Abstract
The objectives of this study are to model the relative effects of positive (childhood intelligence) and negative (magnetic resonance imaging (MRI)-derived white matter hyperintensities (WMH)) predictors of late-life intelligence in two well-characterised normal cohorts aged 68 and 78 and to measure the influence of hypertension on WMH and lifelong cognitive change. The Scottish Mental Surveys of 1932 and 1947 tested the intelligence of almost all school children at age 11. One hundred and one participants born in 1921 and 233 participants born in 1936 had brain MRI, with measurement of WMH using Scheltens‘ scale, and tests of late-life fluid intelligence. Structural equation models of the effect of childhood intelligence and brain WMH on the general intelligence factor ‘g’ in late life in the two samples were constructed using AMOS 18. Similar models were constructed to test the effect of hypertension on WMH and lifelong cognitive change. Fluid intelligence scores were lower and WMH scores were higher in the older samples. Hypertensive participants in both samples had more WMH than normotensive participants. The positive influence of childhood intelligence on ‘g’ was greater in the younger sample. The negative effect of WMH on ‘g’ was linear and greater in the older sample due to greater WMH burden. The negative effect of hypertension on lifelong cognitive ageing was all mediated via MRI-derived brain WMH. The positive relationship between childhood and late-life intelligence decreases with age. The negative relationship between WMH and late-life intelligence is linear and increases with age.
Keywords: Ageing, White matter hyperintensity, Cohort study, Fluid intelligence, Cognitive decline, MRI
Introduction
Understanding factors that interact to increase the risk of dementia requires a life-course approach to cognitive ageing (Singh-Manoux and Kivimaki 2010). Late-life intelligence is influenced by positive and negative factors acting at different stages across the lifespan (Salthouse 2010), modification of which might prevent, postpone or mitigate against dementia (Wang et al. 2002). Childhood intelligence is the strongest predictor of late-life intelligence and accounts for about 40% of the variance in intelligence at age 78 (Deary et al. 2000). Magnetic resonance imaging (MRI)-detected white matter hyperintensities (WMH) (Young et al. 2008) are associated with vascular risk factors (Murray et al. 2005) and with reduced cognitive ability and dementia in older adults (Leaper et al. 2001; Firbank et al. 2007). We previously found that WMH contribute 14% of the variance in cognitive ability at age 78, that WMH are linked to hypertension (Deary et al. 2003), and it is now widely accepted that WMH are an imaging biomarker of vascular disease (Kearney-Schwartz et al. 2009, Raz et al. 2007, Fischer et al. 2007).
Cross-sectional investigations into the relationship between hypertension, WMH and late-life cognition are limited by difficulty in controlling for prior cognitive ability (Leaper et al. 2001, Wright et al. 2008, Breteler et al. 1994). While longitudinal population-based studies confirm the negative effect of WMH on cognitive ageing (Silbert et al. 2008, Gouw et al. 2008), it is unclear whether WMH in late midlife, are as detrimental to the lifelong cognitive trajectory as in the eighth decade.
We have access to two samples, aged 68 and 78 years, whose pre-morbid cognitive abilities were measured in childhood, allowing us to control for original cognitive ability and to measure the influence of key variables on lifelong cognitive ageing. Furthermore, these samples underwent identical study protocols, allowing comparative modelling of the influences on their intelligence to be performed.
The aims of this study were to model the relative effects of the main positive (childhood intelligence) and negative (WMH and hypertension) predictors of late-life intelligence in two cohorts at different ages.
Methods
Scottish mental surveys and Aberdeen birth cohorts
The Scottish Mental Surveys (SMS) of 1932 and 1947 assessed the mental ability of almost all (>95%) children born in 1921 and 1936 and at school on 1st June 1932 or 4th June 1947 (Deary et al. 2008a). The mental ability test used was Godfrey Thomson’s Moray House Test (MHT) No. 12 (Scottish Council for Research in Education 1933), which was normalized for age at time of testing and validated (r = 0.80) against the Stanford–Binet test on 1,000 participants of the SMS of 1932. With the approval of the Local Research Ethics Committee, two samples drawn from local people living independently, who had participated in the SMS of 1932 and 1947, the Aberdeen Birth Cohorts of 1921 and 1936 respectively, (ABC21 and ABC36), were recruited into two follow-up studies of ageing.
