Abstract
Background:
Cutaneous manifestations of diabetes mellitus generally appear subsequent to the development of the disease, but they may be the first presenting signs and in some cases they may precede the primary disease manifestation by many years.
Aims:
The aim of our study was to study the spectrum of dermatoses in diabetics, to know the frequency of dermatoses specific to diabetes mellitus (DM), and to establish the mucocutaneous markers of DM.
Material and Methods:
The study was conducted at a diabetic clinic and our department between September 2008 and June 2010. Two hundred and twenty-four diabetic patients were included in the study group and those with gestational diabetes were excluded. Healthy age- and sex-matched individuals were taken as controls.
Results:
The male to female ratio was 1 : 1.21. Type 2 DM was seen in 89.7% and type 1 DM in 10.3% of the patients. Dermatoses were seen in 88.3% of the diabetics compared to 36% in non-diabetic controls (P<0.05). Cutaneous infections were the most common dermatoses followed by acanthosis nigricans and xerosis in diabetics. Type 2 DM was found to have an increased risk of complications than type 1 DM. Complications of diabetes were seen in 43.7% of the diabetic cases. Diabetic dermopathy, loss of hair over the legs, diabetic foot ulcer, and so on, were found to be the cutaneous markers of DM in our group of cases.
Conclusion:
Dermatoses were more common in diabetics than non-diabetics. Cutaneous infections formed the largest group of dermatoses in DM.
Keywords: Cutaneous markers, dermatoses, diabetes mellitus
Introduction
Diabetes mellitus has emerged as a major public health problem in our country, and our country has a distinction of having the largest number of diabetics in the world.[1] Skin is affected by both acute metabolic derangements and the chronic degenerative complication of diabetes.[2] Multiple factors play a role in the manifestations of cutaneous signs of diabetes mellitus. Abnormalities in the metabolism of carbohydrates, alteration of metabolic pathways, vascular involvement in the form of atherosclerosis, microangiopathy and neuronal involvement in the form of sensory, motor and autonomic neuropathies and impaired host mechanisms, all play a role.[3] The prevalence of a cutaneous disorder appears to be similar between type 1 DM and type 2 DM patients, but with type 2 DM patients developing more frequent cutaneous infections, and type 1 DM patients manifesting more autoimmune-type cutaneous lesions.[4–7] A study of the dermatological manifestations in diabetics may enlighten us on the various manifestations and help in early diagnosis of diabetes, along with effective management. Only few studies of this type from this region of the country exist, hence this study was undertaken.
Material and Methods
The study was conducted in the Outpatient Department of a diabetic clinic, General Medicine and Dermatology and STD Department, of a tertiary care institute, between September 2008 and June 2010. Institute ethical clearance was obtained before the start of the study. Two hundred and twenty four consecutive consenting diabetic patients were included in the study group and those patients not willing to take part in the study and those with gestational diabetes were excluded from the study. Healthy age- and sex-matched individuals attending the Medicine OPD and willing to take part in the study were taken as the control group. Individuals with a family history of diabetes were excluded from the control group. Detailed history including demographic data, duration and type of diabetes, type of treatment taken and chief complaints relating to skin, onset, evolution and duration of the skin lesions, past or family history of similar lesions and associated skin or medical disorders were elicited and recorded on a proforma. A complete cutaneous examination was done in all cases to observe for the presence of any specific or non-specific dermatoses. Dermatoses in the control group were also recorded. The type of skin lesions and the site of involvement were recorded. Relevant systemic examination was carried out. Investigations like fasting and postprandial blood glucose, HbA1c, complete hemogram, liver and renal function tests, lipid profile, 24-hour urinary protein, and fundus examinations were done. Bedside laboratory procedures like the Tzanck smear, KOH mount, and Gram's stain were carried out. To confirm the diagnosis, a skin biopsy was done in a few cases. The results were tabulated and analyzed. Statistical analysis was done using the Graph Pad Instat version 3.06.
