Abstract
Background:
Nitric oxide (NO) is becoming an increasingly important signaling molecule implicated in a growing number of physiological and pathophysiological processes. Research on the effect of NO donors on glucose metabolism in peripheral tissues have grown rapidly in the last decade. This study examined the effects of NGmethyl-L-arginine acetate (L-NMMA) and NGmethyl-L-arginine ester (L-NAME) on fasting and postprandial blood glucose concentrations. The study also investigated if L-NMMA and L-NAME decrease the hyperglycemic effect caused by the NO donor S-nitrosoN-acetylpenicillamine (SNAP) in normoglycemic rats.
Results:
L-NAME and L-NMMA significantly lowered the postprandial blood glucose concentrations. Mean postprandial blood glucose concentrations in rats treated with L-NAME were 5.04 ± 0.07 mmol/L at 120 min, 4.62 ± 0.19 mmol/L at 150 min and 4.36 ± 0.17 mmol/L at 180 min time points compared with 5.46 ± 0.14 (P = 0.029), 5.20 ± 0.17 mmol/L (P = 0.036), and 4.89 ± 0.14 mmol/L (P = 0.015) at the same time points respectively for saline control. Mean blood glucose concentrations in rats treated with L-NMMA were 4.35 ± 0.23 mmol/L (P = 0.0018) at 120 min, 4.60 ± 0.14 mmol/L (P = 0.090) at 150 min and 3.88 ± 0.16 mmol/L (P 0.001) at 180 min. There were significant differences in mean postprandial blood glucose concentrations in rats treated with SNAP, compared with those treated with L-NAME and SNAP at 90 min (P = 0.012), 180 min (P = 0.013) and 210 min (P < 0.0001). In addition, there were significant differences in mean postprandial blood glucose concentrations in rats treated with SNAP compared with those treated with L-NMMA and SNAP at 90 min (P = 0.0011), 180 min (P = 0.015) and 210 min (P = 0.0077).
Conclusion:
The nitric oxide synthase [NOS] inhibitors were effective in reducing postprandial blood glucose concentration in rats treated with SNAP. This suggests that although SNAP is an effective antihypertensive agent it decreases glucose tolerance which can be improved by the use of NOS inhibitors such as L-NMMA or L-NAME. These drugs could be beneficial in controlling blood glucose tolerance in rats administered with SNAP, and possibly in humans.
Keywords: Glucose, NG-methyl-L-arginine acetate, NG-methyl-L-arginine ester, nitric oxide, S-nitro-N-acetylpenicillamine
INTRODUCTION
Nitric oxide (NO) is a novel type of messenger molecule, which is involved in the signal transduction of many different physiological functions.[1,2] There has been increasing evidence of the many and diverse biological functions of endogenous NO in cardiovascular, nervous and immune systems. Nitric oxide is produced in a variety of tissues from the amino acid, L-arginine, through the activation of different isoforms of (NOS).[3] The latter exists in three isoforms including endothelial (eNOS), neuronal (nNOS) and inducible (iNOS) forms.[4] Neuronal NOS is the most abundant isoform found in skeletal muscle and is located preferentially at neuromuscular junctions.[5] Endothelial NOS is present in the vascular endothelium and in skeletal muscles. Both nNOS and eNOS synthesize small amounts of NO and require activation by Ca2+-calmodulin, making them sensitive to agents and processes that increase intracellular calcium.[6]
L-NAME (NGmethyl-L-arginine ester) and L-NMMA (NGmethyl-L-arginine acetate) are used widely as NOS inhibitors.[7,8] They are inhibitors of the formation of NO from L-arginine by the vascular endothelium and smooth muscle. Rees et al., reported that L-NAME was about 10-fold more potent than L-NMMA in increasing blood pressure.[7] While physiological levels of NO can stimulate glucose uptake and oxidation in skeletal muscle and adipocytes,[9] chronic administration of L-NAME in drinking water decreased glucose tolerance in rats.[10] Further, eNOS knockout mice have hyperinsulinemia and impaired insulin-stimulated glucose uptake relative to control mice.[11] These findings suggest that NO could play an important regulatory role in glucose metabolism.
S-Nitrosothiols (RSNO) are an important class of NO donor drugs, which break down to form NO and the corresponding disulfide.[12] Interest in s-nitrosothiols was heightened by the discovery that they occur naturally in vivo, principally as the s-nitrosothiol of plasma albumin, at a concentration of approximately 7 μmol/L.[13] There are a large number of animal and clinical studies demonstrating their advantageous features, especially in the cardiovascular system. S-nitrosoglutathione (GSNO) and S-nitroso-N-acetylpenicillamine (SNAP) are two s-nitrosothiols that are common sources of nitric oxide (NO) in the biomedical field. S-nitrosoglutathione reduces platelet adhesion in bypass grafts,[14] thrombosis following coronary angioplasty[15] and emboli that dissociate from carotid plaques.[16] A three-min intracoronary infusion of SNAP before the ischemic period has been shown to decrease infarct size and improve coronary endothelial function.[17] Research on the effect of NO donors on glucose metabolism in skeletal muscle, cardiac muscle, smooth muscle and adipose tissue has grown rapidly in the last decade. Most research has focused on skeletal muscle and the role of NO in the modulation of skeletal muscle glucose transport remains controversial.[18,19] Further, experimental evidence from animal studies suggested that SNAP had a beneficial effect of reducing blood pressure, however, this was associated with decreased glucose tolerance.[20,21] This study examined the effects of L-NMMA and L-NAME on fasting and postprandial blood glucose concentrations. The study also sought to investigate if L-NMMA and L-NAME decrease the hyperglycemic effect caused by the NO donor S-nitroso-N-acetylpenicillamine (SNAP) in normoglycemic rats.
