Abstract
Recent studies focusing on the memory for temporal order have reported that CA1 plays a critical role in the memory for the sequences of events, in addition to its well-described role in spatial navigation. In contrast, CA3 was found to principally contribute to the memory for the association of items with spatial or contextual information in tasks focusing on spatial memory. Other studies have shown that NMDA signaling in the hippocampus is critical to memory performance in studies that have investigated spatial and temporal order memory independently. However, the role of NMDA signaling separately in CA1 and CA3 in memory that combines both spatial and temporal processing demands (episodic memory) has not been examined. Here we investigated the effect of the deletion of the NR1 subunit of the NMDA receptor in CA1 or CA3 on the spatial and the temporal aspects of episodic memory, using a behavioral task that allows for these two aspects of memory to be evaluated distinctly within the same task. Under these conditions, NMDA signaling in CA1 specifically contributes to the spatial aspect of memory function and is not required to support the memory for temporal order of events.
There is a consensus that the hippocampus plays a crucial role in episodic memory, which combines both the spatial and the temporal components of memory for a particular experience (for review, see Eichenbaum 2004; Rolls and Kesner 2006; Eichenbaum et al. 2007; Squire et al. 2007; Kesner and Hunsaker 2010). In addition, the N-methyl-D-aspartate (NMDA) receptor in the hippocampus is critical to spatial memory and the memory for sequential events (Huerta et al. 2000; McHugh and Tonegawa 2009; for review, see Nakazawa et al. 2004).
Recently, several studies have teased apart the contributions of the hippocampal subfields CA1 and CA3 and have identified the role of NMDA receptor signaling in temporal and spatial aspects of memory (for review, see Nakazawa et al. 2004; Gilbert and Brushfield 2009; Kesner and Hunsaker 2010). These studies have suggested that CA1 is principally involved in the memory for sequential events that are separated by a short delay (Huerta et al. 2000; Gilbert et al. 2001; Kesner et al. 2005; Farovik et al. 2008; Hunsaker et al. 2008; MacDonald et al. 2011), and that the NR1 subunit of the NMDA receptors in CA1 plays a critical role in temporal processing (Huerta et al. 2000). In contrast, CA3 may be primarily involved in the fast encoding of new representations with spatial or contextual content and the learning of arbitrary associations that do not involve a temporal delay (Gilbert and Kesner 2003; Daumas et al. 2004; Kesner et al. 2008; Kesner and Warthen 2010; for review, see Gilbert and Brushfield 2009); NMDA receptor signaling in CA3 plays a key role in this aspect of memory processing (Nakazawa et al. 2002, 2003; Cravens et al. 2006; Rajji et al. 2006; Nakashiba et al. 2008; Fellini et al. 2009; for review, see Gilbert and Brushfield 2009).
All of the above studies have focused either on spatial memory or on the memory for temporal order, but none has evaluated the contribution of NMDA signaling in CA1 or CA3 in memory that combines both the spatial and the temporal dimensions within the same task. Moreover, most studies focusing on episodic memory conducted to date have used tasks that require multiple trials to learn associations between items/events separated by short delays (seconds). However, little is known about the contribution of NMDA signaling in CA1 and CA3 to spatial memory and the memory for the temporal order of items/events separated by longer delays (hours). In this study, we investigated the contributions of NMDA signaling in CA1 and CA3 to memory in an episodic spontaneous object recognition memory task that involves long delays and combines both spatial and temporal aspects at once, but allows for each component to be evaluated separately (Dere et al. 2005).
Results
Selective deletion of the NR1 subunit of the NMDA receptors in CA1 or CA3
To confirm that the NR1 subunit of the NMDA receptors had been knocked out in CA1 in the CA1 NR1 knockout (KO) mice and in CA3 in the CA3 NR1 KO mice, NR1 subunits were detected by in situ hybridization (Fig. 1). Figure 1B shows that the NR1 signal is selectively absent in the CA1 of CA1 NR1 KO mice, whereas it could be detected in all hippocampal subfields in CA1 control mice (Fig. 1A). Likewise, the NR1 signal was not detected in the CA3 of CA3 NR1 KO mice, but was present in all other subfields (Fig. 1D), and the NR1 signal was present in all hippocampal subfields in CA3 control mice (Fig. 1C).
Figure 1.
