Abstract
In a previous cross-sectional study on baseline data, we demonstrated that the volume of brain white matter hyperintensities (WMH) in the splenium of corpus callosum (SCC) predicted the current mobility function of older persons. The primary aim of this follow-up study was to determine the relation of WMH volume change in SCC (SCC-∆WMH) with change in mobility measures. A secondary aim was to characterize the global and regional progression of WMH. Mobility function and WMH burden were evaluated at baseline and at 2 years in 77 community-dwelling individuals (baseline age, 82 ± 4). Regional WMH in SCC, as well as genu and body of corpus callosum, subregions of corona radiata, and superior longitudinal fasciculus were determined using a white matter parcellation atlas. The total WMH volume increased 3.3 ± 3.5 ml/year, mainly through enlargement. Significant WMH increases were observed in all selected regions, particularly within the corona radiata. While at baseline and follow-up we observed correlations between WMH burden and several measures of mobility, longitudinal change correlated only with change in chair rise (CR). SCC-∆WMH showed the highest correlation (r = −0.413, p = 0.0002) and was the best regional predictor of CR decline (OR = 1.5, r2 = 0.3). The SCC-∆WMH was more than five times larger in the CR-decline group compared to the no-decline group (p = 0.0003). The SCC-∆WMH (top quartile) showed a higher sensitivity/specificity for CR decline compared to change in total WMH, 63/88% versus 52/84%, respectively. The findings suggest that accrual of WMHs in posterior areas of the brain supporting inter-hemispheric integration and processing of visual–spatial information is a mechanism contributing to age-related mobility deterioration.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-011-9242-4) contains supplementary material, which is available to authorized users.
Keywords: Aging, Mobility, Brain, White matter hyperintensities, Splenium of corpus callosum, Magnetic resonance imaging
Introduction
Gait and balance abnormalities in elderly individuals often lead to falls and predict further functional decline (Guralnik et al. 1995). Identifiable causes of mobility impairment and falls include medications, deconditioning, arthritis, and pain (Tinetti and Kumar 2010). However, a significant proportion of mobility difficulties occur in otherwise healthy individuals with no obvious cause. Cross-sectional (Masdeu et al. 1989; Baloh et al. 1995; Camicioli et al. 1999; Guttmann et al. 2000; Sachdev et al. 2005; Baezner et al. 2008; Franch et al. 2009) and longitudinal studies (Whitman et al. 2001; Baloh et al. 2003; Wolfson et al. 2005; ten Dam et al. 2007; Silbert et al. 2008; Srikanth et al. 2009) demonstrated an association of total brain white matter hyperintensity (tWMH) volume with mobility impairment as well as other geriatric syndromes (Kuo and Lipsitz 2004; Wakefield et al. 2010). WMHs represent areas of compromised neural connectivity due to demyelination, gliosis, and/or neuronal degeneration (Fazekas et al. 1993; Schmidt et al. 2004) likely caused by ischemia (Pantoni and Garcia 1997) and/or abnormal blood–brain barrier permeability (Wardlaw et al. 2003). Although it is reasonable to believe that tissue damage represented by WMHs compromise sensory motor connectivity/integration supporting mobility (Masdeu et al. 1989; Baloh et al. 2003), little is known about specific tracts involved and the role of WMH distribution in impaired mobility. Both cross-sectional (Whitman et al. 2001; Srikanth et al. 2010) and longitudinal (Silbert et al. 2008) studies showed an association of periventricular WMHs with mobility impairment. These findings are consistent with the presence in these areas of neural tracts supporting motor control, e.g., sensory input or motor output through corona radiata or interhemisperic integration through corpus callosum. In a prior study, we demonstrated that posterior periventricular WMH were associated with impaired mobility (Benson et al. 2002). Consistent with that observation, cross-sectional analysis on baseline data from the current study demonstrated that WMH burden in the splenium of the corpus callosum is the best regional predictor of poor mobility performance (Moscufo et al. 2011). The splenium of the corpus callosum contains commissural neural tracts important for the integration of visual and spatial information. Due to the importance of timely visuospatial processing in motor as well as cognitive functions, morphological changes in this area, which may reflect diminished connectivity/impaired processing, could be a particularly sensitive indicator of functional impairment. Thus, the primary aim of this follow-up analysis was to test/confirm the association of WMH in the splenium of the corpus callosum with mobility impairment on longitudinal data (change versus change). A secondary aim was to characterize WMH progression in the study cohort.
Methods
Subjects
Participants for this longitudinal study were recruited in and around Hartford, Connecticut, from senior centers, retirement communities, and geriatrician referrals and through advertisements in local newspapers and newsletters. Details on recruitment methods and inclusion/exclusion criteria were described previously (Moscufo et al. 2011). Subjects had to walk independently (inclusion) and were excluded if they had diseases compromising mobility or had contraindications for magnetic resonance imaging (MRI). Of the 99 original subjects ≥75-years old, 77 returned for follow-up assessments at 2 years (1.9 ± 0.4 years). Twenty-two individuals (22%) did not return due to pacemaker implant (n = 3), refusal to participate (n = 5), MRI refusal (n = 3), relocation (n = 3), brain tumor (n = 1), myocardial ischemic attack (n = 1), or deceased (n = 6). Ethics committees of participating institutions approved the study.
Mobility assessments
The subject’s ability to perform tasks reflecting mobility independence in daily activities was assessed with Tinetti Balance and Gait scores (Tinetti 1986) and with the three timed components of the Short Physical Performance Battery (Guralnik et al. 1994), i.e., standing balance, rise from chair to stand (CR), and walking speed. Self-paced maximum walking velocity, usual walking velocity, stair descent time, and turn time were also measured during the laboratory mobility testing and included in the analysis (Electronic supplementary materials (ESM) Tables 1 and 2).
