Abstract
Neurogenesis is well-established to occur during adulthood in two regions of the brain, the subventricular zone (SVZ) and the subgranular zone (SGZ) of the dentate gyrus in the hippocampus. Research for more than two decades has implicated a role for adult neurogenesis in several brain functions including learning and effects of antidepressants and antipsychotics. Clear understanding of the players involved in the regulation of adult neurogenesis is emerging. We review evidence for the role of stress, dopamine (DA) and acetylcholine (ACh) as regulators of neurogenesis in the SGZ. Largely, stress decreases neurogenesis, while the effects of ACh and DA depend on the type of receptors mediating their action. Increasingly, the new neurons formed in adulthood are potentially linked to crucial brain processes such as learning and memory. In brain disorders like Alzheimer and Parkinson disease, stress-induced cognitive dysfunction, depression and age-associated dementia, the necessity to restore brain functions is enormous. Activation of the resident stem cells in the adult brain to treat neuropsychiatric disorders has immense potential and understanding the mechanisms of regulation of adult neurogenesis by endogenous and exogenous factors holds the key to develop therapeutic strategies for the debilitating neurological and psychiatric disorders.
Keywords: Acetylcholine, adult neurogenesis, activation of resident stem cells, Alzheimer disease, dopamine, Parkinson disease, stress
INTRODUCTION
The Spanish histologist, psychologist and Nobel laureate said “Once development was ended, the fonts of growth and regeneration of the axons and dendrites dried up irrevocably. In the adult centers, the nerve paths are something fixed, and immutable: Everything may die, nothing may be regenerated”.[1] Until recently, it has been generally believed that the mammalian adult central nervous system (CNS) has a limited regenerative capacity.[2] Further it was considered that the major repair mechanisms in the CNS were post-mitotic, which includes the sprouting of axon terminals, changes in the expression of neurotransmitter-receptors and synaptic reorganization, while replacement of dying/degenerating neurons was not believed to occur.[3] However, more than half a century ago, Altman and Das[4] suggested that neurogenesis continues throughout adulthood, and since then a large body of evidence has demonstrated that new cells that mature to become neurons or glia are indeed born in restricted regions of the adult mammalian CNS.
Altman and Das[4] for the first time used [3H]-thymidine autoradiography and reported the generation of new neurons in a variety of structures in the adult rat and cat including the olfactory bulb, hippocampus and cerebral cortex. Consequently, Michael Kaplan and co-authors showed [3H]-thymidine-labeled cells in the cerebral cortex, dentate gyrus (DG), olfactory bulb of adult rats and mitosis in the subventricular zone (SVZ) of adult macaque monkeys by combining [3H]-thymidine labeling and electron microscopy.[5–8] Since then adult neurogenesis has consistently been found in the SVZ of the lateral ventricle[9,10] and in the hippocampal subgranular zone (SGZ).[11–13] Studies have now confirmed that neuron production, migration and differentiation are major developmental events that continue on a smaller scale, into adult life in a wide range of species from insects to mammals.[14] Elizabeth Gould and her colleagues have found new cells with neuronal characteristics in the neocortex of adult rats and macaques[15] consistent with the earlier findings of Altman[4] and Kaplan.[5] Apart from the rodents and macaques, adult neurogenesis is also reported in the DG of tree shrews, marmoset monkeys and humans.[16–20]
The newly generated neurons in the SGZ migrate to the inner granule cell layer, rapidly extend long axonal projections along the mossy fiber pathway and reach their target CA3 pyramidal neuronal cell layer within 4-10 days after division,[21] form connections with the CA3 neurons, hilar interneurons[21] and release glutamate as their main neurotransmitter[22] thus attaining a functional significance. Newly generated cells in the adult mouse hippocampus are found to exhibit neuronal morphology and display passive membrane properties, action potentials and functional synaptic inputs similar to those found in the mature DG cells.[23] New-born granule neurons in the DG appear to first receive GABAergic synaptic inputs around 1 week after birth and then glutamatergic inputs by 2 weeks.[24,25] Young granule neurons appear to have a high input resistance and a sub-threshold Ca2+-conductance, which finally enable action potential firing with very small excitatory currents.[26] The enhanced excitability might be important for the young neurons when only a few excitatory contacts have been formed. Furthermore, new-born neurons exhibit special properties in synaptic plasticity, such as having a lower threshold for the induction of long-term potentiation (LTP)[26,27] than mature neurons. Figure 1 shows the events of neurogenesis in the DG of the hippocampus.
Figure 1.
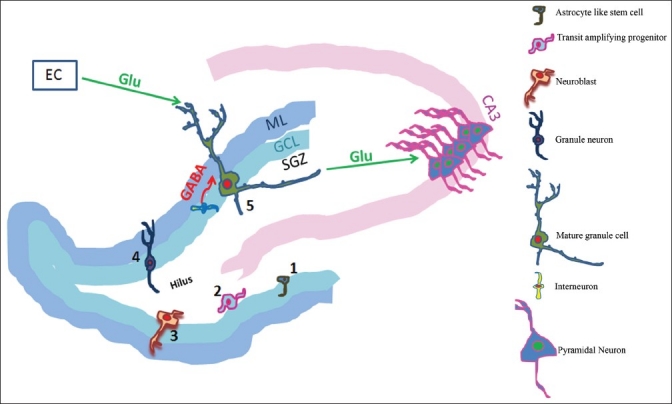
The subgranular zone (SGZ) of the dentate gyrus shelters the astrocyte like stem cells (1), which generate the transit amplifying progenitor cells (2). These transit amplifying cells mature into neuroblasts (3), which migrate (4) in the vicinity into the granule cell layer (GCL) and differentiate into granule neurons (5). These newly generated granule neurons show initial signs of functional interconnections by extending axonal projections along the mossy fiber towards the CA3, and advancing their dendritic branches into the molecular layer (ML). Once these cells mature, they receive glutamatergic (Glu) inputs from the entorhinal cortex (EC), GABAergic inputs from the interneurons and provide glutamatergic inputs to the CA3 neurons.
The discoveries of the phenomenon of adult neurogenesis lead to the search for the behavioral consequence of such a process continuing in adulthood. Subsequent to evidence for a positive relationship between adult neurogenesis and learning in the song system of canaries[28] and zebra finches,[29] several studies have looked into a plausible relationship between learning and adult neurogenesis. Mice with the fewest number of new neurons performed poorly during spatial navigation learning[30] and ablation of neurogenesis by focal irradiation impaired contextual fear conditioning and performance in a hidden platform task,[31,32] while training on associative learning tasks and reactivity to a novel environment enhanced neurogenesis in the adult hippocampus.[19,33] Conditions that increase the number of immature neurons such as estrogen,[34] environmental complexity[35] and physical exercise[36,37] also tend to enhance performance on hippocampal-dependent learning tasks[35,36,38,39] suggesting a potential link between hippocampal neurogenesis and learning. But the issue remains controversial with studies showing that some forms of learning do not recruit adult neurogenesis.[40–43]
METHODOLOGIES FOR INVESTIGATING ADULT NEUROGENESIS
The technical advances in the field of adult neurogenesis have facilitated identification of new-born neurons among the existing neurons in the adult CNS. Four approaches have been explored so far.
Analysis of the incorporation of nucleotide analogs during cell division
During DNA replication in the S-phase of the cell cycle, exogenous nucleotides such as [H3]-thymidine or bromodeoxyuridine (BrdU) are incorporated into newly synthesized DNA and then passed on to cell progeny. [H3]-thymidine is detected using autoradiography and if the exposure times and development procedures used are consistent, reliable stoichiometry can be achieved. BrdU is more extensively used to detect neurogenesis because of the possibility to detect it using immunohistochemistry (not stoichiometric) and more importantly, because it can be co-localized with histological markers for neurons or glia, thus permitting phenotypic analysis. Figure 2 shows the representative micrograph with BrdU-immunoreactivity co-localized with a neuronal (NeuN) or an astrocytic (S100β) marker. However, nucleotide analogs have some limitations. The first one being the requirement of tissue fixation and DNA denaturation that makes it unsuitable for analyzing live cells.[44] Secondly, after several cycles of cell division, dilution of BrdU to undetectable levels can occur.[45] Thirdly, BrdU or other nuclear markers stain only the cell body, so it cannot be used to analyze the synaptic properties of new-born neurons.[46] In addition we must remember that BrdU or [H3]-thymidine incorporation is an indication of DNA synthesis only and not cell division. Incorporation of nucleotide analogs also occurs into nicked, damaged DNA undergoing repair also, although on a smaller scale than during DNA replication.[47] Thus the dose and duration of BrdU pulsing, as well as the detection of BrdU, need to be appropriately controlled to avoid misidentification of repairing/dying cells as new-born cells. A few studies have also used iododeoxyuridine (IdU) and chlorodeoxyuridine (CldU), thymidine analogs with a structure similar to BrdU, to label newly generated neurons.[48–52] However, these analogs do not seem to be as sensitive as BrdU in being incorporated into the newly-born cells at doses similar to those of a saturating BrdU dose.[53] Thus BrdU has been the most widely– and reliably–used marker for survival and differentiation studies in adult neurogenesis.