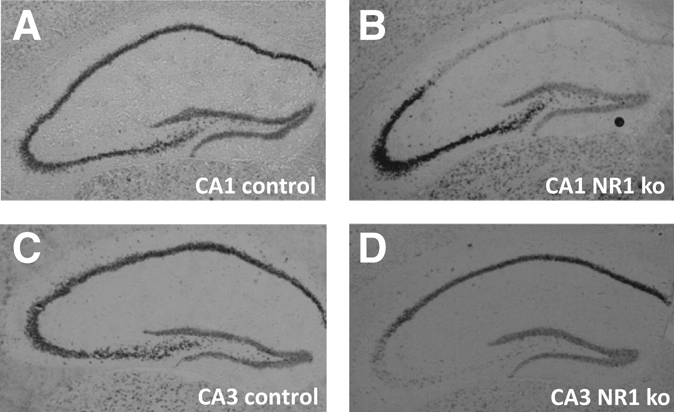
Detection of the NR1 subunit of the NMDA receptors. In control mice, all hippocampal subfields express the NR1 subunit of the NMDA receptor, including CA1 (A) and CA3 (C). As expected, CA1 NR1 KOs lack NR1 subunits specifically in CA1 (B) and CA3 NR1 KOs in CA3 (D).
Impaired ‘what–where’ memory, but spared ‘what–when’ memory in CA1 NR1 KOs
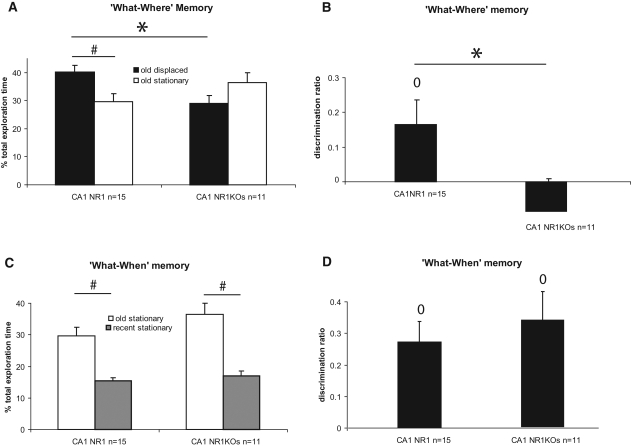
Knocking out the NR1 subunit of the NMDA receptors in CA1 led to a selective impairment of the spatial dimension of episodic memory (“what–where” memory), while sparing the memory for the sequence in which objects had been encountered (“what–when” memory) (Fig. 2).
Figure 2.
Role of CA1 NMDA signaling in episodic recognition memory: percent total exploration time of the “old” displaced, “old” stationary, and “recent” stationary objects. (A) The “what–where” memory of CA1 NR1 KOs was impaired, because KOs failed to detect the spatial displacement of the “old” objects at test, while controls successfully detected it (#). In controls, this difference was due to a higher relative time spent exploring the “old” displaced object and led to a significant between-genotypes difference (*). (B) This finding was confirmed by a discrimination ratio > 0 for controls (o) but not for KOs, leading again to a significant genotype difference (*). (C) The “what–when” memory was spared in CA1 NR1 KOs, because KOs and CA1 control mice successfully discriminated between the “old” and the “recent” stationary objects (#) and (D) showed preference for the “old” stationary object over the “recent” one (o). ±SEM; (#,*,o) all P's < 0.050.
Thus, between-groups comparison of the relative exploration time of the old stationary object with the relative exploration time of the old displaced object revealed that behavioral performance significantly differed between CA1 controls and CA1 NR1 KOs, as shown by a significant “object displacement” by “genotype” interaction effect (two-factor ANOVA [object displacement, genotype], interaction: F(3,48) = 7.58, P = 0.008; genotype effect: F(1,48) = 0.22, P > 0.050; displacement effect: F(1,48) = 0.45, P > 0.050). In addition, further between-groups analysis showed that CA1 NR1 KO mice spent significantly less time exploring the old displaced object than control mice (t(24) = 2.54, P = 0.018, [*]), while groups explored the old stationary object to a similar extent (t(24) = −0.75, P > 0.050) (Fig. 2A). These results were further confirmed by the analysis of the discrimination ratios, which take into consideration only the stationary and displaced old objects (see Materials and Methods), which also revealed a significant between-group difference (t(24) = −2.15, P = 0.041[*]) (Fig. 2B). Furthermore, within-group comparisons of the latter relative exploration times showed that CA1 NR1 KO mice failed to detect the displacement of the “old” object at test, while CA1 control mice successfully detected it (NR1KO: t(10) = −0.99, P = 0.344; NR1: t(14) = 2.20, P = 0.044 [#]) (Fig. 2A). This result was confirmed by a stationary versus displaced old object discrimination ratio significantly superior to 0 for the controls, but not for the NR1 KOs (DR compared with 0; NR1: t(14) = 2.31, P = 0.037 [o]; NR1KO: t(10) = −0.91, P > 0.050) (Fig. 2B). Total exploration time during testing phase did not differ between groups (t(24) = 0.72, P > 0.050) (data not shown).
