Abstract
The association among single nucleotide polymorphisms in inflammatory genes as interleukin-1 alpha (IL-1α), interleukin-1 beta (IL-1β) or tumor necrosis factor alpha (TNF-α) and dementia has been explored mostly in Alzheimer’s disease, while few data addressing their association with dementia in very old people are available. We performed a prospective, door-to-door population-based study of 80 years or older residents in eight municipalities of Varese province, Italy (the Monzino 80-plus study). No difference was found by a cross-sectional approach comparing IL-1α rs1800587, IL-1β rs3087258 and TNF-α rs1799724 genotypic and allelic frequencies between those affected and not affected by dementia. After a 5-year follow-up, the elderly carriers of T-allele of TNF-α rs1799724 were at an increased risk of dementia (p = 0.03). This association was no more significant adjusting for the apolipoprotein E epsilon-4 allele (APOE-ε4, p = 0.26), which was an independent predictor of dementia onset (p = 0.0002). In short, in this Italian population of oldest olds, dementia was associated to the APOE-ε4 allele only.
Keywords: Interleukin-1, Tumor necrosis factor, Apolipoprotein E, Assessment of cognitive disorders/dementia, Association studies in genetics, Neuroinflammation
Introduction
Inflammation has a positive role in successful aging, as an effective inflammatory response is basic to cope with pathogen infections. However, a chronic inflammatory state or an overproduction of inflammatory factors might contribute to age-associated diseases like neurodegenerative disorders, autoimmunity and cancer (Caruso et al. 2004; Van Bodegom et al. 2007). The brain can activate an inflammatory reaction involving the production of proinflammatory cytokines (IL-1β, TNF-α and IL-6) in response to a peripheral immune challenge (Tonelli and Postolache 2005). Dementia and cognitive decline are paralleled by an increased peripheral cytokines production (Weaver et al. 2002; Bruunsgaard et al. 1999; Holmes et al. 2003; van Exel et al. 2003; Dik et al. 2005) that can be interpreted as a consequence of the neurodegenerative process, creating a detrimental feedback loop that further sustains neuronal damage. Moreover, brain inflammatory cytokines can be increased by several different pathological conditions associated with dementia, like cerebrovascular injury (Paganelli et al. 2002), type-2 diabetes (Rizzo et al. 2008) and virus infection (Brabers and Nottet 2006).
Genes coding for the proinflammatory cytokines interleukin-1 alpha (IL1-α, GeneID: 3552), interleukin-1 beta (IL1-β, GeneID: 3553) and tumor necrosis factor alpha (TNF-α, GeneID: 7124) have single nucleotide genetic variants (single nucleotide polymorphism—SNP) in the promoter region that may change the transcription rate and consequently the cytokine production (Dominici et al. 2002; Wen et al. 2006; Prasad et al. 2010). Three of these SNPs, rs1800587 (C/T) for IL-1α, rs3087258 (C/T) for IL-1β and rs1799724 (C/T) for TNF-α, have been evaluated also in the field of Alzheimer’s disease (AD). In this context, their role as a susceptibility factor has been assessed mostly by case–control association studies focused on people aged between 70 and 80 years, with controversial results (Ehl et al. 2003; Sciacca et al. 2003; Wang et al. 2005; Ravaglia et al. 2006; Wehr et al. 2006; Perry et al. 2001; Culpan et al. 2003; Licastro et al. 2007). This scenario holds true for many other AD susceptibility genetic loci, apart from the epsilon-4 allele of the gene coding for the apolipoprotein E (APOE-ε4) located on chromosome 19, that have been consistently reported to be a risk factor (Saunders et al. 1993; Strittmatter et al. 1993; Bertram et al. 2010). Many less investigations are available on the role of IL-1α rs1800587, IL-1β rs3087258 and TNF-α rs1799724 as dementia risk modulators in the very old (>85 years), an age bracket where some of these polymorphisms have been explored in relation to survival (Cavallone et al. 2007). This is an important gap, as most dementia sufferers are 80 years or older. We decided to investigate the association of IL-1α rs1800587, IL-1β rs3087258 and TNF-α rs1799724 with dementia in a prospective door-to-door population-based study (the “Monzino 80-plus study”) enrolling elderly people residing in eight municipalities of Varese province (Italy).
