Abstract
Background:
The pbreakdown of phospholipids lead to accumulation of malondialdehyde (MDA) that is preferred as a surrogate marker of oxidative stress in diabetics.
Objective:
To compare serum MDA levels along with other biochemical parameters between type 2 diabetic patients with and without complications.
Materials and Methods:
This cross-sectional study was carried out in the Department of Biochemistry of a tertiary care teaching hospital in Sikkim on 60 type 2 diabetics and compared with 100 non-diabetic participants. The un-hemolyzed blood samples were used for estimation of biochemical parameters; MDA was estimated in serum by using trichloroacetic acid (TCA) and 1% thiobarbituric acid (TBA). Whole blood was used for estimation of glycated hemoglobin. The comparison of different parameters between cases and control was calculated by using Student's t test.
Results:
In the study and control groups, no significant difference was noted with regard to independent variables. The fasting and postprandial serum glucose along with glycated hemoglobin from whole blood and the lipid profile differed significantly between the study and control groups. Overall, tmean serum MDA level in the study group was significantly higher thanin the controls. Male sex, addiction to tobacco (smoking and smokeless inclusive), longer duration of diabetes (≥5 years), and presence of complications (both microvascular and macrovascular) significantly increased the MDA level.
Conclusion:
To sum up, the serum MDA level was observed to be significantly high in diabetics with and without complication along with other parameters.
Keywords: Glycated hemoglobin, lipid peroxidation, malondialdehyde, type 2 diabetes mellitus
INTRODUCTION
Endogenous reactive oxygen species (ROS) in high quantities overwhelm the innate antioxidant defense system leading to oxidative stress.[1,2] In diabetes mellitus (DM), the glucolipotoxicity is complicated with endothelial dysfunction and susceptible to oxidative stress, as higher blood glucose level is associated with free radical-mediated lipid peroxidation.[3–6] Free oxygen radicals in DM cause peroxidative breakdown of phospholipids that lead to accumulation of malondialdehyde (MDA). MDA plays a key role in modifying low density lipoprotein (LDL), which mediates the patho-physiological changes by non-enzymatic and auto-oxidative glycosylation.[7–10]
Cigarette smokers have elevated levels of oxidized macromolecules owing to heightened ROS production and may act as an additional risk factor in the complications of DM.[11] The distressed equilibrium between pro-oxidants and antioxidants in DM alters the metabolic status of body, leading to development of microvascular and macrovascular complications that may increase with DM duration. Controversy still exists between MDA levels and duration of type 2 DM in the course of persistent rise in metabolic parameters.[3,12–14]
The present study intended to assess the MDA and glycated hemoglobin in patients with type 2 diabetics, with and without complications along with lipid profiles in diabetic participants in comparison to a normal healthy group.
MATERIALS AND METHODS
This cross-sectional study was carried out in the Department of Biochemistry in a tertiary care teaching hospital in Sikkim. All participants were selected randomly from those who visited for treatment at the Diabetic specialty clinic in the Department of Medicine. After careful clinical examination and confirmed diagnosis by a diabetologist, 60 patients presenting with type 2 diabetes mellitus (male = 32, female = 28) were included in the study. These diabetics were divided into four groups: Group A (n=28) with macrovascular complications; Group B (n=19) with microvascular complications; Group C (n=8) with both macro and microvascular complications (overlapped and included in individual complications); and Group D (n=13) diabetics presented without any complication. A total of 100 age- and sex-matched apparently healthy individuals with no symptoms suggestive of DM were taken as control participants.
The participants with co-morbidities, viz, febrile illness, suffering from any other hormonal disorders, benign or malignant disorders, diabetic ketoacidosis, renal failure, transplant rejection, central nervous system disorders, and/or other chronic diseases, were excluded from the study. No patients were on antioxidant supplementation or lipid lowering drugs.