Brain MRI
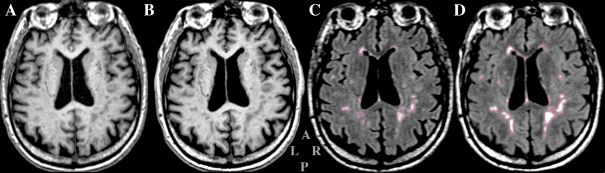
Brain images were acquired on a 3-T scanner (Siemens-Allegra, Erlangen, Germany) using three MRI sequences: T1-weighted magnetization prepared rapid gradient echo (MPRAGE: repetition time (TR)/echo time (TE) = 2,500/2.74 ms, inversion time (TI) = 900 ms, slice thickness = 1 mm, 176 contiguous axial slices) (Fig. 1A and B); 3D Fast Spin-Echo (T2: TR/TE = 2,500/353 ms, slice thickness = 1 mm, 176-contiguous sagittal slices); and fluid-attenuated inversion recovery (FLAIR: TR/TE = 6,000/353 ms, TI = 2,200 ms, slice thickness = 1.3 mm, 128 contiguous sagittal slices) (Fig. 1C and D). Pre-processing included correction of magnetic field-related signal inhomogeneities (Sled et al. 1998) and affine registration (Jenkinson and Smith 2001) of FLAIR and T2 to MPRAGE.
Fig. 1.
Example of magnetic resonance images analyzed in the study. A, B A slice from the T1-weighted MPRAGE series. C, D The same slice in the T2-weighted FLAIR series. In order to show the same anatomical level of the brain, the MR series acquired at baseline (A, C) were aligned to the series acquired at follow-up (B, D). Areas of WMH are outlined in red in the FLAIR images (C, D). Gray letters in the lower middle indicate: anterior (A), posterior (P), left (L), and right (R)
Segmentation of WMH
To identify areas of WMH in FLAIR images, we used the method previously described (Moscufo et al. 2011). We used both MPRAGE and FLAIR series as inputs in the segmentation module of 3D Slicer (Pohl et al. 2004) and the MPRAGE series as input for segmentation in FreeSurfer (Fischl et al. 2002). The two resulting outputs were combined and only WMH regions larger than 3 pixels and with at least 10% spatial overlap between the two methods were retained in the final map (example in Fig. 1c, d). These maps were reviewed for accuracy and manually corrected if necessary. To account for head size variability, total WMH volumes (milliliters, mL) were expressed as percent of the intracranial cavity volume. Frequency and anatomical distribution of WMH were determined as previously described (Moscufo et al. 2011).
Regional WMH burden
We used the brain white matter parcellation atlas (Mori et al. 2008) for automated and objective measurement of regional WMH burden. The atlas was spatially normalized to each subject’s brain at baseline (Moscufo et al. 2011) and then aligned to the same subject’s brain at follow-up by applying the linear and nonlinear transforms obtained from registering the baseline MPRAGE to follow-up MPRAGE images. The selected regions of interest were: corpus callosum—genu, body, splenium (GCC, BCC, SCC); corona radiata—anterior, superior, posterior (ACR, SCR, PCR), and superior longitudinal fasciculus (SLF). These regions contain periventricular fiber tracts that are relevant for motor control. The genu of corpus callosum contains commissural fibers connecting the frontal and prefrontal cortices important for movement planning. The body of corpus callosum contains commissural fibers connecting pre-motor, motor, and somatosensory areas. The splenium of corpus callosum contains fibers connecting the parietal, temporal, and occipital somatosensory and visual cortices sensing body position. The anterior, superior, and posterior parts of the corona radiata contain projection fibers that form the internal capsule traveling to and from the cerebral cortex and include the efferent fibers originating in the motor cortex, giving rise to the corticospinal tract. The superior longitudinal fasciculus contains long association fibers connecting the occipital cortex, mostly visual, to the frontal and prefrontal cortices. Other relevant regions were excluded due to none-to-negligible presence of WMHs (brainstem, cerebellum, corticospinal tract, internal capsule). The WMH burden in each region of interest was expressed as percent of the region’s volume.
WMH segmentation: accuracy and reproducibility
We validated the semi-automated WMH segmentation method by comparison to a “gold standard” obtained by manual outlining of WMHs on FLAIR images of ten randomly selected study subjects by an expert neurologist (ML). Intra-class correlation coefficient was 0.99 (p = 5 × 10−9) and the mean WMH pixel overlap was 82.9 ± 6.6% (min–max = 71.3–92.2). Reproducibility was assessed on ten subjects who participated in a scan–rescan experiment consisting of two MR brain image acquisitions in the same day >1 h apart. The resulting scans were de-identified and processed by an operator blinded to subject identity. For each subject, the two WMH segmentation outputs were compared to assess reproducibility. Intra-class correlation coefficient was 0.99. The percent of pixels reproducibly classified as WMH were 77.5 ± 12.3 (95% CI = 68.7–86.3). Mean percent and standard error in tWMH measurement was 0.02 ± 0.20 (% intracranial cavity volume).