In contrast to the effect of knocking out NR1 in the CA1 on the “what–where” memory, the same manipulation did not affect the “what–when” memory in a significant manner. Thus, comparison of the relative exploration time of the old stationary object with the relative exploration time of the recent objects did not reveal a significant genotype difference, nor a “genotype by order” interaction effect (two-factor ANOVA [genotype, order]: genotype effect: F(1,48) = 2.77, P > 0.050; order effect: F(1,48) = 38.29, P < 0.001; interaction: F(1,48) = 1.18, P > 0.050) (Fig. 2C), and the “old versus recent stationary objects” discrimination ratio did not significantly differ between groups (t(24) = 0.61, P > 0.050) (Fig. 2D). Moreover, within-groups analysis of the relative exploration times and the discrimination ratios (DR) for those objects showed that CA1 controls and CA1 NR1 KOs successfully discriminated between the old stationary and the recent stationary objects, since both groups explored significantly more the old than the recent stationary object (NR1: t(14) = −3.93, P = 0.002, [#]; NR1KO: t(10) = −3.40, P = 0.007, [#]) (Fig. 2C) (DR compared with 0; NR1: t(14) = 4.15, P = 0.001, [o]; NR1KO: t(10) = 3.69, P = 0.004, [o]) (Fig. 2D).
Intact ‘what–where’ and ‘what–when’ memory in CA3 NR1 KOs
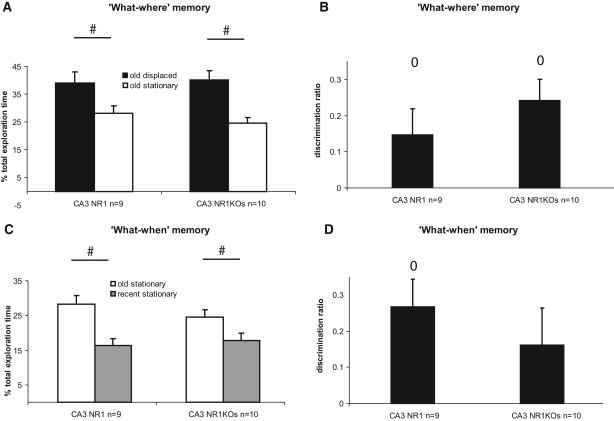
In contrast to CA1, knocking out NR1 subunits of the NMDA receptors in CA3 had only a mild effect on recognition memory performance (Fig. 3).
Figure 3.
Role of CA3 NMDA signaling in episodic recognition memory: percent total exploration time of the “old” displaced, “old” stationary, and “recent” stationary objects. The “what–where” and “what–when” memory were intact in CA3 NR1 KOs. (A) Both KOs and CA3 control mice successfully detected the spatial displacement of the “old” object at test (#) and (C) successfully discriminated between the “old” and the “recent” stationary object (#). (B) Moreover, KOs and controls showed a preference for the “old” displaced objects over the “old” stationary (o). (D) Controls preferred the old stationary object over the new one (o), and despite the fact that the DR did not significantly differ from 0 for the KOs, no genotype differences were found. ±SEM; (#,*,o) all P's < 0.050.
