Abstract
Age-related mechanisms that lead to sarcopenia are not entirely understood. Basal leg blood flow declines with aging by augmented sympathetic vasoconstriction and arterial stiffening, thus a dysfunction in blood vessel dynamics may have an independent role on sarcopenia. We determined whether pulse wave velocity (PWV), marker of arterial stiffness, was associated with skeletal muscle decline. Observational cohort study of older adults(70–79 years) living in Pittsburgh, PA, USA or Memphis, TN, USA. Analyses included 2,405 participants. Correlations among muscle parameters including skeletal muscle density and intermuscular adipose tissue using mid-thigh CT scans were assessed. Linear mixed models tested the association between the change in the sarcopenic index (SI) (assessed by dual energy X-ray absorptiometry) over time and baseline PWV independently of multiple confounders. SI was defined: appendicular lean mass/squared height and calculated at every follow-up (n = 6). Baseline PWV was significantly higher in black women compared to white women (930 ± 431 vs. 843 ± 366; p = 0.0001), while there were no significant differences between black and white men (943 ± 402 vs. 911 ± 375; p = 0.1786). Baseline analyses showed an independent negative association between PWV and muscle parameters after adjusting for confounders in both genders. The PWV-by-race interaction was significant in women and analyses are reported separately by race. Prospective mixed models showed that PWV was an independent determinant of the SI in all men (β = −0.1043; p = 0.0065) and in white women (β = −0.1091; p = 0.0192). In analyses examining the effect of arterial stiffness on limb lean mass over time, PWV correlated with lower leg (β = −0.2196; p = 0.0002)and arm mass (β = −0.0985; p = 0.0011) in all men and lower leg mass(β = −0.1608; p = 0.0027)in white women. In older persons, arterial stiffening is associated with skeletal muscle mass decline differently for race and gender.
Keywords: Aging, Sarcopenia, Pulse wave velocity, Vascular stiffness
Introduction
Sarcopenia, loss of muscle mass, is characterized by a decrease in the total number of skeletal muscle fibers, reduced cross-sectional area of the thigh, and an increase in intramuscular fat content (Evans 1997; Harris 1997). The age-related loss in skeletal muscle mass and the factors that may affect such loss are constantly under investigation. Sarcopenia may also be a common pathway by which multiple diseases contribute to the risk of functional limitation and disability in old age, even though many risk factors have still not been fully identified. There are specific changes in body composition with aging, including a decrease in muscle strength and mass with a parallel increase in fat mass (Visser et al. 2003) that begin to manifest during adulthood and may be partially explained by an imbalance of energy intake and expenditure. Unfortunately, these changes in older adults may be accelerated and lead to sarcopenia. The sarcopenic index is a measure of sarcopenia based on limb lean mass and height (Baumgartner et al. 1998; Newman et al. 2003) and is widely used in older adults.
Even though the pathophysiological pathways leading to sarcopenia in older persons have not been fully identified, a finding from cross-sectional data has suggested that one pathway may be operating in concert between endothelial dysfunction and lean mass in older persons (Sutton-Tyrrell et al. 2001). Due to the fact that basal leg blood flow declines with aging in part due to arterial stiffening, a dysfunction in blood vessel dynamics, may have a predictive role on muscle mass decline. Previous findings have shown an independent association between accelerated arterial stiffness and body weight (Sutton-Tyrrell et al. 2001), however a possible association of accelerated arterial stiffening directly on skeletal muscle mass is lacking. Current methodology for in vivo determination of arterial stiffness involves the estimation of distensibility by pulse wave velocity (PWV), one of the simplest techniques used for evaluating the dynamic properties of arterial wall (Sutton-Tyrell et al. 2005) and faster PWV has been documented as an increased risk factor for many co-morbidities and mortality (Sutton-Tyrell et al. 2005; Laurent et al. 2001).
The purpose of this study was to evaluate the relationship of skeletal muscle mass parameters over time to arterial stiffness estimated by PWV. In this paper, we report cross-sectional and longitudinal analyses between baseline PWV, skeletal muscle density, intermuscular adipose tissue area, and the sarcopenic index over a 6-year follow-up in older participants from the Health, Aging and Body Composition (Health ABC) Study. We hypothesize that baseline PWV will be a determinant of the sarcopenic index, independent of multiple confounders over a 6-year follow-up period.
