Abstract
Brassica vegetables are attracting a great deal of attention as healthy foods because of the fact that they contain substantial amounts of secondary metabolite glucosinolates that are converted into isothiocyanates, such as sulforaphane [(−)1-isothiocyanato-4R-(methylsulfinyl)-butane] (R-SFN), through the actions of chopping or chewing the vegetables. Several studies have analyzed the biological and molecular mechanisms of the anti-cancer activity of synthetic R,S-sulforaphane, which is thought to be a result of its antioxidant properties and its ability to inhibit histone deacetylase enzymes (HDAC). Few studies have addressed the possible antioxidant effects of R-SFN, which could protect cells from the free radical damage that strongly contribute to aging. Moreover, little is known about the effect of R-SFN on stem cells whose longevity is implicated in human aging. We evaluated the effects of R-SFN on the biology on human mesenchymal stem cells (MSCs), which, in addition to their ability to differentiate into mesenchymal tissues, support hematopoiesis, and contribute to the homeostatic maintenance of many organs and tissues. Our investigation found evidence that low doses of R-SFN promote MSCs proliferation and protect them from apoptosis and senescence, while higher doses have a cytotoxic effect, leading to the induction of cell cycle arrest, programmed cell death and senescence. The beneficial effects of R-SFN may be ascribed to its antioxidant properties, which were observed when MSC cultures were incubated with low doses of R-SFN. Its cytotoxic effects, which were observed after treating MSCs with high doses of R-SFN, could be attributed to its HDAC inhibitory activity. In summary, we found that R-SFN, like many other dietary supplements, exhibits a hormetic behavior; it is able to induce biologically opposite effects at different doses.
Keywords: Isothiocyanate, Marrow stromal stem cells, Differentiation, Apoptosis, Senescence, Cell cycle, Histone deacetylase
Introduction
Brassica vegetables (including broccoli and cauliflower) are attracting a great deal of attention as healthy foods because of their content in secondary metabolite glucosinolates as well as the enzyme myrosinase (β-thioglucoside glucohydrolase, EC. 3.2.1.147). When these vegetables are crushed by chopping or chewing, myrosinase catalyzes the rapid conversion of glucosinolates into isothiocyanates, i.e., glucoraphanin (GRA) into sulforaphane [(−)1-isothiocyanato-4R-(methylsulfinyl)-butane] (R-SFN) (Abdull Razis et al. 2010a, b; Fimognari et al. 2002)
A number of studies have documented the chemopreventive properties of sulforaphane (SFN). This compound protects against the onset or reduces the severity of cancer, retinal disease, and skin damage resulting from exogenous agents or genetic predisposition (Ahn et al. 2010; Conaway et al. 2005; Cornblatt et al. 2007; Dinkova-Kostova et al. 2006; Talalay et al. 2007).
Two sets of putative mechanisms have been identified that help to explain the effects of SFN. The first involves the activation of phase II detoxification enzymes (glutathione S-transferase, quinone reductase, and glucuronosyltransferase) and the inhibition of phase I enzymes that activate toxic chemical compounds. In this way, SFN contributes to elevating the cell’s defenses against oxidative damage and promotes the removal of carcinogens (Fimognari et al. 2002; Keum et al. 2004). The second set of putative mechanisms involves the suppression of histone deacetylase activity. It is well known that the remodeling of chromatin proteins is a fundamental epigenetic mechanism for the regulation of gene expression. This process involves post-translational modification of histone tails by acetylation, methylation, phosphorylation and ubiquitination (Spotswood and Turner 2002). Histone deacetylases (HDACs) and histone acetyltransferases determine the pattern of histone acetylation. Accumulating evidence suggests that alterations in acetylated histone levels are associated with several forms of cancer (Di Bernardo et al. 2009; Minucci and Pelicci 2006; Myzak et al. 2006a).
In the last several years, HDAC inhibitors (HDACi) have received attention for their in vivo and in vitro anti-tumoral properties. These anti-cancer actions can be obtained by reverting silenced genes and inducing cell cycle arrest, differentiation, and/or apoptosis (Di Bernardo et al. 2009). SFN may induce HDACi to trigger cell cycle arrest and apoptosis in cancer cells (Ho et al. 2009; Myzak and Dashwood 2006; Myzak et al. 2006a; Myzak et al. 2006b).
The microenvironment of mammalian bone marrow is composed of several different elements that support hematopoiesis and bone homeostasis (Beyer Nardi and da Silva 2006; Bianco et al. 2001; Muller-Sieburg and Deryugina 1995). This microenvironment includes a heterogeneous population of cells, e.g., macrophages, fibroblasts, adipocytes, osteoprogenitors, endothelial cells, and reticular cells. In addition to these cell types, the microenvironment also contains non-hematopoietic stem cells that possess a multilineage potential (Muller-Sieburg and Deryugina 1995; Bianco et al. 2001; Beyer Nardi and da Silva Meirelles 2006). These stem cells are commonly named marrow stromal stem cells or mesenchymal stem cells (MSCs). Mesenchymal cells are primordial cells of mesodermal origin that produce skeletal muscle cells, the blood, vascular, and urogenital systems and connective tissues throughout the body (Beyer Nardi and da Silva Meirelles 2006; Prockop 1997; Sethe et al. 2006).
MSCs are of interest because of their multiple roles in the body. In addition to their ability to differentiate into mesenchymal tissues, they are also capable of differentiating into non-mesenchymal lineages, such as neurons and glia. Furthermore, they support hematopoiesis and can contribute to the homeostatic maintenance of many organs and tissues (Prockop 1997; Sethe et al. 2006; Beyer Nardi and da Silva Meirelles 2006).