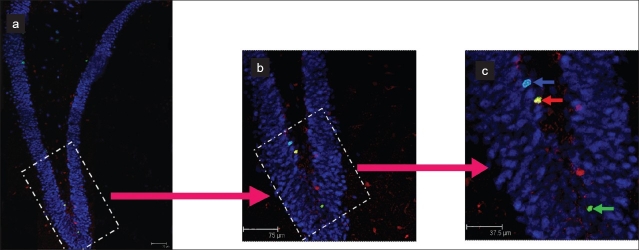
Figure 2.
Representative confocal images of triple labeling for differentiation studies. (a) is the reconstructed image of dentate gyrus in the dorsal hippocampus. (b) represents the 4X zoomed image of the region marked in white dotted rectangle in a, and c represents 8X zoomed image of the region marked in b. The blue arrow in c represents the BrdU-ir cell colocalized with NeuN (BrdU+/NeuN+, appears cyan), the red arrow points at the BrdU-ir cell colocalized with S100β (BrdU+/S100β+, appears yellow) and the green arrow indicates a BrdU-ir cell which is not colocalized with either NeuN nor S100β (BrdU+, appears green; potentially undifferentiated). Scale bar = 75μm in a and b, 37.5μm in c.
Analysis based on expression of specific markers
Developing neurons express distinct markers during their maturation.[54] Proliferating cells are labeled using Ki67, an endogenous marker expressed during most phases of the cell cycle including the S-phase[55,56] or by PCNA (proliferating cell nuclear antigen) that labels cells undergoing division. For immature neurons, PSA-NCAM (poly-sialylated-neural cell-adhesion molecule), Tuj1 (β-tubulin isoform III), CRMP (collaspin response-mediated protein 4, also known as TOAD4), NeuroD and DCX (doublecortin) are commonly used.[23] Among the markers used for mature neurons are MAP-2ab (microtubule associated protein-2 a and b isoforms),[57] NeuN (neuronal nuclei),[11] and Prox-1 (a dentate gyrus cell marker)[58] calbindin[11] and calretinin.[59] New-born neurons can be identified by the presence of immature markers and absence of mature markers of neurons. The requirement for this approach relates to the specificity of the markers since some antibodies to these markers (e.g., Tuj1) also stain non-neuronal cells[60] and some markers (e.g., PSA-NCAM) are re-expressed in pre-existing neurons[61] under certain conditions.
Analysis based on genetic marking with retroviruses
The main drawback with the markers such as BrdU is the inability to perform functional studies. This was overcome by van Praag and colleagues[46] who showed that the new-born cells expressing neuronal markers in the SGZ become functional neurons, using retroviral vector expressing green fluorescent protein (GFP). The use of replication-incompetent retroviruses allow specific labeling of dividing cells and their progeny since they are unable to infect post-mitotic cells, such as neurons.[62] The expression of transgenes from retroviruses requires viral integration into the host genome.[63] For retroviruses that lack nuclear import mechanisms, such as the Muloney murine leukemia virus, viral integration occurs only when the nuclear membrane breaks down during mitosis[63] and serves as a good indicator of cell division. The strategy used to detect neurogenesis is to incorporate the coding sequences of histochemical markers such as GFP into the retroviruses. Since the marker sequence is integrated into the host genome, the problem of dilution (that is seen with BrdU) is eliminated. Retroviruses deliver single copies of the transgenes into their target cells, thus allowing low expression of these proteins since high expression could interfere with the development or function of the synapses.[64] The most important advantage with the retroviral method is that it permits the live imaging of the cells in brain slices allowing the analysis of the structural and functional integration into the host circuitry and also map the physiological maturation of these new-born neurons.[65] The retroviral technique can also be used to knockout specific proteins in the new-born cells, in combination with Cre-loxP technology,[66] or use shRNA[24,67,68] to inhibit expression of the particular protein, only in the labeled cells. However, this method is also not devoid of disadvantages. These retroviruses have to be stereotaxically introduced into the brain and the inflammation due to the injection could alter neurogenesis.[65] Further, fusion of microglia with existing post-mitotic neurons have been reported after combination of retroviruses with microglia.[69] Retroviruses are silenced in certain host cells and the two daughter cells from the same parent cell could undergo differential silencing or could be silenced during differentiation.[70]
Analysis based on fluorescent protein expression under the control of promoter for specific proteins
The generation of transgenic mice expressing fluorescent protein under the control of promoter for specific protein allows visualization and specific manipulation of new-born neurons in the adult CNS with out the disadvantages of stereotaxic surgery and retroviral injection.[71,72] Adult mice expressing GFP under the control of the regulatory regions of the nestin gene reveal both neural progenitors and some immature neurons.[72] With GFP expressed under the transcriptional control of the pro–opiomelanocortin (POMC) genomic sequences, a population of newly-born granule cells of the DG is selectively labeled.[71] In a recent study,[73] GFP expressed under the control of the GAD67 promoter surprisingly labeled only newly-born neurons in the SGZ without labeling the new-born neurons in the SVZ/RMS. These GFP-expressing neurons exhibited histological markers and electrophysiological properties similar to that of immature neurons. Similar to the retroviral technique, this allows the analysis of the morphology of new-born neurons. However, the disadvantage is that most of these markers are expressed only during early maturation.[73,74]
In an interesting breakthrough, a transgenic mouse line with tamoxifen-inducible recombinant CreERT2 under the control of BAC-based promoter of DCX has been described.[75,76] In the DCX-CreER mice, the recombination is temporally controlled by the administration of tamoxifen and the BAC-based promoter of DCX spatially restricts it to the new-born neurons. The labeling is permanent once the recombination occurs and the stable fluorescent protein expression permits detailed morphological and electrophysiological analysis.[75]
Thus, more tools are emerging to study neurogenesis, particularly the functional properties of the new-born neurons. Channelrhodopsin or halorhodopsin could be potentially expressed in a population of the new-born neurons to study the properties of the new-born neurons in vivo or how different factors affect neurogenesis.[77]
FACTORS INFLUENCING ADULT NEUROGENESIS
The rate of neurogenesis is modulated by various physiological and pathological conditions. The newly generated cells may have a function in cognition and brain repair mechanisms. Enriched environment and exercise increases neurogenesis and is associated with improved memory functioning and enhanced synaptic plasticity. Several pathological conditions are known to modulate hippocampal neurogenesis. Neurological diseases, stroke and traumatic brain injury favour neurogenesis, which could be aimed at promoting recovery.[78] On the other hand, ageing,[78] social isolation,[79] alcohol consumption,[80] odor deprivation and maternal deprivation stress are found to have negative effects on neurogenesis in the DG.[81] In this review, we highlight the key findings regarding the effects of stress on adult neurogenesis. While considerable research has been done on the regulation of adult neurogenesis by acetylcholine (ACh) and dopamine (DA), we still do not have a comprehensive understanding of their roles. Accordingly, we review the evidence for the regulation of adult neurogenesis by ACh and DA, which also play an important role in learning and memory and is known to be affected by chronic stress and depression.