Results
Among the 224 diabetic cases, 123 (54.95%) were female and 101 (45.1%) were male. Male to female ratio was 1 : 1.21. The age of the patients ranged from 10–75 years, with a mean age of 49.38±12.78. The oldest male and female patients were 75 and 72 years, respectively. The youngest male was 10 years and female was 20 years. The maximum number of patients was in the age group of 51 to 60 years (29.9%). Type 2 DM was seen in 201 (89.7%) patients, and 23 (10.3%) patients had type 1 DM. Among the diabetic cases, the mean body mass index was found to be 25.9±3.9 (mean±SD), as compared to the controls, who had a mean body mass index of 22.9±2.5 (mean±SD). This difference was found to be statistically significant (P<0.0001).
Dermatoses were seen in 198 (88.3%) diabetic cases and 81 (36%) non-diabetic controls. The increased incidence of dermatoses in diabetic cases was found to be significantly higher as compared to the control group (P<0.05). The various dermatoses observed in the present study, as per the classification of Huntley,[5] are given in Table 1. Cutaneous infections formed the largest group of dermatoses in both the cases and controls, and were observed in 94 (41.9%) cases and 42 (18.8%) controls. The incidence of cutaneous infections in diabetic cases was found to be significantly higher as compared to the non-diabetic controls (P<0.05). Most of the dermatoses occurring in diabetic patients were seen in the fifth decade. The distribution of dermatoses was found to be similar between males and females. There was no significant difference in the distribution of dermatoses between type 1 DM and type 2 DM.
Table 1.
Deramatoses in diabetic cases
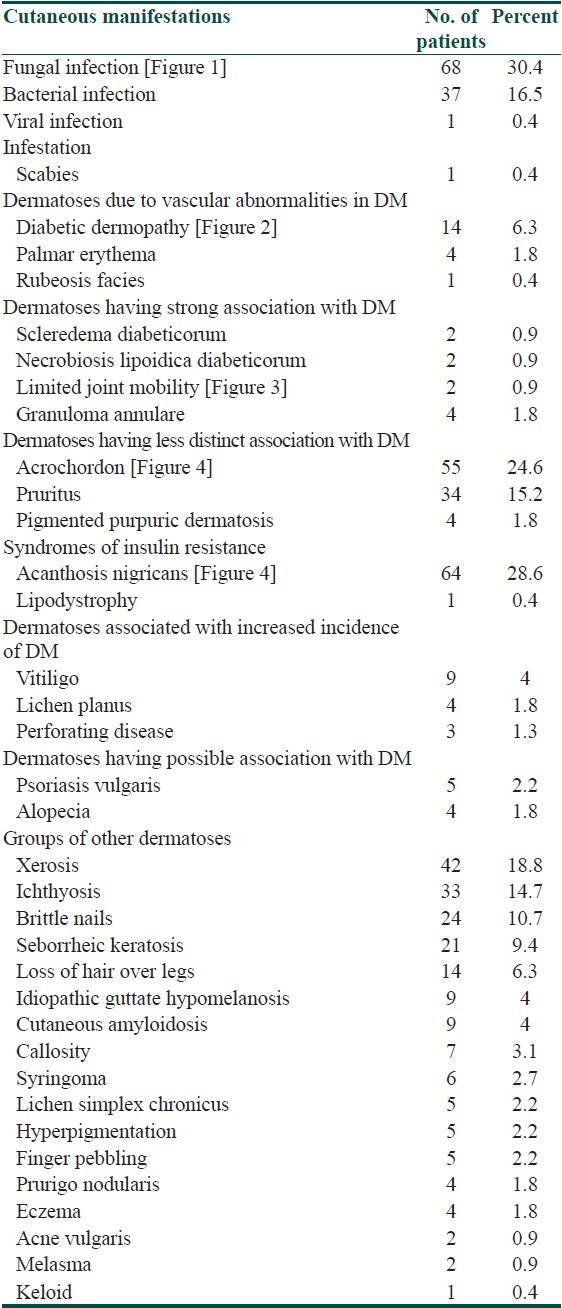
Dermatoses, having a possible association with diabetes mellitus were the first to appear (3.9 years), followed by cutaneous infections (6.1 years). Dermatoses, having a strong association with diabetes mellitus were found to occur in patients with a longer duration of diabetes. Cutaneous manifestations due to the antidiabetic therapy were seen in 3.4% (seven) cases. A maculopapular reaction was found in two patients on oral hypoglycemic agents; five patients had reactions to insulin. Among these, three had lipoatrophy and one each had injection-site infection and keloid formation at the injection-site.
