This graph plots the slope of the linear regression lines of Figure 5 at specific absolute pressures.
In conclusion, our novel experimental design allowed us to increase the pressure of a cell suspension while applying ultrasound. It was demonstrated that increased pressure suppresses the effect of ultrasound on model drug uptake. The data show that the rate of calcein uptake is proportional to exposure time, exposure intensity, and exposure energy and inversely proportional to overpressure. While these experiments have no direct clinical application, the observations presented herein reveal the mechanism of ultrasonic enhanced drug uptake. Since overpressure suppresses both stable and inertial cavitation, these findings suggest that bubble cavitation has an important role in ultrasonic drug delivery. Continued research on the mechanisms and conditions of ultrasound mediated cell permeabilization will advance the effectiveness of in vivo studies and the eventual clinical application.
Acknowledgments
The authors gratefully acknowledge funding from NIH grant CA 98138 for the support of this research. The authors also thank David E. Warden for assistance with the radiation force measurements.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
S. Briant Stringham, Department of Microbiology and Molecular Biology, Brigham Young University, Provo, Utah.
Maria A. Viskovska, Department of Microbiology and Molecular Biology, Brigham Young University, Provo, Utah
Eric S. Richardson, Mechanical Engineering Department, Brigham Young University, Provo, Utah 84602. Department of Biomedical Engineering, University of Minnesota, Minneapolis, MN 55455
Seiga Ohmine, Department of Microbiology and Molecular Biology, Brigham Young University, Provo, Utah.
Ghaleb A. Husseini, Department of Chemical Engineering, Brigham Young University, Provo, Utah 84602. Department of Chemical Engineering, American University of Sharjah, Sharjah, United Arab Emirates
Byron K. Murray, Department of Microbiology and Molecular Biology, Brigham Young University, Provo, Utah
William G. Pitt, Department of Chemical Engineering, Brigham Young University, Provo, Utah 84602
References
- Apfel RE. Acoustic Cavitation: A Possible Consequence of Biomedical Uses of Ultrasound. Br J Cancer. 1982;45(Suppl V):140–6. [PMC free article] [PubMed] [Google Scholar]
- Barnett S. Thresholds for Nonthermal Biofeffects: Theoretical and Experimental Basis for a Threshold Index. Ultrasound Med Biol. 1998;24(S1):S41–S9. doi: 10.1016/s0301-5629(98)80001-x. [DOI] [PubMed] [Google Scholar]
- Bouakaz A, Versluis M, de Jong N. High-speed optical observations of contrast agent destruction. Ultrasound in Medicine and Biology. 2005;31(3):391–9. doi: 10.1016/j.ultrasmedbio.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Brennen CE. Cavitation and Bubble Dynamics. New York: Oxford University Press; 1995. [Google Scholar]
- Chen SG, Silva G, Kinnick R, Fatemi M, Greenleaf JF. Measurement of Static and Dynamic Radiation Force on Spheres. Ieee Ultrasonics Symposium; 2004. pp. 676–9. [Google Scholar]
- Chen SY, Shohet RV, Bekeredjian R, Frenkel P, Grayburn PA. Optimization of ultrasound parameters for cardiac gene delivery of adenoviral or plasmid deoxyribonucleic acid by ultrasound-targeted microbubble destruction. Journal of the American College of Cardiology. 2003;42(2):301–8. doi: 10.1016/s0735-1097(03)00627-2. [DOI] [PubMed] [Google Scholar]
- Church CC. Frequency, pulse length, and the mechanical index. Acoustics Research Letters Online-Arlo. 2005;6(3):162–8. [Google Scholar]
- Ciaravino V, Miller MW, Carstensen EL. Pressure-Mediated Reduction of Ultrasonically Induced Cell Lysis. Radiation Research. 1981;88:209–13. [PubMed] [Google Scholar]