ABC21 cohort recruitment and participation
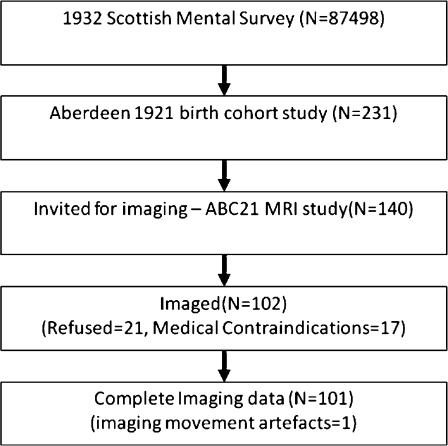
In 1998–1999, 350 local survivors the SMS of 1932 were invited to participate in a longitudinal study of cognitive ageing and health. Details of this study are available elsewhere (Starr et al. 2000). Briefly, 281 participants, aged 78–79, identified through childhood intelligence records volunteered to take part, and of these 231 were healthy enough to enter the study. One hundred forty participants were invited at random to undergo MRI and 119 agreed. Exclusion criteria were neurological disease, dementia, claustrophobia or body metal and were present in 17. Of those who completed MRI, one participant’s images were excluded due to movement artefact. Imaging data for 101 individuals were available for analyses. The recruitment, selection and attrition of the group are shown in Fig. 1.
Fig. 1.
Flowchart detailing how our participants were obtained from the original Scottish Mental Health Survey of 1932
ABC36 cohort recruitment and participation
From 2000–2002, we recruited 483 local survivors of the SMS of 1947, born in 1936, into a longitudinal study of cognitive ageing and health using the same study design. Details of the ABC36 protocol are available elsewhere (Starr et al. 2007). In 2004, 319 participants, selected at random, were invited to undergo MRI. The same exclusion criteria were applied; 243 participants agreed to brain MRI, aged approximately 68 years. Imaging data were available for 233 participants and were used for analyses. The recruitment, selection and attrition of this group are shown in Fig. 2.
Fig. 2.
Flowchart detailing how our participants were obtained from the original Scottish Mental Health Survey of 1947
Cognitive testing
Due to the study design, by definition, age 11 MHT scores were available for all participants (Scottish Council for Research in Education 1933). A psychologist administered the following neuropsychological tests in later life: Raven’s Progressive Matrices (RPM), (Raven 1960), a non-verbal reasoning measure of fluid intelligence; the Auditory Verbal Learning Test (AVLT) (Rey 1964) a measure of immediate and delayed verbal memory; the Uses of Common Objects (UCO) test, a measure of executive function or purposive action (Guilford et al. 1978) and the Digit Symbol test (DS), (Wechsler 1997), a measure of processing speed, attention and visual short-term memory.
Cognitive test score availability
Of 101 ABC21 participants, 98 completed RPM, 93 completed AVLT, 88 completed UCO and 87 completed DS. Eighty-two participants completed all tests. Of the 233 ABC36 participants, 226 completed RPM, 218 completed AVLT, 215 completed UCO and 213 completed DS. Two hundred eleven participants completed all tests.
Extraction of g
The mental tests described above (RPM, AVLT, UCO and DS) are all significantly and positively correlated with each other. The first un-rotated principal component or general cognitive factor (‘g’), used to describe the variance shared between tests, was extracted using principal components analysis. The cognitive scores for both cohorts were combined and data reduction performed. The first un-rotated principal component (‘g’) accounted for 50.2% of the variance in the scores for all subjects. The component loadings were RPM = 0.74, AVLT = 0.66, DS = 0.78 and UCO = 0.65, the standardized variable was multiplied by 15 and added to 100 to create an IQ like score.
Hypertension
Systolic and diastolic blood pressure values were measured after the participant sat resting for 5 min in a warm and quiet room. The mean of three measurements was used for classification. In addition to 43 participants in ABC21 and 73 participants in ABC36 with previously diagnosed hypertension, eight ABC21 and six ABC36 participants had untreated hypertension with systolic blood pressure of more than 150 mmHg and diastolic blood pressure of more than 95 mmHg; thus, 50 (50%) of 101 ABC21 and 79 (34%) of ABC36 were defined as hypertensive.