The study conformed to the Helsinki declaration and approved by Institutional ethics committee. The study was conducted from July 2009 to June 2010. All subjects were explained about the purpose of the study and were ensured that the information collected from them would be confidential and used only for academic purpose. Subsequently, written informed consent was obtained from each participant. They were given the options to withdraw from the study as per their wish. The diabetics with complications were classified on the basis by National Diabetes Data Group (NDDG) of the National Institute of Health (NIH) in 1980/World Health Organization (WHO) criteria.[15–17] Cases of diabetic retinopathy were diagnosed by the Department of Ophthalmology and macrovascular complications were established by the diabetologist. Smokers were defined as consuming ≥5 cigarettes per day and were smoking continuously for a minimum of six months prior to being enrolled.[18] The diabetics were allowed to pursue treatment schedules and regular lifestyles during this study, ie, tobacco addiction.
For the biochemical investigations, two samples of fasting un-hemolyzed blood (after minimum 12 hours of fasting) were collected from the ante-cubital vein of the participants; universal precautions were followed during this procedure, and the participants were followed up for one hour. While whole blood samples were used for the assay of glycated hemoglobin (HbA1C), another set of samples was allowed to clot in aliquots at room temperature for two hours and then centrifuged at 3,000 rpm for 10 minutes to separate the serum. The samples were processed simultaneously for estimations in accordance with the standard operative procedure of the institution. Smokers were instructed to refrain from smoking for one hour before reporting to the laboratory.[18]
The diagnosis of DM was based on WHO criteria, ie, a fasting plasma glucose of 126 mg/dl (7.0 mmole/L) after minimum 12-hour fast, with symptoms of diabetes and two hours of post prandial glucose level of ≥200 mg/dl (11.1 mmole/L) after 75 g oral glucose load. The participants suffering from diabetes for more than five years were included in the study irrespective of their glycemic status. These participants were also confirmed for their present biochemical status. Postprandial sample was drawn two hours after ingestion of 1.75 g per kg body weight with a maximum of 75 g oral glucose in 300 ml water. Fasting sample was used for estimation of all parameters except for postprandial serum glucose estimation.[17]
All biochemical estimations (except MDA) were done by using the RFCL kit (formerly Ranbaxy Fine Chemicals kit) on spectrophotometer Ranbaxy Hospitex REF, LIHD116-SN-161252. Fasting and postprandial serum glucose was estimated quantitatively by glucose oxidase-peroxidase (GOD/POD) technique as described by Trinder (1969).[19] Total cholesterol was estimated quantitatively by cholesterol oxidase-Phenol+aminophenazone (CHOD-PAP) technique as described by Allian (1974).[20] Triacylglycerol was estimated quantitatively by Glycerol-3-phosphate oxidase- Sodium N-ethyl-N-(3-sulfopropyl) m-anisidine (GPO-ESPAS) technique as described by Buccolo and David (1973).[21] Quantitative estimation of high density lipoproteins (HDL) is done by Polyethylene glycol+ Phenol+aminophenazone precipitation (PEG-PAP) method.[22] The lipid profile does not measure LDL level directly but estimates it using the Friedewald formula by subtracting the amount of cholesterol associated with other molecules, like HDL and very low density lipoprotein (VLDL).[23,24]
Oxidative stress was assessed by quantifying thiobarbituric acid (TBA) reactivity as MDA in a spectrophotometer. To 0.5 ml of the serum, 0.5 ml of 30% Trichloroacetic acid (TCA) (Merck) was added and centrifuged at 3,000 rpm for 5 minutes and supernatant was collected. Thereafter, 0.5 ml of supernatant was added to 0.5 ml of 1% TBA (Merck) in a boiling water bath for 30 minutes following which tubes were kept in an ice-cold water bath for 10 minutes. The resulting chromogen absorbance was determined at the wavelength of 532 nm at room temperature against blank reference. The concentration of MDA was read from standard calibration curve plotted using 1, 1, 3, 3’ tetra-ethoxy propane (TEP). The extent of lipid peroxidation was expressed as MDA (μM/L) using a molar extinction coefficient for MDA of 1.56 × 105 M–1 cm–1.[25]
HbA1C was estimated from the whole blood by column chromatography technique. After analysis of groups of normal male and female volunteers, researchers had reported the plasma levels of MDA of 1.076 nmol/ml, ie, 1.076 μmol/L.[26] We also compared HbA1C levels of our study participants with MDA levels.