No significant “genotype” or “genotype by displacement” effects were found when comparing relative exploration times for the old stationary object and for the old displaced object (two-factor ANOVA [displacement, genotype]: displacement effect: F(1,34) = 19.01, P < 0.001; genotype effect: F(1,34) = 0.19, P > 0.050; interaction: F(1,34) = 0.61, P > 0.050) (Fig. 3A), and the DR related to those objects did not differ between groups (t(17) = 1.04, P > 0.050) (Fig. 3B). Furthermore, CA3 control mice and CA3 NR1 KO mice successfully detected that one of the old objects had been displaced at test, since both groups explored significantly more the displaced old object than the stationary one (NR1: t(8) = 2.07, P = 0.032, [#]; NR1KO: t(9) = 4.41, P = 0.002, [#]) (Fig. 3A) (DR compared with 0; NR1: t(8) = 2.37, P = 0.042, [o]; NR1KO: t(9) = 4.12, P = 0.003, [o]) (Fig. 3B). Total exploration time during the testing phase did not significantly differ between groups (t(17) = −1.91, P > 0.050) (data not shown).
Likewise, altering NMDA signaling in CA3 did not significantly affect the memory for temporal sequence, since no “genotype” or “genotype by order” interaction effects were found when comparing relative exploration times for the recent and for the old stationary objects (two-factor ANOVA [genotype, order]: genotype effect: F(1,34) = 0.28, P > 0.050; order effect: F(1,34) = 17.82, P < 0.001; interaction: F(1,34) = 1.31, P > 0.050) (Fig. 3C). This was confirmed by further statistical analysis revealing that controls and KOs spent more time to explore the old than the recent stationary objects (NR1: t(8) = 3.23, P = 0.012, [#]; NR1KO: t(9) = 1.72, P = 0.040, [#]) (Fig. 3C) (DR compared with 0: NR1: t(8) = 3.48, P = 0.008) (Fig. 3D). The discrimination ratio for the KOs failed to reach significance (DR compared with 0: t(9) = 1.58, P > 0.050) (Fig. 3D), but a careful observation of the relative exploration time of the old stationary object of controls and KOs suggests that this result stems from a slightly lower exploration time for this object for the KOs (NR1: RET = 28.21% ± 2.51%; NR1KO: RET = 24.49% ± 2.13%) (Fig. 3C) rather than from a true deficit in the memory for the order in which objects have been encountered, which would be reflected by similar exploration time for the old and recent objects within groups. This absence of significant impairment of the “what–when” memory in CA3 NR1 KOs was further supported by the lack of significant between-group differences (NR1 vs. NR1KOs: “old” stationary; t(17) = −1.14, P > 0.050; “recent” stationary: t(17) = 0.45, P > 0.050; DR: t(17) = −0.80, P > 0.050).
Age difference between groups did not contribute to behavioral phenotype
Indeed, the discrimination ratios of the CA1 and CA3 control groups (CA1NR1 and CA3NR1) did not significantly differ for the “what–where” or the “what–when” memory (t(22) = −0.19, P > 0.050 and t(22) = −0.05, P > 0.050, respectively). In addition, control groups spent a comparable time to explore the “old” displaced, the “old” stationary, and the “recent” stationary objects (RET: t(22) = 0.23, P > 0.050; t(22) = 0.21, P > 0.050; t(22) = −0.47, P > 0.050, respectively), suggesting that there were no significant differences in the pattern of exploration between the two control groups despite the age difference.
Discussion
In this study, we have investigated the contribution of NMDA signaling in CA1 and CA3 to the spatial and temporal aspects of episodic memory, using a task that allows for the evaluation of each feature separately within the same task. Here, we report for the first time that NMDA signaling in CA1 contributes selectively to the spatial aspect of episodic memory (the “what–where” memory), while NMDA signaling in CA3 is not essential. In addition, NMDA signaling in neither CA1 nor CA3 was critical for the temporal aspect of episodic memory (the “what–when” memory) in this paradigm.
NMDA in CA1, but not in CA3, contributes specifically to the spatial dimension of episodic memory
Our findings of a critical involvement of CA1 in the “what–where” component of episodic memory is supported by a recent electrophysiological study, reporting a key role of CA1 in a “what–where” memory paradigm requiring multiple trials to learn odor–context pairs (Komorowski et al. 2009). Moreover, our results are also in line with findings of a recent lesion study that reports impaired spatial episodic recognition memory in CA1-lesioned rats performing an object-cued spatial location recall or a location-cued object recall task (Kesner et al. 2008). Furthermore, our data extend our current understanding of the contribution of CA1 to episodic memory by demonstrating that NMDA signaling is critical to the spatial component of episodic memory. This result, together with the findings that CA1 NR1 KOs are impaired on a spatial version of the Morris water maze (multiple trials learning) (Tsien et al. 1996), strengthens the notion that NMDA signaling is essential for the processing of spatial information in memory in general: episodic and non-episodic. Both the temporo-ammonic pathway and the Schaffer collaterals provide NMDA inputs to CA1, and further investigations will be necessary to decipher which one of those inputs contribute to the “what–where” memory at this level, or whether both are required.