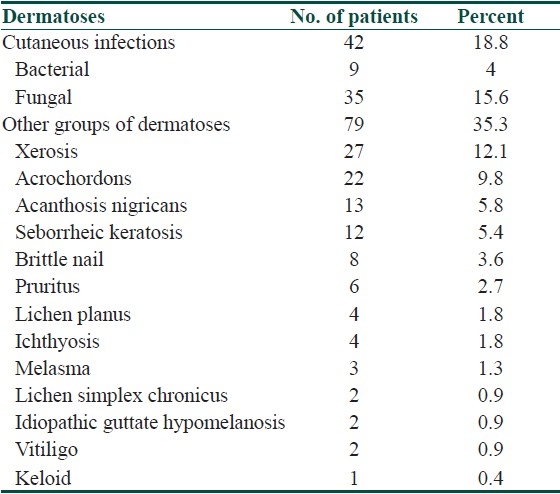
Glycemic control (HbA1C) was checked in 176 patients. Good control (HbA1C 6–7) was seen in 140 patients, fair control (HbA1C 7–8) in 32, and poor control (HbA1C>8) in four patients. No relationship between glycosylated hemoglobin and dermatoses could be found. Dermatoses observed among the control group were as depicted in Table 2.
Table 2.
Dermatoses in the control group
Complications of diabetes mellitus and association
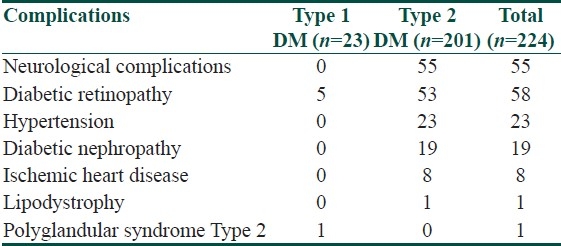
Type 2 DM was associated with an increased risk of complications compared to type 1 DM, with a trend toward statistical significance (P=0.07). Most of the complications occurring in diabetic patients were found to be in the fifth decade. The range of duration of diabetes for most of the complications was found to be between nine and thirteen years. No significant association was found between gender and complications. Distribution of complications according to the type of diabetes is given in Table 3.
Table 3.
Distribution of complications according to the type of diabetes
Cutaneous markers of diabetes mellitus as established in the present study
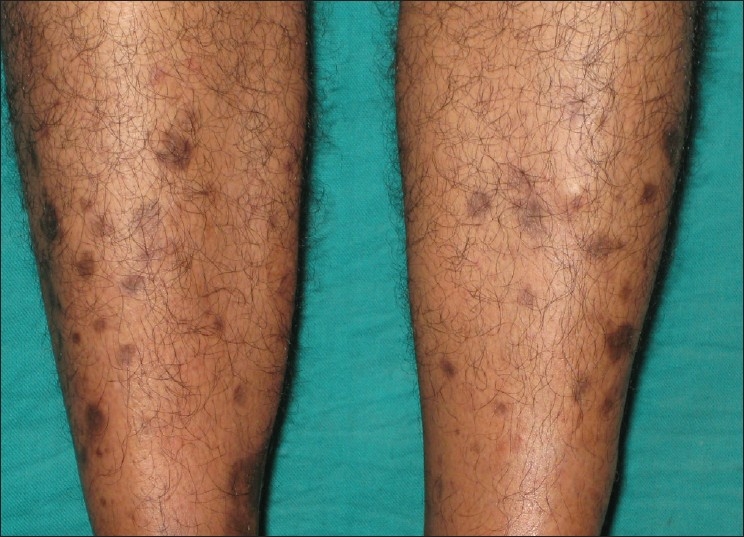
The cutaneous manifestations were analyzed for association with diabetes mellitus using Fischer's exact test. It was found that diabetic dermopathy* [Figure 2], loss of hair over the legs*, acrochordons* [Figure 4], pruritus*, acanthosis nigricans* [Figure 4], ichthyosis*, cutaneous amyloidosis#, syringoma#, callosity#, and brittle nails# were significantly associated with diabetes mellitus (* P<0.001, #<0.05), and thus considered as the cutaneous markers of diabetes mellitus.