Statistics
Statistical analysis was performed using SPSS v.13 application (SPSS Inc., Chicago, Illinois). For each mobility test, we assigned subjects to two categories based on change in performance at follow-up, i.e., decline (=0) or no-decline (=1) relative to baseline. In this report, we indicate change in WMH burden ([follow-up WMH] − [baseline WMH]) as ∆WMH. We used non-parametric Wilcoxon test for paired sample comparisons and Mann–Whitney for non-parametric independent samples comparison. Associations between mobility decline (categorical variable, i.e., decline versus no decline) and ∆WMH (continuous variable) were determined through Spearman correlation (see also footnote in Table 3), and we used family-wise Bonferroni criterion to correct for multiple measurements when correlation tests included the seven regions of interest. We further analyzed associations through logistic regression with ∆WMH (continuous variable) as predictor of mobility decline (categorical variable, i.e., decline versus no decline), controlling for age, gender, mini-mental state score (MMSE), and body mass index (BMI) as confounders, and calculated odds ratios (OR) and (pseudo-)R2 (Nagelkerke). To determine sensitivity, specificity, and positive predictive values of SCC-∆WMH and t∆WMH for mobility performance decline, we divided the cohort into quartiles. Sensitivity (true positive fraction) = true positive/[true positive + false negative]; specificity (true negative fraction) = true negative/[true negative + false positive]; positive predictive value = true positive/[true positive + false positive]. Threshold of statistical significance was p ≤ 0.05 (two-tailed).
Table 3.
Correlation between changes in WMH burden and changes in mobility
| N = 77 | Balance standinga | Chair risea | Walk speeda | Tinetti gaita | Tinetti balancea |
|---|---|---|---|---|---|
| Age | −0.145 (>0.1) | −0.055 (>0.1) | −0.119 (>0.1) | −0.138 (>0.1) | −0.057 (>0.1) |
| BMI | 0.036 (>0.1) | −0.012 (>0.1) | 0.039 (>0.1) | 0.007 (>0.1) | −0.108 (>0.1) |
| Gender | −0.003 (>0.1) | −0.040 (>0.1) | −0.158 (>0.1) | 0.257 (0.026) | 0.039 (>0.1) |
| tWMHb | −0.045 (>0.1) | −0.381 (0.001) | −0.053 (>0.1) | −0.066 (>0.1) | 0.001 (>0.1) |
| GCC-WMHb | −0.083 (>0.1) | −0.297 (0.009) | 0.03 (>0.1) | 0.09 (>0.1) | 0.088 (>0.1) |
| BCC-WMHb | −0.053 (>0.1) | −0.384 (0.001)c | 0.019 (>0.1) | −0.212 (0.068) | −0.031 (>0.1) |
| SCC-WMHb | −0.228 (0.046) | −0.413 (0.0002)c | 0.002 (>0.1) | −0.116 (>0.1) | −0.087 (>0.1) |
| ACR-WMHb | 0.032 (>0.1) | −0.186 (>0.1) | −0.033 (>0.1) | −0.01 (>0.1) | 0.162 (>0.1) |
| SCR-WMHb | −0.056 (>0.1) | −0.300 (0.008) | −0.105 (>0.1) | −0.119 (>0.1) | −0.074 (>0.1) |
| PCR-WMHb | 0.004 (>0.1) | −0.247 (0.031) | 0.056 (>0.1) | 0.006 (>0.1) | −0.028 (>0.1) |
| SLF-WMHb | 0.04 (>0.1) | −0.140 (>0.1) | −0.095 (>0.1) | 0.033 (>0.1) | −0.087 (>0.1) |
Values represent Spearman’s correlation rho (p value). Significant correlations are highlighted in bold. Categorical variables: gender (men = 0, women = 1) and the mobility measures. All other variables are continuous. No significant correlations were observed with other mobility tests, i.e., usual walking velocity, self-paced maximum walking velocity, stair time, and turn time, and therefore are not shown
tWMH total WMH burden. Regional burdens: in corpus callosum genu (GCC-WMH), body (BCC-WMH), and splenium (SCC-WMH); in corona radiata anterior (ACR-WMH), superior (SCR-WMH), and posterior (PCR-WMH); and in superior longitudinal fasciculus (SLF-WMH)
aMobility measures (decline = 0, no-decline = 1)
bDifference = [follow-up − baseline]
cRemained significant after Bonferroni correction for multiple correlations (threshold p <0.007)
Results
Cohort characteristics
Demographic and mobility characteristics of the 77 subjects that returned at follow-up are illustrated in Table 1. Small declines in mobility performance along with inter-subject increased variability were noted. Percentages of subjects showing performance decline were 25% in balance standing, 24% in chair rise, 17% in walking speed, 22% in Tinetti gait, 46% in Tinetti balance, 44% in usual walking velocity, 47% in self-paced maximum velocity, 76% in stair descent time, and 85% in turn time. Both basic and instrumental activities of daily living were not significantly different at follow-up, and changes showed no association with total and regional ∆WMH. The 22 subjects who did not return were older (84 ± 4 versus 82 ± 4 years, p = 0.018) and had lower SPPB mobility score (p = 0.015); no other significant differences were observed. Within the non-returning group, the six deceased subjects were older (88 ± 2 versus 83 ± 4 years, p = 0.018), but no significant differences in tWMH burden or other measures were found.
Table 1.