- Cochran SA, Prausnitz MR. Sonoluminescence as an Indicator of Cell Membrane Disruption by Acoustic Cavitation. Ultrasound in Med & Biol. 2001;27(6):841–50. doi: 10.1016/s0301-5629(01)00382-9. [DOI] [PubMed] [Google Scholar]
- Delius M. Minimal Static Excess Pressure Minimises the Effect of Extracorporeal Shock Waves on Cells and Reduces it on Gallstones. Ultrasound Med Biol. 1997;23(4):611–7. doi: 10.1016/s0301-5629(97)00038-0. [DOI] [PubMed] [Google Scholar]
- Dunn F, Averbuch AJ, Obrien WD. Primary Method for Determination of Ultrasonic Intensity with Elastic Sphere Radiometer. Acustica. 1977;38(1):58–61. [Google Scholar]
- Francis CW. Ultrasound-enhanced thrombolysis. Echocardiography-a Journal of Cardiovascular Ultrasound and Allied Techniques. 2001;18(3):239–46. doi: 10.1046/j.1540-8175.2001.00239.x. [DOI] [PubMed] [Google Scholar]
- Frenkel V. Ultrasound mediated delivery of drugs and genes to solid tumors. Advanced Drug Delivery Reviews. 2008;60(10):1193–208. doi: 10.1016/j.addr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry WJ, Tucker D, Fry FJ, Wulff VJ. Physical Factors Involved in Ultrasonically Induced Changes in Living Systems: II. Amplitude Duration Relations and the Effect of Hydrostatic Pressure for Nerve Tissue. J Acoustical Soc Amer. 1951;23(3):364–8. [Google Scholar]
- Guzman HR, Nguyen DX, Kahn S, Prausnitz MR. Ultrasound-mediated disruption of cell membranes. I. Quantification of molecular uptake and cell viability. J Acoust Soc Am. 2001;110(1):588–96. doi: 10.1121/1.1376131. [DOI] [PubMed] [Google Scholar]
- Guzman HR, Nguyen DX, Kahn S, Prausnitz MR. Ultrasound-mediated disruption of cell membranes. II. Heterogeneous effects on cells. J Acoust Soc Am. 2001;110(1):587–608. doi: 10.1121/1.1376130. [DOI] [PubMed] [Google Scholar]
- Hill CR. Ultrasonic Exposure Thresholds for Changes in Cells and Tissues. J Acoust Soc Am. 1971;52(2):667–72. [Google Scholar]
- Husseini GA, Diaz de la Rosa MA, Gabuji T, Zeng Y, Christensen DA, Pitt WG. Release of Doxorubicin from Unstabilized and Stabilized Micelles Under the Action of Ultrasound. J Nanosci Nanotech. 2007;7(3):1–6. doi: 10.1166/jnn.2007.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husseini GA, Diaz MA, Richardson ES, Christensen DA, Pitt WG. The Role of Cavitation in Acoustically Activated Drug Delivery. J Controlled Release. 2005;107(2):253–61. doi: 10.1016/j.jconrel.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husseini GA, Pitt WG. Micelles and Nanoparicles in Ultrasonic Drug and Gene Delivery. Advanced Drug Delivery Reviews. 2008;60(10):1137–52. doi: 10.1016/j.addr.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynynen K. Ultrasound for Drug and Gene Delivery to the Brain. Advanced Drug Delivery Reviews. 2008;60(10):1209–17. doi: 10.1016/j.addr.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyhani K, Guzman HR, Parsons A, Lewis TN, Prausnitz MR. Intracellular Drug Delivery Using Low-Frequency Ultrasound: Quantification of Molecular Uptake and Cell Viability. Pharm Res. 2001;18(11):1514–20. doi: 10.1023/a:1013066027759. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Targeted delivery of antibodies through the blood-brain barrier by MRI-guided focused ultrasound. Biochemical and Biophysical Research Communications. 2006;340(4):1085–90. doi: 10.1016/j.bbrc.2005.12.112. [DOI] [PubMed] [Google Scholar]
- Korosoglou G, Hardt SE, Bekeredjian R, Jenne J, Konstantin M, Hagenmueller M, Katus HA, Kuecherer H. Ultrasound exposure can increase the membrane permeability of human neutrophil granulocytes containing microbubbles without causing complete cell destruction. Ultrasound in Medicine and Biology. 2006;32(2):297–303. doi: 10.1016/j.ultrasmedbio.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Mayer CR, Bekeredjian R. Ultrasonic Gene and Drug Delivery to the Cardiovascular System. Advanced Drug Delivery Reviews. 2008;60(10):1177–92. doi: 10.1016/j.addr.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Meairs S, Alonso A. Ultrasound, microbubbles and the blood-brain barrier. Progress in Biophysics & Molecular Biology. 2007;93(1–3):354–62. doi: 10.1016/j.pbiomolbio.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Miller DL, Thomas RM. Thresholds for Hemorrhages in Mouse Skin and Intestine Induced by Lithotripter Shock Waves. Ultrasound in Med Biol. 1995;21(2):249–57. doi: 10.1016/s0301-5629(94)00112-x. [DOI] [PubMed] [Google Scholar]