Materials and methods
Study sample and clinical evaluation
The Monzino 80-plus study is an ongoing, prospective, door-to-door population-based survey among all 80 years or older residents in eight neighbouring municipalities in the province of Varese, Italy, and has been fully described elsewhere (Tettamanti et al. 2006). Briefly, details about the participants’ lifestyle, habits, medical history and health status were collected by trained psychologists from both the subject and a family member. The participants were administered a set of neuropsychological tests including CERAD battery (Morris et al. 1989), Mini-Mental State Examination (Folstein et al. 1975), Instrumental Activities of Daily Living (Lawton and Brody 1969), Spontaneous Behavior Interview-bADL (Lucca et al. 1996) and Geriatric Depression Scale (ten-item version) (D’Ath et al. 1994). The diagnosis of dementia was made according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders IV.
Standard protocol approvals, registrations and patient consents
The study procedures were in accordance with the principles outlined in the Declaration of Helsinki of 1964 and the following amendments. The study protocol was submitted to and approved by the Local Research Ethics Committee (Azienda Sanitaria Locale of Varese Province, Varese, Italy). Separate written informed consents for data and blood sample collections were obtained from all participants.
DNA analysis
Blood samples (3 mL) were collected by venipuncture. One aliquot of blood was used to extract genomic DNA (gDNA) using a semi-automated vacuum-based nucleic acids extractor (AB6100, Applied Biosystems). From the subjects who gave their consent for genetic testing (n = 609), gDNA was extracted and genotyped for IL-1α rs1800587, IL-1β rs3087258 and TNF-α rs1799724. To reduce false genotyping rate, each SNP was assessed in duplicate and only unambiguous results were considered. IL-1α, IL-1β and TNF-α genotyping was performed by restriction fragment length polymorphisms using the following primers: IL-1α −889 C/T (rs1800587) F 5′-AAG CTT GTT CTA CCA CCT GAA CTA GGC-3′ and R 5′-TTA CAT ATG AGC CTT CCA TG-3′; IL-1β −511 C/T (rs3087258) F 5′-TGG CAT TGA TCT GGT TCA TC-3′ and R 5′-GTT TAG GAA TCT TCC CAC TT-3′ and TNF-α −850 C/T (rs1799724) F 5′-AAG TCG AGT ATG GGG ACC CCC CGT TAA-3 and R 5′-CCC CAG TGT GGT GCC ATA TCT TCT T-3′ and the restriction enzymes NcoI, AvaI and HindII, respectively (Promega, Madison, WI, USA). The apolipoprotein E (APOE) genotype was assessed as previously described (Albani et al. 2007).
Statistical analysis
The univariate relationship of genotypic or allelic frequency was analysed using χ2 tests, in case of nominal variables (e.g. sex), and t tests or analyses of variance, in case of continuous variables (e.g. age). The logistic regressions were used to study the relationship between the genotypic frequency and the severity of dementia adjusting for age, sex and education level. The association between the genetic trait and the subsequent incidence of dementia was plotted using a Kaplan–Meier survival curve. p values were calculated by means of Cox proportional hazard regression models, adjusting for age, sex, education level and APOE genotype. Our study had 80% power to detect a variation of around 10–12% of the minor allele of IL-1α rs1800587, IL-1β rs3087258 or TNF-α rs1799724 (α = 0.05, effect size small-to-medium). The limit of significance was set at p = 0.05.
Results
Association between IL-1α rs1800587, IL-1β rs3087258, TNF-α rs1799724 and dementia: cross-sectional analysis
The main demographic and cognitive features of the population from the Monzino 80-plus study included for the genetic study are reported in Table 1. The population was divided into participants with dementia (n = 229, 37.6%) and participants without dementia (n = 380, 62.4%) in order to compare genotypic and allelic frequencies in the two groups. The results are shown in Table 2. The genotypic distributions respected Hardy–Weinberg equilibrium (p > 0.05) for all SNPs (both for demented and non-demented). We found no association, either at genotypic or allelic level among the genetic variants considered and dementia presence. The same results were found when we tried to stratify the population according to sex (data not shown) or when the presence of the SNPs rs1800587, rs3087258 or rs1799724 was related to baseline severity of dementia (Table 3). In this case, a possible sex contribution was not taken into account due to limited sample size.