MATERIALS AND METHODS
Rats were obtained from the Basic Medical Sciences Animal House, The University of the West Indies (UWI), Mona. Healthy male and female Sprague-Dawley mixed breed rats were used within the weight range of 250-350 g. The rats were kept in separate cages according to their sex to eliminate the possibility of impregnation. The rats were fed a daily diet of Purina Lab Chow and water administered ad libitum. All procedures were approved by and conducted in accordance with the guidelines of The University of the West Indies Animal Care and the Use Committee. Ethical approval was obtained from University Hospital of the West Indies/University of the West Indies/Faculty of Medical Sciences Ethics Committee.
Sample preparation
A dosage of 30 mg/kg-1 body weight (BW) of L-NAME and L-NMMA [Sigma Chemical Co., St. Louis, MO, USA] was used for analysis. L-NAME and L-NMMA were dissolved in saline (0.3 mL; 0.9% NaCl) just before the beginning of the analysis. The solution was then administered into the tail vein of the rat immediately after the first fasting blood sample was obtained for analysis.
A dosage of 12.5 mg/kg BW of SNAP was used for the analysis. Dimethyl sulfoxide [(DMSO; 0.3 mL, 50%); Sigma Chemical Co., St. Louis, USA] was used to dissolve SNAP just before the beginning of the analysis. The solution was then administered into the tail vein of the rat immediately after the first testing blood sample was obtained for analysis.
Oral glucose tolerance test
The oral glucose tolerance test (OGTT) was carried out using an automated method. Standard solutions were used to calibrate the glucometer from Miles Inc. Diagnostics Division, Indiana, USA. The OGTT was used to determine the effect of L-NAME and L-NMMA via intraveneous administration on blood glucose concentration in normal rats. Rats were fasted for approximately 15 h, during which only water was given ad libitum. The duration of the OGTT was approximately 3½ h. A fasting blood sample (F1, 0 min), was taken from the rat's tail immediately after which the control (saline), inhibitor (L-NAME or L-NMMA) was administered at a dosage of 30 mg/kg-1 BW intravenously in the rat's tail) later, another blood sample was taken at 30 min (F2) and at 60 min (0 h). Immediately after the 60 min (0 h) fasting sample was taken, a glucose load at a dosage of 1.75 g/kg BW was administered orally, after which postprandial blood samples were taken at 0.5-h intervals for a further 2.5 h. Readings were taken in duplicate at each time interval and averaged. In rats treated with SNAP and either L-NAME or L-NMMA, SNAP was administered after the first fasting blood sample was taken at 0 min (F1) and L-NMMA or L-NAME was administered after 30 min (after the second fasting blood sample was taken; F2). Upon completion of the experiments the rats were fed their daily supply of Purina Lab Chow and returned to the UWI animal house. Nitric oxide formation was measured as plasma nitrate/nitrite concentration, using the Griess reaction. Greiss Reagent was then used to quantitatively determine the total nitrite present in deproteinized plasma via spectrophotometry. The absorption maxima was read at a wavelength of 540 nm.[22]
Statistical analysis
Each data point was expressed as mean ± standard error of the mean (SEM). The significance between groups and within groups was determined using the Student's t-test or the Two-Way Analysis of Variance (ANOVA), P ≤ 0.05 was considered to be significant.
RESULTS
Rats administered saline (control solvent) exhibited a typical glucose tolerance curve. There was an increase in blood glucose concentration from a fasting blood sample 0 min (F1) value of 3.51 ± 0.09 mmol/L to a peak of 5.46 ± 0.14 mmol/L at 120 min (1 h, postprandial) after ingestion of a glucose load of 1.75 g/kg BW. This was followed by a gradual decrease to near normal concentration of 4.41 ± 0.11 mmol/L at 210 min [2.5 h, postprandial; Figure 1].
Figure 1.
