Abstract
Background:
Vitiligo is an acquired depigmenting disorder characterized by the loss of functional melanocytes from the epidermis. Although the etiology of vitiligo is unknown, over the last few years, substantial data from clinical research has greatly supported the ‘Autoimmune theory’ and this is supported by the frequent association of vitiligo with disorders that have an autoimmune origin, including Hashimoto's thyroiditis, Graves disease, type 1 insulin-dependent diabetes mellitus, and Addison's disease. As cytokines are important mediators of immunity, there is evidence to suggest that they play a major role in the pathogenesis of autoimmune diseases.
Aim:
Keeping this in view we have assayed sera for cytokine IL-6, IL-2, Tumor necrosis factor (TNF)-α, and IFNγ in 80 cases of vitiligo and compared it with healthy subjects, in order to find out whether they play a role in the pathogenesis of vitiligo or not.
Materials and Methods:
Serum IL-6, IL-2, TNF-α, and IFNγ were done by the indirect enzyme linked immunosorbent assay (ELISA).
Results:
The mean serum IL-6 and IL-2 levels in the patient group were significantly higher when compared with those of the normal controls. The mean serum IFNγ level in patients with vitiligo was significantly lower than that in the control group. There was no significant difference in the serum level of TNF-α between vitiligo and healthy controls.
Conclusion:
An increase in the production of proinflammatory cytokines such as IL-6 and IL-2 in vitiligo patients may play an important role in melanocytic cytotoxicity. Thus, we speculate that the cytokine production of epidermal microenvironment may be involved in vitiligo.
Keywords: Cytokines, IFN-gamma, IL-2, IL-6, TNF-alpha, vitiligo
Introduction
Vitiligo is an acquired depigmenting disorder, characterized by the loss of functional melanocytes from the epidermis. About one to two percent of the world's population suffers from this disorder, without any regard to ethnic, racial, or socioeconomic background.[1] Although the etiology of vitiligo is unknown, over the last few years, substantial data from the clinical research has greatly supported the ‘Autoimmune theory’[2,3] and this is supported by the frequent association of vitiligo with disorders that have an autoimmune origin including Hashimoto's thyroiditis, Graves disease, type 1 insulin-dependent diabetes mellitus, and Addison's disease.[4] As cytokines are important mediators of immunity and there are evidences to suggest that it plays a major role in the pathogenesis of autoimmune diseases including Hashimoto's thyroiditis[5] and insulin-dependent type-1 diabetes mellitus,[6] the alteration in the concentration of various cytokines such as IL-6, IL-8, IL-10, IL-2, TNF-α, and IFNγ have been the subject of intensive investigations in autoimmune disorders,[4,5,7,8] but only a few studies dealt with alterations in the serum concentration of cytokines in vitiligo. Keeping this in view we have assayed sera for cytokine IL-6, IL-2, TNF-α, and IFNγ in 80 cases of vitiligo and compared it with that of healthy subjects, in order to find out whether or not they play a role in the pathogenesis of vitiligo. A total of 80 patients with vitiligo were taken from the Outpatient Department of Dermatology and Venereology of the Sir Sunderlal Hospital, B.H.U., Varanasi, for a period of nine months. About 50 healthy controls from the staff and students of the institute were included in the study with no present or past history of autoimmune or any systemic disease. Clinical diagnosis of the patients was done by the dermatologist. Blood samples were taken when the patients first visited the dermatologist. The study was approved by the ethical committee of this institute and consent was given by all the patients enrolled in the study.
Materials and Methods
Vitiligo patients included 49 cases (61.25%) of generalized vitiligo and 31cases (38.75%) of localized vitiligo. On the basis of stage, 44 cases (55%) were of stable stage (no change in the new lesions within the two months prior to the study, as observed by the patients) and 36 cases (45%) were of active stage (new lesions within the two months prior to the study, as observed by the patients). Exclusion criteria consisted of patients who had diabetes mellitus, thyroiditis, pernicious anemia, psoriasis, atopic dermatitis, and inflammatory skin diseases. The 80 patients included 48 (60%) males and 32 (40%) women.