Methods
Participants
The Health ABC study population consists of 3,075 black (42%) and white, men (48%) and women with an age range of 70–79 years during the recruitment period of March 1997 through July 1998. Eligible participants were identified from a random sample of white Medicare beneficiaries and all age-eligible community-dwelling black residents in designated zip code areas surrounding Pittsburgh, PA and Memphis, TN, USA. All participants gave informed consent to enroll in study protocol. Details of the study enrolment are reported elsewhere (Sutton-Tyrell et al. 2005). The study sample consisted of all participants of the Health ABC cohort after excluding those with missing baseline PWV and DEXA scan data (n = 2,405).
Clinical variables assessed
Pulse wave velocity
Baseline arterial PWV was measured by simultaneous Doppler flow signals obtained from the right carotid and right femoral arteries using nondirectional transcutaneous Doppler flow probes (Park Medical Electronics Inc; Model 810A, 9.0–10.0-MHz probes). Digitized data were recorded by customized programming for subsequent analysis. At least ten beats were averaged for each simultaneous recording site with QRS used for synchronization. Three separate runs were recorded for each participant. The distance between carotid and femoral sites was measured with a metal tape measure. Timing between the onset of flow at the carotid and femoral (defined as foot of the velocity signal at each site) sites was divided by the associated distance to produce flow velocity. Path length was estimated as linear distance from the sternal notch to the femoral artery at the point of applanation. PWV of an arterial segment is inversely related to the square root of the distensibility of that segment and is considered the most reliable measure of large artery stiffness. Stiffer vessels are associated with faster PWV. The National Institute on Aging Laboratory of Cardiovascular Science, Gerontology Research Center (Baltimore, MD, USA) trained and certified all study personnel before data collection, read waveforms, and evaluated data quality. Results from all acceptable runs were averaged.
Covariates
Baseline covariates included: age, height, weight, body mass index (BMI), race, site, diabetes, ankle-brachial index, interleukin-6 (IL-6), peripheral arterial disease (PAD) (defined as a score of <0.90 from the ankle–arm index), systolic blood pressure, smoking status, cardiac heart disease (CHD) status. Smoking status was assessed by a questionnaire and participants were classified as current/former and never. CHD was defined as participant history report of having the diagnosis from a health care provider or bringing medication consistent with the diagnosis to clinic and classified into two groups (none/possible and definite).
Physical activity
Physical activity was defined using the caloric expenditure (Ainsworth et al. 2000) in the past week for self-reported walking, climbing stairs, and exercise. Two categories were created to classify physical activity: (<1,500 and ≥ 1,500 kcal/week; Goodpaster et al. 2001).
Outcome variables
Skeletal muscle adipose measurements
The analyses of lower extremities were performed using the cross-sectional muscle and fat areas of the mid-thigh assessed by CT scan at baseline only (in Pittsburgh: 9,800 Advantage, General Electric, Milwaukee, WI, USA; in Memphis: Somatom Plus 4, Siemens, Erlangen, Germany, and PQ 2000 S, Marconi Medical Systems, Cleveland, OH, USA) and has been previously described (Goodpaster et al. 2001). Muscle and adipose tissue areas were calculated by multiplying the area of a given pixel as extracted from the image header. Muscle density, measured in Hounsfield Units (HU), was included as a marker of intramuscular fat content as validated by Goodpaster et al. (2000). The mean attenuation coefficient values of muscle within the regions outlined on the images were determined by averaging the CT number (pixel intensity) in HU. Skeletal muscle and adipose tissue were distinguished by a bimodal image histogram calculated from the distribution of CT numbers in adipose and muscle. These peaks are readily separable and the areas of the adipose tissue and muscle in the entire image were calculated by the areas under their respective peaks of the histogram (Goodpaster et al. 2000).
Total intermuscular adipose tissue was distinguished from the subcutaneous adipose tissue by manual drawing of a line along the deep fascial plane surrounding the thigh muscles and the area of adipose tissue was calculated in square centimeters.
Peripheral lean mass
DEXA (QDR 4500A, Hologic, Inc., Waltham, MA, USA) was used to measure whole and regional body composition at every follow-up (n = 6). Methods and validation data have been previously described (Visser et al. 1999; Salamone et al. 2000). Appendicular lean mass (aLM) was calculated as the sum of lean mass (LM) in the arms and legs, assuming that all nonfat and nonbone tissue is skeletal muscle.