Study subjects’ characteristics
| N = 77 | Baseline | Follow-up | Change | Mobility decline % of subjects |
|---|---|---|---|---|
| Age (years) | 82 ± 4 (75, 89) | 84 ± 3.9 (77, 92) | 1.9 ± 0.2 (1.6, 2.7) | NA |
| Women (%) | 46 (60%) | 46 (60%) | NA | NA |
| HPTN (%) | 55 (71%) | 54 (70%) | NA | NA |
| BMI | 26.6 ± 4.6 (18.1, 41.7) | 26.3 ± 4.5 (17.4, 40.5)* | −0.3 ± 1.1 (−3.9, 2.2) | NA |
| MMSE | 28.6 ± 1.2 (24, 30) | 27.6 ± 1.7 (21, 30)** | −1.0 ± 1.6 (−5, 3) | NA |
| Standing balancea | 3.5 ± 0.9 (0, 4) | 3.3 ± 1.0 (1, 4) | −0.1 ± 1.2 (−3, 4) | 25% (20/77) |
| Chair risea | 2.3 ± 1.1 (0, 4) | 2.4 ± 1.2 (0, 4) | 0.1 ± 1.0 (−3, 2) | 24% (19/77) |
| Walk speeda | 3.5 ± 0.7 (1, 4) | 3.4 ± 0.8 (1, 4) | −0.05 ± 0.6 (−2, 2) | 17% (13/77) |
| Tinetti gaitb | 11.2 ± 1.2 (5, 12) | 11.2 ± 1.3 (6, 12) | 0.01 ± 1.1 (−4, 4) | 22% (17/77) |
| Tinetti balanceb | 15.0 ± 1.4 (9, 16) | 14.5 ± 1.8 (10, 16)* | −0.57 ± 1.4 (−6, 3) | 46% (35/77) |
Values represent the mean ± SD (min, max) or percent
HPTN hypertension, BMI body mass index, MMSE mini-mental state exam, NA not applicable
*p < 0.05; **p < 0.0001 (Wilcoxon, paired samples)
aScores; components of the short physical performance battery (Guralnik et al. 1994)
bScores; mobility assessment as described in Tinetti (1986)
Changes in WMH burden
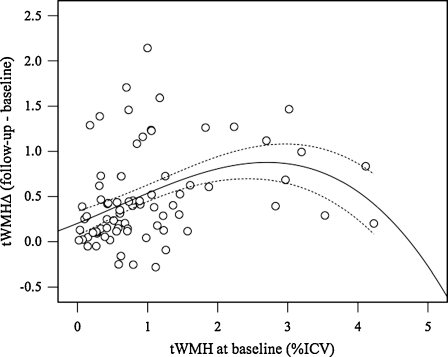
On average, tWMH volume increased from 1% at baseline to 1.5% at 2 years (Table 2), corresponding to 13.9 (±12.8) and 20.4 (±16.2) mL, respectively. Average rate of change was 0.2 ± 0.2% per year (or 3.3 ± 3.5 mL/year). The tWMH increased in 91% of subjects. We observed a correlation between burden at baseline and its increase at follow-up (r = 0.393, p = 0.0004) with a plateau effect apparent at baseline tWMH >2% (Fig. 2). The number of WMH islands did not change significantly (average per subject, 29 at baseline versus 27 at follow-up, p > 0.05). Average volume of WMH islands increased from 0.54 to 0.85 mL (p < 10−5). Regional WMH increases (Table 1) were highest in corona radiata (posterior > superior > anterior) and, as percent of total burden, were 21.0 ± 8.0%, 9.3 ± 4.0%, and 2.5 ± 2.5% in corona radiata, corpus callosum, and superior longitudinal fasciculi, respectively. On average, nearly half (47%) of total new WMH arose in these three brain regions.
Table 2.
Longitudinal changes in total and regional WMH burden
| N = 77 | Baseline | Follow-up | Change |
|---|---|---|---|
| tWMH | 1.0 ± 0.9 (0.02, 4.2) | 1.5 ± 1.2 (0.04, 4.9)* | 0.4 ± 0.5 (−0.2, 2.1) |
| GCC-WMH | 6.0 ± 5.2 (0.1, 25.2) | 9.0 ± 5.8 (0.4, 23.8)* | 3.0 ± 3.3 (−2.6, 12.6) |
| BCC-WMH | 6.3 ± 6.3 (0, 31) | 9.5 ± 7.0 (0.3, 32.2)* | 3.2 ± 3.8 (−8.1, 14.4) |
| SCC-WMH | 1.9 ± 2.7 (0, 12.3) | 3.7 ± 4.5 (0, 19.8)* | 1.7 ± 3.3 (−3.4, 16.3) |
| ACR-WMH | 8.4 ± 8.9 (0, 53.2) | 13.4 ± 10.9 (0, 53.5)* | 5.0 ± 6.3 (−4.8, 30.9) |
| SCR-WMH | 8.1 ± 10.9 (0–60.8) | 14.7 ± 14.6 (0.1, 63.6)* | 6.5 ± 7.5 (−3.6, 40.2) |
| PCR-WMH | 23.5 ± 21.3 (0, 84) | 33.6 ± 24.4 (0, 91.5)* | 10.1 ± 13.0 (−15.4, 71.9) |
| SLF-WMH | 3.5 ± 6.8 (0, 35.4) | 6.7 ± 10.4 (0, 62.4)* | 3.2 ± 5.5 (−0.6, 31.6) |
Values represent percent: mean ± SD (min, max)
tWMH total WMH burden. Regional WMH burdens: in corpus callosum genu (GCC-WMH), body (BCC-WMH), and splenium (SCC-WMH); in corona radiata anterior (ACR-WMH), superior (SCR-WMH), and posterior (PCR-WMH); and in superior longitudinal fasciculus (SLF-WMH)
*p < 0.0001 (Wilcoxon, paired samples)
Fig. 2.