The statistical analysis was done using Graph Pad 3 and the comparison between cases and control was calculated by using Student's t test. One-way analysis of variance (ANOVA) and Bonferroni multiple comparison test was applied to compare multiple independent and dependant variables in MDA levels. The difference was considered to be statistically significant at alpha error of 0.05.
RESULTS
There were no significant difference with regard to age, waist circumference, and body mass index (BMI) in the cases and controls. Between study and control groups, the routine biochemical parameters, fasting and postprandial glucose, along with glycated hemoglobin from whole blood and lipid profile, viz, serum total cholesterol, triacylglycerol, HDL and LDL differed significantly. The mean serum level of MDA in the study group was 2.65±1.33 μmol/L; in the control group, it was 0.93±0.39 μmol/L; MDA level was significantly higher among diabetics. The sensitivity and specificity of MDA level was calculated in comparison with the HbA1C level; these were respectively 95.83 and 91.66. The predictive values of positive and negative tests were respectively 97.87 and 84.61 [Table 1].
Table 1.
Clinico-biochemical correlates of laboratory findings
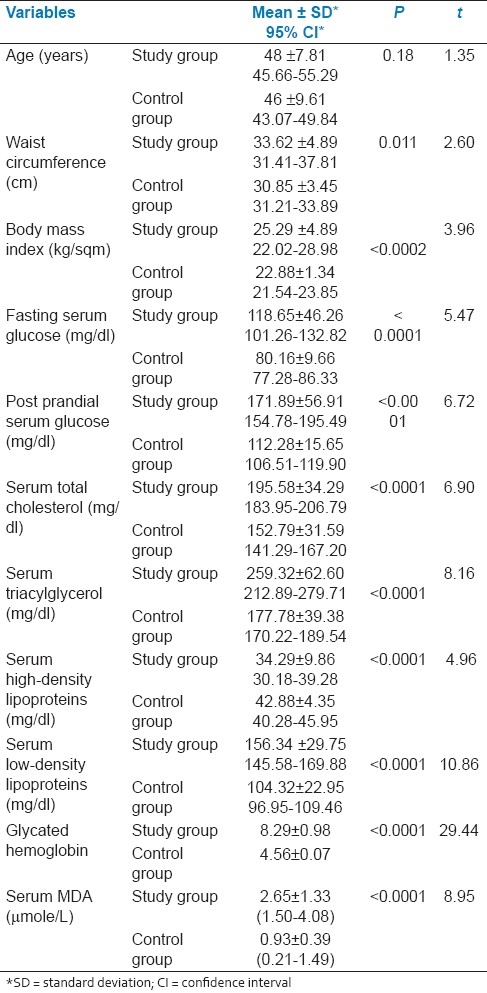
In our study on 32 male and 28 female diabetics, males showed slightly higher value of MDA, which was statistically significant. We had observed the MDA level on the added effect of addiction among diabetics. We found no significant difference in serum MDA levels among smokers (n=22) and smokeless tobacco consumers (n=13). Yet, both groups showed significant increase in serum MDA levels when they were independently compared with non-users of tobacco (n=15). In our study, we noted that the participants (n=34) suffering from diabetes for 5 years or more had significantly higher level of MDA than the diabetics of lesser duration. Serum MDA levels were also evaluated in diabetic participants in terms of macrovascular and microvascular complications in our study. Overall, the difference was extremely significant (P< 0.0001). In Bonferroni multiple comparison test, the MDA levels were significantly higher in participants with complications (macrovascular or microvascular, or both) when compared with diabetics who presented without any complication [Table 2].