Table 1.
Main demographic features of the population considered for genetic testing
| Number of subjects (M:F) | Age (years±SD) [range] | MMSE (mean±SD) | Education level (years±SD) |
|---|---|---|---|
| Non-demented (105:275) | 87.8 ± 4.4 [80–99] | 25.9 ± 2.9 | 5.3 ± 2.4 |
| Demented (32:197) | 90.6 ± 4.1* [81–104] | 13.1 ± 6.3** | 4.6 ± 2.2* |
M males, F females, MMSE Mini-Mental State Examination, SD standard deviation
*p < 0.0001; **p = 0.0006, Student’s t test vs. non-demented
Table 2.
Genotypic and allelic distributions of IL-1α rs1800587, IL-1β rs3087258 and TNF-α rs1799724
| Diagnosis (no. subjects) | Genotype count (%) | χ2 | p | Allele count (%) | χ2 | p | |||
|---|---|---|---|---|---|---|---|---|---|
| C/C | C/T | T/T | C | T | |||||
| IL-1α rs1800587 | |||||||||
| Non-demented (348) | 202 (58.1) | 120 (34.5) | 26 (7.4) | 524 (75.3) | 172 (24.7) | ||||
| Demented (210) | 117 (55.7) | 72 (34.3) | 21 (10.0) | 306 (72.8) | 114 (27.2) | ||||
| Univariate | 1.1 | 0.57a | 0.8 | 0.36b | |||||
| Adjusted | 1.15 | 0.56c | |||||||
| IL-1β rs3087258 | |||||||||
| Non-demented (363) | 205 (56.5) | 126 (34.7) | 32 (8.8) | 536 (73.8) | 190 (26.2) | ||||
| Demented (223) | 122 (54.7) | 78 (34.9) | 23 (10.4) | 322 (72.2) | 124 (27.8) | ||||
| Univariate | 0.41 | 0.81a | 0.3 | 0.54b | |||||
| Adjusted | 0.45 | 0.80c | |||||||
| TNF-α rs1799724 | |||||||||
| Non-demented (356) | 151 (42.4) | 172 (48.3) | 33 (9.3) | 474 (66.6) | 238 (33.4) | ||||
| Demented (215) | 107 (49.8) | 91 (42.3) | 17 (7.9) | 305 (70.9) | 125 (29.1) | ||||
| Univariate | 2.9 | 0.23a | 2.3 | 0.12b | |||||
| Adjusted | 2.1 | 0.34c | |||||||
aAssociated p value for genotypic frequency distribution comparison
bAssociated p value for allelic frequency distribution comparison
cAssociated p value for genotypic frequency distribution comparison after adjusting for age, sex and education level
Table 3.