Plot showing the change in total WMH at follow-up (t∆WMH, y-axis) versus the amount of tWMH at baseline (x-axis). Values represent percent of intracranial cavity volume (ICV). Continuous and dashed lines represent the cubic fit line and 95% confidence intervals of the mean, respectively
Correlation between mobility and WMH
Cross-sectional analyses on baseline and follow-up data confirmed the association of WMH burden with mobility performance. The associations were stronger at baseline compared with the follow-up (ESM Tables 1 and 2). At both assessments, tWMH negatively correlated with Tinetti gait and SCC-WMH negatively correlated with walk score, Tinetti gait, Tinetti balance, self-paced maximum velocity, and usual velocity. Results of correlation analysis on longitudinal data, i.e., ∆WMH versus mobility changes, are shown in Table 3. In this analysis, t∆WMH was significantly associated only with decline in chair rise (r = −0.381, p = 0.001). Significant correlation between decline in chair rise and GCC-∆WMH (r = −0.297, p = 0.009), BCC-∆WMH (r = −0.384, p = 0.001), SCC-∆WMH (r = −0.413, p = 0.0002), SCR-∆WMH (r = −0.300, p = 0.008), and PCR-∆WMH (r = −0.247, p = 0.031) were also observed (Table 3). After Bonferroni correction, only associations with BCC-∆WMH and SCC-∆WMH remained significant. Associations were independent of age, gender, BMI, and MMSE. SCC-∆WMH showed the strongest correlation, higher than that with t∆WMH. Decline in standing balance was also weakly associated with SCC-∆WMH (r = −0.228, p = 0.046), but the association was no longer significant after Bonferroni correction. Men were more likely to show decline in the Tinetti gait test (r = 0.257, p = 0.026). There was no significant association of mobility decline with age and BMI (Table 3). Subjects with lower MMSE score at follow-up were more likely to show a reduced performance in the CR test (r = 0.252, p = 0.029). We performed group analysis to compare WMH burden between chair rise-decliners compared to non-decliners. At baseline, the decline group had higher tWMH volume compared to the no-decline group, but the difference was not statistically significant (Mann–Whitney p = 0.070). PCR-WMH was the only regional burden significantly different (p = 0.050) between the groups. At follow-up, group differences in WMH become significant: tWMH (p = 0.009), BCC-WMH (p = 0.02), SCC-WMH (p = 0.005), ACR-WMH (p = 0.045), SCR-WMH (p = 0.038), and PCR-WMH (p = 0.018). There were no significant differences in age and BMI. Thus, the observed change-versus-change correlations above are driven by the larger WMH increase in the subjects showing chair rise decline, particularly in the SCC.
Larger WMH accrual in SCC predicts CR performance decline
Univariate logistic regression showed that decline in chair rise was significantly predicted by t∆WMH (r2 = 0.229), GCC-∆WMH (r2 = 0.110), BCC-∆WMH (r2 = 0.273), and SCC-∆WMH (r2 = 0.317). SCC-∆WMH explains nearly 32% of the outcome variability, the highest among all the ∆WMH variables. When these variables were entered together using a forward stepwise method, only SCC-∆WMH was retained in the model (OR = 1.5, 95% CI = 1.2–1.9, p = 0.001). Therefore, among the ∆WMH variables, accrual of WMH within the splenium of the corpus callosum best predicted chair rise decline, with a 1.5-fold higher probability of decline for every 1% increase in SCC-∆WMH.
The SCC-∆WMH demonstrated a higher positive predictive value than t∆WMH for chair rise decline in the group with the largest WMH accrual (top quartile), i.e., 63% versus 52% of subjects showed reduced performance. Sensitivity/specificity values (top quartile versus three lower quartiles) of SCC-∆WMH were also higher than t∆WMH, 63%/88% versus 53%/84%, respectively. All together, the above results indicate that SCC-∆WMH is a good predictor of declining mobility, better than t∆WMH.
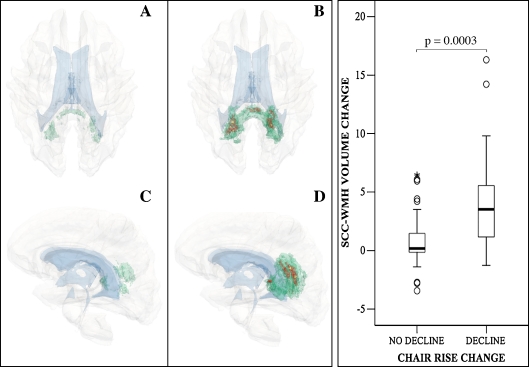
The spatial location and frequency of new WMHs within the SCC are illustrated in Fig. 3. New WMHs occur in a larger area (panels B, D versus A, C) and occur with higher frequency in the decline group (up to 37%) compared to the non-decliners (up to 12%). The SCC-∆WMH is greater than fivefold larger in the decline group compared to the no-decline group (4.5 ± 4.7% versus 0.8 ± 2.0%, p = 0.0003; Fig. 3, boxplots). Additional plots are reported as supplementary data (ESM boxplots).
Fig. 3.