Brain MRI acquisition and image analysis
Brain MRI was obtained in ABC21 from 1999–2000 using a 1.0 T Magnetom Impact (Siemens, Erlangen) with a T2 weighted fast spin echo axial sequence (TR/TE, 4,000/96; slice thickness, 5 mm; space, 1.5 mm). In ABC36 brain MRI was obtained from 2003–2005 using a 1.5 T NVi scanner (General Electric, Milwaukee, Wi) with T2 axial (TR/TE, 4,900/81.4; slice thickness, 5 mm; space, 1.2 mm) and fluid attenuation inversion recovery axial (FLAIR; TR/TE, 9,002/1.33; TI, 2,200; slice thickness, 5 mm; space, 1.2 mm) sequences. WMH were assessed on T2 and FLAIR images using Scheltens’ scale by the same experienced blinded observer. The sum of the regional hyperintensity scores was entered into the analysis.
Statistical analysis and structural equation modelling
All summary and correlation statistical analyses were performed with PASW 18 (SPSS Inc. Chicago). Structural equation models (SEM) were created using the AMOS 18 package (AMOS Development Corporation, Crawfordville). SEMs were based on those previously shown to successfully model the influence on late-life cognition in ABC21 (Deary et al. 2003). We refined this model by using a more sophisticated estimate of lesion burden (Scheltens’ scale) and by including data from ABC36. The four mental tests were hypothesized to share variance attributable to ‘g’. After this latent trait was taken into account, the test scores were hypothesized to be uncorrelated. WMH was hypothesized to contribute to ‘g’ in old age, as was the MHT score at age 11. A further hypothesis was that WMH score, beyond contributing to ‘g’, did not contribute to individual mental abilities. In model 2, hypertension was hypothesized to have a direct effect on ‘g’ and an indirect effect mediated via WMH load.
Using the Hoyle and Panter (1995) guidelines (Hoyle and Panter 1995), the goodness of fit for each model was assessed using chi-square to degrees of freedom ratio (Cmin/df), the Incremental Fit Index (IFI), and the Comparative Fit Index (CFI). A Cmin/df close to 1 and values greater than 0.95 for the IFI and CFI are considered to reflect an acceptable model fit. In addition, the Root Mean Square Error of Approximation (RMSEA) was reported, where a value less than 0.05 indicates a close fit.
Results
Summary statistics
Table 1 shows that the male/female ratio was similar in ABC21 and ABC36. MHT scores age 10.5–11.5 in the ABC36 sample were higher than those in ABC21. Of the tests taken in late life, all scores except AVLT were lower in ABC21, who were around 10 years older at testing. Lesion burden was significantly higher in ABC21. Splitting each cohort by hypertension status and comparing WMH, we found that the hypertensive participants had significantly higher Scheltens’ scores in both cohorts (p < 0.05). Hypertensive participants also had generally poorer cognitive scores in both cohorts although DS score in ABC36 was the only one to reach significance.
Table 1.
Summary of demographic, brain MRI and cognitive test results in ABC36 and ABC21
| Cohort | Mean | Number | SD | P value | |
|---|---|---|---|---|---|
| Age at MRI (year) | ABC36 | 68.6 | 232 | 0.6 | <0.001 |
| ABC21 | 78.6 | 101 | 0.4 | ||
| Sex (male/female) | ABC36 | 123/110 | 0.28a | ||
| ABC21 | 60/41 | ||||
| MHT | ABC36 | 45.1 | 233 | 10.8 | 0.007 |
| ABC21 | 41.6 | 101 | 11.2 | ||
| RPM | ABC36 | 38.1 | 226 | 7.6 | <0.001 |
| ABC21 | 28.4 | 98 | 8.8 | ||
| AVLT | ABC36 | 49.7 | 218 | 12.4 | 0.56 |
| ABC21 | 50.6 | 93 | 13.6 | ||
| DS | ABC36 | 45.1 | 213 | 10.7 | <0.001 |
| ABC21 | 33.6 | 87 | 9.3 | ||
| UCO | ABC36 | 13.3 | 215 | 6.5 | 0.028 |
| ABC21 | 11.6 | 88 | 4.6 | ||
| WMH | ABC36 | 15.3 | 232 | 9.1 | <0.001 |
| ABC21 | 24.5 | 101 | 9.0 | ||
| g | ABC36 | 103.6 | 211 | 13.7 | |
| ABC21 | 90.9 | 82 | 14.4 | ||
| Systolic blood pressure | ABC36 | 138.2 | 232 | 17.7 | <0.001 |
| ABC21 | 147.8 | 98 | 19.9 | ||
| Diastolic blood pressure | ABC36 | 78.3 | 232 | 9.3 | 0.16 |
| ABC21 | 76.1 | 98 | 10.2 | ||
| Blood pressure (hypertensive/other) | ABC36 | 79/151 | 0.01a | ||
| ABC21 | 51/50 | ||||
aFisher’s exact test. Unless stated, differences between cohorts were tested by t test
MHT Moray House Test, RPM Raven’s Progressive Matrices, AVLT Auditory Verbal learning Test, DS Digit Symbol, UCO Uses for Common Objects, WMH brain hyperintensities measured by Scheltens’ scale
Correlations
Table 2 shows significant positive correlations between all adult mental tests taken concurrent with brain imaging, with moderate to large effect sizes in both cohorts. In general, correlation between adult tests was stronger in ABC21. The correlation between age 11 MHT results and adult fluid intelligence was considerably greater in ABC36. There were negative correlations between WMH and fluid intelligence ability as measured by RPM, DS and UCO in ABC36 and between WMH and RPM and UCO in ABC21. There was no relationship between age 11 MHT and WMH in either sample. Using the method of Cohen et al., we found that the correlation coefficients for WMH/g were not different between cohorts (Cohen et al. 2003). The correlation coefficients for MHT/g were significantly different (p < 0.05).
