Abstract
Diet plays a vital role in the management of cancer because they are the source of important physiologically functional components. Scientific observations support the idea that dietary supplement can prevent breast cancer recurrences. Strong correlations are established between the high intake of saturated fat and the incidence of different types of cancer. It is found that chronic alcohol consumption is associated with increased risk of cancers of oral cavity, pharynx, esophagus, and larynx. Again, some evidences are also found regarding phosphorous, glutamate level in the body, and incidence of cancer. Different physiologically functional components are found in the dietary materials. Fibers, the major dietary components, have long been recognized for the unique properties in the treatment of cancer, which are related to its antineoplastic functions. Antioxidant rich diet has been added to the list of cancer-preventing dietary components. Also, recently published research has shown that natural carotenoids in the diet leads to a normalization of body epithelial cells and protects against the risk of stomach and esophagus cancer, and improves the immune system's response. Again, fruit juices, processed vegetable juices, orange peel, green tea, vitamins, flavonoids, and trace materials have cancer inhibitory properties. Clearly, there has been increasing recognition of chemoprotective functions. Now, it can be recognized for another kind of functionality for the improvement of the health of mankind.
Keywords: Antioxidant, chemoprevention, diet, fiber, nutrients
INTRODUCTION
Diet has an etiological role in cancer is well accepted. Strong direct interrelations were found between saturated fat intake and the incidence of breast, colon, and prostate cancers. It is reported that alcohol intake greater than 40 g per day is to be related with cancers of the oral cavity, pharynx, esophagus, and larynx, where alcohol interacts synergistically with smoking to increase risk. Large amount of dietary fibers and other dietary components associated with high intake of grains, vegetables, and fruits significantly reduce the risks of colon cancer and to a lesser but definite extent of breast cancer. Insoluble grain fiber is more strongly associated with decreased risk of cancer than soluble grain fiber. Vitamin A, E, and trace minerals are involved in cancer protection. It is found that a low-density intake of phosphorus increase the plasma levels of 1,25(OH)2D and this lower the risk of prostate cancer in ageing men. Vitamin-D and Calcium are also found to give protection and chemoprevention against colorectal carcinoma. Retinoids can inhibit the process of malignant transformation in epithelial tissues and 1, 3-Cis-Retinoic acid shows best result in the treatment of skin squamous-cell carcinoma and cervical cancer. There have been found inverse relation between high intake of Beta-Carotene rich foods and the incidence of cancer of the esophagus, G.I.T. The type of food habit should be modified accordingly in our country to save populations from cancer.
In clinical nutrition, we are used to deal with the clinical management of patients, but of equal or greater importance is the study of how nutrition affects the development of diseases or modifies its manifestations. This field is complex and links physiologic studies of nutrition to the epidemiological analyses which form the basis of thinking in public health today. Thus, a number of studies have investigated the nutritional risk factors leading to the development of diseases such as heart diseases and cancer. This epidemiological research requires the difficult task of accurately assessing the food consumptions of individuals with poor methodologies; the chances of erroneous results are very high. This has implications for both group and individual comparison. Prentice and Sheppard concluded that no reliable epidemiological methods currently exist for studying the role of diet in the development of cancers that are most critical to public health. They acknowledge, however, the significant role of consistently positive and negative data for epidemiological studies related with diet and cancer and the potential of cautiously, carefully selected and conducted large-scale randomized intervention trials.[1,2]
Physiological studies on the effect of highly controlled changes in food intake on risk factors then allow the epidemiology to be interpreted in metabolic terms. This review article illustrates some of the benefits of metabolic studies and some of the requirements for the successful conduct with particular references to cancer.[3]