Figure 2.
Multiple well-defined hyperpigmented atrophic macules over bilateral shins (shin spots) – Diabetic dermopathy
Figure 4.
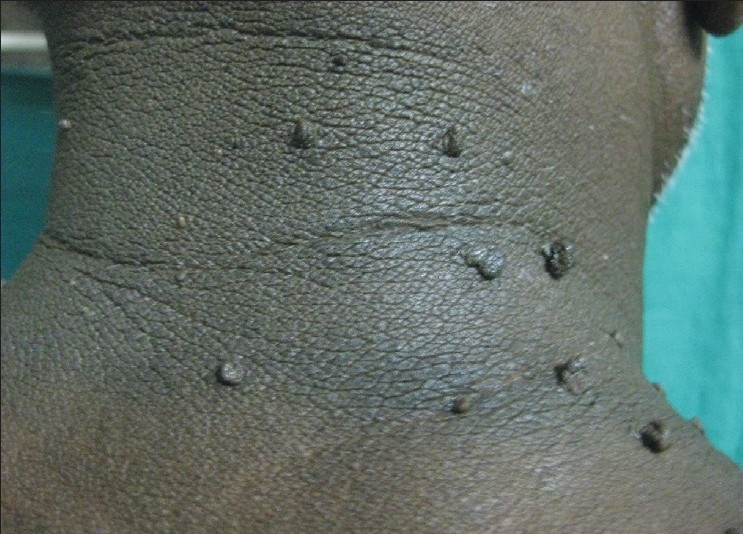
Hyperpigmented velvety plaque topped by skin tags on the nape of the neck – Skin tags and acanthosis nigricans
Figure 1.
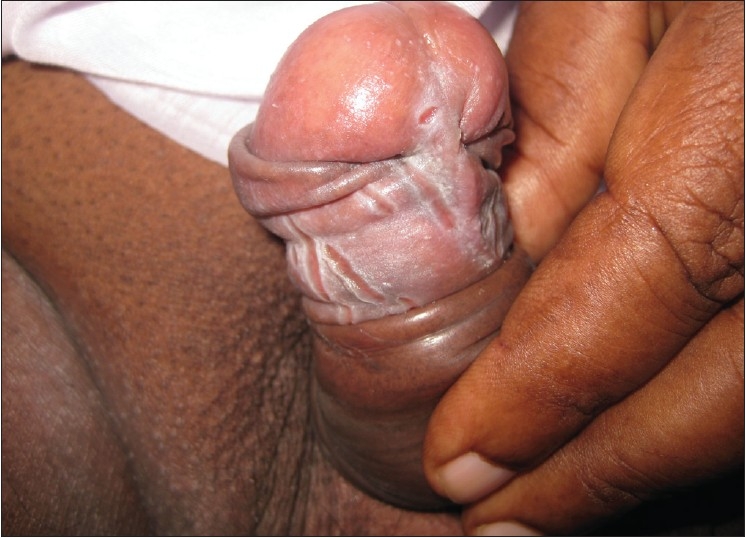
Erythema and fissuring with whitish deposits on the prepuce and the glans penis – Candidal balanoposthitis
Figure 3.