Table 2.
Bivariate correlations between cognitive scores and brain WMH in ABC36 and ABC21
| WMH | MHT | RPM | AVLT | DS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | ABC36 | ABC21 | ABC36 | ABC21 | ABC36 | ABC21 | ABC36 | ABC21 | ABC36 | ABC21 | |
| MHT | r | −0.13 | 0.04 | ||||||||
| n | 232 | 101 | |||||||||
| RPM | r | −0.17* | −0.22* | 0.50** | 0.31** | ||||||
| n | 225 | 98 | 226 | 98 | |||||||
| AVLT | r | −0.13 | −0.10 | 0.24** | 0.23* | 0.29** | 0.33** | ||||
| n | 217 | 93 | 218 | 93 | 216 | 92 | |||||
| DS | r | −0.19** | −0.05 | 0.37** | 0.34** | 0.33** | 0.54** | 0.36** | 0.59** | ||
| n | 212 | 87 | 213 | 87 | 213 | 87 | 213 | 85 | |||
| UCO | r | −0.16* | −0.21 | 0.23** | 0.21* | 0.26** | 0.50** | 0.36** | 0.43** | 0.21** | 0.41** |
| n | 214 | 88 | 215 | 88 | 213 | 88 | 214 | 87 | 211 | 82 | |
| g | r | −0.24** | −0.14 | 0.49** | 0.27* | ||||||
| n | 210 | 82 | 211 | 82 | |||||||
r Pearson’s correlation coefficient, n number of pairs, MHT Moray House Test, RPM Raven’s Progressive Matrices, AVLT Auditory Verbal learning Test, DS Digit Symbol, UCO Uses for Common Objects, WMH brain hyperintensities measured by Scheltens’ scale
*p ≤ 0.05; **p ≤ 0.01 indicate significant correlations
Structural equation modelling
Model adjustment
Preliminary analysis and the modification indices software within AMOS indicated that the model fits would be improved if error terms associated with DS, AVLT and UCO were correlated. No other adjustments to the model were indicated by the analysis.
Model fit
The Cmin/df ratio of models 1 and 2 were 0.57 and 0.82, respectively, the IFI and CFI were 1, and RMSEA 0.00 for both models. These results indicated that the models provided an excellent fit to the data.
Regression weights
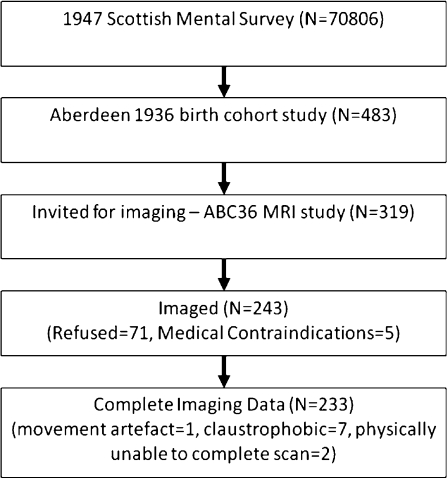
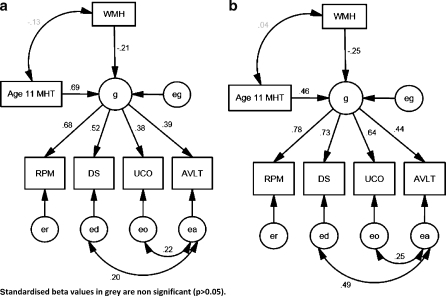
In models (Fig. 3a and b) WMH burden and childhood ability significantly contribute to late-life cognition in both cohorts. Hypertension did not independently contribute to late-life cognition (Fig. 4a and b).