Laboratory Analysis-serum IL-6, IL-2, TNF-α, and IFNγ were done by indirect enzyme-linked immunosorbent assay (ELISA), kit of Immunotech, a Beckman Coulter company of France, supplied by M/S OSB Agencies Delhi, India.
Statistical analysis
The statistical analysis of the data was done using the student's t-test for difference of mean, on SPSS for windows (version 16.0) statistical package (SPSS Inc., Chicago, IL) computer statistics program. P values less than 0.05 were taken as significant.
Results
The mean age of vitiligo patients and controls were 32.16±16.01 and 32.62±11.52 years, respectively. In vitiligo, the age of the patient varied from 10 to 65 years and in healthy volunteers from 19 to 60 years.
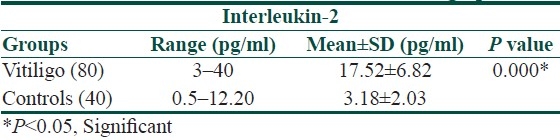
The mean serum IL-6 and IL-2 levels in the patient group were significantly higher when compared with that of the normal controls (13.21±8.79 vs. 5.62±2.25 pg/ml and 17.52±6.82 vs. 3.18±2.03 pg/ml, respectively, [Tables 1 and 2]).
Table 1.
Serum concentration of IL-6 in vitiligo patients
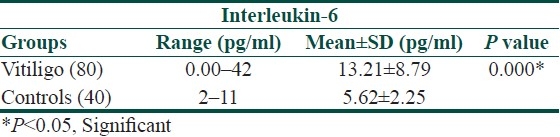
Table 2.
Serum concentration of IL-2 in vitiligo patients
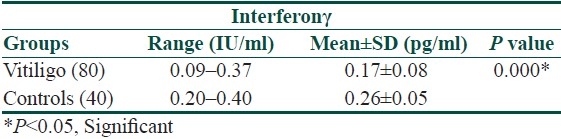
There was a significant relationship in IFNγ between the vitiligo and control groups. The mean serum IFNγ level in patients with vitiligo was significantly lower than in the control group (0.17±0.08 vs. 0.26±0.05 IU/ml, [Table 3]).
Table 3.
Serum concentration of Interferonγ in vitiligo patients
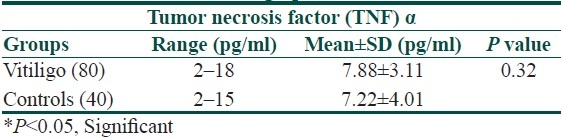
The mean serum TNF-α concentration was increased in the patient group as compared to the control group (7.88±3.11 vs. 7.22±4.01, [Table 4]), but there was no significant difference between the means of the two groups.
Table 4.
Serum concentration of Tumor necrosis factor α in vitiligo patients
There was no significant difference in the serum level of IL-6, IL-2, TNF-α, and IFNγ between the active and stable cases of vitiligo. The mean IL-6 level was significantly elevated in patients with a duration of disease less than 15 years.
Discussion
In our study, the serum concentration of IL-6 was significantly elevated in the patients rather than in the controls. On further analysis it was found that IL-6 significantly increased in patients, in whom the duration of disease was more than 15 years. These results could be substantiated by the study of several workers on vitiligo cases.[9–12] All these workers reported high IL-6 level in vitiligo patients. Il-6 was produced by mononuclear cells, which could induce the expression of ICAM-1 (intercellular cell adhesion molecules) on melanocytes,[13] which might then facilitate leukocyte–melanocyte interactions, leading to polyclonal B-cell activation and subsequently increasing autoantibody production, leading to immunological damage of the melanocytes.[14]
Another interesting finding in this study is a decrease in the production of IFNγ in vitiligo, which may explain the poor cell-mediated immunity in these cases to some unknown antigens.[15] Similar to us, other workers[11] also reported decreased IFNγ in vitiligo.