Table 2.
Serum MDA levels in relation to correlates and complications
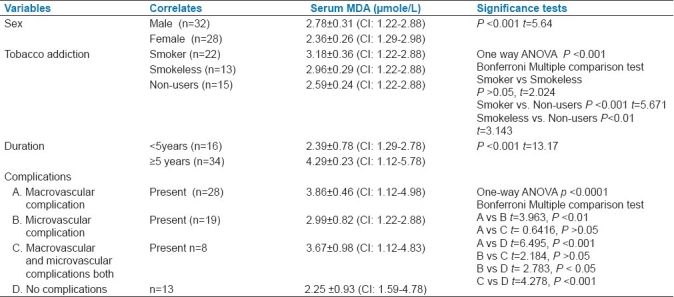
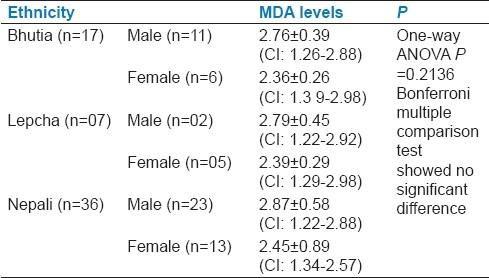
Serum MDA levels of type 2 diabetics in different ethnic populations of Sikkim showed no significant difference [Table 3].
Table 3.
Serum MDA levels of diabetics in different ethnic groups in Sikkim
DISCUSSION
We evaluated the serum MDA level and its relationship with other biochemical findings, ie, fasting blood sugar (FBS), HbA1C, and lipid profiles. The values were compared between diabetic group and control group along with clinical correlates in both groups. The fasting and postprandial serum glucose along with lipid profiles between the study group and control group differed significantly. The mean serum level of MDA in the study group was 2.65±1.33 μmol/L; in the control group it was 0.93±0.39 μmol/L; this difference was statistically significant. Male sex, longer duration of diabetes, addiction to tobacco, and presence of macrovascular and microvascular complications significantly increased the MDA level in the diabetics.
We observed an increase in MDA levels in diabetics who corroborated with the results of studies by our peers in the same field of research.[27–31] The study conducted in Lokmanya Tilak Municipal General Hospital, Mumbai, observed that freshly diagnosed diabetics under no medication had higher MDA levels across sexes compared with the findings in the control group. Researchers concluded that undetected long-term latent diabetes was the reason for the high MDA level.[32] In another study, MDA values increased with an increase in the levels of plasma glucose and duration in patients with diabetes without complications and in diabetics with ischemic heart disease (IHD).[33]
A Spanish study on children and adolescent showed that the level of estimated MDA was higher with the earlier onset and prolonged duration of diabetes, and these levels continued to rise during the course of the disease.[34] An Iranian study observed that elevated MDA levels significantly correlated to duration in diabetics with more than 10 years compared to those with comparatively lesser duration. The association between duration of MDA and DM remained significant after adjustment was made for age, gender, BMI, fasting plasma glucose, and medications.[13]
An Egyptian study reported significant elevation in MDA levels early in type 2 diabetics, before the development of secondary complications. However, no significant correlation was found between MDA levels and patients with a mean DM duration of 10 years.[35] Yet, in another study on diabetics with a mean duration of seven years had higher serum MDA concentrations compared to newly diagnosed cases.[36]
We observed that tobacco addiction had an added significant effect on the MDA level among diabetics. Bloomer found that the MDA level was raised in novice smokers, suggesting that cigarette smoking independently promoted harmful effects related to number of years of smoking.[11]
A study conducted in Thailand noted significant positive correlation between the MDA level and Fasting plasma glucose (FPG) in poorly controlled type 2 DM and type 2 DM complicated with coronary heart disease (CHD).[3] In a western Indian study, the MDA levels were significantly increased in diabetics without complications and non-diabetics with IHD.[37] In a Chinese Harbin Medical University study, the concentrations of MDA in type 2 diabetes mellitus were significantly higher than those in the controls; with retinopathy MDA was significantly raised compared to in those without retinopathy.[38] A Turkish study observed that MDA levels were significantly higher in diabetics with microalbuminuria compared with patients without microalbuminuria.[39] A Gwalior study noted that MDA levels were significantly increased with microvascular complications of diabetes.[40]
The novelty of our study was that the MDA level in type 2 DM with complications was not reported earlier in literature from Sikkim. Further, effects of ethnic variations in MDA levels were also noted among diabetics. This uniqueness will be further investigated by molecular epidemiology in the next phase of research. Moreover, we have compared the HbA1C levels of our study participants with MDA levels to help establish MDA as a marker of diseases in future. It can be considered as an additional marker of prediction of complications in type 2 diabetes.