STRESS AND NEUROGENESIS
The maintenance of homeostasis is one of the key features in normal physiological functioning and stress alters this homeostasis. When stress is severe and prolonged, it can result in impairment in learning and memory and precipitate affective disorders like depression. Chronic exposure of rodents to physical stress or exposure of nonhuman primates to psychosocial stress is reported to cause atrophy of CA3 pyramidal neurons in the hippocampus,[82–88] increase glucocorticoid secretion, activate release of excitatory amino acids, decrease DA levels and increase DA turnover in the hippocampus[89–91] and decrease AChE activity in the hippocampus.[85,89,92]
With respect to hippocampal adult neurogenesis, stress is largely detrimental independent of stressor, species or life stage.[93] Adult neurogenesis is decreased by different types of stressors, including predator odor,[94] social stress,[17,95] acute and chronic restraint stress,[96–100] footshock stress[100,101] and chronic mild stress.[102] It has been observed that different stressors including fox odor, subordination and physical restraint decreases the proliferation and survival of new-born neurons in the adult SGZ of many mammalian species, including rats, tree shrews and marmosets.[15] Proliferation in adult monkeys is diminished in a resident intruder model of stress[18] and in a chronic mild stress model in Wistar rats.[103] Restraint stress of pregnant rats and acoustic startle stress of pregnant macaques produced a lasting suppression of cell proliferation in the DG of the offspring[104,105] and extends into adulthood in rats.[105] Maternal separation in rats during the early post-natal period inhibits cell proliferation and the production of immature neurons in the DG of the adult offspring.[81]
Studies show that stress affects the entire process of neurogenesis. Chronic restraint stress for 3 weeks suppressed cell proliferation[97,98] while acute restraint stress for 2 h did not show any major changes.[96] Rats subjected to chronic or intense uncontrollable stress in adulthood also exhibited prolonged inhibition of cell proliferation in the DG.[101,106,107] By contrast, rats subjected to acute stress in adulthood appear to exhibit a recovery of baseline cell proliferation by the following day.[106] Following chronic stress, increases in the cell cycle inhibitor p27Kip1 parallels the decreased proliferation and apoptosis, indicating that more cells had entered cell cycle arrest and that the granule cell turnover had thus slowed down.[108,109]
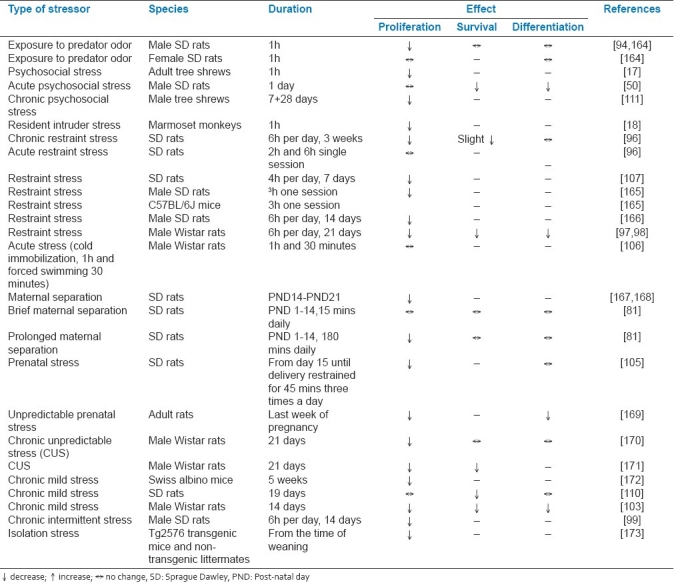
Chronic mild stress or chronic restraint stress was shown to decrease survival of new-born cells in adult rat hippocampus.[97,98,110] Acute psychosocial stress diminishes both short-term and long-term survival of newly differentiated DG neurons.[50,111] Further, stress is shown to differentially affect the differentiation of the newly formed cells. While a few studies support suppression in production of new neurons,[95,96,112] others report enhanced survival and unaffected neuronal maturation[94,101] following stress. All these evidences suggest that stress more often than not has a detrimental effect on hippocampal neurogenesis. Table 1 summarizes the effects of different stressors on the different stages of neurogenesis in the adult DG.
Table 1.
Summary of the effects of various forms of stress on neurogenesis
Although the cascade of events leading to a reduction in adult neurogenesis in the DG following stress is far from understood, substantial evidence suggests that stress hormones play an important role. Gould et al[113] showed that adrenal steroids probably underlie this effect as stress increases adrenal steroid levels and glucocorticoids decrease the rate of neurogenesis.[113] Cameron and McKay extended these findings by showing that the decreased level of neurogenesis in the DG during ageing[11] is due to an increase in glucocorticoid levels and that adrenalectomy could reverse the decline in DG neurogenesis.[114] Increased exogenous corticosterone suppresses neurogenesis both during the early post-natal period and in adulthood.[115,116] By contrast, adrenalectomy with low-dose corticosterone replacement in the drinking water, which prevents the stress-induced rise in corticosterone while maintaining its diurnal rhythm, eliminates the stress’ effect on neurogenesis in the adult DG.[81,94] Collectively, these evidences suggest that stress inhibits cell proliferation and formation of new neurons by increasing glucocorticoids, at least in part.
Also, decreased neurogenesis in response to stress or corticosterone can be blocked by pre-treatment with an N-methyl-D-aspartate (NMDA) receptor antagonist, demonstrating a role for enhanced glutamate transmission.[117] Both, acute treatment with NMDA receptor antagonists and lesion of the entorhinal cortex independently increased the birth of cells in the DG.[118] An earlier study showed that the neuronal vulnerability to chronic restraint stress can be attenuated by entorhinal glutamatergic denervation.[83] Thus, the enhanced glutamate transmission following stress could also contribute to decreased hippocampal neurogenesis. Apart from these, stress is also reported to decrease levels of growth factors including brain-derived neurotrophic factor (BDNF), basic fibroblast growth factor (bFGF), insulin-like growth factor–1 (IGF-1), that are involved in the regulation of adult neurogenesis[119] and various neurochemical changes that could contribute to decreased neurogenesis.[120]
Numerous studies have linked adult neurogenesis with functions of the hippocampus, including learning and memory,[121] as well as with the development of psychopathology and recovery from brain damage. The studies reviewed thus far present the possibility that stress-induced changes in neurogenesis may have cumulative effects that ultimately alter one or more of these brain processes.
NEUROTRANSMITTERS AND NEUROGENESIS
Amongst the myriad of extrinsic factors, many afferent inputs and various neurotransmitters, including classic (such as DA, serotonin, ACh, and glutamate), peptide (e.g., PACAP) and gaseous (e.g., nitric oxide) neurotransmitters have also been implicated in regulating adult neurogenesis.[23,30] The role of glutamate, GABA, serotonin and other neurotransmitters are reviewed elsewhere.[122–125] We discuss the specific role of ACh and DA in the regulation of adult neurogenesis.
Acetylcholine
The hippocampal formation receives abundant regulatory inputs from the basal forebrain cholinergic system, and ACh plays an important role both in learning and in the cognitive deficits associated with ageing and Alzheimer disease (AD).[126,127] Since the cholinergic system is closely associated with learning and memory and evidences have suggested a probable role for neurogenesis in learning, a few studies have investigated the role of ACh on the formation of new hippocampal neurons and its relation to learning and memory. Kaneko et al[128] showed using vChAT, a marker for the cholinergic nerve terminals that a high density of cholinergic innervation is present at the SGZ and the inner granule cell layer. Further, these cholinergic fibers made contact with the PSA-NCAM-positive neurons indicating that these new cells could potentially respond to cholinergic inputs. Neurotoxic lesion of forebrain cholinergic inputs with 192IgG Saporin was found to decrease proliferation, short-term survival,[129] and differentiation of the new-born cells into neurons[130] and increase apoptosis[130] in the hippocampus. This was associated with impaired performance in the water maze task.[129] Partial excitotoxic lesioning of the medial septum by infusion of NMDA significantly reduced survival of newly generated neurons by approximately 40% while the proliferation of progenitors remained unaffected.[131]
Kotani et al[132] showed that chronic treatment with donepezil but not scopolamine increased, the number of BrdU-positive cells in the DG compared to controls without affecting differentiation. Systemic administration of the cholinergic agonist physostigmine increased DG neurogenesis and the new cells were found to express muscarinic M1 and M4 receptors.[129] In an interesting study, it was shown that the muscarinic agonist, oxotremorine rescues the impaired neurogenesis induced by forebrain cholinergic lesions.[133] Homopentameric α7–containing and heteropentameric β2–containing nictonic ACh receptors (nAChR) are expressed by the new-born cells.[128,134] Chronic nicotine exposure results in decreased proliferation and β2 nAChR-knock-out mice display lesser proliferation, but normal survival.[135,136] Recently, it was shown that α7–nAChR are involved in the survival, and morpho-functional maturation of the new-born neurons.[137] These studies suggest that alterations in the cholinergic system plays an important role in the regulation of adult neurogenesis in the hippocampus.
Dopamine
DA is an important neurotransmitter implicated in the regulation of mood, motivation and movement.[137,138] In addition to being a neurotransmitter, it is also found to play a role in the regulation of endogenous neurogenesis in the adult mammalian brain. Early during embryonic development, DA regulates neural precursor cell proliferation[138] and its receptors appear in the highly proliferative germinal zones of the brain.[139,140]
DA has been shown to either activate or inhibit, proliferation of precursor cells in the lateral ganglionic eminence through D1- and D2-like receptors.[141,142] Immunohistochemical and electron microscopy studies in the SVZ have shown that D2-like DAergic receptors are expressed predominately on C-cells (frequently dividing transit-amplifying cells), whereas A-cells (PSA-NCAM- positive-restricted neural precursors) express both D1- and D2-like receptors.[143] Furthermore, immunohistochemical studies with both confocal and electron microscopy[143] have demonstrated that C-cells in the adult SVZ are lodged in a rich network of dopaminergic afferents that form synapse-like structures.