In contrast to the deletion of the NR1 subunit of the NMDA receptor in CA1, deletion of NR1 in CA3 did not significantly affect the spatial dimension of episodic memory. The contribution of CA3 to the memory for the association of items in their locations (the “what–where” memory) in multiple-trials discrimination tasks has been well-described in lesion studies (Gilbert and Kesner 2003; Kesner et al. 2005; Hunsaker et al. 2008; Kesner et al. 2008). However, the role of NMDA signaling in the processing of object-in-place information remained elusive. In the present study, NMDA signaling was specifically altered at the level of CA3, and no significant deficits in the performance reflecting the spatial dimension of episodic memory (the “what–where” memory) could be detected. Hence, our results suggest that the role of CA3 in the memory for object-in-place in episodic recognition memory is not mediated by NMDA signaling. This result is in agreement with findings emerging from the literature on multiple-trials recognition memory tasks that report that constitutive and inducible CA3 NR1 KOs can learn over multiple trials the initial pairing of objects with locations in water maze paradigms or odor–context association tasks (Nakazawa et al. 2002; Rajji et al. 2006). Interestingly, intrahippocampal injections of opioid receptor antagonists targeting the mossy fibers (MF-CA3) pathway impair spatial performance in the water maze task, and disruption of the afferent MF pathway (by injection of intracellular or extracellular zinc chelator in the CA3) led to severe deficits in spatial and contextual fear conditioning (Daumas et al. 2004; Cravens et al. 2006). In summary, our results suggest that NMDA signaling does not play a critical role in the mediation of the spatial dimension of episodic memory at the level of CA3, and the latter studies suggest, in turn, that opiates could be a good candidate for supporting this function, which remains to be tested.
NMDA signaling in CA1 or CA3 is not critical for the temporal dimension of episodic memory
A series of recent studies has underlined the critical role of the perirhinal cortex and the medial prefrontal cortex in the memory for temporal order, and its mediation by the NMDA and the cholinergic systems (for review, see Barker and Warburton 2011). In the hippocampus, cholinergic projections from the medial septum have been shown to significantly contribute to the “what–when” memory assessed with a behavioral paradigm similar to the one we have used in the present study (Schäble et al. 2010). However, the role of hippocampal NMDA signaling in the temporal dimension of episodic memory had not been investigated thoroughly. In the present study, we tested whether NMDA signaling in CA1 or CA3 was involved in the mediation of the “what–where” memory in episodic memory. Our results showed that the contribution of NMDA signaling in CA1 to the “what–when” memory is not critical, since KOs successfully discriminated at test between objects encountered during trial 1 and objects encountered during trial 2. Lesion and electrophysiological studies have reported that CA1 plays a crucial role in the association of distinct stimuli separated by brief delays in multiple-trials learning tasks (Kesner et al. 2005; Farovik et al. 2010; MacDonald et al. 2011), and a mutagenesis study has reported a crucial involvement of the CA1 NMDA receptors in a trace fear-conditioning task (Huerta et al. 2000). Hence, our results suggest that different mechanisms could be underlying qualitatively different types of temporal ordering in CA1: one temporal ordering that involves the association of two stimuli separated by a brief delay (seconds), as it is the case in trace fear-conditioning tasks, which has been shown to be NMDA-dependent (Huerta et al. 2000); and a second, NMDA-independent, which involves the temporal ordering of distinct elaborated representations (episodes) separated by long delays (hours), as it is the case in our study.
In addition, our results also show that NMDA signaling in CA3 is not involved in a critical manner in the processing of temporal information in episodic memory. This is in agreement with previous lesion studies using episodic or multiple-trials recognition memory tasks, and mutagenesis studies investigating trace fear conditioning, which reported that CA3 and NMDA signaling in CA3 do not play a crucial role in temporal order (Kesner et al. 2005; Hunsaker et al. 2008; McHugh and Tonegawa 2009). All together, those data strongly suggest that NMDA signaling in CA1 or CA3 does not mediate the temporal dimension of episodic recognition memory.