Several studies demonstrated that the process of tissue repair is driven by tissue-specific progenitor cells which are replenished and/or stand by MSCs, which migrate from bone marrow and may provide cytokines and a stromal support network (Alexanian and Kurpad 2005; Sethe et al. 2006; Sheng et al. 1998). Involvement of bone marrow-derived stem cells has been demonstrated in the regeneration of a number of organs, such as bone, skin, liver, kidney, and muscle. Consequently, any loss in numbers or functionality of MSCs due to senescence would have profound consequences for the maintenance of tissue viability (Beausejour 2007; Pelicci 2004; Sethe et al. 2006).
Most studies on SFN have analyzed the biological and molecular mechanisms of its anti-cancer activity. Few studies have addressed the possible beneficial effects of SFN on the body’s ability to reproduce healthy cells and protect against DNA damage. As an antioxidant, SFN should protect cells from free radical damage, which is the primary (but not sole) cause of aging. Moreover, little is known about the effect of SFN on stem cells, whose longevity is implicated in human aging.
R-SFN is released from GRA following exposure to the enzyme myrosinase. However, almost all studies have been conducted using the synthetic SFN, racemic at the sulfoxide group, as it is commercially available. As humans are exposed exclusively to R-SFN through the diet (Abdull Razis et al. 2010a, b), we considered interesting to investigate the potential of this enantiomer rather than the racemic mixture of both isomers
On this premise, we evaluated the effects of R-SFN on the biology on human MSCs in vitro. We treated MSCs with R-SFN concentrations lower than those currently used to induce cytotoxic effects in cancer cells (Fimognari et al. 2002; Gingras et al. 2004; Mi et al. 2007; Myzak et al. 2006b; Pledgie-Tracy et al. 2007). The rationale for this choice was that, when administered as a dietary supplement, the bioavailable concentration of R-SFN in serum is far below the concentration used for anti-cancer treatment (Hanlon et al. 2009).
Materials and methods
MSC cultures
Bone marrow was obtained from three healthy donors (age range, 6–10 years old) who had provided informed consent. We separated cells through a Ficoll density gradient (GE Healthcare, Italy), and the mononuclear cell fraction was collected and washed in phosphate-buffered saline (PBS). We seeded 1–2.5 × 105 cells/cm2 in 100-mm dishes with α-modified Eagle’s medium (αMEM) containing 10% fetal bovine serum, 2 ng/ml basic FGF, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (proliferating medium). After 24–48 h, non-adherent cells were discarded, and adherent cells, including mesenchymal stem cells and committed progenitors, were washed twice with PBS. Cells were then incubated for 7–10 days in proliferating medium until they became confluent; they were then extensively propagated for subsequent experiments. All experiments were carried out on cells at passage 3–4 and were repeated at least two times for each donor.
We verified that under our experimental conditions, MSC cultures fulfilled the three proposed criteria to define MSCs: (1) adherence to plastic, (2) specific surface antigen expression, and (3) multipotent differentiation potential. Firstly, MSCs were selected by the plastic-adherence procedure. More than 90% of the MSC population expressed the CD105, CD73 and CD90 antigens. Additionally, we verified that the MSCs were able to differentiate into osteoblasts, adipocytes and chondroblasts (Dominici et al. 2006).
All cell culture reagents were obtained from Euroclone Life Sciences (Italy) and Hyclone (UT, USA) unless stated otherwise.
Sulphoraphane production
R-SFN was generated in situ from natural GRA by myrosinase-catalyzed hydrolysis. GRA was purified from Brassica oleracea L. var. acephala sabellica (Cavolo nero di Toscana) ripe seeds (SUBA & UNICO, Longiano, Italy) as previously described (Haack et al. 2010). Myrosinase, 30 U/mL, was isolated from ripe seeds of white mustard (Sinapis alba L.) as already reported (Pessina et al. 1990). One myrosinase unit is defined as the amount of eznyme hydrolysing 1 μmol sinigrin per minute at a pH of 6.5 and 37°C. Under the experimental conditions employed, i.e. (pH 7.4, 37°C), the R-SFN is the only enzymatic breakdown product of GRA with a yield of more than 99% (Abdull Razis et al. 2010a, b; Fimognari et al. 2003; Fimognari et al. 2002).
Myrosinase was added to cell cultures together with different GRA concentrations to generate equal amounts of R-SFN by in situ catalyzed hydrolysis of GRA (Abdull Razis et al. 2010a, b; Fimognari et al. 2002). Cells were incubated for 72 h with 0.25 μM, 1 μM, 5 μM, or 20 μM GRA and then collected for analysis.
Cell proliferation assay and trypan blue exclusion test of cell viability
The Quick Cell Proliferation Assay Kit II (Biovision, CA, USA) is based on the cleavage of tetrazolium salt to formazan by cellular mitochondrial dehydrogenase. The amount of the dye generated by the activity of dehydrogenase is directly proportional to the number of living cells. The formazan dye produced by viable cells was quantified using a multi-well spectrophotometer by measuring the absorbance of the dye solution at 440 nm. The assay was carried out according to the manufacturer’s instructions. Each determination was performed in quadruplicate.
The trypan blue dye exclusion test was used to determine the number of dead cells (necrotic and apoptotic cells) present in the cell culture following incubation in media with different SFN concentrations. In this test, cell suspensions were mixed with 0.4% trypan blue dye and then visually examined with an inverted light microscope to determine whether cells take up or exclude the dye. In every experiment, at least 1,000 cells were counted across different fields to calculate the percentage of dead cells in each culture.
Detection of apoptotic cells
Apoptotic cells were detected with fluorescein-conjugated annexin V (Roche, Italy) according to the manufacturer’s instructions. Apoptotic cells were observed through a fluorescence microscope (Leica Microsystems Italia, Italy). In every experiment, at least 1,000 cells were counted across different fields to calculate the percentage of dead cells in each culture.
Senescence-associated β-galactosidase assay
Cells were fixed for 10 min with a solution of 2% (v/v) formaldehyde and 0.2% (w/v) glutaraldehyde. Fixed cells were washed with PBS and then incubated at 37°C for at least 2 h with a staining solution (30 mM citric acid/phosphate buffer (pH 6), 5 mM K4Fe(CN)6, 5 mM K3Fe(CN)6, 150 mM NaCl, 2 mM MgCl2, and 1 mg/ml X-Gal solution). The percentage of senescent cells was calculated by dividing the number of blue cells (β-galactosidase-positive cells) observed across multiple fields by the total number of cells in those fields. At least 500 cells across different microscope fields were examined.