Neurospheres have been demonstrated to express D1- and D2-like DAergic receptors.[143–145] Treatment of neurospheres with bromocriptine and apomorphine, significantly increased cell proliferation[143,144] which was blocked by the D2-like antagonist sulpiride,[143,144] suggesting that it was indeed mediated via the D2-like receptors. Neurotoxic lesion of the dopaminergic innervation to the forebrain in mice and rats using MPTP and 6-hydroxydopamine (6-OHDA) decreases global cell proliferation in the SVZ by 30–45%.[143,146,147] While high levels of D3 receptor expression persists in the germinal SVZ,[139] 7-hydroxy-N,N-di-n-propyl-2-aminotetralin (7-OH-DPAT), a preferential D3 receptor agonist does not alter neurogenesis in the SVZ of adult mice following intraventricular infusion.[148]
The inhibition of dopaminergic transmission in adult rats in vivo using the D2-like antagonist haloperidol increased the proliferation of forebrain precursor cells on one hand, while did not alter the number of BrdU-labeled subependymal cells on the other.[145,149] In the same study, the authors also show that addition of DA or quinpirole (a selective D2/3 receptor agonist), but not SKF 38393 (a selective D1 receptor agonist), to neurosphere cultures derived from wild-type mice produced a dose-dependent (and approaching complete) inhibition of neurosphere formation.[145] D2-like agonists like ropinirole or 7-OH-DPAT and levodopa increases precursor cell proliferation in the SVZ in both control and lesioned animals.[143,150]
The presence of DA transporter (DAT)–positive DAergic fibers was reported to be present near a BrdU–positive cell in the SGZ.[143] It was also reported that following MPTP lesion of the nigrostriatal pathway, there is decreased proliferation until 7 days following the lesion.[143] After the administration of haloperidol, no effect,[151,152] decrease,[149] or increase[153] in the proliferation in the SGZ have all been reported. Further, survival and differentiation in the DG is either not affected[152,154] or increased[155] following haloperidol administration. Activation of D2 receptors by quinpirole increased the cell proliferation in the SGZ[156] and in vitro and in vivo experiments show that the effects of quinpirole are mediated by the ciliary neurotrophic factor (CNTF).[156] In animal models of schizophrenia, one study reported that haloperidol restores[153] proliferation while the other did not.[157] Proliferation of the precursor cells in the SGZ decreased following unilateral lesion of substantia nigra pars compacta (SNpc) by 6-OHDA and was restored with subchronic fluoxetine, a selective serotonin reuptake inhibitor but not with maprotiline, a selective norepinephrine reuptake inhibitor.[158] MPTP-lesioned mice had lesser number of PCNA–positive cells indicating decreased precursor cell proliferation in the SGZ.[143] Contrastingly in another study, there was a transient increase in cell proliferation 14 days post-ablation specifically with the amplifying neural progenitor cells and post-mitotic progeny, with no such effect seen after 4 days post-ablation.[159] However, this increase was restored to normal by 30 days of post-ablation. L-DOPA per se did not affect progenitor cell division in the DG and its administration immediately following MPTP treatment did not alter the effect of MPTP. Further, lesion alone did not significantly change the maturation of the precursors, but L-DOPA administration following the lesion enhanced the maturation.[159] Although this suggests a complex regulatory role of DA on neurogenesis, general compensatory mechanisms could also be responsible for the transient activation of neurogenesis following the lesion. Definitely more studies are required to clearly understand the role of DA on neurogenesis in the SGZ.
Evidence for reduced neurogenesis in the SVZ and SGZ in the postmortem brains of Parkinson disease (PD) patients[143] might underlie some of the non-motor cognitive symptoms in PD. A large body of converging data consistently shows that DA stimulates endogenous adult neurogenesis in the SVZ by activating D2-like receptors on transit-amplifying progenitor cells. Further, as precursor cells in the adult mammalian brain are pharmacologically accessible to the systemic administration of dopamimetic drugs, the stimulation of endogenous neurogenesis appears to be a potential strategy for a cell replacement therapy of the brain in diseases in which progenitor cells appear to contribute to repair processes.[160–163]
PERSPECTIVES
Although considerable research has been carried out on adult neurogenesis in the past few years, we are only beginning to have a clear understanding as to how adult neurogenesis is regulated in health and disease, and what its functional implications are. With novel genetic tools available to study the properties of the new-born neurons, we could hope for a clearer picture of the players involved in the regulation of adult neurogenesis in the future. Insight into the basic process and molecular mechanisms of adult neurogenesis not only enriches our knowledge about the unique plastic properties of the adult brain, but also could significantly impact cell replacement therapies for psychiatric and degenerative neurological disorders.
ACKNOWLEDGMENTS
Our work described in this manuscript was supported by grants from Department of Science and Technology (DST) and Department of Biotechnology (DBT), India to BSSR and from Council for Scientific and Industrial Research (CSIR), India to JV.
Footnotes
Source of Support: Department of Science and Technology (DST) and Department of Biotechnology (DBT), India to BSSR and from Council for Scientific and Industrial Research (CSIR), India to JV.
Conflict of Interest: None declared.
REFERENCES
- 1.Ramon Y, Cajal S. Degeneration and regeneration of the nervous system. New York: 1928. [Google Scholar]
- 2.Ortega-Perez I, Murray K, Lledo PM. The how and why of adult neurogenesis. J Mol Histol. 2007;38:555–62. doi: 10.1007/s10735-007-9114-5. [DOI] [PubMed] [Google Scholar]
- 3.Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. Neurogenesis in the adult brain: New strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- 4.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–35. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan MS, Hinds JW. Neurogenesis in the adult rat: Electron microscopic analysis of light radioautographs. Science. 1977;197:1092–4. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan MS. Neurogenesis in the 3-month-old rat visual cortex. J Comp Neurol. 1981;195:323–38. doi: 10.1002/cne.901950211. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan MS, Bell DH. Mitotic neuroblasts in the 9-day-old and 11-month-old rodent hippocampus. J Neurosci. 1984;4:1429–41. doi: 10.1523/JNEUROSCI.04-06-01429.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan MS. Formation and turnover of neurons in young and senescent animals: An electronmicroscopic and morphometric analysis. Ann NY Acad Sci. 1985;457:173–92. doi: 10.1111/j.1749-6632.1985.tb20805.x. [DOI] [PubMed] [Google Scholar]
- 9.varez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22(3):629–34. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–10. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 11.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–33. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmer TD, Takahashi J, Gage FH. The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci. 1997;8:389–404. doi: 10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
- 13.Gage FH, Coates PW, Palmer TD, Kuhn HG, Fisher LJ, Suhonen JO, et al. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci U S A. 1995;92:11879–83. doi: 10.1073/pnas.92.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cayre M, Malaterre J, Scotto-Lomassese S, Strambi C, Strambi A. The common properties of neurogenesis in the adult brain: From invertebrates to vertebrates. Comp Biochem Physiol B Biochem Mol Biol. 2002;132:1–15. doi: 10.1016/s1096-4959(01)00525-5. [DOI] [PubMed] [Google Scholar]