In summary, in this study, we have shown for the first time that NMDA signaling in CA1 specifically mediates the spatial dimension of episodic memory, while it is not the case in CA3. We also report that NMDA does not contribute to the temporal dimension of episodic memory in either CA1 or CA3. Importantly, those conclusions unlikely result from a difference in age between groups, since performance of CA1 and CA3 control mice does not significantly differ for the “what–where” or for the “what–when” memory. Further studies will be necessary to assess whether those findings can be generalized to other types of episodic memory paradigms. Moreover, targeting selectively CA1 NMDA inputs from either the temporo-ammonic pathway or the Schaffer collaterals will be required to elucidate whether both inputs are required for the mediation of the “what–where” memory in such an episodic memory paradigm, or whether one or the other input would suffice.
Materials and Methods
Animals
Male mice that lack the NR1 subunit of the NMDA receptor on a C57BL/6 background and their floxed-NR1 littermates (controls) were provided from the laboratory of S.T. (MIT, Boston, MA) and were previously described (Tsien et al. 1996; Nakazawa et al. 2002). Mice that lack NR1 in CA1 (CA1-NR1 KOs; n = 11) and their controls (CA1NR1; n = 15) were 6–8 wk old. Mice that lack NR1 in CA3 (CA3 NR1 KOs; n = 10) and their controls (CA3-NR1; n = 9) were 18–26 wk of age. At this age, it has been described that NR1 mRNA was not expressed in the CA1 or CA3 pyramidal cells of the KOs (Tsien et al. 1996; Nakazawa et al. 2002), which we confirmed by detecting the NR1 mRNA on brain sections of each experimental animal following behavioral testing (see Fig. 1). Animals were single-caged with food and water ad libitum and tested during their active phase (reversed light/dark cycle; light off at 7 a.m., on at 7 p.m.). All procedures were performed in agreement with the Institutional Animal Care and Use Committee of Boston University and NIH guidelines.
Detection of NR1 mRNA by in situ hybridization
Following behavioral testing, mice were anesthetized with isoflurane and decapitated. Brains were immediately collected, embedded in mounting medium, and frozen. Subsequently, cryostat sections (14 µm) were collected on polylysine-treated slides and postfixed for 5 min in 4% paraformaldehyde in PBS buffer (pH 7.5). As described in Tsien et al. (1996), following rinses and steps reducing unspecific labeling (triethanolamine, acetic anhydride), slices were hybridized for 17 h at 72°C to an NR1 probe labeled with digoxigenine (45-mer probe, sequence 5′-CTCCTCCTCCTCGCTGTTCACCTTAAATCGGCCAAAGGGACT-3′) using hybridization solution (Amersham) to which was added non-labeled nucleotides (100 µg/mL) and denaturated salmon sperm DNA (100 µg/mL). After several washes in 0.2× SSC solution at decreasing temperatures, NR1 signal was detected by adding an anti-DIG-AP antibody in PBS buffer containing a blocking reagent (Roche) and by incubating the slides with NBT/BCIP solution (Roche) (see Fig. 1).
Apparatus and behavioral paradigm
The testing apparatus consisted of an open-topped box (30 × 30 × 40 cm) made of black Plexiglas and fitted with high intra- and extra-maze cues. Behavioral testing followed the protocol described in Dere et al. (2005). Briefly, following four daily sessions during which animals were habituated to the open field and the presence of objects at all later used locations, testing for object recognition took place on day 5. In this spontaneous object recognition memory task, mice placed in an open field explored four objects for three consecutive trials (trial 1, trial 2, and the “test trial”; 10 min of exploration each), and then were returned to their homecage for 50 min between each trial (Fig. 4). Two sets of four identical objects, differing in shape but made of the same material, were used. During trial 1, mice were exposed to a first set of four identical objects (cone-shaped bells) arranged in a triangular shape (Fig. 4A). In trial 2, mice experienced a second set of four identical objects (jingle bells) arranged this time in a square shape (Fig. 4B). Testing for the temporal (“what–when” memory) and the spatial (“what–where” memory) dimensions of episodic recognition memory took place during the test trial (Fig. 4C). During this trial, objects remained arranged in a square pattern, but two duplicates of the second set of objects and one duplicate of the first set of objects remained at the locations where they were experienced previously (“recent” stationary objects and “old” stationary object, respectively), and one duplicate of the first set of objects was placed at a location that differed from where it was originally experienced (“old” displaced object).