Accrual of WMH in the splenium of corpus callosum (SCC-WMH) and decline in chair rise mobility test. Three-dimensional models in A–D show the location and frequency range of new WMH in the no-decline (A, C) compared to the decline group (B, D). For clarity, models were filtered to show only new WMHs within the SCC region. Green indicates the location of new WMH present in up to 12% of subjects (A–D); red indicates the location of new WMH present in 13–24% of subjects (B, D); small areas in yellow indicate the location of new WMH present in 25–37% of subjects (b, d). View is from top in A, B and from left in C, D. Brain is transparent gray, lateral ventricles shown in light blue. Boxplots in the right panel show the range of change of SCC-WMH burden (y-axis) in the two groups indicated in the x-axis. Change in SCC-WMH was calculated as: [follow-up burden] − [baseline burden]. Thick horizontal line in the boxes indicates the group median. Circles, outliers; star, extreme outliers. The statistical significance (Mann–Whitney group comparison) of the difference is shown on top
Discussion
We assessed the relation between change in mobility in older people and change in total and regional lesion burden in brain white matter over 2 years. Our results highlighted the correlation between increased white matter lesion burden within the splenium of the corpus callosum and reduced mobility. The finding confirmed previous observations that periventricular damage in the posterior part of the brain (Benson et al. 2002; Wolfson et al. 2005) and particularly in the callosal splenium (Moscufo et al. 2011) is important in mobility deterioration. The tWMH increased in 91% of the subjects, similar to other studies (Sachdev et al. 2007; Silbert et al. 2008), with a larger increase in subjects with higher tWMH at baseline (Whitman et al. 2001; Taylor et al. 2003; Sachdev et al. 2007; Silbert et al. 2008), but with a “threshold effect” as the WMH accrual tended to plateau in the subjects with highest tWMH burden. WMHs show periventricular distribution, as previously reported (Whitman et al. 2001; Benson et al. 2002; Wen and Sachdev 2004; Sachdev et al. 2007; Silbert et al. 2008; Moscufo et al. 2011). The rate of annual increase is similar to that reported in (Silbert et al. 2008), but higher than those from others (Taylor et al. 2003; Wolfson et al. 2005; Sachdev et al. 2007), although methodological differences make a comparison difficult. The observation of a decrease in number and increase in size of discrete WMHs supports the concept that tWMH increase is due to the expansion of preexisting lesions (Schmidt et al. 2003; DeCarli et al. 2005; Sachdev et al. 2007), and for the vast majority of subjects, the underlying process once established continues. Mechanisms involving astrocytes activated by the abnormal presence of plasma proteins caused by the breakdown of the blood–brain barrier (Baloh and Vinters 1995) and/or ischemic damage (Pantoni and Garcia 1997) have been suggested for the expansion of WMH to periventricular confluence (Schmidt et al. 2003; DeCarli et al. 2005).
Consistent with our previous cross-sectional analysis (Moscufo et al. 2011), among the regions of interest analyzed, most of the accrual occurs in the corona radiata, especially posteriorly, followed by corpus callosum, particularly the genu and body, and in the superior longitudinal fasciculi. These regions contain almost half of the newly occurring WMH. Although WMH accrual in multiple regions correlated with performance decline in chair rise, SCC-∆WMH was the best regional predictor explaining almost a third of the variability, better also than t∆WMH. Thus, SCC, while being the region least affected by accrual of new WMH, is the most strongly associated with compromised mobility. This is consistent with our previous cross-sectional study (Moscufo et al. 2011) and with the observation that parieto-occipital WMHs are the most specific predictor of mobility impairment (Benson et al. 2002).
In the present study, we analyzed mobility data obtained from several widely used tests (Tinetti 1986; Guralnik et al. 1994) as well as others. While we confirmed the association of WMH with various mobility tests in cross-sectional analyses at both baseline and follow-up, in longitudinal analysis on WMH change versus mobility change, we found significant association only with the chair rise mobility test. While lacking a clear explanation, we believe that the absence of association with changes in other mobility tests is related to the nonlinear nature of the latter, which limits their adequacy in assessing change over a relatively short time, to differences in complexity between motor tasks, as well as to individual variability in postural and gait compensatory adaptation. Future analyses on changes assessed over a longer interval on this cohort will help clarify this aspect.
The timed chair rise test measures muscle strength, balance, and coordination in response to visual information. The observed relationship of decreased performance with WMH progression in SCC suggests a mechanism by which damage to local neural pathways and associated reduced connectivity may slow processing/integration of visual–spatial information, thus increasing the time of motor response. However, this remains a speculation that will require direct testing in future work. The sensitivity of mobility to damage in the splenium that we observed in both cross-sectional and longitudinal analyses may be related to the increased reliance on visual inputs for postural control to compensate for reduced proprioception attendant with aging (Pyykko et al. 1990; Borger et al. 1999). Mechanisms involving damage in other tracts (Srikanth et al. 2010) and microstructural non-WMH brain abnormalities (Rosano et al. 2008) are also likely.
Our study is limited by the nonlinearity of the mobility tests, which may reduce their appropriateness for assessing changes in performance over short periods of time. We acknowledge possible measurement errors associated with image processing and analysis and limits to the quantitative representation of regional brain maps and are therefore cautious in data interpretation. Nevertheless, we believe that our approach was effective and objective in carrying out the study. The lack of diffusion data is another limitation, and we are aware of the need to assess the microstructural integrity of the regions we analyzed and their relationship with mobility decline in future studies.
In summary, the results of this longitudinal study suggest an important role for SCC-WMH accrual in diminished mobility performance. The increasing reliance on visual and/or spatial inputs during motor performance in normal aging might explain why this function is particularly sensitive to damage within the SCC. Our findings contribute to a better understanding of the neurological underpinnings and possible mechanisms of a complex and multifactorial process leading to impairment of mobility.