Fig. 3.
Structural equation model of the contributions of brain WMH and childhood intelligence to cognitive ability (g) in (a) ABC36 age 68 and in (b) ABC21 age 78. Standardised beta values in grey are non-significant (p > 0.05)
Fig. 4.
SEM of the contributions of WMH, childhood intelligence and hypertension to cognitive ability (g) in (a) ABC36 age 68 and in (b) ABC21 age 78. Standardised beta values in grey are non-significant (p > 0.05)
Discussion
This study shows that the contribution of childhood intelligence to late-life ability significantly decreases between the ages of 68 and 78 years, after accounting for the effect of WMH burden on the brain. In addition, the results show that the effect of hypertension on late-life cognition is entirely mediated by the accumulation of WMH in the brain. This suggests that hypertension, in its self, does not influence lifelong cognitive change. The differences in WMH burden between the cohorts and the similarity in correlations and regression weights suggests that the effect of WMH burden on cognition is linear.
The results show that the influence of childhood intelligence is greater in the younger sample (ABC36) and that this positive influence decreases with age. This has previously been reported in these cohorts, in a larger sample without imaging (Deary et al. 2004). This could be anticipated, since ABC36 are closer to childhood and the positive effects of education, intellectual engagement and occupational attainment. These activities are probably initiated in part by childhood cognitive abilities (Deary et al. 2008b). Similarly, older participants have had longer to acquire negative influences on cognitive ageing, such as the accumulation of WMH, and are further away from the positive effects of occupational attainment.
Brain WMH burden—as expected—has a significant negative effect on late-life cognition. Our analysis shows no significant difference between the cohorts, with standardized regression weights ranging from 0.18 to 0.25, implying that the impact of WMH burden is additive or linear. We have demonstrated that the main negative effect of hypertension on late-life ability is exerted through WMH, which can be viewed as the visible results of cerebrovascular disease on brain imaging. We found no direct association between hypertension and ‘g’. This differs from our previous publication (Deary et al. 2003) in ABC21. The discrepancy can be explained by the use of a more sensitive WMH assessment method, the Scheltens’ scale, and it may well be that this more successfully captures the effect of hypertension that is cognitively important, than the less sensitive Fazekas scale.
Early-life risk factors for lower cognitive ability are known to include low birth weight, low parental cognitive ability, poor nutrition and environmental stress (Puga et al. 2009). The same factors are related to a higher incidence of vascular disease. The Developmental Origins of Health and Disease Hypothesis, proposes that early-life experiences, including in utero, determine disease risk throughout life and suggest that poor cognitive ability and hypertension share the same risk factors.
The de-differentiation hypothesis (Deary et al. 2004) suggests that the correlation between cognitive ability demonstrated on different tests in late life would increase with age. Our data support this hypothesis and indicate less individual difference in the ability of the participants in ABC21. This implies that measured ability on individual tests is closer to ‘g’. This is entirely consistent with our hypothesis that cerebrovascular disease (or accumulated WMH burden) is becoming one of the over-riding influences on cognitive ageing in the older sample, with the result that fluid intelligence is reduced to a level driven largely by’g’.
The strengths of this study are the availability of a valid measure of childhood intelligence, providing a uniquely valuable baseline measure of childhood cognitive ability. The samples, ABC21 and ABC36, are well characterised and were recruited and assessed in the same way, by the same research team. The tests used were identical and provide a broad assessment of cognitive ability, largely fluid intelligence, recognised to be vulnerable to effects of ageing. The same experienced observer analysed all MRI data using a widely published method, excluding inter-observer variance. The advantage of a structural equation modelling approach over conventional linear regression is that it demonstrates both confirmation of a hypothesis of causality and strength of relationships.