Sarcopenic index
The sarcopenic index is an index that adjusts appendicular lean mass for body size and is calculated by using the following measurements: aLM (from DEXA scan) divided by height-squared (aLM/ht2) (Baumgartner et al. 1998; Newman et al. 2003).
Statistical analyses
In accordance with Newman et al. (2003), all analyses were performed separately by gender. The distribution of PWV and IL-6 values were not normal, therefore, such values were log transformed for data analysis.
Cross-sectional analysis
All descriptive continuous variables were presented as mean (standard deviation) and compared using a student t test, while categorical data were presented as a number (percentage) and compared using χ2 test.
Baseline multivariate linear regressions testing the independent associations between PWV and muscle outcome parameters (lean leg mass, lean arm mass, and sarcopenic index). The models testing lean leg and arm mass with PWV were performed after adjusting for age, diabetes, race, site, height, BMI, systolic blood pressure (SBP), PAD, CHD, smoking, IL-6, physical activity and PWV-by-race interaction. Models testing the association between PWV, muscle density, and intermuscular adipose tissue were also further adjusted for total thigh area. The same analysis was also performed separately by race. Lastly the model testing the sarcopenic index, as the dependent variable included the following covariates: PWV, age, diabetes, race, site, total fat mass, BMI, SBP, PAD, CHD, smoking, IL-6, physical activity, and PWV-by-race interaction. The PWV-by-race interaction term was included in the final model only if it was significant and, in that case, PWV beta estimates were reported separately by race.
Longitudinal analysis
Separate mixed effects models were performed with sarcopenic index, lean leg mass, and lean arm mass as dependent variables in both genders. Mixed effects models use all available data over follow-up and can properly account for correlation between repeated measures (Cnaan et al. 1997). Sarcopenic index models were also adjusted for the following multiple confounders: age, diabetes, time, time2, PWV, race, PWV-by-race interaction, site, total fat mass, smoking, CHD, PAD, IL-6, SBP, and physical activity (high vs. low). The models with lean leg mass and lean arm mass were adjusted for the following multiple confounders: age, diabetes, time, squared time, PWV, race, PWV-by-race interaction, site, height, smoking, CHD, PAD, IL-6, SBP, and physical activity (high vs. low). Also, in this case, the interaction term was included in the final model only if it was significant and, in that case, PWV beta estimates were reported separately by race. Because of the strong correlation between BMI and the sarcopenic index, for descriptive purposes only, two figures were created to observe the variation of the sarcopenic index over time according to PWV tertiles in three different categories of BMI (<25, 25–30, >30). All statistical analyses were performed using SAS version 8.2 (SAS, Inc., Cary, NC, USA). All analyses also included heart rate as an independent confounder do to the fact that it has been shown to be an importantly associated with PWV (Lantelme et al. 2002), however our findings did not vary, thus heart rate was not added to presented models.
Results
Cross-sectional analyses
Characteristics of the study sample are displayed in Table 1 according to race and gender. At baseline, black women had significantly higher arterial stiffness and were heavier than white women. Both black men and black women had significantly better sarcopenic indices than their white counterparts. Table 2 shows the separate and independent associations between PWV and diverse muscle parameters at baseline after adjusting for multiple confounders. A significant PWV-by-race interaction was found only for SI and lean leg mass in women only. PWV was independently associated with the sarcopenic index, lean arm mass, lean leg mass, and skeletal muscle density in men (Table 2). In women, PWV was independently associated with the sarcopenic index and leg lean mass only in white women.
Table 1.