Detection of reactive oxygen species
The generation of reactive oxygen species (ROS) in MSCs was monitored by the conversion of fluorogenic 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) to highly fluorescent dichlorofluorescein diacetate within cells by ROS. In brief, 24 h after treatment with SFN, MSC cultures were incubated for 1 h at 37°C with 20 μM DCFH-DA in PBS. Cells were washed with PBS and then incubated for 30 min with 300 μM H202. Cells were then lysed in a buffer containing 0.1% Triton X-100 for 10 min. Fluorescence was measured with a fluorometric plate reader at 480/530 nm.
Detection of HDAC activity
The Fluor de Lys®-Green HDAC Assay kit (Enzo Life Sciences International, PA, USA) was used to measure HDAC activity in cell extracts. Reactions were carried out according to the manufacturer’s instructions. In brief, the procedure was performed in two stages. First, the components of the deacetylation reaction (MSC cell extracts and HDAC substrate) were mixed. Following an incubation in which substrate deacetylation took place, the developer was added and mixed. This action stopped the deacetylation reaction and generated a fluorophore. The fluorophore was excited with 485-nm light (470–500 nm), and the emitted light (approximately 530 nm) was detected on a fluorometric plate reader.
Immunocytochemistry for HP1 detection
HP1 was detected according to the manufacturer’s protocol. In brief, cells were grown on cover slips, fixed with 4% formaldehyde and permeabilized with methanol. Blocking was carried out with 5% serum for 60 min at room temperature. Slides were incubated overnight with anti-HP1-alpha (1:400) (Millipore Italia, Italy). Afterward, the slides were incubated with goat anti-rabbit secondary antibodies conjugated to FITC (Jackson Immunoresearch, UK) for 45 min at RT.
Following Hoechst 33342 staining, cells were observed using a fluorescence microscope (Leica Italia, Italy). The percentage of HP1-positive cells was calculated by counting at least 500 cells in different microscope fields.
RNA extraction, reverse transcription and real-time PCR
Total RNA was extracted from cell cultures using TRI REAGENT (Molecular Research Center Inc., OH, USA) according to the manufacturer’s protocol. The mRNA levels of the genes analyzed were quantified by real-time reverse transcription (RT)-PCR amplification, as reported previously (Galderisi et al. 1999).
Sequences of mRNAs from the Nucleotide Data Bank (National Center for Biotechnology Information, USA) were used to design primer pairs for real-time RT-PCR reactions (Primer Express, Applied Biosystems, CA, USA). Primer sequences are available upon request. Appropriate regions of HPRT and/or GAPDH cDNA were used as controls. The real-time PCR assays were run on an Opticon 4 machine (MJ Research, Waltham, MA, USA). Reactions were carried out according to the manufacturer’s instructions using a SYBR green PCR master mix.
Western blotting
Cells were lysed in a buffer containing 0.1% Triton X-100 for 30 min at 4−C. Lysates were centrifuged for 10 min at 10,000 g at 4°C. After centrifugation, 10–40 μg of each sample were loaded, resolved by electrophoresis on a polyacrylamide gel, and electroblotted onto a nitrocellulose membrane. Primary antibodies were used according to the manufacturers’ instructions.
Immunoreactive signals were detected with a horseradish peroxidase–conjugated secondary antibody (Santa Cruz, CA, USA) reacting with the ECL Plus reagent (GE Healthcare, Italy).
Statistical analysis
Statistical significance was evaluated by ANOVA analysis followed by a Student’s t test and Bonferroni’s test.
Results
We evaluated the effects of R-SFN on MSCs with exposure to different R-SFN concentrations. The concentrations ranged from 0.25 μM, the bioavailable concentration in serum following dietary supplementation, to 20 μM, the minimal bioactive concentration used as an anti-cancer drug (Fimognari et al. 2002; Hanlon et al. 2009; Mi et al. 2008; Myzak et al. 2006a; Pledgie-Tracy et al. 2007).
R-SFN effects on cell cycle and apoptosis
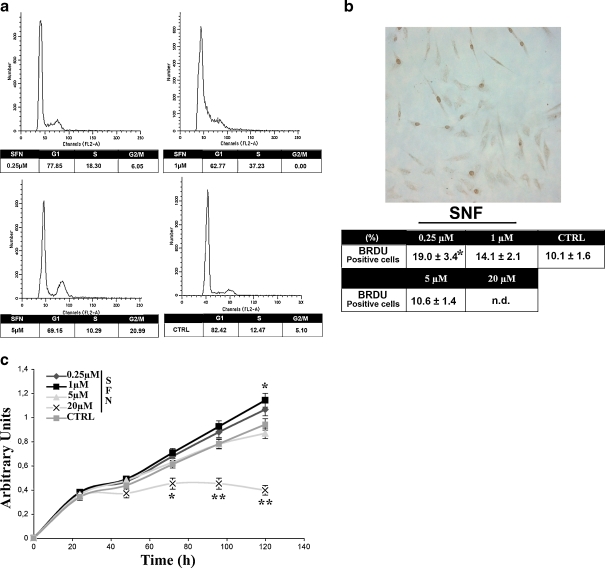
MSC cultures were treated for 3 days with R-SFN and then analyzed by FACS for cell cycle progression. In several experiments (n = 3), we observed a significant increase in S phase cells and a decrease in the number of G1 phase cells in the samples that were treated with 0.25 and 1 μM R-SFN compared with the controls. Conversely, after treatment with 5 μM R-SFN, we observed an increase in the number G2/M phase cells and a reduction in the number of G1 phase cells and no significant variation in the number of cells in S phase (Fig. 1a). The finding that cells accumulated in the G2/M phase is in agreement with other studies (Fimognari et al. 2002; Pledgie-Tracy et al. 2007). We were unable to carry out assays on cells treated with 20 μM SFN because at the end of the treatment period, the vast majority of cells were floating, suggesting that this dose was highly toxic to MSCs.