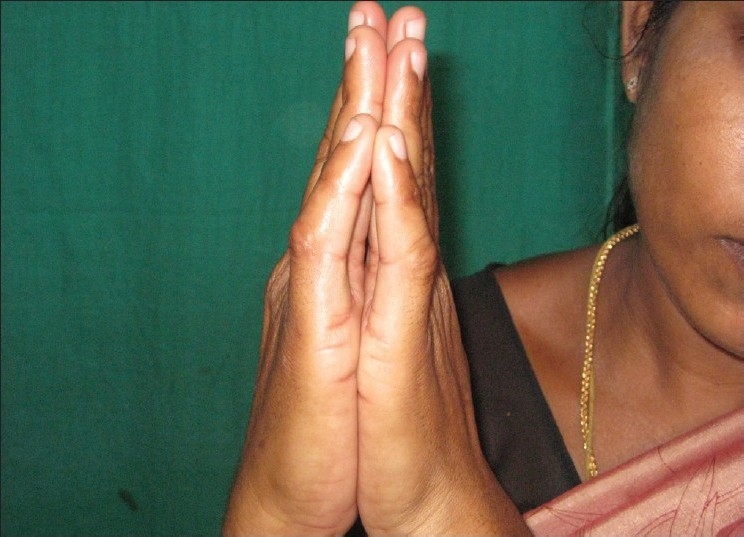
Inability to extend the proximal interphalangeal joints of the fourth and fifth fingers – Prayer sign
Discussion
Cutaneous manifestations of diabetes mellitus generally appear subsequent to the development of the disease, but may be the first presenting signs, and in some cases they may precede the primary disease manifestation by many years.[6] Some diabetes-associated skin conditions are a direct result of the metabolic changes such as hyperglycemia and hyperlipidemia, in addition to other contributing factors, which include, progressive damage to the vascular, neurological or immune system.[7] Bhat et al.,[2] in a study of the cutaneous manifestation of diabetes mellitus in 150 diabetics and age- and sex-matched non-diabetics observed cutaneous manifestation in 66% of the cases and 21.3% of the controls. Nigam and Pande[8] in a study on the pattern of dermatoses in 200 diabetics found that 61% of the diabetics had cutaneous dermatoses. Mahajan et al.,[9] in a study of the cutaneous manifestation of diabetes mellitus in 100 diabetics and 100 age- and sex-matched non-diabetics found that 64% of their cases had cutaneous manifestations as compared to 22% in the control group. In our study, 88.3% (198) of the patients were found to have cutaneous manifestations among the cases as compared to 36% (81) in the controls and this difference was found to be statistically significant.
Type 1 DM has an abrupt onset and is usually seen in cases less than 30 years of age. It occurs mostly in children and the incidence is highest among the 10 to 14-year-old age groups, whereas, type 2 DM has a gradual onset and occurs mainly in the middle-aged and elderly.[10] Bhat et al.[2] and Mahajan et al.,[9] in their studies on diabetes mellitus, documented the most common age group to be of 41 – 50 years in 33.3 and 33% of the patients, respectively. In a study by Mutairi et al.,[11] the most common age group was between 40 and 60 years (45%). Unlike Bhat et al.,[2] our study showed the maximum number of patients to be in the age group of 51 to 60 years (29.9%) followed by 41 to 50 years (27.3%). In our study, most of the dermatoses and complications were seen in the fifth decade. The increase in frequency of cutaneous involvement and complications of diabetes may be attributed to the long duration of diabetes in this group of patients.[11]
Type 1 DM is characterized by insulinopenia, being prone to ketoacidosis, and a lack of ability to produce C peptide, whereas, type 2 DM is characterized by lack of ketoacidosis, an ability to produce C peptide, and a tendency toward obesity, and improvement with weight loss.[12] Type 2 DM was seen in 98 and 97.7% of the patients by Mahajan et al.[9] and Bhat et al.,[2] respectively. In our study 89.7% (201) of the patients had type 2 DM and 10.3% (23) had type 1 DM. In our study, there was no significant difference in the distribution of dermatoses between patients with type 1 DM and type 2 DM, and the results were similar to those observed by Mutairi et al.[11] and Nigam and Pande.[8]
A majority of the patients had a duration of diabetes up to 10 years: 84.5, 60.9, and 83.3% of the patients, as noted by Bhat et al.,[2] Ahmed et al.,[7] and our study, respectively. In our study, the mean duration of diabetes mellitus for most of the dermatoses was found to be between six and nine years and most of the diabetic complications were seen within nine and thirteen years of onset of diabetes mellitus, and this finding was similar to that of Bhat et al.,[2] who noted an increased incidence of cutaneous involvement with increasing years of diabetes mellitus. As the duration of diabetes increased, there was non-enzymatic glycosylation of dermal collagen and mucopolysaccharides, leading to various cutaneous manifestations and complications.[2]
Cutaneous infections occur in 20 to 50% of patients with poorly controlled diabetes, especially type 2 DM. The poor control itself might be the cause or consequence of concurrent infections.[4] The increased incidence of cutaneous infections in diabetes may be related to abnormal microcirculation, hypohidrosis, peripheral vascular disease, diabetic neuropathy, decreased phagocytosis, impaired leukocyte adherence, and delayed chemotaxis.[4,13] In our study, cutaneous infections were the most common cutaneous manifestations seen in 41.9% (94) of the cases, whereas, cutaneous infections were observed in only 18.8% (42) of the controls. This difference in incidence of cutaneous infections was found to be statistically significant (P<0.05). Similar to the observation of our study, Nigam and Pande[8] and Ahmed et al.,[7] found cutaneous infections to be the most common dermatoses in their studies. In our study, cutaneous fungal infections were the most common and were seen in 30.4% (68) of the cases, followed by bacterial 16.5% (37) and viral 0.4% (1) cases.