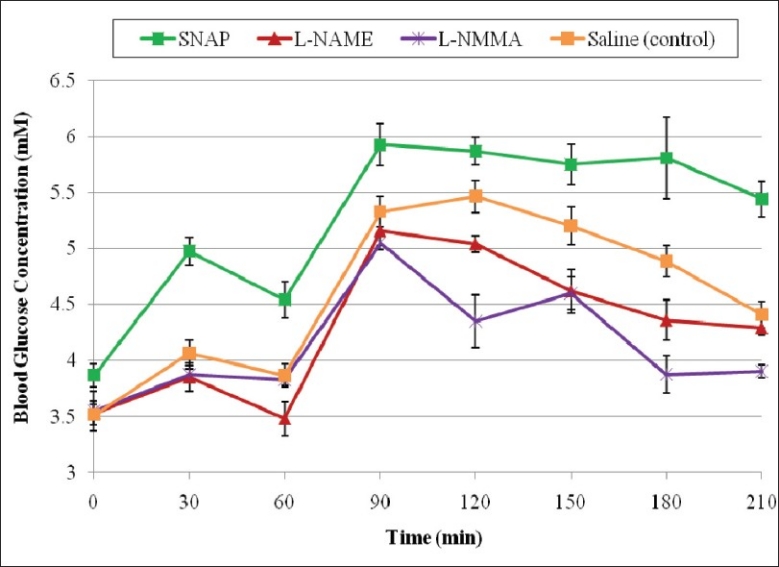
Effect of SNAP, L-NAME and L-NMMA on fasting and postprandial blood glucose concentrations
The NOS inhibitors, L-NAME and L-NMMA significantly lowered the postprandial blood glucose concentrations at the 120 min (1.0 h, postprandial), 150 min (1.5 h, postprandial), 180 min (2 h, postprandial) time points. The significant mean postprandial blood glucose concentrations in rats treated with L-NAME were 5.04 ± 0.07 mmol/L at 120 min, 4.62 ± 0.19 mmol/L at 150 min and 4.36 ± 0.17 mmol/L at 180 min time points compared with 5.46 ± 0.14 (P = 0.029), 5.20 ± 0.17 mmol/L (P = 0.036), and 4.89 ± 0.14 mmol/L (P = 0.015) at the same time points respectively for saline controls [Figure 1]. L-NMMA-treated rats showed greater reduction in blood glucose concentrations compared with L-NAMEtreated rats. Mean blood glucose concentrations in rats treated with L-NMMA were 4.35 ± 0.23 mmol/L (P = 0.0018) at 120 min, 4.60 ± 0.14 mmol/L (P = 0.090) at 150 min and 3.88 ± 0.16 mmol/L (P=0.001) at 180 min compared with those values at the same time points in saline control. The NO donor, SNAP significantly increased the postprandial blood glucose concentrations at the 90 min (0.5 h, postprandial), 120 min (1.0 h, postprandial), 150 min (1.5 h, postprandial), 180 min (2 h, postprandial) time points. The mean postprandial blood glucose concentrations in rats treated with SNAP were 5.92 ± 0.18 mmol/L at 90 min, 5.87 ± 0.13 mmol/L at 120 min, 5.75 ± 0.13 mmol/L at 150 min, and 5.81 ± 0.16 mmol/L at 180 min time points [Figure 1].
Administration of L-NAME and SNAP resulted in significant increase in fasting and postprandial blood glucose concentrations compared with the administration of L-NAME only. The fasting blood glucose concentration at 30 min (F2) was 5.78 ± 0.21 compared with 3.85 ± 0.13 mmol/L (P = 0.0001) in rats treated with only L-NAME. Mean postprandial blood glucose concentrations in rats treated with L-NAME and SNAP were 5.57 ± 0.28 mmol/L at the 150 min time point compared with 4.62 ± 0.19 (P = 0.016) in rats treated with L-NAME only [Figure 2]. There were significant differences between mean postprandial blood glucose concentrations in rats treated with SNAP, compared with those treated with L-NAME and SNAP at the 90 min (P = 0.012), 180 min (P = 0.013) and 210 min time points [P < 0.0001; Figure 2].
Figure 2.
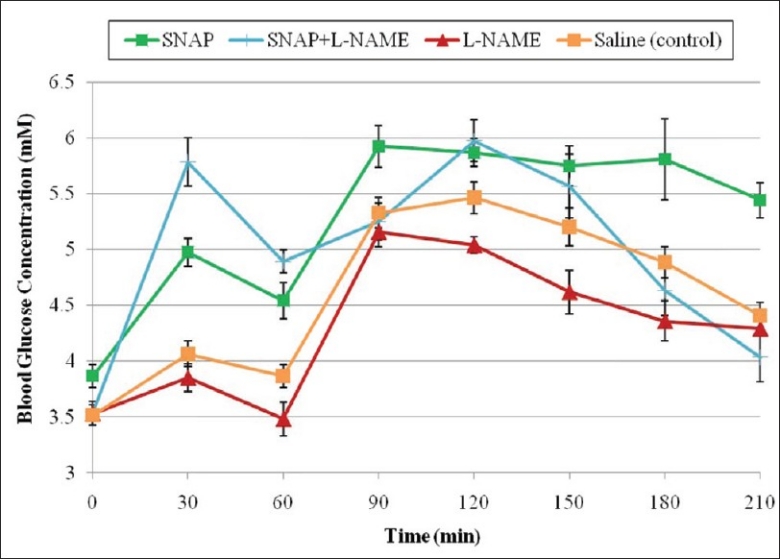
Effect of SNAP, L-NAME, and SNAP and L-NAME on fasting and postprandial blood glucose concentrations
Administration of L-NMMA and SNAP also resulted in significant increase in fasting and postprandial blood glucose concentrations compared with the administration of L-NMMA only. The fasting blood glucose concentration at 30 min (F2) was 4.56 ± 0.15 mmol/L compared with 3.88 ± 0.04 mmol/L (P = 0.019) in rats treated with only L-NMMA. The significant mean postprandial blood glucose concentrations in rats treated with L-NMMA and SNAP were 5.55 ± 0.10 mmol/L at 120 min and 4.78 ± 0.15 mmol/L at 210 min compared with 4.35 ± 0.23 (P = 0.0001) and 3.90 ± 0.05 mmol/L (P = 0.016) at the same time points respectively for rats treated with L-NMMA only [Figure 3]. Further, there were significant differences between mean postprandial blood glucose concentrations in rats treated with SNAP compared with those treated with L-NMMA and SNAP at the 90 min (P = 0.0011), 180 min (P = 0.0150) and 210 min time points [P = 0.0077; Figure 3].
