Abstract
It is still an open question whether subjective memory complaints (SMC) can actually be considered to be clinically relevant predictors for the development of an objective memory impairment and even dementia. There is growing evidence that suggests that SMC are associated with an increased risk of dementia and with the presence of biological correlates of early Alzheimer's disease. In this paper, in order to shed some light on this issue, we try to discern whether subjects with SMC showed a different profile of functional connectivity compared with subjects with mild cognitive impairment (MCI) and healthy elderly subjects. In the present study, we compare the degree of synchronization of brain signals recorded with magnetoencephalography between three groups of subjects (56 in total): 19 with MCI, 12 with SMC and 25 healthy controls during a memory task. Synchronization likelihood, an index based on the theory of nonlinear dynamical systems, was used to measure functional connectivity. Briefly, results show that subjects with SMC have a very similar pattern of connectivity to control group, but on average, they present a lower synchronization value. These results could indicate that SMC are representing an initial stage with a hypo-synchronization (in comparison with the control group) where the brain system is still not compensating for the failing memory networks, but behaving as controls when compared with the MCI subjects.
Keywords: Subjective memory complaints, Mild cognitive impairment, Magnetoencephalography, Alzheimer's disease, Functional connectivity, Synchronization Likelihood
Introduction
Subjective memory complaints (SMC) represent a subjective noticeable decline from previous level of memory functioning (Vestberg et al. 2009) and are common in older people both with and without objective evidence of memory impairment (Mitchell, 2008a). It is estimated that between 17% and 57% of elderly people report them (Ganguli et al. 2004; Jessen et al. 2007; Mitchell 2008b), with their occurrence being more frequent in females (Gagnon et al. 1994). This percentage increases with age, rising to 43% for those aged 65–74, 51% for those aged 75–84, and 88% for those aged 85 and older (Bassett and Folstein 1993). A meta-analysis made by Mitchell (2008b) indicates that SMCs are present in 42.8% of patients with dementia, in 38.2% of those with mild cognitive impairment (MCI) and in 17.4% of healthy elderly controls.
Over recent years, many studies involving different populations have reported that memory complaints appear to be associated with depression, mainly in samples based on volunteers and self-referrals (Jonker et al. 2000). However, in community-based samples, a significant relationship was observed between self-reported memory problems and poor memory performance independently of depressive symptomatology (Jonker et al. 1996). Apart from the depression, the SMC have also been related to anxiety and personality traits (Jorm et al. 2004). However, there is also growing evidence to suggest that SMC are associated with an increased risk of dementia and with the presence of biological correlates of early Alzheimer's disease (Rodda et al. 2009).
Some cross-sectional studies have found associations between SMC and objective memory performance (Jonker et al. 1996; Gagnon et al. 1994), although others did not (Bolla et al. 1991; Minett et al. 2007). Nevertheless, longitudinal studies seem to show this relationship more clearly (Tobiansky et al. 1995; Schmand et al. 1996)(see Jonker et al. 2000 for a review). Although subjects with objective memory impairment normally report SMC, there are other elderly subjects that perform as well as healthy controls on memory tests but still report complaints about their memory. It is still an open question whether these SMCs can actually be considered to be clinically relevant predictors for the development of an objective memory impairment and even dementia (Roberts et al. 2009; Riedel-Heller and Matschinger 1999; Glodzik-Sobanska et al. 2007; Treves et al. 2005; Wang et al. 2004).
Thus, although differences are not found in neuropsychological test scores in comparison with a ‘healthy’ group, some neuroimaging studies in people with SMC showed a smaller entorhinal cortex (Jessen et al. 2006), a reduced hippocampal volume (Van der Flier et al. 2004), reduced metabolism (Mosconi et al. 2008) and subcortical parieto-occipital white matter lesions (Stenset et al. 2008). Studies of brain activity during cognitive tasks note that subjects with SMC present an increase in activity in comparison with the control group (Rodda et al. 2009; Rodda et al. 2010; Maestu et al. 2010; Jessen et al. 2010; Elfgren et al. 2010; Gallassi et al. 2010; Benito-León et al. 2010; Luck et al. 2010). All these studies have evaluated the spatial or the spatiotemporal profiles of activity, but none of them assess whether the functional connectivity between brain regions was spared or not in subjects with SMC. Functional connectivity measures the statistical interdependencies between two brain signals and seems to be related to the brain ability to communicate information between brain regions (Varela et al. 2001). Functional connectivity is, probably, an essential tool for the study of brain functioning, with its deviation from healthy reference being an indication of lesion (Schnitzler and Gross 2005, Guggisberg et al. 2008). The evaluation of functional connectivity in subjects with SMC is a relevant issue due to the ideas developed by Braak and Braak (Braak and Braak 1991), which indicate pathophysiological changes up to decades before the diagnosis of dementia. Furthermore, the idea that views Alzheimer's disease (AD) as a disconnection syndrome (Morris and Becker 1994) has not yet been tested in subjects with SMC.