- Mitragotri S. Innovation - Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nature Reviews Drug Discovery. 2005;4(3):255–60. doi: 10.1038/nrd1662. [DOI] [PubMed] [Google Scholar]
- Morton KI, Haar GRt, Stratford IJ, Hill CR. Subharmonic Emission as an Indicator of Ultrasonically-induced Biological Damage. Ultrasound Med Biol. 1983;9(6):629–33. doi: 10.1016/0301-5629(83)90008-x. [DOI] [PubMed] [Google Scholar]
- Muller OJ, Katus HA, Bekeredjian R. Targeting the heart with gene therapy-optimized gene delivery methods. Cardiovascular Research. 2007;73(3):453–62. doi: 10.1016/j.cardiores.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Nelson JL, Roeder BL, Carmen JC, Roloff F, Pitt WG. Ultrasonically Activated Chemotherapeutic Drug Delivery in a Rat Model. Cancer Res. 2002;62:7280–3. [PubMed] [Google Scholar]
- Nyborg WL. Biological Effects of Ultrasound: Development of Safety Guidelines. Part II: General Review. Ultrasound Med Biol. 2001;27(3):301–33. doi: 10.1016/s0301-5629(00)00333-1. [DOI] [PubMed] [Google Scholar]
- Pitt WG, Husseini GA, Staples BJ. Ultrasonic drug delivery - a general review. Expert Opin Drug Delivery. 2004;1(1):37–56. doi: 10.1517/17425247.1.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice P, Cuschierp A, Dholakia K, Prausnitz M, Campbell P. Membrane disruption by optically controlled microbubble cavitation. Nature Physics. 2005;1(2):107–10. [Google Scholar]
- Reddy LH. Drug delivery to tumours: recent strategies. Journal of Pharmacy and Pharmacology. 2005;57(10):1231–42. doi: 10.1211/jpp.57.10.0001. [DOI] [PubMed] [Google Scholar]
- Richardson ES, Pitt WG, Woodbury DJ. The Role of Cavitation in Liposome Formation. Biophysical Journal. 2007;93(12):4100–7. doi: 10.1529/biophysj.107.104042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney JA. Hemolysis Near an Ultrasonically Pulsating Gas Bubble. Science. 1970;169:869–71. doi: 10.1126/science.169.3948.869. [DOI] [PubMed] [Google Scholar]
- Schlicher RK, Radhakrishna H, Tolentino TP, Apkarian RP, Zarnitsyn V, Prausnitz MR. Mechanism of intracellular delivery by acoustic cavitation. Ultrasound in Medicine and Biology. 2006;32(6):915–24. doi: 10.1016/j.ultrasmedbio.2006.02.1416. [DOI] [PubMed] [Google Scholar]
- Tachibana K, Uchida T, Ogawa K, yamashita N, Tamura K. Induction of cell-membrane porosity by ultrasound. Lancet. 1999;353:1409. doi: 10.1016/S0140-6736(99)01244-1. [DOI] [PubMed] [Google Scholar]
- Tang H, Mitragotri S, Blankschtein D, Langer R. Theoretical Description of Transdermal Transport of Hydrophilic Permeants: Application to Low-Frequency Sonophoresis. J Pharm Sci. 2001;90(5):545–68. doi: 10.1002/1520-6017(200105)90:5<545::aid-jps1012>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Tezel A, Sens A, Mitragotri S. Investigations of the role of cavitation in low-frequency sonophoresis using acoustic spectroscopy. J Pharm Sci. 2002;91(2):444–53. doi: 10.1002/jps.10024. [DOI] [PubMed] [Google Scholar]
- Van Wamel A, Bouakaz A, Versluis M, De Jong N. Micromanipulation of endothelial cells: Ultrasound-microbubble-cell interaction. Ultrasound in Medicine and Biology. 2004;30(9):1255–8. doi: 10.1016/j.ultrasmedbio.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Williams AR. Ultrasound: Biological Effects and Potential Hazards. London: Academic Press; 1983. [Google Scholar]