Assessment of the association between dementia severity at baseline and IL-1α rs1800587, IL-1β rs3087258 or TNF-α rs1799724 genotype
| Genotype count (%) | F | p | |||
|---|---|---|---|---|---|
| C/C | C/T | T/T | |||
| IL-1α rs1800587 | |||||
| No. of subjects | 117 | 72 | 21 | 1.5 | 0.22 |
| Age (mean±SD) | 89.9 ± 4.2 | 90.8 ± 3.6 | 90.8 ± 4.7 | ||
| MMSE (mean±SD) | 13.1 ± 5.5 | 11.4 ± 6.0 | 12.5 ± 6.0 | ||
| IL-1β rs3087258 | |||||
| No. of subjects | 122 | 75 | 26 | 1.0 | 0.36 |
| Age (mean±SD) | 90.0 ± 3.9 | 90.9 ± 4.3 | 92.0 ± 4.6 | ||
| MMSE (mean±SD) | 13.1 ± 5.5 | 12.5 ± 6.3 | 10.7 ± 5.5 | ||
| TNF-α rs1799724 | |||||
| No. of subjects | 107 | 91 | 17 | 0.2 | 0.8 |
| Age (mean±SD) | 90.8 ± 3.9 | 90.0 ± 4.3 | 88.8 ± 3.4 | ||
| MMSE (mean±SD) | 12.2 ± 6.3 | 12.8 ± 5.1 | 11.9 ± 6.2 | ||
MMSE Mini-Mental State Examination, SD standard deviation
Association between IL-1α rs1800587, IL-1β rs3087258, TNF-α rs1799724 and dementia: longitudinal analysis
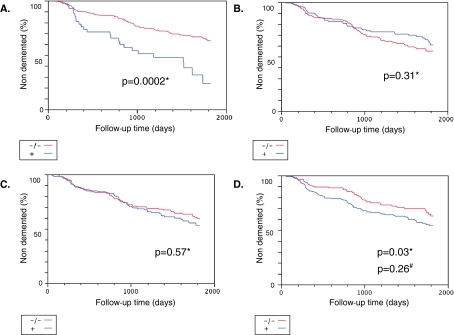
Taking advantage of the availability of a longitudinal follow-up of the participants to the Monzino 80-plus study, we decided to evaluate the association between dementia onset over time and IL-1α rs1800587, IL-1β rs3087258, TNF-α rs1799724 or APOE genotype, considering as a risk factor the presence of at least one T-allele or, in the case of the APOE genotype, assuming that the epsilon-4 (ε4) allele should increase dementia incidence in comparison to ε4-non-carriers. The curves are shown in Fig. 1, where the p values were corrected for age, sex and education level using Cox regression models. While for IL-1α rs1800587 and IL-1β rs3087258 no difference between C-carriers and T-carriers was detectable, for the TNF-α rs1799724 T-allele carriers, an increased risk of developing dementia was present [OR (95%CI): 1.5 (1.03–2.2)]. The APOE-ε4 carriers were at an increased risk of dementia, with an OR (95%CI) of 2.96 (1.81–4.61). Finally, we verified whether the TNF-α rs1799724 T-allele carriers had an increased risk of developing incident dementia independently from the APOE genotype. When we adjusted our multivariate analysis also for APOE-ε4 presence, the association was no longer significant [OR (95%CI): 1.2 (0.8–1.9), p = 0.26].
Fig. 1.
Incident cases of dementia in the Monzino 80-plus study. Incident dementia cases were stratified according to the genetic variants of APOE, IL-1α, IL-1β and TNF-α. The participants to the study were followed up for 5 years, and the occurrence of dementia during this period was registered. In each plot, the comparison is made between the carriers of at least one risk allele (blue line, +) and non-carriers of the same allele (red line, −/−) according to a dominant model. aAPOE. The APOE-epsilon 4 (APOE-ε4) allele was considered as risk allele. bIL-1α −889 C/T (rs1800587). cIL-1β −511 C/T (rs3087258). dTNF-α −850 C/T (rs1799724). For these three genetic variants, the minor T-allele was considered as the risk allele. Asterisk sign indicates the adjusted p value after correcting for age, sex and education. Number sign indicates the adjusted p value after correcting for age, sex, education and APOE-ε4 status
Discussion
The neurodegenerative disorders affect the nervous system, and the neuroinflammation is a common trait that parallels the disease onset and progression. The role of inflammation as disease-triggering factor is controversial; however, in most cases, it contributes to worsen the pathology, while a chronic inflammatory response in the brain might predispose to dementia. The individual genetic background might play a role in modulating the extent of neuroinflammation, in particular for genetic variants influencing the production of key proinflammatory cytokines like IL-1α, IL-1β and TNF-α. We have performed the genotyping of three SNPs located in the promoter region of the just cited genes in an Italian general population aged 80 years or older, in an attempt to evaluate an association between these genetic variants and dementia and to demonstrate whether the presence of these SNPs correlated with the severity of dementia. Our cross-sectional negative findings are not supportive of a major contribution of this genetic background to dementia status or severity, at least in our Italian population of the oldest old. Our results are in agreement with the others that have addressed the role of the examined SNPs in Alzheimer’s disease in the Italian population, finding very limited or no association (Terreni et al. 2003; Licastro et al. 2007; Tedde et al. 2008; Serretti et al. 2009). However, positive associations have been reported, too (Bosco et al. 2004; Seripa et al. 2005; Grimaldi et al. 2000). A potential limitation of our analysis is the use of a diagnosis of dementia syndrome instead of the dementia type. However, while the great majority of the persons affected by dementia are 80 years or older, classification of dementias in the very old remains a big challenge even at autopsy stage, where an improvement of diagnostic criteria is a matter of investigation (Neuropathology Group: Medical Research Council Cognitive Function and Aging Study 2001; Sinka et al. 2010). The association between pathological features of Alzheimer’s disease and dementia is stronger in younger old persons than in older persons (Savva et al. 2009), while mixed pathologies account for most dementia cases in very old persons (Jellinger and Attems 2010). Moreover, our study was able to detect only relatively high changes of the minor allele frequency of the three SNPs tested (10–12%), so we are not able to exclude that minor genetic variation from the explored SNPs can exist, even if in this case their contribution to a very frequent phenotype as dementia in the very old would be of limited impact.