In the present study, we try to overcome some of the limitations of the previous studies by (1) removing variables that could confound the origins of SMC such as psychiatric conditions; (2) using a structured questionnaire with more than 20 questions as opposed to a list of 2 or 3 questions used in most previous studies; (3) recording the brain magnetic activity with magnetoencephalography (MEG) during a memory task in three different groups of elderly people: healthy volunteers with neither subjective nor objective memory impairment, subjects with SMC without objective memory impairment and MCI subjects with both subjective and objective memory impairments with the aim to evaluate differences in brain activity between these three groups; (4) evaluating the functional interaction between brain regions in different frequency bands through the use of MEG signals. We predict that subjects with SMC will show a differential profile of functional connectivity compared with both MCI and healthy elderly subjects indicating some preliminary signs of neurophysiological impairment.
Methods
Participants
Fifty-six, right handed, elderly participants recruited from the Geriatric Unit of the ‘Hospital Universitario San Carlos Madrid’ and the ‘Centro de Prevención del Deterioro Cognitivo, Ayuntamiento de Madrid’, participated in the study. Participants were divided into three groups based on their clinical profiles: 19 participants were considered as multidomain MCI patients, 25 as elderly control participants and 12 as SMC participants.
The SMC group was composed of 12 (9 women, average 72.5 years old) elderly participants who came, on their own initiative, to the ‘Centre for the Prevention of Cognitive Decline’ and reported experiencing memory deficits. This is a public health centre in Madrid (Spain), which runs memory training programmes for both healthy elders and MCI patients. Participants for the SMC group were selected following the criteria proposed by Abdularab and Heun (2008): (1) Patient stating that their memory function has deteriorated compared to earlier stages in life; (2) time of onset being in adulthood; (3) providing a valid example; (4) memory deterioration confirmed by an informant (close relative or friend); (5) normal objective memory performance. The assessment was based on structured interview and a neuropsychological assessment. To ensure that memory complaints were not caused by a psychiatric condition, all patients were interviewed by an experienced psychiatrist (PM) and had to score below 9 in the geriatric depression scale (Yesavage and Brooks 1991). Additionally, to confirm the memory complaints, participants from this group had to score higher than 13 (mean 27.6) in the memory failures of everyday test (Sunderland et al. 1983). Given that the association between subjective ratings and future cognitive decline is stronger when complaints have been confirmed by an informant (Farias et al. 2005), we required confirmation from relatives or close friends. None of these patients met the criteria for MCI and had no history of psychiatric or neurological disorders. Most SMC patients were following educational courses at local social centres.
MCI diagnosis was established according to the criteria proposed by Petersen et al. (Grundman et al. 2004; Petersen 2004). Thus, MCI patients fulfilled the following criteria: (1) cognitive complaint corroborated by an informant (a person who stays with the patient at least for half a day at least 4 days a week); (2) objective cognitive impairment, documented by delayed recall in the logical memory II subtest of the revised Wechsler Memory Scale (score ≤16/50 for patients with more than 15 years of education; score ≤8/50 for patients with 8–15 years of education); (3) normal general cognitive function, as assessed by a clinician during a structured interview with the patient and an informant and, additionally, a mini mental state examination (MMSE) score greater than 24; (4) relatively preserved daily living activities as measured by the Lawton scale; (5) not sufficiently impaired, cognitively and functionally to meet criteria for dementia. Age and years of education were matched to the SMC group. According to their clinical and neuropsychological profile, all patients in this group were considered multi-domain MCI patients (see (Petersen 2004). As for the geriatric depression scale, none of the MCI showed depression (score lower than 9) (Yesavage and Brooks 1991).
Twenty-five age-matched healthy elderly participants were included as a control group. Age and years of education were matched to the SMC group (see Table 1). To confirm the absence of memory complaints, a score of 0 was required in a four-question questionnaire (see (Mitchell 2008a)). None of the participants had a history of neurological or psychiatric condition. To summarize, MCI patients showed both subjective and objective memory impairment, SMC participants presented only with memory complaints with a normal score on the memory test and healthy elders showed neither subjective nor objective memory impairment.
Table 1.