Diabetic dermopathy (shin spots) is considered as the most common cutaneous lesion in diabetes, although it is not pathognomonic of diabetes mellitus.[4,14] It affects 7–70% of the diabetics, and is more common in men over 50 years of age.[4] Diabetic dermopathy was seen in 11.3% by Bhat et al.,[2] in 7% by Mahajan et al.,[9] in 5.6% by Mutairi et al.,[11] in 4.2% by Ahmed et al.,[7] and in 3% by Nigam and Pande.[8] In our study 6.3% (14) of the patients had diabetic dermopathy in the cases and none in the control. The increased incidence of diabetic dermopathy in the cases was found to be statistically significant. On the other hand, rubeosis is more prominent in fair-skinned people and usually involves the face, neck, hands, and feet. Good diabetic control may improve the erythema.[14] Rubeosis has been reported in 3 – 59% of the diabetics.[4] In our study 2.2% of the diabetics had rubeosis, whereas, none of the controls had it.
Limited joint mobility (LJM) can be found in 30 – 50% of childhood and adolescence diabetes and it is clinically the earliest apparent complication. It usually begins as the extension deficit of the fifth finger of each hand.[15] In a study by Frost et al.,[15] on 335 type 1 DM patients, 33.7% (113) had limited joint mobility (LJM). In our study 8.6% (two) of 23 type 1 DM patients had LJM, whereas, none of the patients with type 2 DM and controls had LJM. Granuloma annulare is a necrobiotic disorder[13] usually seen in children and young adults.[5] The association of granuloma annulare with diabetes is not clear.[13] We observed granuloma annulare in 1.8% (four) diabetic patients and none in the control group. Necrobiosis lipoidica (NL) is a necrobiotic disorder[13] and the lesions can be found on the hands, forearms, abdomen, face and scalp, but about 85% are seen on the legs. When NL is seen in other areas than the lower legs, the patient is less likely to have diabetes mellitus.[5] Nerobiosis lipoidica occurs in 0.3–1.6% of diabetics, but can occur in non-diabetic conditions also.[4] In our study, NL was seen in 0.9% (two) of the cases and none in the control. Of late, an association between scleredema diabeticorum and longstanding diabetes was observed.[13] As granuloma annulare, necrobiosis lipoidica, and scleredema diabeticorum were observed only in a few cases, the association between these diseases and diabetes could not be ascertained in this study.
Generalized pruritus is not specifically associated with diabetes mellitus, although pruritus vulvae and balanitis may be the presenting symptoms of diabetes.[13] Pruritus was reported in 10% of the diabetics by Mahajan et al.,[9] and in 4.5% by Nigam and Pande.[8] We observed pruritus in 15.2% (34) of the cases and 7.4% (six) of the controls. There was a statistically significant (P<0.05) increased incidence of pruritus in diabetics than non-diabetics. Acrochordons have been established as cutaneous markers of diabetes.[5] We observed acrochordons in 24.6% (55) of the cases as compared to 9.8% (22) of the controls, and this was found to be statistically significant (P<0.05). Acanthosis nigricans is usually seen in situations of insulin resistance, such as type 2 DM, obesity, and total lipodystrophy.[4] Acanthosis nigricans was seen in 5.3% (eight) of the diabetic patients by Bhat et al.,[2] 4.7% (five) by Mutairi et al.,[11] 3% (three) by Mahajan et al.[9] and 2.8% (ten) by Ahmed et al.[7] We noted acanthosis nigricans in 28.6% (64) of the cases and 5.8% (13) of the controls and this was found to be statistically significant (P<0.05).