When we analysed the same genetic data using the prospective approach, we found at first an association between the presence of TNF-α rs1799724 T-allele and the development of dementia, with a small effect (OR: 1.5). Moreover, we confirmed a role as risk modulator for the APOE-ε4 allele that in our longitudinal analysis clearly increased the risk for dementia development in the population (OR: 2.9). However, the TNF-α rs1799724 T-allele effect was not independent from APOE-ε4 presence, and to better dissect its contribution to incident dementia, an increase in the sample size is probably required to have a sufficient number of T-allele carriers without the APOE-ε4 allele (that in our population were 38 subjects only). Ravaglia et al. analysed in a younger Italian population IL-1β rs3087258 and incident dementia (any type and AD), with a follow-up of 4 years, and they found no association (Ravaglia et al. 2006). Our negative results on IL-1β rs3087258 polymorphism are in agreement with this study and our contribution reinforces the finding by Ravaglia et al. but in an older population.
In summary, we did not find an association between dementia status or severity and rs1800587, rs3087258 or rs1799724 in a well-described population of Italian very old people, while the longitudinal approach gave evidence of the role of the APOE-ε4 allele as a risk factor for dementia syndrome development also in the oldest olds.
Acknowledgements
We are grateful to all the patients who participated in this study that was supported by a grant from “Fondazione Italo Monzino” (Milan, Italy). L.P. is a recipient of a fellowship from “Golgi Cenci” Foundation, Abbiategrasso (Milan, Italy). We also thank Judith Baggott for English editing.
Contributor Information
Diego Albani, Phone: +39-02-39014594, FAX: +39-02-3546277, Email: diego.albani@marionegri.it.
Mauro Tettamanti, Email: mauro.tettamanti@marionegri.it.
Sara Batelli, Email: batelli@genzentrum.lmu.de.
Letizia Polito, Email: letizia.polito@marionegri.it.
Sabrina Dusi, Email: sabrina.dusi@istituto-besta.it.
Eleonora Ateri, Email: eleonora.ateri@marionegri.it.
Gianluigi Forloni, Email: gianluigi.forloni@marionegri.it.
Ugo Lucca, Email: ugo.lucca@marionegri.it.