Distribution of age and cognitive test scores in each group
| Age | MMSE | GDS | LM2 | Hits | |
|---|---|---|---|---|---|
| Control | 72 ± 8 | 29.5 ± 0.7 | 1 | 27 ± 7 | 100 ± 25 |
| SMC | 72 ± 6 | 29 ± 1 | 1 | 24 ± 10 | 109 ± 22 |
| MCI | 75 ± 3 | 28 ± 1 | 3 | 14 ± 6 | 102 ± 31 |
MMSE mini mental state examination, GDS global deterioration scale, LM2 logical memory delayed free recall, Hits mean of correct responses to the target stimuli, SMC subjective memory complaints group, MCI mild cognitive impairment group
MCI patients, SMC subjects and healthy participants underwent a neuropsychological assessment, in order to establish their cognitive status with respect to multiple cognitive functions. Specifically, memory impairment was assessed by the logical memory test (immediate and delayed) from the Wechsler Memory Scale-III-R. Two scales of cognitive and functional status were applied as well: the Spanish version of the MMSE (Lobo et al. 1979), and the Global Deterioration Scale/Functional Assessment Staging GDS/FAST. Participants were selected so that the number of years of education was as similar as possible for the three groups (MCI patients 8.5, SMC patients 8.3 and control participants 8.9 on average). Before the MEG recording, all participants or their legal representatives gave written informed consent to participate in the study, which was approved by the local ethics committee.
Stimuli and task
MEG scans were obtained in the context of a modified version of the Sternberg's letter-probe task (deToledo-Morrell et al. 1991; Maestu et al. 2001) in which a set of five letters was presented and participants were asked to keep the letters in mind. After the presentation of the five-letter set, a series of single letters (500 ms in duration with a random ISI between 2 and 3 s) was introduced one at a time, and participants were asked to press a button with their right hand when a member of the previous set was detected. The list consisted of 250 letters in which half were targets (previously presented letters) and half distracters (not previously presented letters). Participants undertook a training series before the actual test, which did not start until the participant demonstrated that he/she remembered the five-letter set. Letters were projected through a LCD video-projector (SONY VPL-X600E), situated outside the shielded-room onto a series of in-room mirrors, the last of which was suspended approximately 1* metre above the participant's face. Letters subtended 1.8° and 3° of horizontal and vertical visual angle, respectively.
MEG data collection
The MEG signal was recorded with a 254-Hz sampling frequency and a band pass of 0.5–50 Hz, using a 148-channel whole-head magnetometer (MAGNES® 2500 WH, 4-D Neuroimaging) confined in a magnetically shielded room. An environmental noise reduction algorithm using reference channels at a distance from the MEG sensors was applied to the data. Thereafter, single-trial epochs were visually inspected by an experienced investigator, and epochs containing visible blinks, eye movements or muscular artefacts were excluded from further analysis. Artefact-free epochs from each channel were then classified into four different categories according to the subject's performance in the experiment: hits, false alarms, correct rejections and omissions. Only hits were considered for further analysis because we were interested in evaluating the functional connectivity patterns which support recognition success. Thirty-five epochs (1 s each one) were used to calculate synchronization likelihood (SL) values. This lower bound was determined by the participant with least epochs. To have an equal number of epochs across participants, 35 epochs were randomly chosen from each of the other participants.
In-house Fortran code was used to implement the SL algorithm as described by Stam and van Dijk (2002). The SL algorithm was applied to the 35 extracted artefact-free 1-s epochs for each subject. For each frequency band, optimal SL parameter values were chosen according to Montez et al. (2006) for each frequency band and 1-s length:
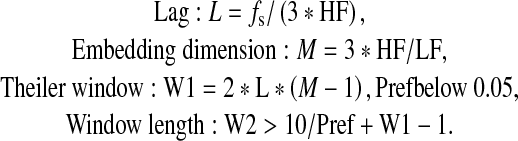 |
Where fs sampling rate, and HF and LF are the high- and low-frequency bands, respectively.
The following frequency bands were considered: alpha1 (α1, 8–11 Hz), alpha2 (α2, 11–14 Hz), beta1 (β1, 14–25 Hz), beta2 (β2, 25–35 Hz), gamma (γ, 35–45 Hz). The SL index was not computed for bands under 8 Hz as the epoch length and sampling rate do not allow an accurate enough estimation (Montez et al. 2006).
All epochs were digitally filtered off-line at the above frequency bands. Subsequently, the SL was calculated for each of the 35 1-s epochs with 148*147/2 channel pairs for each frequency band, unfiltered epochs and each subject (25 controls, 19 MCIs and 12 SMCs).
Statistical analysis
To compare the level of SL between the three groups, SL values were first averaged across epochs for each participant and channel pair. Then, false discovery rate Benjamini and Yekutieli 2001; Genovese et al. 2002) was applied to find channel pairs with significant differences between each couple of groups (MCI vs. control, MCI vs. SMC and control vs. SMC). For each channel pair, a between-groups Kruskal–Wallis (non-parametric) test was calculated. From the resulting p values, a significance threshold was calculated with a corresponding q = 0.2 using the type I false discovery rate implementation.