Figure 3.
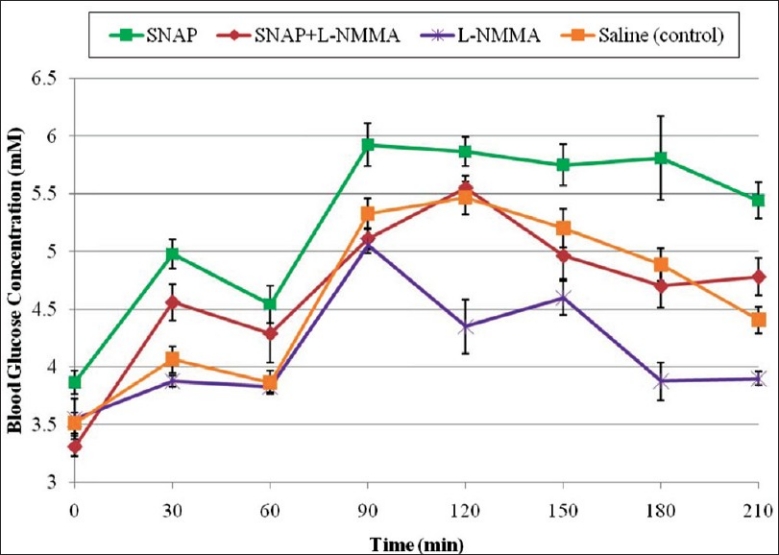
Effect of SNAP, L-NMMA, and SNAP and L-NMMA on fasting and postprandial blood glucose concentrations
Rats treated with SNAP and L-NAME had higher blood glucose concentration than those treated with SNAP and L-NMMA at all except the 180 min and 210 min time points. Administration of the NOS inhibitor L-NAME gave a total NO2– concentration of 22.47 ± 3.29 μmol/L and L-NMMA, 15.39 ± 3.09 μmol/L. Administration of L-NAME and SNAP (56.82 ± 13.23 μmol/L), and L-NMMA and SNAP (40.27 ± 7.8 μmol/L) showed that total NO2– concentrations were significantly elevated compared with only L-NAME and L-NMMA respectively [P < 0.05; Figure 4].
Figure 4.
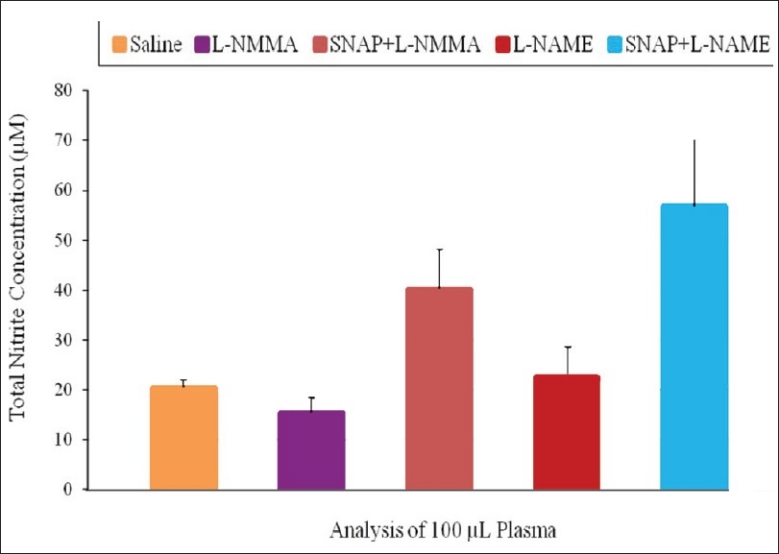
Effect of SNAP, L-NAME and L-NMMA on total nitrite concentration
DISCUSSION
This study found that the NOS inhibitors, L-NAME and L-NMMA significantly lowered postprandial blood glucose concentration. Significant differences in blood glucose concentrations were observed at the 120 min (1.0 h, postprandial), 150 min (1.5 h, postprandial) and 180 min (2.0 h, postprandial) time points for both NOS inhibitors. L-NMMA-treated rats showed greater reduction in postprandial blood glucose concentration in comparison to L-NAME–treated rats at these time points. There are conflicting reports in the literature on the effect of NOS inhibitors on glucose concentration, tolerance, and uptake with most studies investigating the latter.[10,18] Kingwell and colleagues examined the potential role of NO in the regulation of skeletal muscle glucose uptake during exercise. They found that infusion of L-NMMA into the femoral artery during cycling exercise substantially attenuated the increase in leg glucose uptake in healthy young individuals and especially in people with Type 2 diabetes.[18] Rat studies from other laboratories have yielded conflicting results.[19,23] For example, two studies found NOS inhibition prevented increases in contraction-stimulated glucose uptake[23,24] but two other studies reported no effect of NOS inhibition on contraction-stimulated glucose uptake.[19,25] The findings of improved glucose tolerance by the NOS inhibitors are more in keeping with the findings of Kingwell and colleagues.