References
- Albani D, Roiter I, Artuso V, Batelli S, Prato F, Pesaresi M, Galimberti D, Scarpini E, Bruni A, Franceschi M, Piras MR, Confaloni A, Forloni G. Presenilin-1 mutation E318G and familial Alzheimer's disease in the Italian population. Neurobiol Aging. 2007;28:1682–1688. doi: 10.1016/j.neurobiolaging.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: back to the future. Neuron. 2010;68:270–281. doi: 10.1016/j.neuron.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Bosco P, Guéant-Rodríguez RM, Anello G, Romano A, Namour B, Spada RS, Caraci F, Tringali G, Ferri R, Guéant JL. Association of IL-1 RN*2 allele and methionine synthase 2756 AA genotype with dementia severity of sporadic Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2004;75:1036–1038. doi: 10.1136/jnnp.2003.025866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabers NA, Nottet HS. Role of the pro-inflammatory cytokines TNF-alpha and IL-1beta in HIV-associated dementia. Eur J Clin Invest. 2006;36:447–458. doi: 10.1111/j.1365-2362.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H, Andersen-Ranberg K, Jeune B, Pedersen AN, Skinhøj P, Pedersen BK. A high plasma concentration of TNF-alpha is associated with dementia in centenarians. J Gerontol A Biol Sci Med Sci. 1999;54:M357–M364. doi: 10.1093/gerona/54.7.M357. [DOI] [PubMed] [Google Scholar]
- Caruso C, Lio D, Cavallone L, Franceschi C. Aging, longevity, inflammation, and cancer. Ann NY Acad Sci. 2004;1028:1–13. doi: 10.1196/annals.1322.001. [DOI] [PubMed] [Google Scholar]
- Cavallone L, Bonafè M, Olivieri F, Cardelli M, Marchegiani F, Giovagnetti S, Stasio G, Giampieri C, Mugianesi E, Stecconi R, Sciacca F, Grimaldi LM, Benedictis G, Lio D, Cederholm T, Persson M, Andersson P, Stenvinkel P, Nordfors L, Madden J, Vedin I, Wretlind B, Grimble RF, Palmblad J. Polymorphisms in cytokine genes influence long-term survival differently in elderly male and female patients. J Intern Med. 2007;262:215–223. doi: 10.1111/j.1365-2796.2007.01803.x. [DOI] [PubMed] [Google Scholar]
- Culpan D, MacGowan SH, Ford JM, Nicoll JA, Griffin WS, Dewar D, Cairns NJ, Hughes A, Kehoe PG, Wilcock GK. Tumour necrosis factor alpha gene polymorphisms and Alzheimer’s disease. Neurosci Lett. 2003;350:61–65. doi: 10.1016/S0304-3940(03)00854-1. [DOI] [PubMed] [Google Scholar]
- D’Ath P, Katona P, Mullan E, Evans S, Katona C. Screening, detection and management of depression in elderly primary care attenders. I: the acceptability and performance of the 15 item Geriatric Depression Scale (GDS15) and the development of short versions. Fam Pract. 1994;11:260–266. doi: 10.1093/fampra/11.3.260. [DOI] [PubMed] [Google Scholar]
- Dik MG, Jonker C, Hack CE, Smit JH, Comijs HC, Eikelenboom P. Serum inflammatory proteins and cognitive decline in older persons. Neurology. 2005;64:1371–1377. doi: 10.1212/01.WNL.0000158281.08946.68. [DOI] [PubMed] [Google Scholar]
- Dominici R, Cattaneo M, Malferrari G, Archi D, Mariani C, Grimaldi LM, Biunno I. Cloning and functional analysis of the allelic polymorphism in the transcription regulatory region of interleukin-1 alpha. Immunogenetics. 2002;54:82–86. doi: 10.1007/s00251-002-0445-9. [DOI] [PubMed] [Google Scholar]
- Ehl C, Kölsch H, Ptok U, Jessen F, Schmitz S, Frahnert C, Schlösser R, Rao ML, Maier W, Heun R. Association of an interleukin-1beta gene polymorphism at position-511 with Alzheimer’s disease. Int J Mol Med. 2003;11:235–238. [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Grimaldi LM, Casadei VM, Ferri C, Veglia F, Licastro F, Annoni G, Biunno I, Bellis G, Sorbi S, Mariani C, Canal N, Griffin WS, Franceschi M. Association of early-onset Alzheimer's disease with an interleukin-1alpha gene polymorphism. Ann Neurol. 