Results
MCI vs. control participants
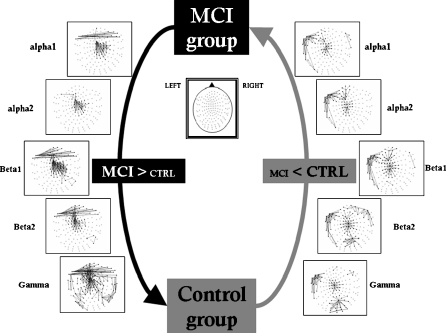
Comparing these two groups (see Fig. 1), MCI patients showed a clear cluster of higher synchronization values over the anterior and central regions in all frequency bands. Additionally, MCI patients showed higher inter-hemispheric SL values than the control group between left and right temporo-frontal sensors in all frequency bands (except in band α2).
Fig. 1.
Significant differences in synchronization likelihood between electrode pairs for different frequency bands. (mild cognitive impairment > control and mild cognitive impairment < control). False discovery rate type I was applied
Two non-functionally related clusters of local interactions showed higher SL values in the control group: one among left temporal sensors and another one in central-posterior channels. Both of them were found in all frequency bands. Additionally, mainly in γ band, there is a higher posterior synchronization in controls compared to MCI patients.
MCI vs. SMC
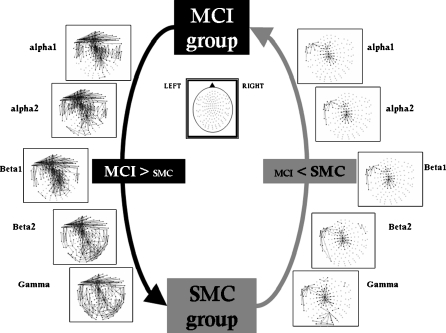
Functional connectivity profile is very similar to the one showed previously when MCI and controls were compared. However, in the case of MCI/SMC comparison, there is an increased number of statistical differences. This increase means that the SMC group presents, on average, lower synchronization than control group when compared with MCI patients (see Fig. 2).
Fig. 2.
Significant differences in synchronization likelihood between electrode pairs for different frequency bands. (mild cognitive impairment > subjective memory complaints and mild cognitive impairment < subjective memory complaints). False discovery rate type I was applied
Control participants vs. SMC
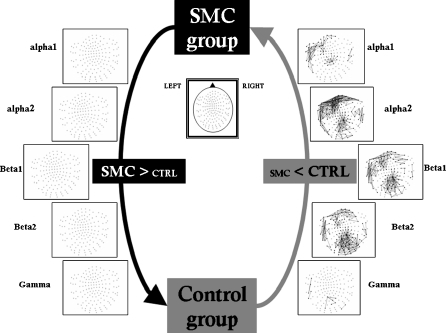
When comparing control group and SMC, only the control group showed higher synchronization values (see Fig. 3). These differences achieve statistically significant values over the anterior regions, the left temporal lobe and the posterior regions. All these differences were limited to frequencies between α1 and β2 as differences in the γ band did not achieve statistically significant values. Such higher synchronization tends to be increased in posterior regions and in the frontal region, mainly in the α2 frequency band.
Fig. 3.
Significant differences in synchronization likelihood between electrode pairs for different frequency bands. (control > subjective memory complaints). False discovery rate type I was applied
Discussion
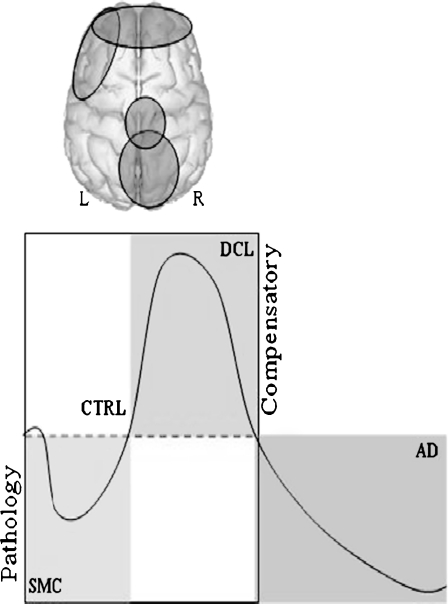
In this paper, we try to show whether subjects with SMC but not objective memory impairment, showed a different profile of functional connectivity compared with subjects with MCI and healthy elderly subjects. In different frequency bands (α to γ), SMC subjects showed lower synchronization values than MCI and healthy elderly subjects, although with a different topology. Conversely, SMC subjects showed only higher synchronization values in comparison with the MCI subjects. In fact, their profile, in comparison with the MCI subjects, was very similar to the one showed when MCI and controls were compared. Regardless of whether or not we understand SMC as the initial stage of a cognitive continuum decline, all these results could indicate that SMCs are representing an initial stage with a hypo-synchronization (in comparison with the control group) where the brain system is not still compensating for the failure of the memory networks, but behaving as controls when compared with the MCI subjects (see Fig. 4).