L-NAME is metabolized by an esterase to yield the active NOS inhibitor, NG-nitroL-arginine[26] and its fasting hypoglycemic effect observed in this study may be due to fact that L-NAME inhibits glucagon release even in the absence of glucose, thus suggesting that NO is a positive modulator of glucagon release. Similar reports have shown that low concentration of L-NMMA inhibits glucagon release[27] which may account for the its observed lowering of the fasting blood glucose concentration, and glucagon is reportedly an initial amplifier of glucosestimulated insulin release.[28]
The postprandial hypoglycemic effect of L-NAME and L-NMMA could also be due to their effect on insulin. Studies have shown that 5 mmol/L of L-NAME inhibited cNOS activity in isolated, intact islets by approximately 60% due to it exerting non-selective effects on insulin secretory mechanisms. Further, 10 mmol/L of L-NAME itself is capable of stimulating insulin release from isolated islets in the presence of a basal, physiological concentration of glucose (7 mmol/L).[29] It has been proposed that high concentrations of L-NAME (>5 mmol/L) may increase insulin release by directly blocking the β-cell K+ ATP channels.[30]
Skeletal muscle is the primary target tissue for insulin stimulation of glucose transport, a regulatory mechanism vital for glucose homeostasis. The insulin released due to L-NAME and L-NMMA may increase glucose transport in this tissue mainly by activating the translocation of glucose transporter 4 (GLUT-4) from an occluded intracellular tubulo-vesicular reservoir to the cell surface.[31] The increase in muscle perfusion[32] is thought to increase the delivery of glucose to muscle cells.[33] Baron and colleagues suggested that approximately 30% of insulin's effects on glucose uptake can be accounted for by increases in muscle perfusion.[33] Further, insulin increases blood flow and glucose delivery in skeletal muscle of both humans[34] and rats[35] and insulin stimulation of glucose uptake in skeletal muscles is NO-dependent.
Rats treated with SNAP and L-NAME, and L-NMMA had higher blood glucose concentration than those treated with L-NAME or L-NMMA only. Further, rats treated with SNAP and L-NAME had higher blood glucose concentration than those treated with SNAP and L-NMMA at all except the 180 min and 210 min time points. Researchers have proposed[36,37] that the inhibitory action of NO released from s-nitrosothiols such as SNAP on glucose-induced insulin release might be exerted through the formation of s-nitrosothiols,[38] thereby impairing thiol groups essential for the nutrient-induced insulin secretory process.[39,40] Further, in vitro studies have shown that NO released from SNAP can have direct effects on isolated hepatocytes, which results in an inhibition of gluconeogenesis, a reduction in glycogen synthesis and an increase in glucose output.[41,42] This could account for the hyperglycemia observed in rats treated with SNAP. In addition, GSNO and sodium nitroprusside (SNP) enhance both glucose transport and GLUT-4 translocation in human vascular smooth muscle cells, bypassing the inhibitory effect of L-NAME[43] which confirms the finding of this study.
The hyperglycemic effect of SNAP is in agreement with previous reports by McGrowder and colleagues who showed that GSNO and SNAP increased postprandial blood glucose concentration in normoglycemic dogs. These investigators showed decreased postprandial plasma insulin concentrations[44] and increased plasma glucagon concentration.[20] Further evidence that could account for the hyperglycemic effect of SNAP is reported by a study by Horton et al which demonstrated that the NO donors SNAP and SIN-1 inhibit gluconeogenesis from isolated rat hepatocytes in a time and dose-dependent manner.[41] They suggested that the mechanism by which this occurs involves a decrease in the amount of phosphoenolpyruvate carboxykinase (PEPCK) protein. On the other hand, Sprangers et al showed that glycogen synthesis from glucose in rat hepatocytes was inhibited by SNAP due to decreased glycogen synthase activity (less conversion of glycogen synthase b into a by synthase phosphatase).[45] They also found that glycogen synthesis is more sensitive to inhibition by NO than is gluconeogenesis.[45]
The observed hyperglycemic effect of SNAP in the presence of L-NAME or L-NMMA in normoglycemic rats could arise as a result of a number of factors, including (1) inhibition of the secretory process by NO, such that insufficient insulin is secreted in response to a glucose challenge; (2) a decrease in the vascular permeability of cell membrane by NO, resulting in decreased glucose and insulin delivery to the tissues; (3) the action of NO released from SNAP on the pancreatic islet cells, impairing the first or early phase of the glucose-stimulated insulin release into the blood and (4) NO inhibition of a number of crucial enzymes in the glycolytic pathway and electron chain such as glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and aconitase respectively resulting in a decrease in glycolysis and glucose oxidation.[46] Of these mechanisms proposed, it is believed that the inhibitory effect of NO on GAPDH plays a significant role in the observed hyperglycemic effect by SNAP.[21]
The excretory form of NO is usually in the form of nitrates (NO3-) and nitrites (NO2-) and the conversion of one form to the next readily takes place in vivo.[22] However, the total nitrite concentration incorporates both NO2- and NO3- species. Plasma NO2– concentration is used as a biological marker of NO formation in the body. Rats treated with SNAP and L-NAME, and SNAP and L-NMMA showed significantly higher total nitrite concentration than those administered with only L-NAME and L-NMMA. The decomposition of SNAP caused an increase in NO concentration which may have been responsible for the observed hyperglycemic effect.