In our study, the serum concentration of TNF-α was increased in the vitiligo group, but statistically it was not significant. A weak, but existing relation between the serum levels and a possible intercutaneous role of TNF-α in vitiligo may be suggested. Similarly, Moretti et al., reported an increased TNF-α in the epidermis from vitiligo biopsies.[9,16] TNF-α could contribute to keratinocyte apoptosis, which may result in autoimmune response and ultimately melanocyte disappearance.[15] It has also been reported that TNF-α leads to mitochondria-dependent cell death and activation of the inflammatory gene.[17] Contrary to this, Yu et al.[11] reported a significant decrease in TNF-α level in 12 non-segmental vitiligo patients.
According to our study, the mean value of serum IL-2 has been significantly increased in vitiligo cases as compared to the controls. IL-2 is primarily produced by recently activated T cells, which act as growth and death factors for antigen-activated T lymphocytes and also promote the development of T regulatory cells.[18]
The studies by Yeo et al.[19] and Galadari,[20] on 79 and 32 vitiligo patients, respectively, showed that the serum levels of sIL-R (soluble interleukin-2 receptor) were elevated in the patient group as compared to the controls. The sIL-2R was correlated with the amount of IL-2R expressed on the T cell, which in turn was stimulated by IL-2.[21–23]
Conclusion
An increase in the production of pro-inflammatory cytokines, such as IL-6 and IL-2, in vitiligo patients, may play an important role in melanocytic cytotoxicity. Thus, we speculate that the cytokine production of the epidermal microenvironment may be involved in vitiligo. Decreased concentration of IFNγ and a normal level of TNF-α suggest that probably TH1-mediated cell-mediated immunity is not involved in the pathogenesis of vitiligo; however, the rise of IL-2 cannot be explained. Further studies with a larger sample size are suggested, to elucidate these issues in future.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Lerner AB. Vitiligo. J Invest Dermatol. 1959;32:285–310. [PubMed] [Google Scholar]
- 2.Ongenae K, van Geel N, Naeyaert JM. Evidence for an autoimmune pathogenesis of vitiligo. Pigment cell Res. 2003;16:90–100. doi: 10.1034/j.1600-0749.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- 3.van den Wijngaard R, Wankowicz Kalinska A, Pal S, Weening J, Das P. Autoimmune melanocyte destruction in vitiligo. Lab Invest. 2001;81:1061–7. doi: 10.1038/labinvest.3780318. [DOI] [PubMed] [Google Scholar]
- 4.Alkahateeb A, Fain PR, Thody A, Bennett DC, Spritz RA. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16:208–14. doi: 10.1034/j.1600-0749.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- 5.Paolieri F, Salmaso C, Battifora M, Montagna P, Pesce G, Bagnasco M. Possible pathogenetic relevance of interleukin-1β in ‘destructive’ organ-specific autoimmune disease (Hashimoto's thyroiditis) Ann New York Acad Sci. 1999;876:221–8. doi: 10.1111/j.1749-6632.1999.tb07642.x. [DOI] [PubMed] [Google Scholar]
- 6.Espersen GT, Mathiesen O, Grunnet N, Jensen S, Ditzel J. Cytokine plasma levels and lymphocyte subsets in patients with newly diagnosed insulin-dependent (type 1) diabetes mellitus before and following initial insulin treatment. APMIS. 1993;101:703–6. doi: 10.1111/j.1699-0463.1993.tb00168.x. [DOI] [PubMed] [Google Scholar]
- 7.Ko YC, Kawai T. Interleukin-8. Rinsho Byori. 1995;43:329–34. [PubMed] [Google Scholar]