Fig. 4.
Cognitive decline continuum: from pathology to compensatory phenomena. Although SMC subjects show a different profile of functional connectivity pattern than MCI and healthy controls, synchronization changes are focused on the anterior, central, posterior and left temporal regions. MCI subject showed a hyper-synchronization (compensatory effect), whereas SMC a hypo-synchronization (the brain is not still compensating for the fails of the memory networks, being a pathological or dementia effect). AD patients show an impairment of their functional connectivity (Stam et al. 2009)
When functional connectivity profiles from subjects with SMC were compared with those of the MCI group, SMC showed, in all frequency bands, both a hypo and hyper-synchronization, but with a different network topology. Thus, MCI subjects showed higher synchronization over the anterior and central regions as well as in the left and right temporal regions. In the β2 and γ, this profile of higher synchronization is even more widely distributed to include the posterior regions bilaterally. This could indicate that at the time when the memory impairment is severe enough to be detected by memory test, MCI subjects tend to integrate information between the left and right frontal lobes to develop better memory strategies (Cabeza et al. 2002) and thus achieve a similar level of performance to the rest of the groups. Conversely, subjects with SMCs showed higher synchronization than MCIs in the central and left temporal sensors. It is interesting to point out that these profiles of functional connectivity are mirroring those found when comparing MCI and controls. Thus, subjects with SMC behave this time as controls demonstrating that they have less severe neurophysiological impairment than that showed by the MCI subjects.
When functional connectivity profiles were compared between SMC and controls, the control group showed higher synchronization values. These differences achieve statistically significant values over the anterior regions, the left temporal lobe and the posterior regions. All these differences were limited to frequencies between α1 and β2 since differences in the γ band did not achieve statistically significant values. There are some more differences in the profile of synchronization between controls and SMC. For example, in the β band, while the comparison between controls and MCI showed higher synchronization in favour of the MCIs over anterior and central regions, these patterns are widely distributed when comparing MCIs and SMC, which accounts for their diminution in the synchronization values along the whole sensor area. This hypo-synchronization could underlie the memory difficulties in the day-to-day activities reported by SMC subjects. Thus, the difficulties in achieving a correct coupling between brain regions diminish the probability of an efficient transmission of information (Stam et al. 2009) leading to memory failures. A compensation mechanism showed by MCI subjects, seems to be unnecessary at this point since memory difficulties are not yet severe enough.
As far as we know, there are only three previous papers published with functional neuroimaging in patients with SMC (Rodda et al. 2010; Rodda et al. 2009; Maestu et al. 2010). In all three of them, SMC subjects showed higher activation than healthy controls. This result seems to be contradictory with the profile of lower synchronization found in this study. These three previous papers used measurements of the magnitude of the signal, such as the increased or decreased blood flow or power of the magnetic field in particular regions of the brain. However, none of them calculated functional connectivity between brain regions. The fact that one particular brain region increases in blood flow or magnetic field does not necessarily imply that it is having better communication with other brain regions. Furthermore, functional connectivity is evaluating how brain regions are communicating with each other rather than just assessing the magnitude of the signal in a particular brain region. So here, we try to test the integration rather than the segregation of the functional activity. Thus, it seems like while subjects with SMC are increasing their local activity, they fail in their ability of communicating information between brain regions. In any case, we think that fMRI and MEG findings are complementary because they may suggest the presence of early functional changes in SMC.
In conclusion, the profiles of functional connectivity described in this work, point toward a model of how the brain mechanisms behave in different stages of a possible cognitive decline continuum. Taking the healthy elderly subjects' profile of functional connectivity as a standard at the SMC stage, subjects show a hypo-synchronization of their memory-related networks and at the MCI stage, memory networks increase their coupling as a compensatory mechanism (Bajo et al. 2010) and finally AD patients show an impairment of their functional connectivity (Stam et al. 2009). Thus, at the stage of SMC, compensatory mechanisms are still not necessary because the memory deficit is not severe enough to be detected by memory tests and does not dramatically disturb daily living activities. However, when the memory impairment is severe enough to be detected by the neuropsychological assessment, then the brain compensatory mechanisms are required to enhance information communication by means of synchronization. Finally, due to the severe damage of the biochemical and anatomical structure of the brain at the AD stage, all these compensatory mechanisms are no longer possible. Future studies should consider a follow-up of the SMC subjects to determine which of these subjects develop an objective memory impairment, with the aim of describing profiles of prediction from SMC to MCI.