The study involved the use of rat models where there was the co-administration of the NO donor SNAP with NOS inhibitors L-NAME or L-NMMA on fasting and postprandial blood glucose concentrations. This experimental approach of using both NO donors and NOS inhibitors has been used in other studies such as that of Sarma et al who reported that coadministration of SIN-1 with L-NAME and hemoglobin increased the nociceptive threshold in rats.[47]
CONCLUSION
This study found that the NOS inhibitors, L-NAME and L-NMMA significantly lowered postprandial blood glucose concentration in normoglycemic rats while SNAP had the opposite effect. The NOS inhibitors were effective in reducing postprandial blood glucose concentrations in rats treated with SNAP. The observed hyperglycemic effect on administration of SNAP may limit its use as an antihypertensive agent. The decreased glucose tolerance due to SNAP administration could be improved by the use of NOS inhibitors such as L-NMMA or L-NAME.
ACKNOWLEDGEMENT
The authors are grateful to the Office of Graduate Studies and Research, University of the West Indies for providing funding for this research.
Footnotes
Source of Support: Office of Graduate Studies and Research, University of the West Indies
Conflict of Interest: None declared.
REFERENCES
- 1.Kerwin JF, Heller M. The arginine-nitric oxide pathway: A target for new drugs. Med Res Rev. 1994;14:23–7. doi: 10.1002/med.2610140103. [DOI] [PubMed] [Google Scholar]
- 2.Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994;298:249–58. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–12. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 4.Kobzik L, Bredt DS, Lowenstein CJ, Drazen J, Gaston B, Sugarbaker D, et al. Nitric oxide synthase in human and rat lung: Immunocytochemical and histochemical localization. Am J Respir Cell Mol Biol. 1993;9:371–7. doi: 10.1165/ajrcmb/9.4.371. [DOI] [PubMed] [Google Scholar]
- 5.Kusner LL, Kasminski HJ. Nitric oxide synthase is concentrated at the skeletal muscle endplate. Brain Res. 1996;730:238–42. doi: 10.1016/0006-8993(96)00675-0. [DOI] [PubMed] [Google Scholar]
- 6.Abu-Sound HM, Rousseau DL, Stuehr DJ. Nitric oxide binding to the heme of neuronal nitric-oxide synthase links its activity to changes in oxygen tension. J Biol Chem. 1996;271:32515–8. doi: 10.1074/jbc.271.51.32515. [DOI] [PubMed] [Google Scholar]
- 7.Rees DD, Palmer RM, Schulz R, Hodson HF, Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990;101:746–52. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCall TB, Feelisch M, Palmer RM, Moncada S. Identification of N-iminoethy-l-Lornithine as an irreversible inhibitor of nitric oxide synthase in phagocytic cells. Br J Pharmacol. 1991;102:234–8. doi: 10.1111/j.1476-5381.1991.tb12159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roy D, Perreault M, Marette A. Insulin stimulation of glucose uptake in skeletal muscles and adipose tissues in vivo is NO dependent. Am J Physiol. 1998;274:E692–E9. doi: 10.1152/ajpendo.1998.274.4.E692. [DOI] [PubMed] [Google Scholar]
- 10.Balon TW, Jasman AP, Young JC. Effects of chronic N(omega)nitro-L-arginine methyl ester administration on glucose tolerance and skeletal muscle glucose transport in the rat. Nitric Oxide. 1999;3:312–20. doi: 10.1006/niox.1999.0235. [DOI] [PubMed] [Google Scholar]
- 11.Duplain H, Burcelin R, Sartori C, Cook S, Egli M, Lepori M, et al. Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation. 2001;104:342–5. doi: 10.1161/01.cir.104.3.342. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen T, Brunson D, Crespi CL, Penman BW, Wishnok JS, Tannenbaum SR. DNA damage and mutation in human cells exposed to nitric oxide in vitro. Proc Natl Acad Sci USA. 1992;89:3030–4. doi: 10.1073/pnas.89.7.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stamler JS, Jaraki O, Osborne JA, Simon DI, Keaney J, Vita J, et al. Nitric oxide circulates in mammalian plasma primarily as an S-nitrosoadduct of serum albumin. Proc Natl Acad Sci USA. 1992;89:7674–7. doi: 10.1073/pnas.89.16.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salas E, Langford EJ, Marrinan MT, Martin JF, Moncada S, de Belder AJ. S-nitrosoglutathione inhibits platelet activation and deposition in coronary artery saphenous vein grafts in vitro and in vivo. Heart. 1998;80:146–50. doi: 10.1136/hrt.80.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langford EJ, Brown AS, Wainwright RJ, de Belder AJ, Thomas MR, Smith RE, et al. Inhibition of platelet activity by S-nitrosoglutathione during coronary angioplasty. Lancet. 1994;344:1458–60. doi: 10.1016/s0140-6736(94)90287-9. [DOI] [PubMed] [Google Scholar]