- 15.Gould E, Gross CG. Neurogenesis in adult mammals: Some progress and problems. J Neurosci. 2002;22:619–23. doi: 10.1523/JNEUROSCI.22-03-00619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–7. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 17.Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–8. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci U S A. 1998;95:3168–71. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–5. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 20.Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci U S A. 1999;96:5768–73. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hastings NB, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. J Comp Neurol. 1999;413:146–54. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 22.Toni N, Teng EM, Bushong EA, Aimone JB, Zhao C, Consiglio A, et al. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci. 2007;10:727–34. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- 23.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–50. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 24.Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–93. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ge S, Sailor KA, Ming GL, Song H. Synaptic integration and plasticity of new neurons in the adult hippocampus. J Physiol. 2008;586:3759–65. doi: 10.1113/jphysiol.2008.155655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–7. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 27.Wang HL, Tsai LY, Lee EH. Corticotropin-releasing factor produces a protein synthesis-dependent long-lasting potentiation in dentate gyrus neurons. J Neurophysiol. 2000;83:343–9. doi: 10.1152/jn.2000.83.1.343. [DOI] [PubMed] [Google Scholar]
- 28.Alvarez-Buylla A, Theelen M, Nottebohm F. Birth of projection neurons in the higher vocal center of the canary forebrain before, during, and after song learning. Proc Natl Acad Sci U S A. 1988;85:8722–6. doi: 10.1073/pnas.85.22.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nordeen KW, Nordeen EJ. Projection neurons within a vocal motor pathway are born during song learning in zebra finches. Nature. 1988;334:149–51. doi: 10.1038/334149a0. [DOI] [PubMed] [Google Scholar]
- 30.Kempermann G, Gage FH. Genetic determinants of adult hippocampal neurogenesis correlate with acquisition, but not probe trial performance, in the water maze task. Eur J Neurosci. 2002;16:129–36. doi: 10.1046/j.1460-9568.2002.02042.x. [DOI] [PubMed] [Google Scholar]
- 31.Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–6. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–52. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 33.Lemaire V, Aurousseau C, Le MM, Abrous DN. Behavioural trait of reactivity to novelty is related to hippocampal neurogenesis. Eur J Neurosci. 1999;11:4006–14. doi: 10.1046/j.1460-9568.1999.00833.x. [DOI] [PubMed] [Google Scholar]
- 34.Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–5. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 36.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–70. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 38.Leuner B, Mendolia-Loffredo S, Shors TJ. High levels of estrogen enhance associative memory formation in ovariectomized females. Psychoneuroendocrinology. 2004;29:883–90. doi: 10.1016/j.psyneuen.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm Behav. 1998;34:149–62. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- 40.Madsen TM, Kristjansen PE, Bolwig TG, Wortwein G. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–42. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 41.Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, et al. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci. 2006;9:729–31. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- 42.Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 43.Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 2001;85:2423–31. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- 44.Rakic P. Neurogenesis in adult primate neocortex: An evaluation of the evidence. Nat Rev Neurosci. 2002;3:65–71. doi: 10.1038/nrn700. [DOI] [PubMed] [Google Scholar]
- 45.Hayes NL, Nowakowski RS. Dynamics of cell proliferation in the adult dentate gyrus of two inbred strains of mice. Brain Res Dev Brain Res. 2002;134:77–85. doi: 10.1016/s0165-3806(01)00324-8. [DOI] [PubMed] [Google Scholar]
- 46.van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–4. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Selden JR, Dolbeare F, Clair JH, Nichols WW, Miller JE. Statistical confirmation that immunofluorescent detection of DNA repair in human fibroblasts by measurement of bromodeoxyuridine incorporation is stoichiometric and sensitive. Cytometry. 1993;14:154–67. doi: 10.1002/cyto.990140207. [DOI] [PubMed] [Google Scholar]
- 48.Dupret D, Fabre A, Dobrossy MD, Panatier A, Rodriguez JJ, Lamarque S, et al. Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biol. 2007;5:e214. doi: 10.1371/journal.pbio.0050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vega CJ, Peterson DA. Stem cell proliferative history in tissue revealed by temporal halogenated thymidine analog discrimination. Nat Methods. 2005;2:167–9. doi: 10.1038/nmeth741. [DOI] [PubMed] [Google Scholar]
- 50.Thomas RM, Hotsenpiller G, Peterson DA. Acute psychosocial stress reduces cell survival in adult hippocampal neurogenesis without altering proliferation. J Neurosci. 2007;27:2734–43. doi: 10.1523/JNEUROSCI.3849-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burns KA, Kuan CY. Low doses of bromo- and iododeoxyuridine produce near-saturation labeling of adult proliferative populations in the dentate gyrus. Eur J Neurosci. 2005;21:803–7. doi: 10.1111/j.1460-9568.2005.03907.x. [DOI] [PubMed] [Google Scholar]
- 52.Bauer S, Patterson PH. The cell cycle-apoptosis connection revisited in the adult brain. J Cell Biol. 2005;171:641–50. doi: 10.1083/jcb.200505072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leuner B, Glasper ER, Gould E. Thymidine analog methods for studies of adult neurogenesis are not equally sensitive. J Comp Neurol. 2009;517:123–33. doi: 10.1002/cne.22107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–52. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 55.Kee N, Sivalingam S, Boonstra R, Wojtowicz JM. The utility of Ki-67 and brdu as proliferative markers of adult neurogenesis. J Neurosci Methods. 2002;115:97–105. doi: 10.1016/s0165-0270(02)00007-9. [DOI] [PubMed] [Google Scholar]
- 56.Eisch AJ, Mandyam CD. Adult neurogenesis: Can analysis of cell cycle proteins move us “Beyond Brdu”? Curr Pharm Biotechnol. 2007;8:147–65. doi: 10.2174/138920107780906540. [DOI] [PubMed] [Google Scholar]
- 57.Zigova T, Pencea V, Betarbet R, Wiegand SJ, Alexander C, Bakay RA, et al. Neuronal progenitor cells of the neonatal subventricular zone differentiate and disperse following transplantation into the adult rat striatum. Cell Transplant. 1998;7:137–56. doi: 10.1177/096368979800700209. [DOI] [PubMed] [Google Scholar]
- 58.Liu M, Pleasure SJ, Collins AE, Noebels JL, Naya FJ, Tsai MJ, et al. Loss of BETA 2/ neuro D leads to malformation of the dentate gyrus and epilepsy. Proc Natl Acad Sci U S A. 2000;97:865–70. doi: 10.1073/pnas.97.2.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Corotto FS, Henegar JA, Maruniak JA. Neurogenesis persists in the subependymal layer of the adult mouse brain. Neurosci Lett. 1993;149:111–4. doi: 10.1016/0304-3940(93)90748-a. [DOI] [PubMed] [Google Scholar]
- 60.Katsetos CD, Del VL, Geddes JF, Assimakopoulou M, Legido A, Boyd JC, et al. Aberrant localization of the neuronal class iii beta III- tubulin in astrocytomas. Arch Pathol Lab Med. 2001;125:613–24. doi: 10.5858/2001-125-0613-ALOTNC. [DOI] [PubMed] [Google Scholar]
- 61.Charles P, Reynolds R, Seilhean D, Rougon G, Aigrot MS, Niezgoda A, et al. Re-expression of PSA-NCAM by demyelinated axons: An inhibitor of remyelination in multiple sclerosis? Brain. 2002;125:1972–9. doi: 10.1093/brain/awf216. [DOI] [PubMed] [Google Scholar]
- 62.Roe T, Reynolds TC, Yu G, Brown PO. Integration of murine leukemia virus DNA depends on mitosis. EMBO J. 1993;12:2099–108. doi: 10.1002/j.1460-2075.1993.tb05858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lewis PF, Emerman M. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J Virol. 1994;68:510–6. doi: 10.1128/jvi.68.1.510-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kelsch W, Lin CW, Lois C. Sequential development of synapses in dendritic domains during adult neurogenesis. Proc Natl Acad Sci U S A. 2008;105:16803–8. doi: 10.1073/pnas.0807970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao C. Retrovirus-mediated cell labeling. In: Gage FH, Kempermann G, Song H, editors. Adult neurogenesis. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2008. pp. 101–18. [Google Scholar]