The foremost limitation to our study concerns the use of cross-sectional data, which prevented us from drawing causal relationships. Longitudinal studies are thus required to confirm our results. Moreover, we estimated free MDA levels in serum that have inherent limitations in the analysis of results. Besides, spectrophotometric analysis of MDA content has limited specificity. Our study confirms that there is an increased oxidative stress in diabetics compared to nondiabetic counterparts and emphasizes the importance of assessing these markers for early diagnosis and therapeutic interventions in the Sikkimese population.
Our findings strongly confirmed the evidence that diabetic patients were susceptible to oxidative stress and higher blood glucose level had an association with free radical-mediated lipid peroxidation.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004;24:816–23. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Mehta JL. Role of oxidative stress in coronary heart disease. Indian Heart J. 2004;56:163–73. [PubMed] [Google Scholar]
- 3.Likidlilid A, Patchanans N, Peerapatdit T, Sriratanasathavorn C. Lipid peroxidation and antioxidant enzyme activities in erythrocytes of type 2 diabetic patients. J Med Assoc Thai. 2010;93:682–93. [PubMed] [Google Scholar]
- 4.Su Y, Liu XM, Sun YM, Jin HB, Fu R, Wang YY, et al. The relationship between endothelial dysfunction and oxidative stress in diabetes and prediabetes. Int J Clin Pract. 2008;62:877–82. doi: 10.1111/j.1742-1241.2008.01776.x. [DOI] [PubMed] [Google Scholar]
- 5.Wright E, Jr, Scism-Bacon JL, Glass LC. Oxidative stress in type 2diabetes: The role of fasting and postprandial glycaemia. Int J Clin Pract. 2006;60:308–14. doi: 10.1111/j.1368-5031.2006.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newsholme P, Haber EP, Hirabara SM, Rebelato EL, Procopio J, Morgan D, et al. Diabetes associated cell stress and dysfunction: Role of mitochondrial and non-mitochondrial ROS production and activity. J Physiol. 2007;583:9–24. doi: 10.1113/jphysiol.2007.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palinski W, Ord VA, Plump AS, Breslow JL, Steinberg D, Witztum JL. ApoE-deficient mice are a model of lipoprotein oxidation in atherogenesis.Demonstration of oxidation-specific epitopes in lesions and high titers of autoantibodies to malondialdehyde-lysine in serum. Arterioscler Thromb. 1994;14:605–16. doi: 10.1161/01.atv.14.4.605. [DOI] [PubMed] [Google Scholar]
- 8.Lazzarino G, Raatikainen P, Nuutinen M, Nissinen J, Tavazzi B, Pierro DD, et al. Myocardial release of malondialdehyde and purine compounds during coronary bypass surgery. Circulation. 1994;90:291–7. doi: 10.1161/01.cir.90.1.291. [DOI] [PubMed] [Google Scholar]
- 9.Kesavulu MM, Giri R, Kameswara Rao B, Apparao C. Lipid peroxidation and antioxidant enzyme levels in type 2 diabetics with microvascular complications. Diabetes Metab. 2000;26:387–92. [PubMed] [Google Scholar]
- 10.Peerapatdit T, Patchanans N, Likidlilid A, Poldee S, Sriratanasathavorn C. Plasma lipid peroxidation and antioxidiant nutrients in type 2 diabetic patients. J Med Assoc Thai. 2006;89:S147–55. [PubMed] [Google Scholar]