Vitiligo has been associated with both type 1 and type 2 diabetes mellitus and there is an increased association of vitiligo with a proven or presumed autoimmune disease.[13] Patients with vitiligo, lichen planus, and cutaneous perforating dermatosis are reported to have an increased incidence of abnormal glucose tolerance or frank diabetes mellitus.[5] In our study, vitiligo was seen in 4% (nine) of the cases, and 0.9% (two) controls. Psoriasis has been reported to occur with an increased frequency in diabetics in the past, but several reports exist that have not confirmed these earlier observations.[13] In our study psoriasis was seen in 2.2% (five) patients and none in the control. Most cutaneous reactions to oral antidiabetic medications have been reported with first generation sulfonylureas, and 1 – 5% of the patients taking sulfonylureas develop cutaneous reactions within two months of therapy, in the form of maculopapular drug rash;[4] cutaneous reaction to insulin occurs in 5–10% of the patients and is usually local.[5] In our study, 0.9% (two) patients developed maculopapular reaction due to oral hypoglycemic agents, and five patients had reactions due to insulin, 1.3%, (three) of whom had lipoatrophy and 0.4% each had injection-site infection (one) and keloid formation (one) at the injection site.
The complications of diabetes mellitus are many and inter-related and are mostly a result of poor glycemic control, hypertension, hyperlipidemia, obesity, and smoking.[16] There has been no significant statistical association between diabetic dermoangiopathy and diabetic retinopathy, neuropathy, nephropathy, or hypertension (P>0.05) in our study and similar results have been obtained by Mahajan et al.[9] In our study, type 2 DM has been associated with increased risk of diabetic complications compared to type 1 DM, although the increased frequency had not achieved statistical significance.
Kahana et al.,[17] screened 216 patients with skin tags and found overt DM in 26.3% (57) of the patients and impaired glucose tolerance test was found in 7.9% (17) and similarly Thappa[18] found that 62.8% of the patients with skin tags had diabetes, and thus both the studies concluded that skin tags may serve as markers for diabetes mellitus. Requena et al.,[19] found clear-cell porocarcinoma as a cutaneous marker of diabetes mellitus by histopathology, in which the clear-cell appearance of neoplastic cells was due to glycogen accumulation within their cytoplasm and it was a result of phosphorylase activity deficiency in diabetic patients. Grandhe et al.[20] found acanthosis nigricans to be an independent cutaneous marker of type 2 diabetes mellitus. As there was a statistically significant increased frequency of diabetic dermopathy, loss of hair over the legs, acrochordons, pruritus, acanthosis nigricans, ichthyosis, cutaneous amyloidosis, syringoma, callosity, and brittle nails in diabetics than in non-diabetics, in our study, these cutaneous manifestations may be considered as the cutaneous markers of diabetes.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Sahay BK. Diabetes mellitus-basic considerations. In: Saha SN, editor. API Text of Medicine. 8th ed. Mumbai: The Association Of Physicians of India; 2008. pp. 1042–43. [Google Scholar]
- 2.Bhat YJ, Gupta V, Kudyar RP. Cutaneous manifestations of diabetes mellitus. Int J Diab Dev Ctries. 2006;26:152–5. [Google Scholar]