Fig. 1.
a Flow cytometry analysis. Representative FACS profiles of MSCs treated with different concentration of R-SFN compared with controls (CTRL). The table shows the mean expression values. b BrdU assay. The micrograph shows a representative field of BrdU incorporation in cells treated with different concentrations of R-SFN relative to CTRL. The table shows the mean expression values and standard deviations (SD; n = 3, *p < 0.05). n.d. not detected. c Tetrazolium salt-based proliferation assay. One thousand cells were seeded in 96-multi-well plates and cell proliferation was analyzed in the presence of increasing concentrations of R-SFN for 120 h. Number of living cells in culture is directly proportional to the absorbance at 440 nm. The graph shows the mean expression values and SD (n = 3, *p < 0.05; **p < 0.01)
Data obtained with BrdU incorporation overlapped with the flow cytometry results. After treatment with 0.25, 1, and 5 μM R-SFN, we observed an increase in BrdU incorporation compared with controls, suggesting an increase in the number of S phase cells. We were unable to carry out the BrdU assay for cells treated with 20 μM R-SFN (Fig. 1b).
We performed a growth curve of MSCs plated in the presence of increasing concentrations of R-SFN (Fig. 1c). Cells incubated with 0.25 and 1 μM R-SFN exhibited higher proliferation rate compared with control, whereas 20 μM R-SFN induced a significant reduction of proliferation index. These results are in agreement with BrdU incorporation and cell death assays (see below).
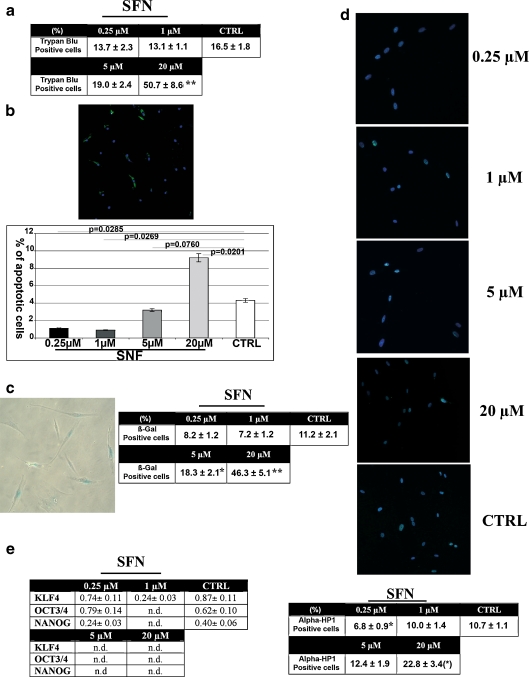
It should be noted that low concentrations of R-SFN reduced the numbers of dead cells in MSC cultures, while treatment with 5 μM and 20 μM R-SFN increased the numbers of dead cells, suggesting a cytotoxic effect (Fig. 2a). As trypan blue staining cannot distinguish between necrotic and apoptotic cells, we also carried out an annexin assay to assess the degree of apoptosis. Low concentrations of R-SFN (up to 5 μM) reduced the percentage of apoptotic cells in MSC cultures, whereas incubation with 20 μM induced a significant increase in the number of apoptotic cells (Fig. 2b).
Fig. 2.
a Trypan bleu assay. The table shows the mean expression values of trypan bleu-positive cells in cultures treated with R-SFN compared with the control (n = 3, **p < 0.01). b Annexin assay. The fluorescence photomicrograph shows a representative field of cells stained with annexin V (green). Nuclei were counterstained with Hoechst 33342 (blue). The histogram shows the mean expression values of apoptotic cells (±SD; n = 3). c Senescence-associated β-galactosidase assay performed on MSCs. Table shows percentage of senescent cells (±SD; n = 3; *p < 0.05; **p < 0.01). d Fluorescence photomicrographs show cells stained with anti-HP1-gamma (green) and with Hoechst 33342 (blue). A representative microscopic field for each treatment is shown. In the table, the average fluorescence pixel intensity in HP1-positive cells (±SD; n = 3) is indicated. In this assay, we counted at least 500 HP1-positive cells for each treatment. The intensity of staining for each positive cell was acquired with a CCD camera and analyzed with Quantity One 1-D analysis software (Biorad Laboratories, CA, USA). We calculated the sum of fluorescent pixel values of HP1 positive cells and then determined the average fluorescent pixel intensity that was expressed as arbitrary units (n = 3; *p < 0.05; **p < 0.01). e RT-PCR analysis of stemness-related genes is shown in the table. The change in the mRNA of cells incubated with R-SFN was compared with control cultures (CTRL). The mRNA levels were normalized with respect to HPRT and are expressed as “arbitrary units”; n.d. not detected. We used the comparative cycle threshold method as quantitative approach
High doses of R-SFN induced cellular senescence
Perturbations of cell biology, such as those induced by high doses of R-SFN, can drive cells to different possible fates, including apoptosis and senescence. These outcomes are not mutually exclusive, despite the fact that some cellular stresses preferentially induce one or the other of these two fates (Campisi and d’Adda di Fagagna 2007). The factors that determine whether cells undergo senescence or apoptosis are unclear. Determinants may include the cell type and the nature and intensity of the stress/damage (Campisi and d’Adda di Fagagna 2007). We investigated whether a high concentration of R-SFN could induce senescence of MSCs in addition to triggering cell death, as demonstrated in this study and others (Choi and Singh 2005; Fimognari et al. 2002; Karmakar et al. 2006; Mi et al. 2007). Treatment with 5 μM and 20 μM R-SFN resulted in a significant increase in senescent cells, as detected by the acid-β-galactosidase assay (Fig. 2c).