Characteristics of study population at baseline
| Men | Women | |||||
|---|---|---|---|---|---|---|
| White (n = 716) | Black (n = 420) | P value | White (n = 716) | Black (n = 420) | P value | |
| Age (years) | 74.1 (3) | 73.6 (3) | 0.0163 | 73.6 (3) | 73.5 (3) | 0.4449 |
| Pulse wave velocity (cm/s) | 911 (375) | 943 (402) | 0.1786 | 843 (366) | 930 (431) | 0.0001 |
| SBP (mmHg) | 134 (20) | 140 (21) | <0.0001 | 134 (19) | 140 (22) | <0.0001 |
| DBP (mmHg) | 72 (11) | 76 (12) | <0.0001 | 69 (11) | 73 (12) | <0.0001 |
| BMI (kg/m2) | 27.0 (3.7) | 27.0 (4.3) | 0.8285 | 26.2 (4.6) | 29.6 (5.7) | <0.0001 |
| Diabetes (%) | 13.8 | 19.9 | 0.0076 | 7.6 | 21.4 | <0.0001 |
| Ever smokers (%) | 69.0 | 70.9 | 0.4881 | 41.2 | 44.2 | 0.2819 |
| PAD (%) | 5.4 | 15.2 | <0.0001 | 5.9 | 16.0 | <0.0001 |
| CHD (%) | 25.1 | 19.3 | 0.0236 | 10.7 | 16.0 | 0.0049 |
| High physical activity (%) | 36.0 | 19.3 | <0.0001 | 15.4 | 9.5 | 0.0018 |
| IL-6 (pg/ml) | 2.3 (1.8) | 2.6 (1.8) | 0.0133 | 2.1 (1.7) | 2.5 (2.1) | <0.0001 |
| Total LBM (kg) | 56.2 (6.8) | 57.4 (7.9) | 0.0079 | 39.1 (5.3) | 43.4 (6.3) | <0.0001 |
| Total fat mass (kg) | 24.6 (7.0) | 23.0 (7.6) | 0.0003 | 27.2 (8.0) | 31.7 (9.8) | <0.0001 |
| Total percent fat (%) | 29.9 (4.8) | 28.0 (5.3) | <0.0001 | 40.2 (5.4) | 41.1 (5.8) | 0.0042 |
| Arm lean mass (kg) | 3.4 (0.5) | 3.8 (0.6) | <0.0001 | 2.0 (0.3) | 2.4 (0.4) | <0.0001 |
| Leg lean mass (kg) | 8.8 (1.2) | 9.4 (1.5) | <0.0001 | 6.1 (1.0) | 7.2 (1.3) | <0.0001 |
| SI (kg/m2) | 7.7 (0.9) | 8.3 (1.1) | <0.0001 | 6.0 (0.8) | 7.1 (1.1) | <0.0001 |
| CT thigh scan | ||||||
| Intermuscular adipose tissue area (cm2) | 9.2 (6.1) | 10.9 (8.1) | <0.0001 | 8.7 (4.6) | 12.2 (6.8) | <0.0001 |
| Muscle density (HU) | 37.7 (6.4) | 37.1 (6.4) | 0.1613 | 34.5 (6.8) | 32.7 (7.1) | <0.0001 |
| Thigh area (cm2) | 195 (35) | 211 (43) | <0.0001 | 200 (48) | 243 (62) | <0.0001 |
Values are expressed as means (SD) unless otherwise specified
SBP systolic blood pressure, DBP diastolic blood pressure, BMI body mass index, PAD peripheral arterial disease, CHD coronary heart diesease
Table 2.
Multivariate linear regression models of muscle parameters at baseline and log (PWV) after adjusting for multiple confoundersa
| SIa (kg/m2) | Lean arm mass (kg)b | Lean leg mass (kg)b | Muscle density (HU)c | Intermuscular adipose tissuec (cm2) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Beta | P value | Beta | P value | Beta | P value | Beta | P value | Beta | P value | |
| Men | ||||||||||
| PWV-by-race interaction | 0.4915 | 0.2823 | 0.3178 | 0.5007 | 0.4404 | |||||
| PWV (cm/s) | −0.0990 | 0.0100 | −0.1064 | 0.0005 | −0.2170 | 0.0003 | −1.4746 | 0.0019 | −0.0027 | 0.9957 |
| Women | ||||||||||
| PWV-by-race interaction | 0.0349 | 0.1139 | 0.0094 | 0.5456 | 0.4403 | |||||
| PWV (cm/s) | −0.0082 | 0.6849 | −0.2754 | 0.5555 | 0.2387 | 0.4897 | ||||
| PWV in whites (cm/s) | −0.1208 | 0.0105 | −0.1691 | 0.0021 | ||||||
| PWV in blacks (cm/s) | 0.0235 | 0.6473 | 0.0388 | 0.5186 | ||||||
When PWV-by-race interaction was statistically significant, PWV beta estimates were reported separately by race
aCovariates in the models included: age, diabetes, site, total fat mass, BMI, SBP, PAD, CHD, smoking, IL-6, physical activity, race, pwv
bCovariates included in the models: age, diabetes, site, height, BMI, SBP, PAD, CHD, smoking, IL-6, physical activity,race, pwv
cCovariates included in the models: age, diabetes, site, height, BMI, SBP, PAD, CHD, smoking, IL-6, physical activity, thigh area, race, pwv
Longitudinal analyses
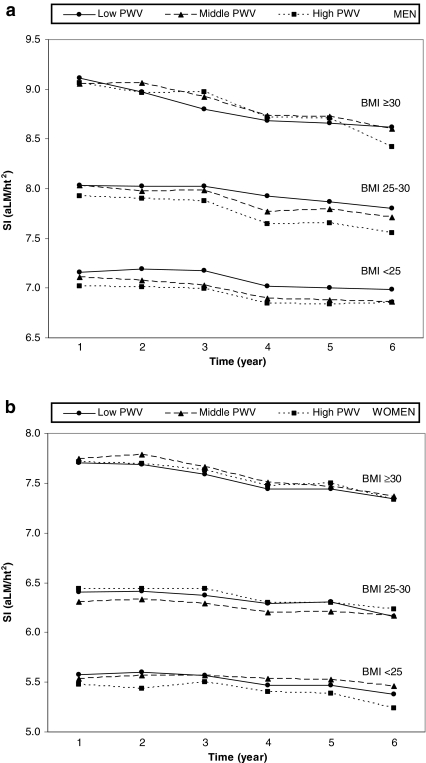