- 11.Bloomer RJ. Decreased blood antioxidant capacity and increased lipid peroxidation in young cigarett e smokers compared to nonsmokers: Impact of dietary intake. [Last accessed 2010 on October 23];Nutrition Journal. 2007 6:39. doi: 10.1186/1475-2891-6-39. Available from: http://www.nutritionj.com/content/6/1/39 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brownlee M, Cerami A, Viassara H. Advanced glycosylation end produts in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1998;318:1315–21. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- 13.Nakhjavani M, Esteghamati A, Nowroozi S, Asgarani F, Rashidi A, Khalilzadeh O. Type 2 diabetes mellitus duration: An independent predictor of serum malondialdehyde levels. Singapore Med J. 2010;51:582–5. [PubMed] [Google Scholar]
- 14.Ohtsuki T, Malasumoto M, Suzuki K, Taniguchi N, Kadanada T. Mitochondrial lipid peroxidation and superoxide dismutase in rat hypertensive target organs. Am J Physiol. 1995;268:1418–21. doi: 10.1152/ajpheart.1995.268.4.H1418. [DOI] [PubMed] [Google Scholar]
- 15.Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979;28:1039–57. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 16.WHO Technical Report Series 646. Geneva, Switzerlan: 1980. World Health Organization. Second report of the WHO expert committ ee on diabetes. [PubMed] [Google Scholar]
- 17.WHO Technical Report Series 727. Geneva, Switzerland: 1985. World Health Organization. Diabetes mellitus, report of a WHO study group. [PubMed] [Google Scholar]
- 18.Dietrich M, Block G, Norkus EP, Hudes M, Traber MG, Cross CE, et al. Smoking and exposure to environmental tobacco smoke decrease some plasma antioxidants and increase gamma-tocopherol in vivo after adjustment for dietary antioxidant intakes. Am J Clin Nutr. 2003;77:160–6. doi: 10.1093/ajcn/77.1.160. [DOI] [PubMed] [Google Scholar]
- 19.Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen receptor. Ann Clin Biochem. 1969;6:24–7. [Google Scholar]
- 20.Allian CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–5. [PubMed] [Google Scholar]
- 21.Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973;19:476–82. [PubMed] [Google Scholar]
- 22.Izzo C, Grlllo F, Murador E. Improved method for determination of high density lipoprotein cholesterol, I: Isolation of high density lipoprotein by use of polyethylene glycol 6000. Clin Chem. 1981;27:371–4. [PubMed] [Google Scholar]
- 23.Warnick GR, Knopp RH, Fitzpatrick V, Branson L. Estimating low-density lipoprotein cholesterol by the Friedewald equation is adequate for classifying patients on the basis of nationally recommended cut points. Clin Chem. 1990;36:15–9. [PubMed] [Google Scholar]
- 24.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 25.Konukoğlu D, Akçay T, Dinçer Y, Hatemi H. The susceptibility of red blood cells to autoxidation in type 2 diabetic patients with angiopathy. Metabolism. 1999;48:1481–4. doi: 10.1016/s0026-0495(99)90233-0. [DOI] [PubMed] [Google Scholar]
- 26.Volpi N, Tarugi P. Improvement in the high-performance liquid chromatography malondialdehyde level determination in normal human plasma. J Chromatogr B Biomed Sci Appl. 1998;713:433–7. doi: 10.1016/s0378-4347(98)00195-9. [DOI] [PubMed] [Google Scholar]