- 16.Kaposzta Z, Martin JF, Markus HS. Switching off embolization from symptomatic carotid plaque using S-nitrosoglutathione. Circulation. 2002;105:1480–4. doi: 10.1161/01.cir.0000012347.47001.97. [DOI] [PubMed] [Google Scholar]
- 17.Gourine AV, Bulhak AA, Gonon AT, Pernow J, Sjoquist PO. Cardioprotective effect induced by brief exposure to nitric oxide before myocardial ischemiareperfusion in vivo. Nitric Oxide. 2002;7:210–6. doi: 10.1016/s1089-8603(02)00114-3. [DOI] [PubMed] [Google Scholar]
- 18.Kingwell B, Formosa M, Muhlmann M, Bradley S, McConell G. Nitric oxide synthase inhibition reduces glucose uptake during exercise in individuals with type 2 diabetes more than in control subjects. Diabetes. 2002;51:2572–80. doi: 10.2337/diabetes.51.8.2572. [DOI] [PubMed] [Google Scholar]
- 19.Higaki Y, Hirshman MF, Fujii N, Goodyear LJ. Nitric oxide increases glucose uptake through a mechanism that is distinct from the insulin and contraction pathways in rat skeletal muscle. Diabetes. 2001;50:241–7. doi: 10.2337/diabetes.50.2.241. [DOI] [PubMed] [Google Scholar]
- 20.McGrowder D, Ragoobirsingh D, Dasgupta T. Effects of S-Nitroso-N-acetylpenicillamine administration on glucose tolerance and plasma levels of insulin and glucagon in the dog. Nitric Oxide. 2001;5:402–12. doi: 10.1006/niox.2001.0360. [DOI] [PubMed] [Google Scholar]
- 21.Campbell S, AlexanderLindo R, Dasgupta T, McGrowder D. The effect of S-nitrosocaptopril and S-nitrosoNacetyl-D, L-penincillamine on blood glucose concentration and haemodynamic parameters. J Appl Biomed. 2009;7:123–31. [Google Scholar]
- 22.Weitzberg E, Lundberg JO. Nonenzymatic NO production in humans. Nitric Oxide. 1998;2:17. doi: 10.1006/niox.1997.0162. [DOI] [PubMed] [Google Scholar]
- 23.Roberts CK, Barnard RJ, Scheck SH, Balon TW. Exercise-stimulated glucose transport in skeletal muscle is nitric oxide dependent. Am J Physiol. 1997;273:E220–5. doi: 10.1152/ajpendo.1997.273.1.E220. [DOI] [PubMed] [Google Scholar]
- 24.Roy D, Perreault M, Marette A. Insulin stimulation of glucose uptake in skeletal muscles and adipose tissues in vivo is NO dependent. Am J Physiol. 1998;274:E692–9. doi: 10.1152/ajpendo.1998.274.4.E692. [DOI] [PubMed] [Google Scholar]
- 25.Etgen GJ, Fryburg DA, Gibbs EM. Nitric oxide stimulates skeletal muscle glucose transport through a calcium/contraction- and phosphatidylinositol-3-kinase-independent pathway. Diabetes. 1997;46:1915–9. doi: 10.2337/diab.46.11.1915. [DOI] [PubMed] [Google Scholar]
- 26.Southan GJ, Szabo’ C. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem Pharmacol. 1996;51:383–94. doi: 10.1016/0006-2952(95)02099-3. [DOI] [PubMed] [Google Scholar]
- 27.Arkesson B, Lundquist I. Influence of nitric oxide modulators on cholinergically stimulated hormone release from mouse islets. J Physiol. 1999;515:463–73. doi: 10.1111/j.1469-7793.1999.463ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuit FC, Pipeleers DG. Regulation of adenosine 3’,5’ monophosphate levels in the pancreatic B cell. Endocrinology. 1985;117:834–40. doi: 10.1210/endo-117-3-834. [DOI] [PubMed] [Google Scholar]
- 29.Panagiotidis G, Arkesson B, Rydell EL, Lundquist I. Influence of nitric oxide synthase inhibition, nitric oxide and hydroperoxide on insulin release induced by various secretagogues. Br J Pharmacol. 1995;114:289–96. doi: 10.1111/j.1476-5381.1995.tb13225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krippeit-Drews P, Welker S, Drews G. Effects of the nitric oxide synthase inhibitor NGnitro-L-arginine methyl ester on electrical activity and ion channels of mouse pancreatic B cells. Biochem Biophys Res Commun. 1996;224:199–205. doi: 10.1006/bbrc.1996.1007. [DOI] [PubMed] [Google Scholar]