Electronic supplementary materials
Boxplots show the distribution of WMH volume changes (y-axis) in the two chair rise mobility groups (decline and no-decline, x-axis). The WMH variables shown are those that, in addition to the splenium of corpus callosum, showed significant correlation with decline in the chair rise test (see Table 3). Change was calculated as: [WMH burden at follow-up] − [WMH burden at baseline] (see also “Methods”). Thick horizontal line in the boxes indicates the group median. Circles, outliers; star, extreme outliers. The statistical significance of the group difference (Mann–Whitney group comparison test) is shown on top (JPEG 9 kb)
High resolution image file. (TIFF 1427 kb)
(DOC 89 kb)
Acknowledgments
Study supported by the National Institute on Aging—AG022092 (LW) and AG022092-01A1S1 (NM)—University of Connecticut Health Center General Clinical Research Center Grant M01 RR06192; NIH 5 P41 RR13218. We wish to thank Istvan Csapo for his expert advice on image analysis and method validation, Yang Duang for expert neuroradiological assistance, Antal Kucsai for assistance with computer network and archiving of digital MR images, Brian Healy for advice on statistical analyses, and Julie Raulukaitis, Greg Book, and Russell Starankewicz for assistance with MR image acquisition and transfer.
References
- Baezner H, Blahak C, Poggesi A, Pantoni L, Inzitari D, Chabriat H, Erkinjuntti T, Fazekas F, Ferro JM, Langhorne P, O'Brien J, Scheltens P, Visser MC, Wahlund LO, Waldemar G, Wallin A, Hennerici MG. Association of gait and balance disorders with age-related white matter changes: the LADIS Study. Neurology. 2008;70(12):935–942. doi: 10.1212/01.wnl.0000305959.46197.e6. [DOI] [PubMed] [Google Scholar]
- Baloh RW, Vinters HV. White matter lesions and disequilibrium in older people. II. Clinicopathologic correlation. Arch Neurol. 1995;52(10):975–981. doi: 10.1001/archneur.1995.00540340067014. [DOI] [PubMed] [Google Scholar]
- Baloh RW, Yue Q, Socotch TM, Jacobson KM. White matter lesions and disequilibrium in older people. I. Case–control comparison. Arch Neurol. 1995;52(10):970–974. doi: 10.1001/archneur.1995.00540340062013. [DOI] [PubMed] [Google Scholar]
- Baloh RW, Ying SH, Jacobson KM. A longitudinal study of gait and balance dysfunction in normal older people. Arch Neurol. 2003;60(6):835–839. doi: 10.1001/archneur.60.6.835. [DOI] [PubMed] [Google Scholar]
- Benson RR, Guttmann CR, Wei X, Warfield SK, Hall C, Schmidt JA, Kikinis R, Wolfson LI. Older people with impaired mobility have specific loci of periventricular abnormality on MRI. Neurology. 2002;58(1):48–55. doi: 10.1212/wnl.58.1.48. [DOI] [PubMed] [Google Scholar]
- Borger LL, Whitney SL, Redfern MS, Furman JM. The influence of dynamic visual environments on postural sway in the elderly. J Vestib Res. 1999;9(3):197–205. [PubMed] [Google Scholar]
- Camicioli R, Moore MM, Sexton G, Howieson DB, Kaye JA. Age-related brain changes associated with motor function in healthy older people. J Am Geriatr Soc. 1999;47(3):330–334. doi: 10.1111/j.1532-5415.1999.tb02997.x. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ. Anatomical mapping of white matter hyperintensities (WMH): exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke. 2005;36(1):50–55. doi: 10.1161/01.STR.0000150668.58689.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, Radner H, Lechner H. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43(9):1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/S0896-6273(02)00569-X. [DOI] [PubMed] [Google Scholar]
- Franch O, Calandre L, Alvarez-Linera J, Louis ED, Bermejo-Pareja F, Benito-Leon J. Gait disorders of unknown cause in the elderly: clinical and MRI findings. J Neurol Sci. 2009;280(1–2):84–86. doi: 10.1016/j.jns.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttmann CR, Benson R, Warfield SK, Wei X, Anderson MC, Hall CB, Abu-Hasaballah K, Mugler JP, 3rd, Wolfson L. White matter abnormalities in mobility-impaired older persons. Neurology. 2000;54(6):1277–1283. doi: 10.1212/wnl.54.6.1277. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–156. doi: 10.1016/S1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kuo HK, Lipsitz LA. Cerebral white matter changes and geriatric syndromes: is there a link? J Gerontol A Biol Sci Med Sci. 2004;59(8):818–826. doi: 10.1093/gerona/59.8.M818. [DOI] [PubMed] [Google Scholar]
- Masdeu JC, Wolfson L, Lantos G, Tobin JN, Grober E, Whipple R, Amerman P. Brain white-matter changes in the elderly prone to falling. Arch Neurol. 1989;46(12):1292–1296. doi: 10.1001/archneur.1989.00520480034016. [DOI] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, Zijl P, Mazziotta J. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40(2):570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscufo N, Guttmann CRG, Meier D, Csapo I, Hildenbrand PG, Healy BC, Schmidt JA, Wolfson L (2011) Brain regional lesion burden and impaired mobility in the elderly. Neurobiol Aging 32(4):646–654 [DOI] [PMC free article] [PubMed]
- Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke. 1997;28(3):652–659. doi: 10.1161/01.STR.28.3.652. [DOI] [PubMed] [Google Scholar]
- Pohl KM, Bouix S, Kikinis R, Grimson WEL (2004) Anatomical guided segmentation with non-stationary tissue class distributions in an expectation–maximization framework. IEEE International Symposium on Biomedical Imaging: From Nano to Macro., Arlington, VA, USA, pp 81–84 [DOI] [PMC free article] [PubMed]