However, potential weaknesses of this study should also be highlighted. Because this is an investigation comparing two historical cohorts, and not a true longitudinal study, it is not possible to state definitively that differences demonstrated between ABC21 and ABC36 are solely attributable to ageing. We have compared two cohorts who differed in age at testing by around ten years. While, because of consistency in other methods and measures, age is very likely to be the major determinant of differences demonstrated, other factors might have influenced results. People born in 1921 experienced greater deprivation in childhood than those born in 1936. Counter-intuitively, ABC36 participants who were children during World War II, received better nutrition, as a result of the food rationing regimen they experienced, than the participants of ABC21, who were children during the great depression. Cognitive ability age 11 years differed between the two cohorts, with ABC21 scoring a mean of 41.6 and ABC36 a mean of 45.1 on the MHT. This may reflect educational differences between 1932 and 1947 or indicate a true improvement in childhood ability. Although the two cohorts were imaged on different MRI scanners operating at different field strengths, the resulting MRI images were scored using the same method by the same observer, excluding inter-observer variance. The measured mean Scheltens’ score was higher (24.5 versus 15.3) in the older ABC21, as expected for a biomarker of an age-related pathology. Use of a visual semi-quantitative method of MRI assessment could be criticised as being prone to ceiling effects (van Straaten et al. 2006), but although voxel-based method may avoid this artefact, Scheltens’ scale has been shown to be a robust method for assessing WMH (Kapeller et al. 2003). The age of images acquired in the older sample precluded voxel-based image analysis.
Another potential weakness is lack of comprehensive modelling of all components of the metabolic syndrome and their influence on brain MRI and cognitive change. The metabolic syndrome, comprising impaired glucose tolerance, abdominal or central obesity, hypertension, hypertriglyceridemia and reduced high-density lipoprotein cholesterol, has been associated with age-related cognitive decline, mild cognitive impairment and dementia (both vascular and Alzheimer’s disease) (see (Frisardi et al. 2010) and (Panza et al. 2010) for reviews). However, comprehensive data on these components was not available in the samples presented here.
Here, we have demonstrated that the positive relationship between childhood and late-life intelligence decreases with age and that there is a reciprocally greater negative effect of measures of cerebrovascular disease in the older sample. This provides insights into the relative influence of positive and negative contributors to cognitive ageing. Future work should examine whether the differences demonstrated here can be replicated in a longitudinal data set, should preferably use a voxel-based method of image analysis, include brain volumes and should measure sub-visible changes to brain structure using diffusion tensor imaging. Future work should also model the influence of components of the metabolic syndrome on brain structure and lifelong cognitive ageing.
Acknowledgements
The authors would like to thank the participants of the Aberdeen 1921 and 1936 Birth Cohorts, without whom this research would not have been possible. Image acquisition and image analysis for ABC21 and ABC36 were funded by The Chief Scientist Office, Scottish Executive and Alzheimer’s Research Trust (now Alzheimer’s Research UK) respectively.
Drs Murray, Staff, McNeil and Salarirad are part of SINAPSE, the Scottish Imaging Network
Disclosures
Dr Alison D Murray, Dr Roger T Staff, Dr Chris J McNeil, Dr Sima Salarirad, Prof John M Starr Prof Ian J Deary and Prof Lawrence J Whalley report no disclosures.
Contributor Information
Alison D. Murray, Email: a.d.murray@abdn.ac.uk
Roger T. Staff, Email: r.staff@abdn.ac.uk
Chris J. McNeil, Email: c.mcneil@abdn.ac.uk
Sima Salarirad, Email: s.salari@abdn.ac.uk.