2000;47:361–365. doi: 10.1002/1531-8249(200003)47:3<361::AID-ANA12>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Holmes C, El-Okl M, Williams AL, Cunningham C, Wilcockson D, Perry VH. Systemic infection, interleukin 1beta, and cognitive decline in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2003;74:788–789. doi: 10.1136/jnnp.74.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA, Attems J. Prevalence of dementia disorders in the oldest-old: an autopsy study. Acta Neuropathol. 2010;119:421–433. doi: 10.1007/s00401-010-0654-5. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. doi: 10.1093/geront/9.3_Part_1.179. [DOI] [PubMed] [Google Scholar]
- Licastro F, Porcellini E, Caruso C, Lio D, Corder EH. Genetic risk profiles for Alzheimer's disease: integration of APOE genotype and variants that up-regulate inflammation. Neurobiol Aging. 2007;11:1637–1643. doi: 10.1016/j.neurobiolaging.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Lucca U, Tettamanti M, Martelli P, Lucchelli F, Alberoni M, Spagnoli A. Spontaneous behaviour interview: from test performance to daily life-based Alzheimer patient evaluation (Abstract). The challenge of dementias. Edinburgh: The Lancet; 1996. p. 58. [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, Belle G, Fillenbaum G, Mellits ED, Clark C, the CERAD investigators The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Neuropathology Group: Medical Research Council Cognitive Function and Aging Study Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet. 2001;357:169–175. doi: 10.1016/S0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- Paganelli R, Iorio A, Patricelli L, Ripani F, Sparvieri E, Faricelli R, Iarlori C, Porreca E, Gioacchino M, Abate G. Proinflammatory cytokines in sera of elderly patients with dementia: levels in vascular injury are higher than those of mild-moderate Alzheimer's disease patients. Exp Gerontol. 2002;37:257–263. doi: 10.1016/S0531-5565(01)00191-7. [DOI] [PubMed] [Google Scholar]
- Perry RT, Collins JS, Wiener H, Acton R, Go RC. The role of TNF and its receptors in Alzheimer’s disease. Neurobiol Aging. 2001;22:873–883. doi: 10.1016/S0197-4580(01)00291-3. [DOI] [PubMed] [Google Scholar]
- Prasad KN, Nyati KK, Verma A, Rizwan A, Paliwal VK. Tumor necrosis factor-alpha polymorphisms and expression in Guillain–Barré syndrome. Hum Immunol. 2010;71:905–910. doi: 10.1016/j.humimm.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Ravaglia G, Paola F, Maioli F, Martelli M, Montesi F, Bastagli L, Bianchin M, Chiappelli M, Tumini E, Bolondi L, Licastro F. Interleukin-1beta and interleukin-6 gene polymorphisms as risk factors for AD: a prospective study. Exp Gerontol. 2006;41:85–92. doi: 10.1016/j.exger.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Rizzo MR, Abbatecola AM, Barbieri M, Vietri MT, Cioffi M, Grella R, Molinari A, Forsey R, Powell J, Paolisso G. Evidence for anti-inflammatory effects of combined administration of vitamin E and C in older persons with impaired fasting glucose: impact on insulin action. J Am Coll Nutr. 2008;27:505–511. doi: 10.1080/07315724.2008.10719732. [DOI] [PubMed] [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C, Medical Research Council Cognitive Function and Ageing Study Age, neuropathology, and dementia. N Engl J Med. 2009;360:2302–2309. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- Sciacca FL, Ferri C, Licastro F, Veglia F, Biunno I, Gavazzi A, Calabrese E, Martinelli Boneschi F, Sorbi S, Mariani C, Franceschi M, Grimaldi LM. Interleukin-1B polymorphism is associated with age at onset of Alzheimer’s disease. Neurobiol Aging. 2003;24:927–931. doi: 10.1016/S0197-4580(03)00011-3. [DOI] [PubMed] [Google Scholar]