In Fig. 1a–b, the behavior of the sarcopenic index over time is shown in both men and women grouped according to PWV tertiles in three different categories of BMI. As expected, BMI status was associated with the sarcopenic index. In men, there was a tendency for the highest tertile of baseline PWV to be associated with a worse sarcopenic index over time across all levels of BMI (see Fig. 1a), while such tendency in women was not as clear (Fig. 1b). We observed that the sarcopenic index decreased in both men and women respectively from year 1 (7.9 ± 1.0; 6.5 ± 1.1) to year 6 (7.6 ± 0.9; 6.2 ± 1.0).
Fig. 1.
a The SI is stratified according to tertiles of PWV in three different categories of BMI at every follow-up in men. b The SI is stratified according to tertiles of PWV in three different categories of BMI at every follow-up in women
Using the mixed effects regression models with the sarcopenic index as the dependent variable we found that age, site, BMI, total fat mass, race, Il-6, and PWV at baseline were independently associated with the sarcopenic index in both men and women over an observation period of 6 years, while high physical activity was a determinant of sarcopenic index in men (data not shown). PWV was independently associated with the sarcopenic index in men, while in women a significant PWV-by-race interaction was found, and PWV was significantly associated with SI only in white women (Table 3). We also tested the PWV-by-time interaction term, which did not reach statistical significance in either gender (Table 3).
Table 3.
Mixed effects regression analyses with SI (kg/ht2), lean mass (arm and leg) as dependent variable over 6 year follow-up
| SI (kg/m2)a | Lean arm mass (kg)b | Lean leg mass (kg)b | ||||
|---|---|---|---|---|---|---|
| Beta | P value | Beta | P value | Beta | P value | |
| Men (n = 1,136) | ||||||
| PWV-by-race interaction | 0.5030 | 0.3195 | 0.3465 | |||
| PWV (log (cm/s)) | −0.1043 | 0.0065 | −0.0985 | 0.0011 | −0.2196 | 0.0002 |
| Time | −0.0269 | 0.0074 | −0.0148 | 0.0036 | −0.0358 | 0.0030 |
| Time2 | −0.0057 | <0.0001 | −0.0017 | 0.0149 | −0.0068 | <0.0001 |
| Women (n = 1,269) | ||||||
| PWV-by-race interaction | 0.0215 | 0.1306 | 0.0047 | |||
| PWV (log (cm/s)) | 0.0044 | 0.8175 | ||||
| PWV in whites (cm/s) | −0.1091 | 0.0192 | −0.1608 | 0.0027 | ||
| PWV in blacks (cm/s) | 0.0460 | 0.3634 | 0.0599 | 0.3064 | ||
| Time | −0.0080 | <0.0001 | 0.0069 | 0.0492 | 0.0006 | 0.9471 |
| Time2 | −0.0080 | <0.0001 | −0.0025 | <0.0001 | −0.0078 | <0.0001 |
When PWV-by-race interaction was statistically significant, PWV beta estimates were reported separately by race
aCovariates included in the models: age, site, BMI, SBP, PAD, CHD, IL-6, physical activity, total fat mass, time, time2, pwv, race
bCovariates included in the models: age, site, height, BMI, SBP, PAD, CHD, IL-6, physical activity, time, time2, pwv, race
Table 3 also reports the results from the mixed effects models with the arm LM, and leg LM as dependent variables over time. We report that baseline PWV was an independent determinant of arm and leg LM in men over time, while PWV was significantly correlated with leg LM in white women only. We did not find any significant correlations between PWV and arm LM in women. In all men, we also found that age, height, BMI, smoking status, Il-6, and high physical activity were significant determinants of arm and leg LM (data not shown). Interestingly in all women, we found that age, height, BMI, and diabetes were significant determinants of both arm and leg LM, while Il-6 continued to correlated with leg LM (data not shown).