- Pyykko I, Jantti P, Aalto H. Postural control in elderly subjects. Age Ageing. 1990;19(3):215–221. doi: 10.1093/ageing/19.3.215. [DOI] [PubMed] [Google Scholar]
- Rosano C, Sigurdsson S, Siggeirsdottir K, Phillips CL, Garcia M, Jonsson PV, Eiriksdottir G, Newman AB, Harris TB, Buchem MA, Gudnason V, Launer LJ. Magnetization transfer imaging, white matter hyperintensities, brain atrophy and slower gait in older men and women. Neurobiol Aging. 2008;31(7):1197–1204. doi: 10.1016/j.neurobiolaging.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev PS, Wen W, Christensen H, Jorm AF. White matter hyperintensities are related to physical disability and poor motor function. J Neurol Neurosurg Psychiatry. 2005;76(3):362–367. doi: 10.1136/jnnp.2004.042945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev P, Wen W, Chen X, Brodaty H. Progression of white matter hyperintensities in elderly individuals over 3 years. Neurology. 2007;68(3):214–222. doi: 10.1212/01.wnl.0000251302.55202.73. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Enzinger C, Ropele S, Schmidt H, Fazekas F. Progression of cerebral white matter lesions: 6-year results of the Austrian Stroke Prevention Study. Lancet. 2003;361(9374):2046–2048. doi: 10.1016/S0140-6736(03)13616-1. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Scheltens P, Erkinjuntti T, Pantoni L, Markus HS, Wallin A, Barkhof F, Fazekas F. White matter lesion progression: a surrogate endpoint for trials in cerebral small-vessel disease. Neurology. 2004;63(1):139–144. doi: 10.1212/01.wnl.0000132635.75819.e5. [DOI] [PubMed] [Google Scholar]
- Silbert LC, Nelson C, Howieson DB, Moore MM, Kaye JA. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology. 2008;71(2):108–113. doi: 10.1212/01.wnl.0000316799.86917.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Srikanth V, Beare R, Blizzard L, Phan T, Stapleton J, Chen J, Callisaya M, Martin K, Reutens D. Cerebral white matter lesions, gait, and the risk of incident falls: a prospective population-based study. Stroke. 2009;40(1):175–180. doi: 10.1161/STROKEAHA.108.524355. [DOI] [PubMed] [Google Scholar]
- Srikanth V, Phan TG, Chen J, Beare R, Stapleton JM, Reutens DC. The location of white matter lesions and gait—a voxel-based study. Ann Neurol. 2010;67(2):265–269. doi: 10.1002/ana.21826. [DOI] [PubMed] [Google Scholar]
- Taylor WD, MacFall JR, Provenzale JM, Payne ME, McQuoid DR, Steffens DC, Krishnan KR. Serial MR imaging of volumes of hyperintense white matter lesions in elderly patients: correlation with vascular risk factors. AJR Am J Roentgenol. 2003;181(2):571–576. doi: 10.2214/ajr.181.2.1810571. [DOI] [PubMed] [Google Scholar]
- Dam VH, Heuvel DM, Craen AJ, Bollen EL, Murray HM, Westendorp RG, Blauw GJ, Buchem MA. Decline in total cerebral blood flow is linked with increase in periventricular but not deep white matter hyperintensities. Radiology. 2007;243(1):198–203. doi: 10.1148/radiol.2431052111. [DOI] [PubMed] [Google Scholar]
- Tinetti M. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc. 1986;34(2):119–126. doi: 10.1111/j.1532-5415.1986.tb05480.x. [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Kumar C. The patient who falls: "It's always a trade-off". Jama. 2010;303(3):258–266. doi: 10.1001/jama.2009.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield DB, Moscufo N, Guttmann CR, Kuchel GA, Kaplan RF, Pearlson G, Wolfson L. White matter hyperintensities predict functional decline in voiding, mobility, and cognition in older adults. J Am Geriatr Soc. 2010;58(2):275–281. doi: 10.1111/j.1532-5415.2009.02699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw JM, Sandercock PA, Dennis MS, Starr J. Is breakdown of the blood–brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke. 2003;34(3):806–812. doi: 10.1161/01.STR.0000058480.77236.B3. [DOI] [PubMed] [Google Scholar]
- Wen W, Sachdev P. The topography of white matter hyperintensities on brain MRI in healthy 60- to 64-year-old individuals. Neuroimage. 2004;22(1):144–154. doi: 10.1016/j.neuroimage.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Whitman GT, Tang Y, Lin A, Baloh RW. A prospective study of cerebral white matter abnormalities in older people with gait dysfunction. Neurology. 2001;57(6):990–994. doi: 10.1212/wnl.57.6.990. [DOI] [PubMed] [Google Scholar]
- Wolfson L, Wei X, Hall CB, Panzer V, Wakefield D, Benson RR, Schmidt JA, Warfield SK, Guttmann CR. Accrual of MRI white matter abnormalities in elderly with normal and impaired mobility. J Neurol Sci. 2005;232(1–2):23–27. doi: 10.1016/j.jns.2004.12.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Boxplots show the distribution of WMH volume changes (y-axis) in the two chair rise mobility groups (decline and no-decline, x-axis). The WMH variables shown are those that, in addition to the splenium of corpus callosum, showed significant correlation with decline in the chair rise test (see Table 3). Change was calculated as: [WMH burden at follow-up] − [WMH burden at baseline] (see also “Methods”). Thick horizontal line in the boxes indicates the group median. Circles, outliers; star, extreme outliers. The statistical significance of the group difference (Mann–Whitney group comparison test) is shown on top (JPEG 9 kb)
High resolution image file. (TIFF 1427 kb)
(DOC 89 kb)