Acknowledgements
This study was supported by two grants for the Spanish Ministry of Science and Innovation (SEJ-2006-07560; PSI2009-14415-C03-01).
References
- Abdulrab K, Heun R. Subjective memory impairment. A review of its definitions indicates the need for a comprehensive set of standardized and validated criteria. Eur Psychiatr. 2008;23:321–330. doi: 10.1016/j.eurpsy.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Bajo R, Maestú F, Nevado A, Sancho M, et al. Functional connectivity in mild cognitive impairment during a memory task: implications for the disconnection hypothesis. J Alzheimers Dis. 2010;22:183–193. doi: 10.3233/JAD-2010-100177. [DOI] [PubMed] [Google Scholar]
- Bassett SS, Folstein MF: memory complaint, memory performance performance, and psychiatric diagnosis: a community study. J Geriatr Psychiatr Neurol. 1993;6:105–111. doi: 10.1177/089198879300600207. [DOI] [PubMed] [Google Scholar]
- Benito-León J, Mitchell AJ, Vega S, Bermejo-Pareja F. A population-based study of cognitive function in older people with subjective memory complaints. J Alzheimers Dis. 2010;22(1):159–170. doi: 10.3233/JAD-2010-100972. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29(4):1165–1188. doi: 10.1214/aos/1013699998. [DOI] [Google Scholar]
- Bolla KI, Lindgren KN, Bonaccorsy C, Bleecker ML. Memory complaints in older adults. Arch Neurol. 1991;48:61–64. doi: 10.1001/archneur.1991.00530130069022. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- deToledo-Morrell L, Evers S, Hoeppner TJ, Morrell F, Garron DC, Fox JH. A ‘stress’ test for memory dysfunction. Electrophysiologic manifestations of early Alzheimer's disease. Arch Neurol. 1991;48:605–609. doi: 10.1001/archneur.1991.00530180061018. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Dolcos F, Graham R, Nyberg L. Similarities and differences in the neural correlates of episodic memory retrieval and working memory. Neuroimage. 2002;16:317–330. doi: 10.1006/nimg.2002.1063. [DOI] [PubMed] [Google Scholar]
- Elfgren C, Gustafson L, Vestberg S, Passant U. Subjective memory complaints, neuropsychological performance and psychiatric variables in memory clinic attendees: a 3-year follow-up study. Arch Gerontol Geriatr. 2010;51(3):e110–e114. doi: 10.1016/j.archger.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Farias ST, Mungas D, Jagust W. Degree of discrepancy between self and other- reported everyday functioning by cognitive status: dementia, mild cognitive impairment and healthy elders. Int J Geriatr Psychiatry. 2005;20:827–834. doi: 10.1002/gps.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon M, Dartigues JF, Mazaux JM, Dequae L, Letenneur L, Giroire JM, Barberger-Gateau P. Self-reported memory complaints and memory performance in elderly French community residents: results of the PAQUID research program. Neuroepidemiology. 1994;13(4):145–154. doi: 10.1159/000110373. [DOI] [PubMed] [Google Scholar]
- Gallassi R, Oppi F, Poda R, Scortichini S, Stanzani Maserati M, Marano G, Sambati L. Are subjective cognitive complaints a risk factor for dementia? Neurol Sci. 2010;31(3):327–336. doi: 10.1007/s10072-010-0224-6. [DOI] [PubMed] [Google Scholar]
- Ganguli M, Dodge HH, Shen C, DeKosky ST. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology. 2004;63:115–121. doi: 10.1212/01.wnl.0000132523.27540.81. [DOI] [PubMed] [Google Scholar]
- Genovese R, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Glodzik-Sobanska L, Reisberg B, Santi S, Babb JS, Pirraglia E, Rich KE, Brys M, Leon MJ. Subjective memory complaints: presence, severity and future outcome in normal older subjects. Dement Geriatr Cogn Disord. 2007;24:177–184. doi: 10.1159/000105604. [DOI] [PubMed] [Google Scholar]