- 66.Tashiro A, Zhao C, Gage FH. Retrovirus-mediated single-cell gene knockout technique in adult newborn neurons in vivo. Nat Protoc. 2006;1:3049–55. doi: 10.1038/nprot.2006.473. [DOI] [PubMed] [Google Scholar]
- 67.Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, et al. Disrupted-in-schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–58. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim PM, Duan X, Huang AS, Liu CY, Ming GL, Song H, et al. Aspartate racemase, generating neuronal D-aspartate, regulates adult neurogenesis. Proc Natl Acad Sci U S A. 2010;107:3175–9. doi: 10.1073/pnas.0914706107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ackman JB, Siddiqi F, Walikonis RS, LoTurco JJ. Fusion of microglia with pyramidal neurons after retroviral infection. J Neurosci. 2006;26:11413–22. doi: 10.1523/JNEUROSCI.3340-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ellis J. Silencing and variegation of gammaretrovirus and lentivirus vectors. Hum Gene Ther. 2005;16:1241–6. doi: 10.1089/hum.2005.16.1241. [DOI] [PubMed] [Google Scholar]
- 71.Overstreet LS, Hentges ST, Bumaschny VF, de Souza FS, Smart JL, Santangelo AM, et al. A transgenic marker for newly born granule cells in dentate gyrus. J Neurosci. 2004;24:3251–9. doi: 10.1523/JNEUROSCI.5173-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamaguchi M, Saito H, Suzuki M, Mori K. Visualization of neurogenesis in the central nervous system using nestin promoter-gfp transgenic mice. Neuroreport. 2000;11:1991–6. doi: 10.1097/00001756-200006260-00037. [DOI] [PubMed] [Google Scholar]
- 73.Zhao S, Zhou Y, Gross J, Miao P, Qiu L, Wang D, et al. Fluorescent labeling of newborn dentate granule cells in GAD 67- GFP transgenic mice: A genetic tool for the study of adult neurogenesis. PLoS One. 2010;5:e12506. doi: 10.1371/journal.pone.0012506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Crino PB, Trojanowski JQ, Eberwine J. Internexin, MAP 1B, and nestin in cortical dysplasia as markers of developmental maturity. Acta Neuropathol. 1997;93:619–27. doi: 10.1007/s004010050660. [DOI] [PubMed] [Google Scholar]
- 75.Cheng X, Li Y, Huang Y, Feng X, Feng G, Xiong ZQ. Pulse labeling and long-term tracing of newborn neurons in the adult subgranular zone. Cell Res. 2011;21:338–49. doi: 10.1038/cr.2010.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jablonska B, Aguirre A, Raymond M, Szabo G, Kitabatake Y, Sailor KA, et al. Chordin-induced lineage plasticity of adult SVZ neuroblasts after demyelination. Nat Neurosci. 2010;13:541–50. doi: 10.1038/nn.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang Z, Ming GL, Song H. Genetically targeting new neurons in the adult hippocampus. Cell Res. 2011;21:220–2. doi: 10.1038/cr.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abrous DN, Koehl M, Le MM. Adult neurogenesis: From precursors to network and physiology. Physiol Rev. 2005;85:523–69. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- 79.Lu L, Bao G, Chen H, Xia P, Fan X, Zhang J, et al. Modification of hippocampal neurogenesis and neuroplasticity by social environments. Exp Neurol. 2003;183:600–9. doi: 10.1016/s0014-4886(03)00248-6. [DOI] [PubMed] [Google Scholar]
- 80.Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem. 2002;83:1087–93. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- 81.Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat Neurosci. 2004;7:841–6. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- 82.Govindarajan A, Shankaranarayana Rao BS, Nair D, Trinh M, Mawjee N, Tonegawa S, et al. Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc Natl Acad Sci U S A. 2006;103:13208–13. doi: 10.1073/pnas.0605180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sunanda, Meti BL, Raju TR. Entorhinal cortex lesioning protects hippocampal CA3 neurons from stress-induced damage. Brain Res. 1997;770:302–6. doi: 10.1016/s0006-8993(97)00888-3. [DOI] [PubMed] [Google Scholar]
- 84.Sunanda, Rao MS, Raju TR. Effect of chronic restraint stress on dendritic spines and excrescences of hippocampal CA3 pyramidal neurons-a quantitative study. Brain Res. 1995;694:312–7. doi: 10.1016/0006-8993(95)00822-8. [DOI] [PubMed] [Google Scholar]
- 85.Ramkumar K, Srikumar BN, Rao BS Shankaranarayana, Raju TR. Self-stimulation rewarding experience restores stress-induced CA3 dendritic atrophy, spatial memory deficits and alterations in the levels of neurotransmitters in the hippocampus. Neurochem Res. 2008;33:1651–62. doi: 10.1007/s11064-007-9511-x. [DOI] [PubMed] [Google Scholar]
- 86.McEwen BS, Conrad CD, Kuroda Y, Frankfurt M, Magarinos AM, McKittrick C. Prevention of stress-induced morphological and cognitive consequences. Eur Neuropsychopharmacol. 1997;7:S323–8. doi: 10.1016/s0924-977x(97)00064-3. [DOI] [PubMed] [Google Scholar]
- 87.Rao BS Shankaranarayana, Madhavi R, Sunanda, Raju TR. Complete reversal of dendritic atrophy in CA3 neurons of the hippocampus by rehabilitation in restraint stressed rats. Current Science. 2001;80:653–9. [Google Scholar]
- 88.Vyas A, Mitra R, Rao BS Shankaranarayana, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–8. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sunanda, Rao BS Shankaranarayana, Raju TR. Restraint stress-induced alterations in the levels of biogenic amines, amino acids, and AChE activity in the hippocampus. Neurochem Res. 2000;25:1547–52. doi: 10.1023/a:1026606201069. [DOI] [PubMed] [Google Scholar]
- 90.Torres IL, Gamaro GD, Vasconcellos AP, Silveira R, Dalmaz C. Effects of chronic restraint stress on feeding behavior and on monoamine levels in different brain structures in rats. Neurochem Res. 2002;27:519–25. doi: 10.1023/a:1019856821430. [DOI] [PubMed] [Google Scholar]
- 91.Srikumar BN, Raju TR, Rao BS Shankaranarayana. Contrasting effects of bromocriptine on learning of a partially baited radial arm maze task in the presence and absence of restraint stress. Psychopharmacology (Berl) 2007;193:363–74. doi: 10.1007/s00213-007-0801-4. [DOI] [PubMed] [Google Scholar]
- 92.Srikumar BN, Raju TR, Rao BS Shankaranarayana. The involvement of cholinergic and noradrenergic systems in behavioral recovery following oxotremorine treatment to chronically stressed rats. Neuroscience. 2006;143:679–88. doi: 10.1016/j.neuroscience.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 93.Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16:233–8. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- 94.Tanapat P, Hastings NB, Rydel TA, Galea LA, Gould E. Exposure to fox odor inhibits cell proliferation in the hippocampus of adult rats via an adrenal hormone-dependent mechanism. J Comp Neurol. 2001;437:496–504. doi: 10.1002/cne.1297. [DOI] [PubMed] [Google Scholar]
- 95.Czeh B, Welt T, Fischer AK, Erhardt A, Schmitt W, Muller MB, et al. Chronic psychosocial stress and concomitant repetitive transcranial magnetic stimulation: Effects on stress hormone levels and adult hippocampal neurogenesis. Biol Psychiatry. 2002;52:1057–65. doi: 10.1016/s0006-3223(02)01457-9. [DOI] [PubMed] [Google Scholar]
- 96.Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 2003;17:879–86. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- 97.Veena J, Srikumar BN, Mahati K, Bhagya V, Raju TR, Rao BS Shankaranarayana. Enriched environment restores hippocampal cell proliferation and ameliorates cognitive deficits in chronically stressed rats. J Neurosci Res. 2009;87:831–43. doi: 10.1002/jnr.21907. [DOI] [PubMed] [Google Scholar]
- 98.Veena J, Srikumar BN, Raju TR, Rao BS Shankaranarayana. Exposure to enriched environment restores the survival and differentiation of new born cells in the hippocampus and ameliorates depressive symptoms in chronically stressed rats. Neurosci Lett. 2009;455:178–82. doi: 10.1016/j.neulet.2009.03.059. [DOI] [PubMed] [Google Scholar]
- 99.Rosenbrock H, Koros E, Bloching A, Podhorna J, Borsini F. Effect of chronic intermittent restraint stress on hippocampal expression of marker proteins for synaptic plasticity and progenitor cell proliferation in rats. Brain Res. 2005;1040:55–63. doi: 10.1016/j.brainres.2005.01.065. [DOI] [PubMed] [Google Scholar]
- 100.Vollmayr B, Simonis C, Weber S, Gass P, Henn F. Reduced cell proliferation in the dentate gyrus is not correlated with the development of learned helplessness. Biol Psychiatry. 2003;54:1035–40. doi: 10.1016/s0006-3223(03)00527-4. [DOI] [PubMed] [Google Scholar]