- 3.Rao GS, Pai GS. Cutaneous manifestation of diabetes mellitus. Indian J Dermatol Venereol Leprol. 1997;63:232–4. [PubMed] [Google Scholar]
- 4.Feringer T, Miller FO. Cutaneous manifestation of diabetes mellitus. Dermatol Clin. 2002;20:483–92. doi: 10.1016/s0733-8635(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 5.Huntley AC. The cutaneous manifestations of diabetes mellitus. J Am Acad Dermatol. 1982;7:427–55. doi: 10.1016/s0190-9622(82)80248-x. [DOI] [PubMed] [Google Scholar]
- 6.Pavlović MD, Milenković T, Dinić M, Misović M, Daković D, Todorović S, et al. The prevalence of cutaneous manifestations in young patients with type 1 diabetes. Diabetes Care. 2007;30:1964–7. doi: 10.2337/dc07-0267. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed K, Muhammad Z, Qayum I. Prevalence of cutaneous manifestations of diabetes mellitus. J Ayub Med Coll Abbottabad. 2009;21:76–9. [PubMed] [Google Scholar]
- 8.Nigam PK, Pande S. Pattern of dermatoses in diabetics. Indian J Dermatol Venereol Leprol. 2003;69:83–5. [PubMed] [Google Scholar]
- 9.Mahajan S, Karanne RV, Sharma SK. Cutaneous manifestation of diabetes mellitus. Indian J Dermatol Venereol Leprol. 2003;69:105–8. [PubMed] [Google Scholar]
- 10.Park K. Parks Textbook of Preventive and Social Medicine. 19th ed. Jabalpur: Banarsidas bhanot Publishers; 2007. Epidemiology of chronic non-communicable diseases and conditions; pp. 327–31. [Google Scholar]
- 11.Al-Mutairi N, Zaki A, Sharma AK, Al-Sheltawi M. Cutaneous manifestations of diabetes mellitus. Med Princ Pract. 2006;15:427–30. doi: 10.1159/000095488. [DOI] [PubMed] [Google Scholar]
- 12.Sarkeny RPE, Breathnach S, Seymoor CA, Weismann, Burns D. Metabolic and nutritional disorder. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rooks Textbook of Dermatology. 7th ed. Vol. 4. Massachusettes: Blackwell Science Ltd; 2004. pp. 106–9. [Google Scholar]
- 13.Sibbald RG, Schachter RK. The skin and diabetes mellitus. Int J Dermatol. 1984;23:567–84. doi: 10.1111/j.1365-4362.1984.tb05694.x. [DOI] [PubMed] [Google Scholar]
- 14.Owen VJ. Diabetes mellitus. Br J Dermatol. 1969;81:9–13. doi: 10.1111/j.1365-2133.1969.tb12839.x. [DOI] [PubMed] [Google Scholar]
- 15.Frost D, Beischer W. Limited joint mobility in type I diabetic patients. Diabetes care. 2001;24:95–9. doi: 10.2337/diacare.24.1.95. [DOI] [PubMed] [Google Scholar]
- 16.Kalish JA, LoGerfo . Diabetic foot and vascular complications. In: DeGroot LJ, Jameson JL, editors. Endocrinology. 5th ed. Philadelphia: Elsevier Saunders; 2005. pp. 1367–72. [Google Scholar]
- 17.Kahana M, Grossman E, Feinstein A, Ronnen M, Cohen M, Millet MS. Skin tags: A cutaneous marker for diabetes mellitus. Acta Dermatol Venereol. 1987;67:175–7. [PubMed] [Google Scholar]
- 18.Thappa DM. Skin tags as markers of diabetes mellitus: An epidemiological study in India. J Dermatol. 1995;22:729–31. doi: 10.1111/j.1346-8138.1995.tb03910.x. [DOI] [PubMed] [Google Scholar]
- 19.Requena L, Sarasa JL, Piqué E, Fariña MC, Olivares M, Martín L. Clear-cell porocarcinoma: Another cutaneous marker of diabetes mellitus. Am J Dermatopathol. 1997;19:540–4. doi: 10.1097/00000372-199710000-00110. [DOI] [PubMed] [Google Scholar]
- 20.Grandhe NP, Bhansali A, Dogra S, Kumar B. Acanthosis nigricans: Relation with type 2 diabetes mellitus, anthropometric variables, and body mass in Indians. Postgrad Med J. 2005;81:541–4. doi: 10.1136/pgmj.2004.028308. [DOI] [PMC free article] [PubMed] [Google Scholar]