Figure 4.
Episodic recognition memory paradigm (Dere et al. 2005). Mice explored objects for 10 min three times in a row, each trial separated by 50 min. (A) Trial 1: Mice explored four identical objects arranged in a triangle shape. (B) Trial 2: Mice explored a different set of objects arranged in a square shape. (C) In the test trial, mice explored two duplicates of the objects encountered during trial 2 placed at the same location (“recent” stationary objects) and two duplicates of the objects encountered during trial 1: one at the same location (“old” stationary) and one displaced (“old” displaced).
Data analysis
The performance of the animals was videotaped, and the time spent exploring the objects was scored off-line by two independent experimenters who were blind to genotypes. Only exploration directed toward the object was scored (not the exploration of the arena or the time spent sitting on the objects without object exploration, which was minimal). Exploratory behavior was defined as the animal directing its nose toward the object at a distance <1 cm, which still allowed for its vibrissae to be in contact with the object. All animals explored for >18 sec per trial, or the data were excluded. Object exploration times were averaged across experimenters, and relative exploration time (RET, e.g., percentage of the total exploration time), which took into consideration the exploration times for the four objects, was calculated for each object. Successful recognition of the spatial displacement of the “old” object (the “what–where” memory) is reflected by an RET significantly higher for the “old” displaced object than for the “old” stationary one. Successful recognition of the temporal order in which objects have been experienced (the “what–when” memory) is reflected by an RET significantly higher for the “old” stationary object when compared with the average of the RET of the “recent” stationary object. In addition, a standard memory index used to study spontaneous object recognition memory was also calculated. This discrimination ratio takes into consideration the exploration times of only two objects at once (instead of four in the case of the RET): either the two objects that can reflect the spatial aspect of episodic memory: the “old” displaced and the “old” stationary for the “what–where” memory (DRwhat–where), or the exploration times of the two objects that would reflect the temporal aspect of episodic memory: the “old” and the “recent” stationary objects for the “what–when” memory (DRwhat–when). DRs are calculated as follows:
DR = 0 indicates no preference; DRwhat–where significantly greater than 0 indicates a preference for the “old” displaced object versus the “old” stationary one, and DRwhat–when significantly greater than 0 a preference for the “old” stationary object versus the “recent” stationary one (Ennaceur and Delacour 1988).
Statistical comparisons of the RET across objects for “what–where” and “what–when” performance were made using two-factor ANOVAs. Further between-groups statistical analysis relied on two-tailed unpaired t-tests, and further within-groups analysis on two-tailed paired t-tests. One sample two-tailed t-test analysis was used to compare the DRs with zero.
Acknowledgments
This work was supported U.S. National Institute of Mental Health grants NIMH MH60450 and the Mercator Stiftung.