Discussion
These data highlight for the first time a possible role of arterial stiffening on age-related muscle mass decline, and in particular on sarcopenia. We investigated the occurrence of a specific association between arterial stiffening, as seen by PWV and peripheral skeletal muscle mass. In addition, we demonstrated that baseline PWV is specifically associated with the sarcopenic index over time in older white men and women, independently of age, chronic inflammation, peripheral arterial disease, smoking, and cardiac heart disease status. As expected, we found a protective role of body mass index, total fat mass, and high physical activity for sarcopenic index, while IL-6 and cardiac heart disease were associated with muscle mass decline over time.
PWV is a widely used technique for assessing arterial stiffness and has been shown to predict cardiovascular events in the general population (Mattace-Raso et al. 2006), as well as in the elderly (Murakami et al. 2005). In fact, arterial stiffness is also considered an important indicator for the degree of endothelial dysfunction and is associated with reduced blood flow volume in the lower extremities of older persons (Suzuki et al. 2001). Such data underline that higher PWV, as an expression of increased rigidity of the vessel wall, may contribute not only to increased arterial resistance to blood flow, but also to decreased flow volume of the lower extremity vessel dynamics. Our findings expand such data and provide evidence indicating that PWV is associated with the change in muscle mass decline over time. Why PWV should be involved in the development/worsening of the sarcopenic index is still unclear. Nevertheless the relationship between PWV and muscle mass decline might be partially explained by an age-related reduction in limb blood flow which, in turn, could be related to lower cardiac output or lower vascular conductance due to elevated sympathetic vasoconstrictor nerve activity (Dinenno et al. 1999). Due to the fact that we showed negative associations of arterial stiffness with skeletal muscle density, as well as peripheral lean mass, our data provide further insight to additional physiopathologic mechanisms involving both peripheral lean mass and intramuscular fat infiltration. Furthermore, a recent study demonstrated that increased arterial stiffness is associated with forearm vascular resistance, as a measure of microvascular dysfunction (Mitchell et al. 2005). Therefore, a role of microvascular dysfunction on muscle mass decline in older persons cannot be ruled out. Studies have suggested that increased aortic stiffness may stimulate hypertrophy, remodeling, or rarefaction in the microcirculation, leading to resistance to blood flow (Pepine et al. 1979; Ting et al. 1986).
Changes in tissue quality, such as an increase in connective tissue and peri-cellular fat infiltration, may also contribute to altered vascular limb dynamics. A previous finding from Health ABC study suggested that visceral fat may accelerate arterial stiffness (Sutton-Tyrell et al. 2005). One previous study has demonstrated a direct association between the severity of atherosclerosis and peripheral lean mass as well as truncal fat mass, however in a marginal and reciprocal manner (Alexandersen et al. 2006). With regard to the relationship between PWV and muscle density, PWV may also have an effect on muscle mass and metabolism since intramuscular fat infiltration has been shown to significantly rise with aging (Madsen et al. 1997) and with an invasion of pro-inflammatory cytokines (Barbieri et al. 2003). The final effect could interfere with muscle contraction thus, contributing to the genesis of muscle mass deterioration.
As result of both pathophysiologic mechanisms of atherosclerosis and obesity, a common pathway consisting in a rise of muscular inflammation could drive PWV toward sarcopenia. Such possibility is strengthened by evidence that pro-inflammatory cytokines could alter blood vessel dynamics which in turn, can alter muscle metabolism and activate a vicious catabolic cycle resulting in IL-6 production and further skeletal muscle breakdown. Indeed, we confirmed a significant role played by chronic inflammation, as seen by IL-6 levels, on muscle mass decline over time in men.