It has been demonstrated that distinct heterochromatin structures accumulate during senescence and could represent a hallmark of this process (Campisi and d’Adda di Fagagna 2007; Squillaro et al. 2010). The gamma isoform of heterochromatic protein 1 (HP1-gamma) is a heterochromatic adaptor molecule involved in higher order chromatin structure (Maison and Almouzni 2004). It is widely used to identify heterochromatic foci in cell nuclei. In MSCs, following incubation with 20 μM R-SFN, we detected a significant increase (p < 0.05) in HP1-positive foci in the nuclei (Fig. 2d).
To further investigate this finding, we analyzed the effect of a high dose of R-SFN on the expression of “stemness” genes. These are genes participating in the control of stem cell properties such as self-renewal and retention of an uncommitted state. These genes were first identified in embryonic stem cells (Mikkers and Frisen 2005; Ramalho-Santos et al. 2002; Takahashi and Yamanaka 2006). In adult stem cells, some “stemness genes” are not expressed. In particular, in a previous study, we detected the expression of OCT3/4, NANOG, and KLF4, which are part of the core transcriptional circuitry for regulating stem cell properties, in proliferating MSCs (Masui et al. 2007; Matoba et al. 2006; Squillaro et al. 2010). The highest doses of R-SFN decreased the expression levels of these genes (Fig. 2e).
Overall, these data provide evidence that R-SFN may alter cellular biology in a way that triggers senescence phenomena. To our knowledge, this is the first study to elucidate the effects of R-SFN on the senescence process. Triggering senescence could contribute to the anti-cancer effects claimed for SFN. Nevertheless, the therapeutic use of SFN affects the physiology of normal stem cells, which contribute to the body’s homeostasis, and may induce severe side effects in cancer patients.
Low doses of R-SFN protect cells from in vitro senescence
As demonstrated with the cytotoxic and apoptosis assays, low doses of R-SFN provide a beneficial effect for MSCs by reducing the number of senescent cells as detected with the acid-β-galactosidase assay (Fig. 2b). This finding was in agreement with the reduced formation of heterochromatin and with data regarding “stemness” gene expression (Fig. 2d, and e).
Low doses of R-SFN showed antioxidant properties, but high doses resulted in minimal HDAC inhibitory activity
Our data suggest that R-SFN may induce biologically opposite effects at different doses. It is reasonable to hypothesize that R-SFN may act with different mechanisms to produce different outcomes. For this reason, we decided to evaluate defenses against oxidative damage and suppression of histone deacetylase activity in MSC cultures following treatment with different concentrations of R-SFN.
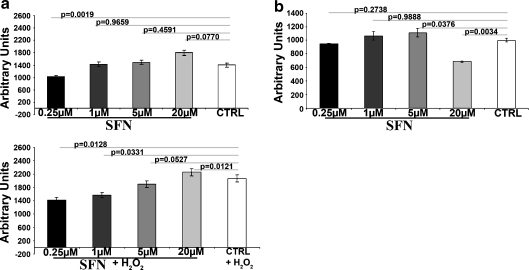
The effect of R-SFN on the production of cellular ROS was investigated under the basal condition and under a condition of oxidative stress (300 μM H202). Low doses of R-SFN reduced the production of ROS both in the basal state and under stress conditions (Fig. 3a). At a 20-μM concentration, the protective action of R-SFN was lost. This finding may be related to the observation that, paradoxically, high doses of isothiocyanate may have pro-oxidant properties due to their ability to cause glutathione depletion and superoxide production in cells (Valgimigli and Iori 2009).
Fig. 3.
a Detection of reactive oxygen species in MSCs incubated with R-SFN under the basal condition (R-SFN) and oxidative stress (R-SFN + 300 μM H202). The histogram shows the mean expression values of ROS expressed as arbitrary units. SD indicates standard deviations (n = 3). b Detection of HDAC activity in cell extracts. The histogram shows the mean expression values of HDAC activity in MSCs incubated with R-SFN relative to control. HDAC activity was expressed as arbitrary units. SD standard deviations (n = 3)
Suppression of HDAC activity by R-SFN was monitored with the Fluor de Lys®-Green HDAC assay. Our data demonstrated that minimal HDAC inhibitory activity could be detected at the highest concentration tested (Fig. 3b). This finding is in agreement with other reports showing that high concentrations of SFN (>10 μM) may affect HDAC activity (Ho et al. 2009; Myzak and Dashwood 2006; Myzak et al. 2006a; Myzak et al. 2006b).
Taken together, our results suggest that the protective effect of R-SFN may be attributed to its antioxidant properties, while impairment in cell biology processes (apoptosis, senescence) may be due to HDAC inhibition. This latter conclusion is in agreement with our previous research showing that inhibition of histone deacetylase enzymes triggers senescence and apoptosis in mesenchymal stem cells (Di Bernardo et al. 2010; Di Bernardo et al. 2009).
Molecular analysis of SFN treatment
After analyzing the biological consequences of R-SFN in MSCs, we attempted to elucidate the molecular pathways involved in SFN-induced phenomena. We analyzed the expression of the P53-P21-P16 pathways, which are known to control cell cycle arrest, differentiation, apoptosis, and/or senescence processes (Campisi and d’Adda di Fagagna 2007; Galderisi et al. 2006; Oberdoerffer and Sinclair 2007).
High concentrations of R-SFN (5 and 20 μM) induced a strong upregulation of P53 and P21 proteins (Fig. 4). This finding is in agreement with studies showing the increase in the expression of these genes during G2/M arrest and apoptosis (Foijer and te Riele 2006; Taylor and Stark 2001).
Fig. 4.