- 101.Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: Reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–71. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- 102.Alonso R, Griebel G, Pavone G, Stemmelin J, Le FG, Soubrie P. Blockade of CRF (1) or V (b) receptors reverses stress-induced suppression of neurogenesis in a mouse model of depression. Mol Psychiatry. 2004;9:278–86. doi: 10.1038/sj.mp.4001464. [DOI] [PubMed] [Google Scholar]
- 103.Silva R, Mesquita AR, Bessa J, Sousa JC, Sotiropoulos I, Leao P, et al. Lithium blocks stress-induced changes in depressive-like behavior and hippocampal cell fate: The role of glycogen-synthase-kinase-3beta. Neuroscience. 2008;152:656–69. doi: 10.1016/j.neuroscience.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 104.Coe CL, Kramer M, Czeh B, Gould E, Reeves AJ, Kirschbaum C, et al. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol Psychiatry. 2003;54:1025–34. doi: 10.1016/s0006-3223(03)00698-x. [DOI] [PubMed] [Google Scholar]
- 105.Lemaire V, Koehl M, Le MM, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci U S A. 2000;97:11032–7. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heine VM, Maslam S, Joels M, Lucassen PJ. Prominent decline of newborn cell proliferation, differentiation, and apoptosis in the aging dentate gyrus, in absence of an age-related hypothalamus-pituitary-adrenal axis activation. Neurobiol Aging. 2004;25:361–75. doi: 10.1016/S0197-4580(03)00090-3. [DOI] [PubMed] [Google Scholar]
- 107.Luo C, Xu H, Li XM. Quetiapine reverses the suppression of hippocampal neurogenesis caused by repeated restraint stress. Brain Res. 2005;1063:32–9. doi: 10.1016/j.brainres.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 108.Heine VM, Maslam S, Joels M, Lucassen PJ. Increased P27 KiP1 protein expression in the dentate gyrus of chronically stressed rats indicates G1 arrest involvement. Neuroscience. 2004;129:593–601. doi: 10.1016/j.neuroscience.2004.07.048. [DOI] [PubMed] [Google Scholar]
- 109.Heine VM, Maslam S, Zareno J, Joels M, Lucassen PJ. Suppressed proliferation and apoptotic changes in the rat dentate gyrus after acute and chronic stress are reversible. Eur J Neurosci. 2004;19:131–44. doi: 10.1046/j.1460-9568.2003.03100.x. [DOI] [PubMed] [Google Scholar]
- 110.Lee KJ, Kim SJ, Kim SW, Choi SH, Shin YC, Park SH, et al. Chronic mild stress decreases survival, but not proliferation, of new-born cells in adult rat hippocampus. Exp Mol Med. 2006;38:44–54. doi: 10.1038/emm.2006.6. [DOI] [PubMed] [Google Scholar]
- 111.Czeh B, Michaelis T, Watanabe T, Frahm J, de BG, van KM, et al. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci U S A. 2001;98:12796–801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Westenbroek C, Den Boer JA, Veenhuis M, Ter Horst GJ. Chronic stress and social housing differentially affect neurogenesis in male and female rats. Brain Res Bull. 2004;64:303–8. doi: 10.1016/j.brainresbull.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 113.Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psychiatry. 1999;46:1472–9. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- 114.Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894–7. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- 115.Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–9. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 116.Gould E, Woolley CS, McEwen BS. Adrenal steroids regulate postnatal development of the rat dentate gyrus: I. Effects of glucocorticoids on cell death. J Comp Neurol. 1991;313:479–85. doi: 10.1002/cne.903130308. [DOI] [PubMed] [Google Scholar]
- 117.Cameron HA, Tanapat P, Gould E. Adrenal steroids and N-methyl-D-aspartate receptor activation regulate neurogenesis in the dentate gyrus of adult rats through a common pathway. Neuroscience. 1998;82:349–54. doi: 10.1016/s0306-4522(97)00303-5. [DOI] [PubMed] [Google Scholar]
- 118.Cameron HA, McEwen BS, Gould E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci. 1995;15:4687–92. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- 120.Lucassen PJ, Meerlo P, Naylor AS, van Dam AM, Dayer AG, Fuchs E, et al. Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: Implications for depression and antidepressant action. Eur Neuropsychopharmacol. 2010;20:1–17. doi: 10.1016/j.euroneuro.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 121.Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–24. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- 122.Gardier AM. Mutant mouse models and antidepressant drug research: Focus on serotonin and brain-derived neurotrophic factor. Behav Pharmacol. 2009;20:18–32. doi: 10.1097/FBP.0b013e3283243fcd. [DOI] [PubMed] [Google Scholar]
- 123.Markwardt S, Overstreet-Wadiche L. Gabaergic signalling to adult-generated neurons. J Physiol. 2008;586:3745–9. doi: 10.1113/jphysiol.2008.155713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pathania M, Yan LD, Bordey A. A symphony of signals conducts early and late stages of adult neurogenesis. Neuropharmacology. 2010;58:865–76. doi: 10.1016/j.neuropharm.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Duman RS, Nakagawa S, Malberg J. Regulation of adult neurogenesis by antidepressant treatment. Neuropsychopharmacology. 2001;25:836–44. doi: 10.1016/S0893-133X(01)00358-X. [DOI] [PubMed] [Google Scholar]
- 126.Bartus RT, Dean RL, III, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–14. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 127.Winkler J, Thal LJ, Gage FH, Fisher LJ. Cholinergic strategies for alzheimer's disease. J Mol Med. 1998;76:555–67. doi: 10.1007/s001090050250. [DOI] [PubMed] [Google Scholar]
- 128.Kaneko N, Okano H, Sawamoto K. Role of the cholinergic system in regulating survival of newborn neurons in the adult mouse dentate gyrus and olfactory bulb. Genes Cells. 2006;11:1145–59. doi: 10.1111/j.1365-2443.2006.01010.x. [DOI] [PubMed] [Google Scholar]
- 129.Mohapel P, Leanza G, Kokaia M, Lindvall O. Forebrain acetylcholine regulates adult hippocampal neurogenesis and learning. Neurobiol Aging. 2005;26:939–46. doi: 10.1016/j.neurobiolaging.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 130.Cooper-Kuhn CM, Winkler J, Kuhn HG. Decreased neurogenesis after cholinergic forebrain lesion in the adult rat. J Neurosci Res. 2004;77:155–65. doi: 10.1002/jnr.20116. [DOI] [PubMed] [Google Scholar]
- 131.Van der BK, Mulder J, Keijser JN, Eggen BJ, Luiten PG, Van der Zee EA. Input from the medial septum regulates adult hippocampal neurogenesis. Brain Res Bull. 2005;67:117–25. doi: 10.1016/j.brainresbull.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 132.Kotani S, Yamauchi T, Teramoto T, Ogura H. Pharmacological evidence of cholinergic involvement in adult hippocampal neurogenesis in rats. Neuroscience. 2006;142:505–14. doi: 10.1016/j.neuroscience.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 133.Van Kampen JM, Eckman CB. Agonist-induced restoration of hippocampal neurogenesis and cognitive improvement in a model of cholinergic denervation. Neuropharmacology. 2010;58:921–9. doi: 10.1016/j.neuropharm.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ide Y, Fujiyama F, Okamoto-Furuta K, Tamamaki N, Kaneko T, Hisatsune T. Rapid integration of young newborn dentate gyrus granule cells in the adult hippocampal circuitry. Eur J Neurosci. 2008;28:2381–92. doi: 10.1111/j.1460-9568.2008.06548.x. [DOI] [PubMed] [Google Scholar]
- 135.Scerri C, Stewart CA, Breen KC, Balfour DJ. The effects of chronic nicotine on spatial learning and bromodeoxyuridine incorporation into the dentate gyrus of the rat. Psychopharmacology (Berl) 2006;184:540–6. doi: 10.1007/s00213-005-0086-4. [DOI] [PubMed] [Google Scholar]
- 136.Shingo AS, Kito S. Effects of nicotine on neurogenesis and plasticity of hippocampal neurons. J Neural Transm. 2005;112:1475–8. doi: 10.1007/s00702-005-0370-2. [DOI] [PubMed] [Google Scholar]
- 137.Campbell NR, Fernandes CC, Halff AW, Berg DK. Endogenous signaling through alpha7-containing nicotinic receptors promotes maturation and integration of adult-born neurons in the hippocampus. J Neurosci. 2010;30:8734–44. doi: 10.1523/JNEUROSCI.0931-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Borta A, Hoglinger GU. Dopamine and adult neurogenesis. J Neurochem. 2007;100:587–95. doi: 10.1111/j.1471-4159.2006.04241.x. [DOI] [PubMed] [Google Scholar]