- Seripa D, Matera MG, Dal Forno G, Gravina C, Masullo C, Daniele A, Binetti G, Bonvicini C, Squitti R, Palermo MT, Davis DG, Antuono P, Wekstein DR, Dobrina A, Gennarelli M, Fazio VM. Genotypes and haplotypes in the IL-1 gene cluster: analysis of two genetically and diagnostically distinct groups of Alzheimer patients. Neurobiol Aging. 2005;26:455–464. doi: 10.1016/j.neurobiolaging.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Serretti A, Olgiati P, Politis A, Malitas P, Albani D, Dusi S, Polito L, Mauro S, Zisaki A, Piperi C, Liappas I, Stamouli E, Mailis A, Atti AR, Morri M, Ujkaj M, Batelli S, Forloni G, Soldatos CR, Papadimitriou GN, Ronchi D, Kalofoutis A. Lack of association between interleukin-1 alpha rs1800587 polymorphism and Alzheimer's disease in two Independent European samples. J Alzheimers Dis. 2009;16:181–187. doi: 10.3233/JAD-2009-0946. [DOI] [PubMed] [Google Scholar]
- Sinka L, Kövari E, Gold G, Hof PR, Herrmann FR, Bouras C, Giannakopoulos P. Small vascular and Alzheimer disease-related pathologic determinants of dementia in the oldest-old. J Neuropathol Exp Neurol. 2010;69:1247–1255. doi: 10.1097/NEN.0b013e3181ffc3b9. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedde A, Putignano AL, Nacmias B, Bagnoli S, Cellini E, Sorbi S. Lack of association between TNF-alpha polymorphisms and Alzheimer's disease in an Italian cohort. Neurosci Lett. 2008;446:139–142. doi: 10.1016/j.neulet.2008.09.044. [DOI] [PubMed] [Google Scholar]
- Terreni L, Fogliarino S, Quadri P, Ruggieri RM, Piccoli F, Tettamanti M, Lucca U, Forloni G. Tumor necrosis factor alpha polymorphism C-850T is not associated with Alzheimer's disease and vascular dementia in an Italian population. Neurosci Lett. 2003;344:135–137. doi: 10.1016/S0304-3940(03)00434-8. [DOI] [PubMed] [Google Scholar]
- Tettamanti M, Garrì MT, Nobili A, Riva E, Lucca U. Low folate and the risk of cognitive and functional deficits in the very old: the Monzino 80-plus study. J Am Coll Nutr. 2006;25:502–508. doi: 10.1080/07315724.2006.10719565. [DOI] [PubMed] [Google Scholar]
- Tonelli LH, Postolache TT. Tumor necrosis factor alpha, interleukin-1 beta, interleukin-6 and major histocompatibility complex molecules in the normal brain and after peripheral immune challenge. Neurol Res. 2005;27:679–684. doi: 10.1179/016164105X49463. [DOI] [PubMed] [Google Scholar]
- Bodegom D, May L, Meij HJ, Westendorp RG. Regulation of human life histories: the role of the inflammatory host response. Ann NY Acad Sci. 2007;1100:84–97. doi: 10.1196/annals.1395.007. [DOI] [PubMed] [Google Scholar]
- Exel E, Craen AJ, Remarque EJ, Gussekloo J, Houx P, Bootsma-van der Wiel A, Frölich M, Macfarlane PW, Blauw GJ, Westendorp RG. Interaction of atherosclerosis and inflammation in elderly subjects with poor cognitive function. Neurology. 2003;61:1695–1701. doi: 10.1212/01.wnl.0000098877.07653.7c. [DOI] [PubMed] [Google Scholar]
- Wang WF, Liao YC, Wu SL, Tsai FJ, Lee CC, Hua CS. Association of interleukin-I beta and receptor antagonist gene polymorphisms with late onset Alzheimer’s disease in Taiwan Chinese. Eur J Neurol. 2005;12:609–613. doi: 10.1111/j.1468-1331.2005.01033.x. [DOI] [PubMed] [Google Scholar]
- Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59:371–378. doi: 10.1212/wnl.59.3.371. [DOI] [PubMed] [Google Scholar]
- Wehr H, Bednarska-Makaruk M, Łojkowska W, Graban A, Hoffman-Zacharska D, Kuczyńska-Zardzewiały A, Mrugała J, Rodo M, Bochyńska A, Sułek A, Ryglewicz D. Differences in risk factors for dementia with neurodegenerative traits and for vascular dementia. Dement Geriatr Cogn Disord. 2006;22:1–7. doi: 10.1159/000092845. [DOI] [PubMed] [Google Scholar]
- Wen AQ, Wang J, Feng K, Zhu PF, Wang ZG, Jiang JX. Effects of haplotypes in the interleukin 1beta promoter on lipopolysaccharide-induced interleukin 1beta expression. Shock. 2006;26:25–30. doi: 10.1097/01.shk.0000223125.56888.c7. [DOI] [PubMed] [Google Scholar]