John M. Starr, Email: jstarr@staffmail.ed.ac.uk
Ian J. Deary, Email: i.deary@ed.ac.uk
Lawrence J. Whalley, Email: l.j.whalley@abdn.ac.uk
References
- Breteler MM, Amerongen NM, Swieten JC, Claus JJ, Grobbee DE, Gijn J, Hofman A, Harskamp F. Cognitive correlates of ventricular enlargement and cerebral white matter lesions on magnetic resonance imaging. The Rotterdam Study. Stroke. 1994;256:1109–1115. doi: 10.1161/01.STR.25.6.1109. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Nelson HE. Applied multiple regression/correlation analysis for the behavioral sciences. 3. Hillsdale: Lawrence Erlbaum Associates; 2003. [Google Scholar]
- Deary, I.J., Whalley, L.J., Lemmon, H., Crawford, J.R. & Starr, J.M. 2000, “The stability of individual differences in mental ability from childhood to old age: follow-up of the 1932 Scottish mental survey”, Intelligence 28:49–55
- Deary IJ, Leaper SA, Murray AD, Staff RT, Whalley LJ. Cerebral white matter abnormalities and lifetime cognitive change: a 67-year follow-up of the Scottish Mental Survey of 1932. Psychol Aging. 2003;181:140–148. doi: 10.1037/0882-7974.18.1.140. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Whiteman MC, Starr JM, Whalley LJ, Fox HC. The impact of childhood intelligence on later life: following up the Scottish mental surveys of 1932 and 1947. J Pers Soc Psychol. 2004;861:130–147. doi: 10.1037/0022-3514.86.1.130. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Batty GD, Gale CR. Bright children become enlightened adults. Psychol Sci. 2008;191:1–6. doi: 10.1111/j.1467-9280.2008.02036.x. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Whalley LJ, Starr JM. A Lifetime of Intelligence. Follow up studies of the Scottish mental surveys of 1932 and 1947. Washington: American Psychological Association; 2008. [Google Scholar]
- Firbank MJ, Wiseman RM, Burton EJ, Saxby BK, O’Brien JT, Ford GA. Brain atrophy and white matter hyperintensity change in older adults and relationship to blood pressure. Brain atrophy, WMH change and blood pressure". J Neurol. 2007;2546:713–721. doi: 10.1007/s00415-006-0238-4. [DOI] [PubMed] [Google Scholar]
- Fischer P, Krampla W, Mostafaie N, Zehetmayer S, Rainer M, Jungwirth S, Huber K, Bauer K, Hruby W, Riederer P, Tragl KH. VITA study: white matter hyperintensities of vascular and degenerative origin in the elderly. J Neural Transm Suppl. 2007;72(72):181–188. doi: 10.1007/978-3-211-73574-9_23. [DOI] [PubMed] [Google Scholar]
- Frisardi V, Solfrizzi V, Seripa D, Capurso C, Santamato A, Sancarlo D, Vendemiale G, Pilotto A, Panza F. Metabolic-cognitive syndrome: a cross-talk between metabolic syndrome and Alzheimer’s disease. Ageing Res Rev. 2010;94:399–417. doi: 10.1016/j.arr.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Gouw AA, Flier WM, Fazekas F, Straaten EC, Pantoni L, Poggesi A, Inzitari D, Erkinjuntti T, Wahlund LO, Waldemar G, Schmidt R, Scheltens P, Barkhof F, LADIS Study Group Progression of white matter hyperintensities and incidence of new lacunes over a 3-year period: the Leukoaraiosis and Disability study. Stroke. 2008;395:1414–1420. doi: 10.1161/STROKEAHA.107.498535. [DOI] [PubMed] [Google Scholar]
- Guilford JP, Christensen PR, Merrifield PR, Wilson RC. Alternate uses: manual of instructions and interpretation. Orange: Sheridan Psychological Services; 1978. [Google Scholar]
- Hoyle RH, Panter AT. Writing about structural equation models. In: Hoyle RH, editor. Structural equation modeling: concepts, issues, and applications. Thousand Oaks: Sage Publications; 1995. pp. 158–176. [Google Scholar]
- Kapeller P, Barber R, Vermeulen RJ, Ader H, Scheltens P, Freidl W, Almkvist O, Moretti M, Ser T, Vaghfeldt P, Enzinger C, Barkhof F, Inzitari D, Erkinjunti T, Schmidt R, Fazekas F, Force ET, European Task Force of Age Related White Matter Changes Visual rating of age-related white matter changes on magnetic resonance imaging: scale comparison, interrater agreement, and correlations with quantitative measurements. Stroke. 2003;342:441–445. doi: 10.1161/01.STR.0000049766.26453.E9. [DOI] [PubMed] [Google Scholar]