Western blot analysis of P53, P21, and P16 in MSCs treated with R-SFN. The table shows the protein mean expression values (±SD; n = 3). (*p < 0.05)
Surprisingly, P16 protein levels did not change significantly in cells treated with 1 and 5 μM R-SFN as expected in cells undergoing senescence. It should be pointed out that senescence triggered by different stimuli may occur in a P16-independent way (Haferkamp et al. 2009; Kim et al. 2009; Klimova et al. 2009; Yu et al. 2009). The downregulation of P16 protein level in cells treated with 20 μM R-SFN may be associated with huge toxic effect observed at this concentration.
Discussion
Several studies have demonstrated that aging is partly the result of an age-associated decline in stem cell self-renewal, replication, and lineage commitment (Sharpless and DePinho 2007). Senescence can be induced by different stressors, including dysfunctional telomeres, excessive mitogen signaling, perturbations in chromatin organization, and DNA-damaging agents such as ROS.
ROS are among the most potent inducers of cellular senescence, which in turn contribute to aging. Free radical scavengers and antioxidants are key components in the war against aging, and several dietary supplements are claimed to be effective in reducing the risk of cancer and slowing the aging process. Isothiocyanates such as SFN are currently under investigation as anti-cancer agents, but few studies have tried to address their anti-aging properties.
Our study found that low doses of R-SFN promoted MSC proliferation and protected them from apoptosis and senescence (Figs. 1 and 2), while higher doses had a cytotoxic effect, leading to the induction of cell cycle arrest, programmed cell death, and senescence (Figs. 1 and 2). The beneficial effects of R-SFN may be ascribed to its antioxidant properties, which were observed after incubating MSC cultures with low doses of R-SFN (Fig. 3a). Its toxic effects, which were observed after treating MSCs with high doses of R-SFN, could be attributed to pro-oxidant properties through its ability to cause glutathione depletion and superoxide production in cells. In fact, the assay to detect ROS production by MSC cultures showed that low doses of R-SFN reduced ROS formation, while high doses were not effective in reducing ROS (Fig. 3a). Moreover, a high concentration of R-SFN led to HDAC inhibitory activity (Fig. 3b). This latter property may be associated with the induction of apoptosis and senescence in MSCs, as our previous research clearly demonstrated that inhibition of HDAC had detrimental effects on MSC biology (Di Bernardo et al. 2010; Di Bernardo et al. 2009).
Our studies clearly demonstrate that MSCs have a hormetic dose response to R-SFN. The term hormesis has long been used to describe the phenomenon in which a specific chemical is able to induce biologically opposite effects at different doses; most commonly, there is a stimulatory or beneficial effect at low doses and an inhibitory or toxic effect at high doses (Son et al. 2008). In the case of natural compounds, an example of hormesis is vitamin A, which is essential for normal development in low amounts, but in high amounts, it can cause anorexia, altered mental states, and other symptoms (Son et al. 2008). Our research suggests that R-SFN may be considered a hormetic dietary supplement; at low doses, it may exert protective effects on stem cells (at least in vitro), while at higher doses, it shows cytostatic or cytotoxic effects, as previously demonstrated on cancer cells.
In conclusion, our study suggests that the use of R-SFN as an anti-cancer agent must be carefully evaluated because this compound may impair the functions of normal stem cells, including MSCs. Adverse effects on MSCs can have profound consequences because they support hematopoiesis and can contribute to the homeostatic maintenance of many organs and tissues.
However, low intake of R-SFN, as can be obtained by eating Brassica vegetables, may be beneficial for health because it seems to have protective effects on stem cells.
References
- Abdull Razis AF, Bagatta M, Nicola GR, et al. Up-regulation of cytochrome P450 and phase II enzyme systems in rat precision-cut rat lung slices by the intact glucosinolates, glucoraphanin and glucoerucin. Lung Cancer. 2010a;71(3):298–305. doi: 10.1016/j.lungcan.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Abdull Razis AF, Iori R, Ioannides C (2010b) The natural chemopreventive phytochemical R-sulforaphane is a far more potent inducer of the carcinogen-detoxifying enzyme systems in rat liver and lung than the S-isomer. Int J Cancer. doi:10.1002/ijc.25620 [DOI] [PubMed]
- Ahn YH, Hwang Y, Liu H, et al. Electrophilic tuning of the chemoprotective natural product sulforaphane. Proc Natl Acad Sci U S A. 2010;107(21):9590–9595. doi: 10.1073/pnas.1004104107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexanian AR, Kurpad SN. Quiescent neural cells regain multipotent stem cell characteristics influenced by adult neural stem cells in co-culture. Exp Neurol. 2005;191(1):193–197. doi: 10.1016/j.expneurol.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Beausejour C. Bone marrow-derived cells: the influence of aging and cellular senescence. Handb Exp Pharmacol. 2007;180:67–88. doi: 10.1007/978-3-540-68976-8_4. [DOI] [PubMed] [Google Scholar]
- Beyer Nardi N, Silva ML. Mesenchymal stem cells: isolation, in vitro expansion and characterization. Handb Exp Pharmacol. 2006;174:249–282. [PubMed] [Google Scholar]