- Grundman M, Petersen RC, Ferris SH, Thomas RG, Aisen PS, Bennett DA, et al. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- Guggisberg AG, Honma SM, Findlay A, Dalal SS, Kirsch HE, Berger MS, et al. Mapping functional connectivity in patients with brain lesions. Ann Neurol. 2008;63:193–203. doi: 10.1002/ana.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F, Feyen L, Freymann K, Tepest R, Maier W, Heun R, et al. Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiol Aging. 2006;27:1751–1756. doi: 10.1016/j.neurobiolaging.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Jessen F, Wiese B, Cvetanovska G, Fuchs A, Kaduszkiewicz H, Kolsch H, et al. Patterns of subjective memory impairment in the elderly: association with memory performance. Psychol Med. 2007;37:1753–1762. doi: 10.1017/S0033291707001122. [DOI] [PubMed] [Google Scholar]
- Jessen F, Wiese B, Bachmann C, Eifflaender-Gorfer S, Haller F, Kölsch H, Luck T, Mösch E, Bussche H, Wagner M, Wollny A, Zimmermann T, Pentzek M, Riedel-Heller SG, Romberg HP, Weyerer S, Kaduszkiewicz H, Maier W, Bickel H. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatr. 2010;67(4):414–422. doi: 10.1001/archgenpsychiatry.2010.30. [DOI] [PubMed] [Google Scholar]
- Jonker C, Launer LJ, Hooijer C, Lindeboom J. Memory complaints and memory impairment in older individuals. J Am Geriatr Soc. 1996;44(1):44–49. doi: 10.1111/j.1532-5415.1996.tb05636.x. [DOI] [PubMed] [Google Scholar]
- Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population- based studies. Int J Geriatr Psychiatry. 2000;15(11):983–991. doi: 10.1002/1099-1166(200011)15:11<983::AID-GPS238>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Butterworth P, Anstey KJ, et al. Memory complaints in a community sample aged. 60–64 years: associations with cognitive functioning, psychiatric symptoms, medical conditions, APOE genotype, hippocampus and amygdala volumes, and white-matter hyperintensities. Psychol Med. 2004;34(8):1495–1506. doi: 10.1017/S0033291704003162. [DOI] [PubMed] [Google Scholar]
- Lobo A, Ezquerra J, Gomez BF, Sala JM, Seva DA. Cognocitive mini-test (a simple practical test to detect intellectual changes in medical patients) Actas Luso Esp Neurol Psiquiatr Cienc Afines. 1979;7:189–202. [PubMed] [Google Scholar]
- Luck T, Riedel-Heller SG, Luppa M, Wiese B, Wollny A, Wagner M, Bickel H, Weyerer S, Pentzek M, Haller F, Moesch E, Werle J, Eisele M, Maier W, Bussche H, Kaduszkiewicz H. Risk factors for incident mild cognitive impairment—results from the German study on ageing, cognition and dementia in primary care patients (AgeCoDe) Acta Psychiatr Scand. 2010;121(4):260–272. doi: 10.1111/j.1600-0447.2009.01481.x. [DOI] [PubMed] [Google Scholar]
- Maestu F, Fernandez A, Simos PG, Gil-Gregorio P, Amo C, Rodriguez R, Arrazola J, Ortiz T. Spatio-temporal patterns of brain magnetic activity during a memory task in Alzheimer's disease. Neuroreport. 2001;12:3917–3922. doi: 10.1097/00001756-200112210-00013. [DOI] [PubMed] [Google Scholar]
- Maestu F, Baykova E, Ruiz JM, Montejo P, Montenegro M, Llanero M, Solesio E, Gil P, Yubero R, Paul N, Pozo F, Nevado A. Increased biomagnetic activity in healthy elderly with subjective memory complaints. Clin Neurophysiol. 2011;122:499–505. doi: 10.1016/j.clinph.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Minett TSC, Silva RV, Ortiz KZ, Bertolucci PHF. Subjective memory complaints in an elderly sample: a cross- sectional study. Int J Geriatr Psychiatry. 2007;23:49–54. doi: 10.1002/gps.1836. [DOI] [PubMed] [Google Scholar]
- Mitchell AJ. Is it time to separate subjective cognitive complaints from the diagnosis of mild cognitive impairment? Age Ageing. 2008a;37:497–499. doi: 10.1093/ageing/afn147. [DOI] [PubMed] [Google Scholar]
- Mitchell AJ. The clinical significance of subjective memory complaints in the diagnosis of mild cognitive impairment and dementia: a meta-analysis. Int J Geriatr Psychiatry. 2008b;23:1191–1202. doi: 10.1002/gps.2053. [DOI] [PubMed] [Google Scholar]
- Montez T, Linkenkaer-Hansen K, Dijk BW, Stam CJ. Synchronization likelihood with explicit time-frequency priors. Neuroimage. 2006;33:1117–1125. doi: 10.1016/j.neuroimage.2006.06.066. [DOI] [PubMed] [Google Scholar]
- Morris R, Becker J (1994) Cognitive Neuropsychology of Alzheimer Disease. Oxford University Press