- 27.Ramakrishna V, Jailkhani R. Oxidative stress in non-insulin-dependent diabetes mellitus (NIDDM) patients. Acta Diabetol. 2008;45:41–6. doi: 10.1007/s00592-007-0018-3. [DOI] [PubMed] [Google Scholar]
- 28.Whiting PH, Kalansooriya A, Holbrook I, Haddad F, Jennings PE. The relationship between chronic glycaemic control and oxidative stress in type 2 diabetes mellitus. Br J Biomed Sci. 2008;65:71–4. doi: 10.1080/09674845.2008.11732800. [DOI] [PubMed] [Google Scholar]
- 29.Hanachi P, Moghadam RH, Latiffah AL. Investigation of Lipid Profiles and Lipid Peroxidation in patients with Type 2 Diabetes. Eur J Sci Res. 2009;28:6–13. [Google Scholar]
- 30.Kalaivanam KN, Dharmalingam M, Marcus SR. Lipid peroxidation in type 2 diabetes mellitus. Int J Diabetes Dev Ctries. 2006;26:30–2. [Google Scholar]
- 31.Surekha Rani H, Madhavi G, Ramachandra Rao V, Sahay BK, Jyothy A. Risk factors for coronary heart disease in type II diabetes Mellitus. Indian J Clin Biochem. 2005;20:75–80. doi: 10.1007/BF02867404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reshamwala SM, Patil ND. Biochemical changes in erythrocytemembrane in uncontrolled type 2 diabetes mellitus. Indian J Biochem Biophys. 2005;42:250–3. [PubMed] [Google Scholar]
- 33.Sundaram RK, Bhaskar A, Vijayalingam S. Antioxidant status and lipid peroxidation in type II diabetes mellitus with and without complications. Clin Sci. 1996;90:255–60. doi: 10.1042/cs0900255. [DOI] [PubMed] [Google Scholar]
- 34.Domínguez C, Ruiz E, Gussinye M. Oxidative Stress at Onset and in Early Stages of Type 1 Diabetes in Children and Adolescents. Diabetes Care. 1998;21:1736–42. doi: 10.2337/diacare.21.10.1736. [DOI] [PubMed] [Google Scholar]
- 35.Soliman GZ. Blood lipid peroxidation (superoxide dismutase, malondialdehyde, glutathione) levels in Egyptian type 2 diabetic patients. Singapore Med J. 2008;49:129–36. [PubMed] [Google Scholar]
- 36.Pasaoglu H, Sancak B, Bukan N. Lipid peroxidation and resistance to oxidation in patients with type 2 diabetes mellitus. Tohoku J Exp Med. 2004;203:211–8. doi: 10.1620/tjem.203.211. [DOI] [PubMed] [Google Scholar]
- 37.Gupta M, Suresh Chari S. Proxidant and antioxidant status in patients of type II Diabetes Mellitus with IHD. Indian J Clin Biochem. 2006;21:118–22. doi: 10.1007/BF02912925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan HZ, Zhang H, Chang D, Li H, Sui H. The change of oxidative stress products in diabetes mellitus and diabetic retinopathy. Br J Ophthalmol. 2008;92:548–51. doi: 10.1136/bjo.2007.130542. [DOI] [PubMed] [Google Scholar]
- 39.Ozdemir G, Ozden M, Maral H, Kuskay S, Cetinalp P, Tarkun I. Malondialdehyde, glutathione, glutathione peroxidase and homocysteine levels in type 2 diabetic patients with and without microalbuminuria. Ann Clin Biochem. 2005;42:99–104. doi: 10.1258/0004563053492838. [DOI] [PubMed] [Google Scholar]
- 40.Kumawat M, Pahwa MB, Gahlaut VS, Singh N. Status of Antioxidant Enzymes and Lipid Peroxidation in Type 2 Diabetes Mellitus with Micro Vascular Complications. Open Endocrinol J. 2009;3:12–5. [Google Scholar]