- 8.Ajjan RA, Watson PF, McIntosh RS, Weetman AP. Intrathyroidal cytokine gene expression in Hashimoto's thyroiditis. Clin Exp Immunol. 1996;105:523–8. doi: 10.1046/j.1365-2249.1996.d01-784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moretti S, Spallanzani A, Amato L, Hautmann G, Gallerani I, Fabiani M, et al. New insights into the pathogenesis of vitiligo: Imbalance of epidermal cytokines at sites of lesions. Pigment Cell Res. 2002;15:87–92. doi: 10.1034/j.1600-0749.2002.1o049.x. [DOI] [PubMed] [Google Scholar]
- 10.Pichler R, Sfetsos K, Badics B, Gutenbrunner S, Berg J, Auböck J. Lymphocyte imbalance in vitiligo patients indicated by elevated CD4C/CD8C T-cell ratio. Wien Med Wochenschr. 2009;159:337–41. doi: 10.1007/s10354-009-0699-z. [DOI] [PubMed] [Google Scholar]
- 11.Yu HS, Chang KL, Yu CL, Li HF, Wu MT, Wu CS, et al. Alterations in IL-6, IL-8, GM-CSF, TNF-alpha, and IFN-gamma release by peripheral mononuclear cells in patients with active vitiligo. J Invest Dermatol. 1997;108:527–9. doi: 10.1111/1523-1747.ep12289743. [DOI] [PubMed] [Google Scholar]
- 12.Zailaie MZ. Decreased proinflammatory cytokine production by peripheral blood mononuclear cells from vitiligo patients following aspirin treatment. Saudi Med J. 2005;26:799–805. [PubMed] [Google Scholar]
- 13.Kirnbauer R, Charvat B, Schauer K, Kock A, Urbanshi A, Forster E, et al. Modulation of intercellular adhesion molecule-1 expression on human melanocytes and melanoma cells: Evidence for a regulatory role of IL-6, IL-7, TNF-α and UVB light. J Invest Dermatol. 1992;98:320–6. doi: 10.1111/1523-1747.ep12499793. [DOI] [PubMed] [Google Scholar]
- 14.Morelli JG, Norris DA. Influence of inflammatory mediators and cytokines on human melanocyte function. J Invest Dermatol. 1993;100:191–5. [PubMed] [Google Scholar]
- 15.Kao CH, Yu HS. Depletion and repopulation of Langerhans cells in non-segmental type vitiligo. J Dermatol. 1990;17:287–96. doi: 10.1111/j.1346-8138.1990.tb01643.x. [DOI] [PubMed] [Google Scholar]
- 16.Moretti S, Fabbri P, Baroni G, Berti S, Bani D, Berti E, et al. Keratinocyte dysfunction in vitiligo epidermis: Cytokine microenvironment and correlation to keratinocyte apoptosis. Histol Histopathol. 2009;24:849–57. doi: 10.14670/HH-24.849. [DOI] [PubMed] [Google Scholar]
- 17.Wankowicz-Kalinska A, van Den Wijngaard RM, Tigges BJ, Westerhof W, Ogg GS, Cerundolo V, et al. Immunopolarization of CD4 + and Cd8+T cells to type-1-like is associated with melanocyte loss in human vitiligo. Lab Invest. 2003;83:683–95. doi: 10.1097/01.lab.0000069521.42488.1b. [DOI] [PubMed] [Google Scholar]
- 18.Malek TR. The main function of IL-2 is to promote the development of T regulatory cells. J Leukoc Biol. 2003;74:961–5. doi: 10.1189/jlb.0603272. [DOI] [PubMed] [Google Scholar]
- 19.Yeo UC, Yang YS, Park KB, Sung HT, Jung SY, Lee ES, et al. Serum concentration of the soluble interleukin-2 receptor in vitiligo patients. J Dermatol Sci. 1999;19:182–8. doi: 10.1016/s0923-1811(98)00070-x. [DOI] [PubMed] [Google Scholar]
- 20.Galadari I. Serum levels of the soluble interleukin-2 receptor in vitiligo patients in UAE. Eur Ann Allergy Clin Immunol. 2005;37:109–11. [PubMed] [Google Scholar]
- 21.Goldsmith MA, Greene WC. Interleukin-2 and the interleukin-2 receptor. In: Thomson A, editor. The Cytokine Handbook. 2nd ed. London: Academic Press; 1994. pp. 55–80. [Google Scholar]
- 22.Gaulton GN, Williamson P. Interleukin-2 and the interleukin-2 receptor complex. Chem Immunol. 1994;59:91–114. [PubMed] [Google Scholar]
- 23.Rubin LA, Nelson DL. The soluble interleukin-2 receptor: Biology, function, and clinical application. Ann Intern Med. 1990;113:619–27. doi: 10.7326/0003-4819-113-8-619. [DOI] [PubMed] [Google Scholar]