- Kearney-Schwartz A, Rossignol P, Bracard S, Felblinger J, Fay R, Boivin JM, Lecompte T, Lacolley P, Benetos A, Zannad F. Vascular structure and function is correlated to cognitive performance and white matter hyperintensities in older hypertensive patients with subjective memory complaints. Stroke. 2009;404:1229–1236. doi: 10.1161/STROKEAHA.108.532853. [DOI] [PubMed] [Google Scholar]
- Leaper SA, Murray AD, Lemmon HA, Staff RT, Deary IJ, Crawford JR, Whalley LJ. Neuropsychologic correlates of brain white matter lesions depicted on MR images: 1921 Aberdeen Birth Cohort. Radiology. 2001;2211:51–55. doi: 10.1148/radiol.2211010086. [DOI] [PubMed] [Google Scholar]
- Murray AD, Staff RT, Shenkin SD, Deary IJ, Starr JM, Whalley LJ. Brain white matter hyperintensities: relative importance of vascular risk factors in nondemented elderly people. Radiology. 2005;2371:251–257. doi: 10.1148/radiol.2371041496. [DOI] [PubMed] [Google Scholar]
- Panza F, Frisardi V, Capurso C, Imbimbo BP, Vendemiale G, Santamato A, D’Onofrio G, Seripa D, Sancarlo D, Pilotto A, Solfrizzi V. Metabolic syndrome and cognitive impairment: current epidemiology and possible underlying mechanisms. J Alzheimers Dis. 2010;213:691–724. doi: 10.3233/JAD-2010-091669. [DOI] [PubMed] [Google Scholar]
- Puga B, Puga PG, Arriba A, Armendariz Y, Labarta JI, Longas AF. Psychomotor and intellectual development (Neurocognitive Function) of children born small for gestational age (SGA). Transversal and longitudinal study. Pediatr Endocrinol Rev. 2009;6(Suppl 3358):370. [PubMed] [Google Scholar]
- Raven JC, Court JH, Raven J (1977) Manual for Raven’s Progressive Matrices and Vocabulary Scales. London, United Kingdom, Lewis
- Raz N, Rodrigue KM, Kennedy KM, Acker JD. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007;212:149–157. doi: 10.1037/0894-4105.21.2.149. [DOI] [PubMed] [Google Scholar]
- Rey A. L’examen clinique en psychologie. Paris: Presses universitair de France; 1964. [Google Scholar]
- Salthouse, T.A. 2010, “Selective review of cognitive aging”. J Int Neuropsychol Soc, 1–7 [DOI] [PMC free article] [PubMed]
- The intelligence of Scottish school children: a national survey of an age group. London: University of London Press; 1933. [Google Scholar]
- Silbert LC, Nelson C, Howieson DB, Moore MM, Kaye JA. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology. 2008;712:108–113. doi: 10.1212/01.wnl.0000316799.86917.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Manoux A, Kivimaki M. The importance of cognitive ageing for understanding dementia. Age (Dordr) 2010;324:509–512. doi: 10.1007/s11357-010-9147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr JM, Deary IJ, Lemmon H, Whalley LJ. Mental ability age 11 years and health status age 77 years. Age Ageing. 2000;296:523–528. doi: 10.1093/ageing/29.6.523. [DOI] [PubMed] [Google Scholar]
- Starr JM, Deary IJ, Fox H, Whalley LJ. Blood pressure and cognition in the Aberdeen 1936 birth cohort. Gerontology. 2007;536:432–437. doi: 10.1159/000111696. [DOI] [PubMed] [Google Scholar]
- Straaten EC, Fazekas F, Rostrup E, Scheltens P, Schmidt R, Pantoni L, Inzitari D, Waldemar G, Erkinjuntti T, Mantyla R, Wahlund LO, Barkhof F, LADIS Group Impact of white matter hyperintensities scoring method on correlations with clinical data: the LADIS study. Stroke. 2006;373:836–840. doi: 10.1161/01.STR.0000202585.26325.74. [DOI] [PubMed] [Google Scholar]
- Wang HX, Karp A, Winblad B, Fratiglioni L. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: a longitudinal study from the Kungsholmen project. Am J Epidemiol. 2002;15512:1081–1087. doi: 10.1093/aje/155.12.1081. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale-iii. New York: The Psychological Corp; 1997. [Google Scholar]
- Wright CB, Festa JR, Paik MC, Schmiedigen A, Brown TR, Yoshita M, DeCarli C, Sacco R, Stern Y. White matter hyperintensities and subclinical infarction: associations with psychomotor speed and cognitive flexibility. Stroke. 2008;393:800–805. doi: 10.1161/STROKEAHA.107.484147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young VG, Halliday GM, Kril JJ. Neuropathologic correlates of white matter hyperintensities. Neurology. 2008;71(11):804–811. doi: 10.1212/01.wnl.0000319691.50117.54. [DOI] [PubMed] [Google Scholar]