- Bianco P, Riminucci M, Gronthos S, Gehron Robey P. Bone marrow stromal stem cells: nature, biology and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- Campisi J, di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- Choi S, Singh SV. Bax and Bak are required for apoptosis induction by sulforaphane, a cruciferous vegetable-derived cancer chemopreventive agent. Cancer Res. 2005;65(5):2035–2043. doi: 10.1158/0008-5472.CAN-04-3616. [DOI] [PubMed] [Google Scholar]
- Conaway CC, Wang CX, Pittman B, Yang YM, Schwartz JE, Tian D, McIntee EJ, Hecht SS, Chung FL. Phenethyl isothiocyanate and sulforaphane and their N-acetylcysteine conjugates inhibit malignant progression of lung adenomas induced by tobacco carcinogens in A/J mice. Cancer Res. 2005;65(18):8548–8557. doi: 10.1158/0008-5472.CAN-05-0237. [DOI] [PubMed] [Google Scholar]
- Cornblatt BS, Ye L, Dinkova-Kostova AT, Erb M, Fahey JW, Singh NK, Chen MS, Stierer T, Garrett-Mayer E, Argani P, Davidson NE, Talalay P, Kensler TW, Visvanathan K. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis. 2007;28(7):1485–1490. doi: 10.1093/carcin/bgm049. [DOI] [PubMed] [Google Scholar]
- Bernardo G, Squillaro T, Dell’Aversana C, Miceli M, Cipollaro M, Cascino A, Altucci L, Galderisi U. Histone deacetylase inhibitors promote apoptosis and senescence in human mesenchymal stem cells. Stem Cells Dev. 2009;18(4):573–581. doi: 10.1089/scd.2008.0172. [DOI] [PubMed] [Google Scholar]
- Bernardo G, Alessio N, Dell’Aversana C, et al. Impact of histone deacetylase inhibitors SAHA and MS-275 on DNA repair pathways in human mesenchymal stem cells. J Cell Physiol. 2010;225(2):537–544. doi: 10.1002/jcp.22236. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Jenkins SN, Fahey JW, Ye L, Wehage SL, Liby KT, Stephenson KK, Wade KL, Talalay P. Protection against UV-light-induced skin carcinogenesis in SKH-1 high-risk mice by sulforaphane-containing broccoli sprout extracts. Cancer Lett. 2006;240(2):243–252. doi: 10.1016/j.canlet.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Dominici M, Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Fimognari C, Nusse M, Cesari R, Iori R, Cantelli-Forti G, Hrelia P. Growth inhibition, cell-cycle arrest and apoptosis in human T-cell leukemia by the isothiocyanate sulforaphane. Carcinogenesis. 2002;23(4):581–586. doi: 10.1093/carcin/23.4.581. [DOI] [PubMed] [Google Scholar]
- Fimognari C, Nusse M, Berti F, Iori R, Cantelli-Forti G, Hrelia P. Sulforaphane modulates cell cycle and apoptosis in transformed and non-transformed human T lymphocytes. Ann NY Acad Sci. 2003;1010:393–398. doi: 10.1196/annals.1299.072. [DOI] [PubMed] [Google Scholar]
- Foijer F, Riele H. Check, double check: the G2 barrier to cancer. Cell Cycle. 2006;5(8):831–836. doi: 10.4161/cc.5.8.2687. [DOI] [PubMed] [Google Scholar]
- Galderisi U, Bernardo G, Cipollaro M, Jori FP, Piegari E, Cascino A, Peluso G, Melone MAB. Induction of apoptosis and differentiation in neuroblastoma and astrocytoma cells by the overexpression of Bin1, a novel Myc interacting protein. J Cell Biochem. 1999;74:313–22. doi: 10.1002/(SICI)1097-4644(19990901)74:3<313::AID-JCB1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Galderisi U, Cipollaro M, Giordano A. The retinoblastoma gene is involved in multiple aspects of stem cell biology. Oncogene. 2006;25(38):5250–5256. doi: 10.1038/sj.onc.1209736. [DOI] [PubMed] [Google Scholar]
- Gingras D, Gendron M, Boivin D, Moghrabi A, Theoret Y, Beliveau R. Induction of medulloblastoma cell apoptosis by sulforaphane, a dietary anticarcinogen from Brassica vegetables. Cancer Lett. 2004;203(1):35–43. doi: 10.1016/j.canlet.2003.08.025. [DOI] [PubMed] [Google Scholar]
- Haack M, Lowinger M, Lippmann D, et al. Breakdown products of neoglucobrassicin inhibit activation of Nrf2 target genes mediated by myrosinase-derived glucoraphanin hydrolysis products. Biol Chem. 2010;391(11):1281–1293. doi: 10.1515/BC.2010.134. [DOI] [PubMed] [Google Scholar]
- Haferkamp S, Scurr LL, Becker TM, Frausto M, Kefford RF, Rizos H. Oncogene-induced senescence does not require the p16(INK4a) or p14ARF melanoma tumor suppressors. J Invest Dermatol. 2009;129(8):1983–1991. doi: 10.1038/jid.2009.5. [DOI] [PubMed] [Google Scholar]
- Hanlon N, Coldham N, Gielbert A, Sauer MJ, Ioannides C. Repeated intake of broccoli does not lead to higher plasma levels of sulforaphane in human volunteers. Cancer Lett. 2009;284(1):15–20. doi: 10.1016/j.canlet.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Ho E, Clarke JD, Dashwood RH. Dietary sulforaphane, a histone deacetylase inhibitor for cancer prevention. J Nutr. 2009;139(12):2393–2396. doi: 10.3945/jn.109.113332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmakar S, Weinberg MS, Banik NL, Patel SJ, Ray SK. Activation of multiple molecular mechanisms for apoptosis in human malignant glioblastoma T98G and U87MG cells treated with sulforaphane. Neuroscience. 2006;141(3):1265–1280. doi: 10.1016/j.neuroscience.2006.04.075. [DOI] [PubMed] [Google Scholar]
- Keum YS, Jeong WS, Kong AN. Chemoprevention by isothiocyanates and their underlying molecular signaling mechanisms. Mutat Res. 2004;555(1–2):191–202. doi: 10.1016/j.mrfmmm.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Kim KS, Kang KW, Seu YB, Baek SH, Kim JR. Interferon-gamma induces cellular senescence through p53-dependent DNA damage signaling in human endothelial cells. Mech Ageing Dev. 2009;130(3):179–188. doi: 10.1016/j.mad.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Klimova TA, Bell EL, Shroff EH, Weinberg FD, Snyder CM, Dimri GP, Schumacker PT, Budinger GR, Chandel NS. Hyperoxia-induced premature senescence requires p53 and pRb, but not mitochondrial matrix ROS. FASEB J. 2009;23(3):783–794. doi: 10.1096/fj.08-114256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison C, Almouzni G. HP1 and the dynamics of heterochromatin maintenance. Nat Rev Mol Cell Biol. 2004;5(4):296–304. doi: 10.1038/nrm1355. [DOI] [PubMed] [Google Scholar]