References
- Barker GR, Warburton EC 2011. Evaluating the neural basis of temporal order memory for visual stimuli in the rat. Eur J Neurosci 33: 705–716 [DOI] [PubMed] [Google Scholar]
- Cravens CJ, Vargas-Pinto N, Christian KM, Nakazawa K 2006. CA3 NMDA receptors are crucial for rapid and automatic representation of context memory. Eur J Neurosci 24: 1771–1780 [DOI] [PubMed] [Google Scholar]
- Daumas S, Halley H, Lassalle JM 2004. Disruption of hippocampal CA3 network: Effects on episodic-like memory processing in C57BL/6J mice. Eur J Neurosci 20: 597–600 [DOI] [PubMed] [Google Scholar]
- Dere E, Huston JP, De Souza Silva MA 2005. Episodic-like memory in mice: Simultaneous assessment of object, place and temporal order memory. Brain Res Brain Res Protoc 16: 10–19 [DOI] [PubMed] [Google Scholar]
- Eichenbaum H 2004. Hippocampus: Cognitive processes and neural representations that underlie declarative memory. Neuron 44: 109–120 [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C 2007. The medial temporal lobe and recognition memory. Annu Rev Neurosci 30: 123–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J 1988. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res 31: 47–59 [DOI] [PubMed] [Google Scholar]
- Farovik A, Dupont LM, Arce M, Eichenbaum H 2008. Medial prefrontal cortex supports recollection, but not familiarity, in the rat. J Neurosci 28: 13428–13434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farovik A, Dupont LM, Eichenbaum H 2010. Distinct roles for dorsal CA3 and CA1 in memory for sequential nonspatial events. Learn Mem 17: 12–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellini L, Florian C, Courtey J, Roullet P 2009. Pharmacological intervention of hippocampal CA3 NMDA receptors impairs acquisition and long-term memory retrieval of spatial pattern completion task. Learn Mem 16: 387–394 [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Brushfield AM 2009. The role of the CA3 hippocampal subregion in spatial memory: A process oriented behavioral assessment. Prog Neuropsychopharmacol Biol Psych 33: 774–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP 2003. Localization of function within the dorsal hippocampus: The role of the CA3 subregion in paired-associate learning. Behav Neurosci 117: 1385–1394 [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, Lee I 2001. Dissociating hippocampal subregions: Double dissociation between dentate gyrus and CA1. Hippocampus 11: 626–636 [DOI] [PubMed] [Google Scholar]
- Huerta PT, Sun LD, Wilson MA, Tonegawa S 2000. Formation of temporal memory requires NMDA receptors within CA1 pyramidal neurons. Neuron 25: 473–480 [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Lee B, Kesner RP 2008. Evaluating the temporal context of episodic memory: The role of CA3 and CA1. Behav Brain Res 188: 310–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Hunsaker MR 2010. The temporal attributes of episodic memory. Behav Brain Res 215: 299–309 [DOI] [PubMed] [Google Scholar]
- Kesner RP, Warthen DK 2010. Implications of CA3 NMDA and opiate receptors for spatial pattern completion in rats. Hippocampus 20: 550–557 [DOI] [PubMed] [Google Scholar]
- Kesner RP, Hunsaker MR, Gilbert PE 2005. The role of CA1 in the acquisition of an object–trace-odor paired associate task. Behav Neurosci 119: 781–786 [DOI] [PubMed] [Google Scholar]
- Kesner RP, Hunsaker MR, Warthen MW 2008. The CA3 subregion of the hippocampus is critical for episodic memory processing by means of relational encoding in rats. Behav Neurosci 122: 1217–1225 [DOI] [PubMed] [Google Scholar]
- Komorowski RW, Manns JR, Eichenbaum H 2009. Robust conjunctive item-place coding by hippocampal neurons parallels learning what happens where. J Neurosci 29: 9918–9929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H 2011. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron 71: 737–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh TJ, Tonegawa S 2009. CA3 NMDA receptors are required for the rapid formation of a salient contextual representation. Hippocampus 19: 1153–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T, Young JZ, McHugh TJ, Buhl DL, Tonegawa S 2008. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science 319: 1260–1264 [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, Johnston D, Wilson MA, et al. 2002. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science 297: 211–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Sun LD, Quirk MC, Rondi-Reig L, Wilson MA, Tonegawa S 2003. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron 38: 305–315 [DOI] [PubMed] [Google Scholar]
- Nakazawa K, McHugh TJ, Wilson MA, Tonegawa S 2004. NMDA receptors, place cells and hippocampal spatial memory. Nat Rev Neurosci 5: 361–372 [DOI] [PubMed] [Google Scholar]
- Rajji T, Chapman D, Eichenbaum H, Greene R 2006. The role of CA3 hippocampal NMDA receptors in paired associate learning. J Neurosci 26: 908–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Kesner RP 2006. A computational theory of hippocampal function, and empirical tests of the theory. Prog Neurobiol 79: 1–48 [DOI] [PubMed] [Google Scholar]
- Schäble S, Huston JP, Brandao ML, Dere E, de Souza Silva MA 2010. Neurokinin-2 receptor antagonism in medial septum influences temporal-order memory for objects and forebrain cholinergic activity. Peptides 31: 108–115 [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE 2007. Recognition memory and the medial temporal lobe: A new perspective. Nat Rev Neurosci 8: 872–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S 1996. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 87: 1327–1338 [DOI] [PubMed] [Google Scholar]