Our data show that faster PWV was associated with a significant decline in peripheral skeletal muscle mass in the entire cohort of men and white women, while PWV did not correlate with any muscle parameters in black women. An interesting finding was that the PWV-by-race interaction term was significant in women and not in men in regards to the sarcopenic index and lower leg mass. Such findings might be interpreted by the fact baseline PWV was significantly higher in black compared to white women. Our data also confirm those findings from a previous study underlining that arterial stiffness was significantly more accelerated in blacks than whites, however such authors did not consider differences in gender (Din-Dzietham et al. 2004). Indeed, our data add further insight not only to racial differences, but also to differences seen by gender, demonstrating that white women from this cohort may be more susceptible to muscle mass decline due to arterial stiffening. Furthermore, a protective role played by body weight on muscle mass decline may be operating due to the fact that black women were significantly heavier than white women at baseline (Visser et al. 2002). Interestingly, black women from this study had a significantly lower skeletal muscle density and higher intermuscular adipose tissue which may play an unknown role on muscle mass change. Only future intervention studies will determine the mechanisms explaining the difference among women of different races on muscle mass decline over time.
Some limitations of the present study must be acknowledged. There was a large number of participants with missing data at baseline on PWV and some follow-up subjects were lost over time (n = 139). However, our analyses used mixed effects models which allowed to maximize population available. Furthermore, our cohort consists of a large group of older persons, therefore data highlighting an association between sarcopenia and arterial stiffness in younger persons were unavailable. Nevertheless, muscle mass decline is a more common finding in the elderly. We also did not have available follow-up data on CT scans regarding muscle density and intermuscular adipose tissue of the lower extremities. Indeed, such data will be of great interest for a study continuation protocol. Skeletal muscle attenuation, as determined by CT, is a non-invasive measure of muscle density and higher muscle attenuation values have been associated with greater specific force production (Goodpaster et al. 2001). Therefore, the inverse correlation between PWV and muscle density in men would be interpreted that higher arterial stiffening is associated with lower muscle density. Our cross-sectional data also showed an inverse relationship between lean mass and arterial stiffness.
In conclusion, our data indicate that an arterial stiffness parameter, PWV, is associated with increases in the sarcopenic index over time independent of age, total body fat, peripheral arterial disease, chronic inflammation and cardiac heart disease. Future intervention studies will be needed in order to investigate the effect of improvement in arterial stiffness on muscle mass decline in older persons.
Acknowledgements
Study concept and design: Abbatecola, Paolisso, Harris.Had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis: Abbatecola, Chiodini, Gallo, Paolisso, Harris Drafting of the manuscript: Abbatecola, Chiodini Statistical analysis: Abbatecola, Chiodini, Gallo Critical revision of the manuscript for important intellectual content: Gallo, Paolisso, Harris, Lakatta, Sutton-Tyrrell, Tylavsky, Goodpaster, Schwartz, de Rekeneire Study supervision: Harris
This study was by the following contracts: National Institute on Aging at the National Institute of Health with the following contract numbers: N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106. This research was supported in part by the Intramural Research Program of the National Institute of Health, National Institute on Aging
References
- Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- Alexandersen P, Tanko LB, Bagger YZ, Jespersen J, Skouby SO, Christiansen C. Associations between aortic calcification and components of body composition in elderly men. Obesity. 2006;14:1571–1578. doi: 10.1038/oby.2006.181. [DOI] [PubMed] [Google Scholar]
- Barbieri M, Ferrrucci L, Ragno E, et al. Chronic inflammation and the effect of IGF-1 on muscle strength and power in older persons. Am J Physiol Metab. 2003;284:E481–E487. doi: 10.1152/ajpendo.00319.2002. [DOI] [PubMed] [Google Scholar]
- Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- Cnaan A, Laird NM, Slasor P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med. 1997;16:2349–2380. doi: 10.1002/(SICI)1097-0258(19971030)16:20<2349::AID-SIM667>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Din-Dzietham R, Couper D, Evans G, Arnett DK, Jones DW. Arterial stiffness is greater in African Americans than in whites: evidence from the Forsyth County, North Carolina, ARIC cohort. Am J Hypertens. 2004;17:304–313. doi: 10.1016/j.amjhyper.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Jones PP, Seals DR, Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans. Relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation. 1999;100:164–170. doi: 10.1161/01.cir.100.2.164. [DOI] [PubMed] [Google Scholar]
- Evans W. Functional and metabolic consequences of sarcopenia. J Nutr. 1997;127:998S–1003S. doi: 10.1093/jn/127.5.998S. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 2000;89(1):104–110. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: The health ABC study. J Appl Physiol. 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- Harris T. Muscle mass and strength: relation to function in population studies. J Nutr. 1997;127:1004S–1006S. doi: 10.1093/jn/127.5.1004S. [DOI] [PubMed] [Google Scholar]
- Lantelme P, Mestre C, Lievre M, Gressard A, Milon H. Heart rate: an important confounder of pulse wave velocity assessment. Hypertension. 2002;39:1083–1087. doi: 10.1161/01.HYP.0000019132.41066.95. [DOI] [PubMed] [Google Scholar]
- Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- Madsen OR, Lauridsen UB, Hartkopp A, Sorensen OH. Muscle strength and soft tissue composition as measured by dual energy X-ray absorptiometry in women aged 18–87 years. Eur J Appl Physiol Occup Physiol. 1997;76:239–245. doi: 10.1007/s004210050154. [DOI] [PubMed] [Google Scholar]
- Mattace-Raso FU, Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- Mitchell GF, Lacourcière Y, Arnold JM, Dunlap ME, Conlin PR, Izzo JL., Jr Changes in aortic stiffness and augmentation index after acute converting enzyme or vasopeptidase inhibition. Hypertension. 2005;46:1111–1117. doi: 10.1161/01.HYP.0000186331.47557.ae. [DOI] [PubMed] [Google Scholar]
- Murakami S, Otsuka K, Hotta N, et al. Common carotid intima-media thickness is predictive of all-cause and cardiovascular mortality in elderly community-dwelling people: longitudinal investigation for the longevity and aging in Hokkaido County (LILAC) study. Biomed Pharmacother. 2005;59:S49–S53. doi: 10.1016/S0753-3322(05)80010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman A, Kupelian V, Visser M, Health ABC Study Investigators et al. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–1609. doi: 10.1046/j.1532-5415.2003.51534.x. [DOI] [PubMed] [Google Scholar]
- Pepine CJ, Nichols WW, Curry RC, Jr, Conti CR. Aortic input impedance during nitroprusside infusion. A reconsideration of afterload reduction and beneficial action. J Clin Invest. 1979;64:643–654. doi: 10.1172/JCI109505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone LM, Fuerst T, Visser M, et al. Measurement of fat mass using DEXA: a validation study in elderly adults. J Appl Physiol. 2000;89:345–352. doi: 10.1152/jappl.2000.89.1.345. [DOI] [PubMed] [Google Scholar]
- Sutton-Tyrell K, Najjar SS, Boudreau RM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- Sutton-Tyrrell K, Newman A, Simonsick EM, et al. Aortic stiffness is associated with visceral adiposity in older adults enrolled in the study of health, aging, and body composition. Hypertension. 2001;38:429–433. doi: 10.1161/01.hyp.38.3.429. [DOI] [PubMed] [Google Scholar]
- Suzuki E, Kashiwagi A, Nishio Y, et al. Increased arterial wall stiffness limits flow volume in the lower extremities in type 2 diabetic patients. Diabetes Care. 2001;24:2107–2114. doi: 10.2337/diacare.24.12.2107. [DOI] [PubMed] [Google Scholar]
- Ting CT, Brin KP, Lin SJ, et al. Arterial hemodynamics in human hypertension. J Clin Invest. 1986;78:1462–1471. doi: 10.1172/JCI112737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M, Fuerst T, Lang T, Salamone L, Harris TB. Validity of fan-beam dual-energy X-ray absorptiometry for measuring fat-free mass and leg muscle mass. Health, aging, and body composition study–dual-energy X-ray absorptiometry and body composition working group. J Appl Physiol. 1999;87:1513–1520. doi: 10.1152/jappl.1999.87.4.1513. [DOI] [PubMed] [Google Scholar]
- Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- Visser M, Pahor M, Tylavsky F, et al. One- and two-year change in body composition as measured by DXA in a population based cohort of older men and women. J Appl Physiol. 2003;94:2368–2374. doi: 10.1063/1.1594811. [DOI] [PubMed] [Google Scholar]