- Mosconi L, Santi S, Brys M, Tsui WH, Pirraglia E, Glodzik-Sobanska L, et al. Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein E4 carriers with subjective memory complaints. Biol Psychiatr. 2008;63:609–618. doi: 10.1016/j.biopsych.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Riedel-Heller SG, Matschinger H. Do memory complaints indicate the presence of cognitive impairment? Results of a field study. Eur Arch Psychiatr Neurol Sci. 1999;249:197–204. doi: 10.1007/s004060050087. [DOI] [PubMed] [Google Scholar]
- Roberts JL, Clare L, Woods RT. Subjective memory complaints and awareness of memory functioning in mild cognitive impairment: a systematic review. Dement Geriatr Cognit Disord. 2009;28:95–109. doi: 10.1159/000234911. [DOI] [PubMed] [Google Scholar]
- Rodda JE, Dannhauser TM, Cutinha DJ, Shergill SS, Walker Z. Subjective cognitive impairment: increased prefrontal cortex activation to control during an encoding task. Int J Geriatr Psychiatry. 2009;24:865–874. doi: 10.1002/gps.2207. [DOI] [PubMed] [Google Scholar]
- Rodda J, Dannhauser T, Cutinha DJ, Shergill SS, Walker Z. Subjective cognitive impairment: functional MRI during a divided attention task. Eur Psychiatr. 2010 doi: 10.1016/j.eurpsy.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Schmand B, Jonker C, Hooijer C, Lindeboom J. Subjective memory complaints may announce dementia. Neurology. 1996;46(1):121–125. doi: 10.1212/wnl.46.1.121. [DOI] [PubMed] [Google Scholar]
- Schnitzler A, Gross J. Normal and pathological oscillatory communication in the brain. Nat Rev Neurosci. 2005;6:285–296. doi: 10.1038/nrn1650. [DOI] [PubMed] [Google Scholar]
- Stam CJ, Dijk BW. Synchronization likelihood: an unbiased measure of generalized synchronization in multivariate data sets. Physica D. 2002;163:236–241. doi: 10.1016/S0167-2789(01)00386-4. [DOI] [Google Scholar]
- Stam CJ, Haan W, Daffertshofer A, Jones BF, Manshanden I, Cappellen van Walsum AM, et al. Graph theoretical analysis of magnetoencephalographic functional connectivity in Alzheimer's disease. Brain. 2009;132:213–224. doi: 10.1093/brain/awn262. [DOI] [PubMed] [Google Scholar]
- Stenset V, Hofoss D, Berstad AE, Negaard A, Gjerstad L, Fladby T. White matter lesion subtypes and cognitive deficits in patients with memory impairment. Dement Geriatr Cognit Disord. 2008;26:424–431. doi: 10.1159/000165355. [DOI] [PubMed] [Google Scholar]
- Sunderland A, Harris JE, Baddeley AD. Do laboratory tests predict everyday memory? A neuropsychological study. Journal of Verbal Learning and Verbal Behavior. 1983;22(3):341–357. doi: 10.1016/S0022-5371(83)90229-3. [DOI] [Google Scholar]
- Tobiansky R, Blizard R, Livingston G, Mann A. The gospel oak study stage IV: the clinical relevance of subjective memory impairment in older people. Psychol Med. 1995;25:779–786. doi: 10.1017/S0033291700035029. [DOI] [PubMed] [Google Scholar]
- Treves TA, Verchovsky R, Klimovitzky S, Korczyn AD. Incidence of dementia in patients with subjective memory complaints. Int Psychogeriatr. 2005;17:265–273. doi: 10.1017/S1041610205001596. [DOI] [PubMed] [Google Scholar]
- Flier WM, Buchem MA, Weverling-Rijnsburger AW, Mutsaers ER, Bollen EL, Admiraal-Behloul F, et al. Memory complaints in patients with normal cognition are associated with smaller hippocampal volumes. J Neurol. 2004;251(6):671–675. doi: 10.1007/s00415-004-0390-7. [DOI] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Vestberg S, Passant U, Elfgren C. Stability in the clinical characteristics of patients with memory complaints. Arch Gerontol Geriatr. 2010;50:e26–e30. doi: 10.1016/j.archger.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Wang L, Belle G, Crane PK, Kukull WA, Bowen JD, McCormick WC, Larson EB. Subjective memory deterioration and future dementia in people aged 65 and older. J Am Geriatr Soc. 2004;52:2045–2051. doi: 10.1111/j.1532-5415.2004.52568.x. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brooks JO. On the importance of longitudinal research in Alzheimer's disease. J Am Geriatr Soc. 1991;39(9):942–944. doi: 10.1111/j.1532-5415.1991.tb04464.x. [DOI] [PubMed] [Google Scholar]