- Masui S, Nakatake Y, Toyooka Y, Shimosato D, Yagi R, Takahashi K, Okochi H, Okuda A, Matoba R, Sharov AA, Ko MS, Niwa H. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol. 2007;9(6):625–635. doi: 10.1038/ncb1589. [DOI] [PubMed] [Google Scholar]
- Matoba R, Niwa H, Masui S, Ohtsuka S, Carter MG, Sharov AA, Ko MS. Dissecting Oct3/4-regulated gene networks in embryonic stem cells by expression profiling. PLoS ONE. 2006;1:e26. doi: 10.1371/journal.pone.0000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi L, Wang X, Govind S, Hood BL, Veenstra TD, Conrads TP, Saha DT, Goldman R, Chung FL. The role of protein binding in induction of apoptosis by phenethyl isothiocyanate and sulforaphane in human non-small lung cancer cells. Cancer Res. 2007;67(13):6409–6416. doi: 10.1158/0008-5472.CAN-07-0340. [DOI] [PubMed] [Google Scholar]
- Mi L, Xiao Z, Hood BL, Dakshanamurthy S, Wang X, Govind S, Conrads TP, Veenstra TD, Chung FL. Covalent binding to tubulin by isothiocyanates. A mechanism of cell growth arrest and apoptosis. J Biol Chem. 2008;283(32):22136–22146. doi: 10.1074/jbc.M802330200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkers H, Frisen J. Deconstructing stemness. EMBO J. 2005;24(15):2715–2719. doi: 10.1038/sj.emboj.7600749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6(1):38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- Muller-Sieburg CE, Deryugina E. The stromal cells’ guide to the stem cell universe. Stem Cells. 1995;13(5):477–486. doi: 10.1002/stem.5530130505. [DOI] [PubMed] [Google Scholar]
- Myzak MC, Dashwood RH. Histone deacetylases as targets for dietary cancer preventive agents: lessons learned with butyrate, diallyl disulfide, and sulforaphane. Curr Drug Targets. 2006;7(4):443–452. doi: 10.2174/138945006776359467. [DOI] [PubMed] [Google Scholar]
- Myzak MC, Dashwood WM, Orner GA, Ho E, Dashwood RH. Sulforaphane inhibits histone deacetylase in vivo and suppresses tumorigenesis in Apc-minus mice. FASEB J. 2006;20(3):506–508. doi: 10.1096/fj.05-4785fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myzak MC, Ho E, Dashwood RH. Dietary agents as histone deacetylase inhibitors. Mol Carcinog. 2006;45(6):443–446. doi: 10.1002/mc.20224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdoerffer P, Sinclair DA. The role of nuclear architecture in genomic instability and ageing. Nat Rev Mol Cell Biol. 2007;8(9):692–702. doi: 10.1038/nrm2238. [DOI] [PubMed] [Google Scholar]
- Pelicci PG. Do tumor-suppressive mechanisms contribute to organism aging by inducing stem cell senescence? J Clin Invest. 2004;113(1):4–7. doi: 10.1172/JCI200420750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessina A, Thomas RM, Palmieri S, Luisi PL. An improved method for the purification of myrosinase and its physicochemical characterization. Arch Biochem Biophys. 1990;280(2):383–389. doi: 10.1016/0003-9861(90)90346-Z. [DOI] [PubMed] [Google Scholar]
- Pledgie-Tracy A, Sobolewski MD, Davidson NE. Sulforaphane induces cell type-specific apoptosis in human breast cancer cell lines. Mol Cancer Ther. 2007;6(3):1013–1021. doi: 10.1158/1535-7163.MCT-06-0494. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA. "Stemness": transcriptional profiling of embryonic and adult stem cells. Science. 2002;298(5593):597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev. 2006;5(1):91–116. doi: 10.1016/j.arr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol. 2007;8(9):703–713. doi: 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
- Sheng JG, Mrak RE, Griffin WS. Enlarged and phagocytic, but not primed, interleukin-1 alpha-immunoreactive microglia increase with age in normal human brain. Acta Neuropathol. 1998;95(3):229–234. doi: 10.1007/s004010050792. [DOI] [PubMed] [Google Scholar]
- Son TG, Camandola S, Mattson MP. Hormetic dietary phytochemicals. Neuromolecular Med. 2008;10(4):236–246. doi: 10.1007/s12017-008-8037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spotswood HT, Turner BM. An increasingly complex code. J Clin Invest. 2002;110(5):577–582. doi: 10.1172/JCI16547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squillaro T, Alessio N, Cipollaro M, Renieri A, Giordano A, Galderisi U. Partial silencing of methyl cytosine protein binding 2 (MECP2) in mesenchymal stem cells induces senescence with an increase in damaged DNA. FASEB J. 2010;24(5):1593–1603. doi: 10.1096/fj.09-143057. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Talalay P, Fahey JW, Healy ZR, Wehage SL, Benedict AL, Min C, Dinkova-Kostova AT. Sulforaphane mobilizes cellular defenses that protect skin against damage by UV radiation. Proc Natl Acad Sci USA. 2007;104(44):17500–17505. doi: 10.1073/pnas.0708710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene. 2001;20(15):1803–1815. doi: 10.1038/sj.onc.1204252. [DOI] [PubMed] [Google Scholar]
- Valgimigli L, Iori R. Antioxidant and pro-oxidant capacities of ITCs. Environ Mol Mutagen. 2009;50(3):222–237. doi: 10.1002/em.20468. [DOI] [PubMed] [Google Scholar]
- Yu AL, Fuchshofer R, Kook D, Kampik A, Bloemendal H, Welge-Lussen U. Subtoxic oxidative stress induces senescence in retinal pigment epithelial cells via TGF-beta release. Investig Ophthalmol Vis Sci. 2009;50(2):926–935. doi: 10.1167/iovs.07-1003. [DOI] [PubMed] [Google Scholar]