- 31.Kahn BB. Facilitative glucose transporters: Regulatory mechanisms and dysregulation in diabetes. J Clin Invest. 1992;89:1367–74. doi: 10.1172/JCI115724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baron AD, BrechtelHook G, Johnson A, Cronin J, Leaming R, Steinberg HO. Effect of perfusion rate on the time course of insulin-mediated skeletal muscle glucose uptake. Am J Physiol. 1996;271:E1067–72. doi: 10.1152/ajpendo.1996.271.6.E1067. [DOI] [PubMed] [Google Scholar]
- 33.Baron AD, Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G. Insulin-mediated skeletal muscle vasodilation contributes to both insulin sensitivity and responsiveness in lean humans. J Clin Invest. 1995;96:786–92. doi: 10.1172/JCI118124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baron AD. Hemodynamic actions of insulin. Am J Physiol. 1994;267:E187–202. doi: 10.1152/ajpendo.1994.267.2.E187. [DOI] [PubMed] [Google Scholar]
- 35.Pıtre M, Nadeau A, Bachelard H. Insulin sensitivity and hemodynamic responses to insulin in WistarKyoto and spontaneously hypertensive rats. Am J Physiol. 1996;271:E658–68. doi: 10.1152/ajpendo.1996.271.4.E658. [DOI] [PubMed] [Google Scholar]
- 36.Panagiotidis G, Akesson B, Rydell EL, Lundquist I. Influence of nitric oxide synthase inhibition, nitric oxide and hydroperoxide on insulin release induced by various secretagogues. Br J Pharmacol. 1995;114:289–96. doi: 10.1111/j.1476-5381.1995.tb13225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panagiotidis G, Panagiotidis G, Stanstrom A, Lundquist I. In vivo action of cyclic AMP modulating secretagogues on islet monoamine oxidase activity and insulin release. Endocrine. 1994;2:571–6. [Google Scholar]
- 38.Stamler JS, Simon DI, Osborne JA, Mullins ME, Jaraki O, Michel T, et al. S-nitrosylation of proteins with nitric oxide: Synthesis and characterization of biologically active compounds. Proc Natl Acad Sci USA. 1992;89:444–8. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hellman B, Idahl LA, Lernmark A, Sehlin J, Taljedal IB. Membrane sulfhydryl groups and pancreatic beta cell recognition of insulin secretagogues. Excerpt Med Int Congr Ser. 1974;312:657–8. [Google Scholar]
- 40.Ammon HP, Mark M. Thiols and pancreatic b-cell function: A review. Cell Biochem Funct. 1985;3:157–71. doi: 10.1002/cbf.290030302. [DOI] [PubMed] [Google Scholar]
- 41.Horton RA, Ceppi ED, Knowles RG, Titheradge MA. Inhibition of hepatic gluconeogenesis by nitric oxide: A comparison with endotoxic shock. Biochem J. 1994;299:735–9. doi: 10.1042/bj2990735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seredycz LI, Lautt WW. Hemorrhage results in hepatic insulin-sensitizing substance-dependent insulin resistance mediated by somatostatin in rats. Neuroendocrinology. 2006;84:94–102. doi: 10.1159/000097484. [DOI] [PubMed] [Google Scholar]
- 43.Bergandi LF, Silvagno I, Russo I, Riganti C, Anfossi G, Aldieri E, et al. Insulin stimulates glucose transport via nitric oxide/cyclic GMP pathway in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003;23:2215–21. doi: 10.1161/01.ATV.0000107028.20478.8e. [DOI] [PubMed] [Google Scholar]
- 44.McGrowder D, Ragoobirsingh D, Dasgupta T. The hyperglycemic effect of S-nitrosoglutathione in the dog. Nitric oxide. 1999;3:481–91. doi: 10.1006/niox.1999.0254. [DOI] [PubMed] [Google Scholar]
- 45.Sprangers F, Jellema WT, Lopuhaa CE, Endert E, Ackermans MT, Van Lieshout JJ, et al. Partial inhibition of nitric oxide synthesis in vivo does not inhibit glucose production in man. Metabolism. 2002;51:57–64. doi: 10.1053/meta.2002.28958. [DOI] [PubMed] [Google Scholar]
- 46.Ishii T, Sunami O, Nakajima H, Nishio H, Takeuchi T, Hata F. Critical role of sulphenic acid formation of thiols in the inactivation of glyceraldehyde-3-phosphate dehydrogenase by nitric oxide. Biochem Pharmacol. 1999;58:133–43. doi: 10.1016/s0006-2952(99)00060-x. [DOI] [PubMed] [Google Scholar]
- 47.Sarma J, Tandan SK, Hajare SW, Kumar D, Raviprakash V. Effect of centrally administered nitric oxide modulators in Brewer's yeast-induced nociception in rats. Indian J Exp Biol. 2000;38:1123–8. [PubMed] [Google Scholar]