- 139.Diaz J, Ridray S, Mignon V, Griffon N, Schwartz JC, Sokoloff P. Selective expression of dopamine D3 receptor mrna in proliferative zones during embryonic development of the rat brain. J Neurosci. 1997;17:4282–92. doi: 10.1523/JNEUROSCI.17-11-04282.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rakic P, Lidow MS. Distribution and density of monoamine receptors in the primate visual cortex devoid of retinal input from early embryonic stages. J Neurosci. 1995;15:2561–74. doi: 10.1523/JNEUROSCI.15-03-02561.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ohtani N, Goto T, Waeber C, Bhide PG. Dopamine modulates cell cycle in the lateral ganglionic eminence. J Neurosci. 2003;23:2840–50. doi: 10.1523/JNEUROSCI.23-07-02840.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Popolo M, McCarthy DM, Bhide PG. Influence of dopamine on precursor cell proliferation and differentiation in the embryonic mouse telencephalon. Dev Neurosci. 2004;26:229–44. doi: 10.1159/000082140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, et al. Dopamine depletion impairs precursor cell proliferation in parkinson disease. Nat Neurosci. 2004;7:726–35. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- 144.Coronas V, Bantubungi K, Fombonne J, Krantic S, Schiffmann SN, Roger M. Dopamine D3 receptor stimulation promotes the proliferation of cells derived from the post-natal subventricular zone. J Neurochem. 2004;91:1292–301. doi: 10.1111/j.1471-4159.2004.02823.x. [DOI] [PubMed] [Google Scholar]
- 145.Kippin TE, Kapur S, van der KD. Dopamine specifically inhibits forebrain neural stem cell proliferation, suggesting a novel effect of antipsychotic drugs. J Neurosci. 2005;25:5815–23. doi: 10.1523/JNEUROSCI.1120-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Baker SA, Baker KA, Hagg T. Dopaminergic nigrostriatal projections regulate neural precursor proliferation in the adult mouse subventricular zone. Eur J Neurosci. 2004;20:575–9. doi: 10.1111/j.1460-9568.2004.03486.x. [DOI] [PubMed] [Google Scholar]
- 147.Winner B, Geyer M, Couillard-Despres S, Aigner R, Bogdahn U, Aigner L, et al. Striatal deafferentation increases dopaminergic neurogenesis in the adult olfactory bulb. Exp Neurol. 2006;197:113–21. doi: 10.1016/j.expneurol.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 148.Baker SA, Baker KA, Hagg T. D3 dopamine receptors do not regulate neurogenesis in the subventricular zone of adult mice. Neurobiol Dis. 2005;18:523–7. doi: 10.1016/j.nbd.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 149.Wakade CG, Mahadik SP, Waller JL, Chiu FC. Atypical neuroleptics stimulate neurogenesis in adult rat brain. J Neurosci Res. 2002;69:72–9. doi: 10.1002/jnr.10281. [DOI] [PubMed] [Google Scholar]
- 150.Van Kampen JM, Hagg T, Robertson HA. Induction of neurogenesis in the adult rat subventricular zone and neostriatum following dopamine D3 receptor stimulation. Eur J Neurosci. 2004;19:2377–87. doi: 10.1111/j.0953-816X.2004.03342.x. [DOI] [PubMed] [Google Scholar]
- 151.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–10. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang HD, Dunnavant FD, Jarman T, Deutch AY. Effects of antipsychotic drugs on neurogenesis in the forebrain of the adult rat. Neuropsychopharmacology. 2004;29:1230–8. doi: 10.1038/sj.npp.1300449. [DOI] [PubMed] [Google Scholar]
- 153.Keilhoff G, Grecksch G, Becker A. Haloperidol normalized prenatal vitamin D depletion-induced reduction of hippocampal cell proliferation in adult rats. Neurosci Lett. 2010;476:94–8. doi: 10.1016/j.neulet.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 154.Halim ND, Weickert CS, McClintock BW, Weinberger DR, Lipska BK. Effects of chronic haloperidol and clozapine treatment on neurogenesis in the adult rat hippocampus. Neuropsychopharmacology. 2004;29:1063–9. doi: 10.1038/sj.npp.1300422. [DOI] [PubMed] [Google Scholar]
- 155.Keilhoff G, Grecksch G, Bernstein HG, Roskoden T, Becker A. Risperidone and haloperidol promote survival of stem cells in the rat hippocampus. Eur Arch Psychiatry Clin Neurosci. 2010;260:151–62. doi: 10.1007/s00406-009-0033-1. [DOI] [PubMed] [Google Scholar]
- 156.Yang P, Arnold SA, Habas A, Hetman M, Hagg T. Ciliary neurotrophic factor mediates dopamine D2 receptor-induced CNS neurogenesis in adult mice. J Neurosci. 2008;28:2231–41. doi: 10.1523/JNEUROSCI.3574-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Maeda K, Sugino H, Hirose T, Kitagawa H, Nagai T, Mizoguchi H, et al. Clozapine prevents a decrease in neurogenesis in mice repeatedly treated with phencyclidine. J Pharmacol Sci. 2007;103:299–308. doi: 10.1254/jphs.fp0061424. [DOI] [PubMed] [Google Scholar]
- 158.Suzuki K, Okada K, Wakuda T, Shinmura C, Kameno Y, Iwata K, et al. Destruction of dopaminergic neurons in the midbrain by 6-hydroxydopamine decreases hippocampal cell proliferation in rats: Reversal by fluoxetine. PLoS One. 2010;5:e9260. doi: 10.1371/journal.pone.0009260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Park JH, Enikolopov G. Transient elevation of adult hippocampal neurogenesis after dopamine depletion. Exp Neurol. 2010;222:267–76. doi: 10.1016/j.expneurol.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–70. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 161.Curtis MA, Penney EB, Pearson AG, van Roon-Mom WM, Butterworth NJ, Dragunow M, et al. Increased cell proliferation and neurogenesis in the adult human huntington's disease brain. Proc Natl Acad Sci USA. 2003;100:9023–7. doi: 10.1073/pnas.1532244100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jin K, Sun Y, Xie L, Childs J, Mao XO, Greenberg DA. Post-ischemic administration of heparin-binding epidermal growth factor-like growth factor (HB-EGF) reduces infarct size and modifies neurogenesis after focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 2004;24:399–408. doi: 10.1097/00004647-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 163.Picard-Riera N, Decker L, Delarasse C, Goude K, Nait-Oumesmar B, Liblau R, et al. Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc Natl Acad Sci USA. 2002;99:13211–6. doi: 10.1073/pnas.192314199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Falconer EM, Galea LA. Sex differences in cell proliferation, cell death and defensive behavior following acute predator odor stress in adult rats. Brain Res. 2003;975:22–36. doi: 10.1016/s0006-8993(03)02542-3. [DOI] [PubMed] [Google Scholar]
- 165.Bain MJ, Dwyer SM, Rusak B. Restraint stress affects hippocampal cell proliferation differently in rats and mice. Neurosci Lett. 2004;368:7–10. doi: 10.1016/j.neulet.2004.04.096. [DOI] [PubMed] [Google Scholar]
- 166.Xu H, Chen Z, He J, Haimanot S, Li X, Dyck L, et al. Synergetic effects of quetiapine and venlafaxine in preventing the chronic restraint stress-induced decrease in cell proliferation and BDNF expression in rat hippocampus. Hippocampus. 2006;16:551–9. doi: 10.1002/hipo.20184. [DOI] [PubMed] [Google Scholar]
- 167.Lee HJ, Kim JW, Yim SV, Kim MJ, Kim SA, Kim YJ, et al. Fluoxetine enhances cell proliferation and prevents apoptosis in dentate gyrus of maternally separated rats. Mol Psychiatry. 2001;6(610):725–8. doi: 10.1038/sj.mp.4000954. [DOI] [PubMed] [Google Scholar]
- 168.Park HJ, Lim S, Lee HS, Lee HJ, Yoo YM, Kim SA, et al. Acupuncture enhances cell proliferation in dentate gyrus of maternally-separated rats. Neurosci Lett. 2002;319:153–6. doi: 10.1016/s0304-3940(01)02581-2. [DOI] [PubMed] [Google Scholar]
- 169.Mandyam CD, Crawford EF, Eisch AJ, Rivier CL, Richardson HN. Stress experienced in utero reduces sexual dichotomies in neurogenesis, microenvironment, and cell death in the adult rat hippocampus. Dev Neurobiol. 2008;68:575–89. doi: 10.1002/dneu.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Heine VM, Zareno J, Maslam S, Joels M, Lucassen PJ. Chronic stress in the adult dentate gyrus reduces cell proliferation near the vasculature and VEGF and Flk-1 protein expression. Eur J Neurosci. 2005;21:1304–14. doi: 10.1111/j.1460-9568.2005.03951.x. [DOI] [PubMed] [Google Scholar]
- 171.Oomen CA, Mayer JL, de Kloet ER, Joels M, Lucassen PJ. Brief treatment with the glucocorticoid receptor antagonist mifepristone normalizes the reduction in neurogenesis after chronic stress. Eur J Neurosci. 2007;26:3395–401. doi: 10.1111/j.1460-9568.2007.05972.x. [DOI] [PubMed] [Google Scholar]
- 172.Li S, Wang C, Wang W, Dong H, Hou P, Tang Y. Chronic mild stress impairs cognition in mice: From brain homeostasis to behavior. Life Sci. 2008;82:934–42. doi: 10.1016/j.lfs.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 173.Dong H, Goico B, Martin M, Csernansky CA, Bertchume A, Csernansky JG. Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsW (Tg 2576) mutant mice by isolation stress. Neuroscience. 2004;127:601–9. doi: 10.1016/j.neuroscience.2004.05.040. [DOI] [PubMed] [Google Scholar]