The chemicals which are used with food at the time of processing are strictly examined for toxic effect and if in any cases toxicity is found, they are strictly controlled. Despite all these precautions, a considerable number of North America human cancer cases are found to be associated with diet and nutrition. The possible utilization of excess calories, natural contaminants, and naturally occurring carcinogens within the food supply to this horrendous burden of cancer is taken into account. The theoretically possible use of bioengineering techniques to modify the constituents of food and vegetables and thus to minimize the level of noxious agents and carcinogens in the food supply has become an important consideration in this context.[4]
The idea that diet and nutrition have an important influence on health is an age-old one. Its relation with cancer was mentioned in Chinese medical writings in the 12th century. Recent interest in this subject started with animal studies in 1930. Some of the methodological issues in human studies include the inherent problems in estimating dietary intakes, the result of compounding and interaction, and the low risks associated with particular food items or nutrients.[5]
One hundred four consecutive patients with newly diagnosed small-cell lung cancer, metastatic breast cancer, and ovarian cancer in good physical fitness condition were studied carefully by the National Food and Agency, Denmark. This study has pointed out that many ambulatory cancer patients do not eat sufficient to maintain weight. It is also found that a moderate weight loss is associated with physiological distress and lower quality of life.[6,7]
DIETARY FAT AND CANCER
Although there are some inconsistencies in the reports relating dietary fat to cancer incidence, animal studies support a cancer-promoting role for fat and International epidemiologic studies strongly suggests that high amount of dietary fat intake may be associated with increased incidence and mortality of cancers of the breast, colon, rectum, and prostate. Cancers of the ovaries, the endometrium, and the pancreas also have been linked to fat intake, but the evidence needs further research to draw conclusion.[8–10]
Some differences relating cancer mortality and food consumption patterns among Southern European countries may be focused. An increase of all cancer site mortality is shown in Southern European countries, whereas in England and Wales, a decrease in colorectal cancer among women is observed. The marked divergent fat consumption pattern between Northern and Southern Europe is associated with their substantial differences in ovarian and colorectal cancer and breast cancer mortality trends.[8]
DIETARY FAT AND BREAST CANCER
Current information links nutrition and dietary factors to the risk of developing breast cancer, as well as to survival and outcomes after a diagnosis of breast cancer. Epidemiological and other data suggest that foods rich in fat and lower in fibers are associated with the high incidence of breast cancer. Several pilot intervention studies have been performed in women with breast cancer by providing decreased amount of dietary fat. Other studies suggest that weight gain after breast cancer is generally found, which may be detrimental for survival. All these reports suggest that further studies are essential for better understanding the relationship between nutritional factors and the breast cancer.[11] Figure 1 illustrates the great differences in breast cancer mortality among countries and the direct correlation of breast cancer mortality with dietary fat intake. Rates are highest in North America and Western Europe and lowest in Asia with dramatic differences.[12] A hospital-based study in Northern Italy and Southern France on host-related risk factors and breast cancer reported that high-density lipoprotein (HDL)-cholesterol levels should be specially checked in women who are more than 59 years old or in premenopausal women presenting a low basal metabolic rate, or in postmenopausal women with an early menopause as HDL-Cholesterol level appears to be related to estrogen metabolism.[13]
Figure 1.
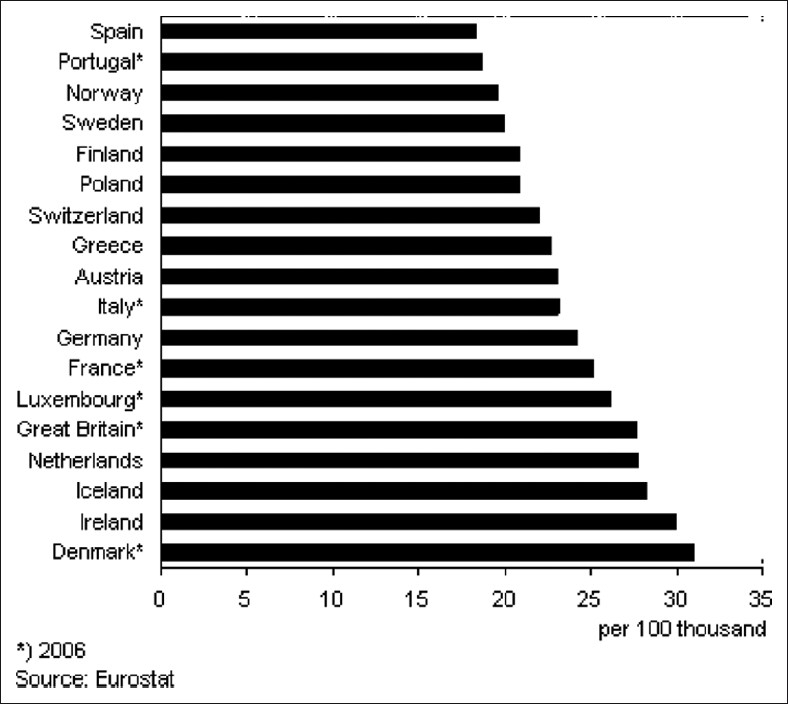
Differences in breast cancer mortality among countries
Strong direct interrelations were found between saturated fat intake and the incidence of breast, colon, and prostate cancers and between intakes of total polyunsaturated fatty acids and the occurrence of breast and prostate cancers. Not only the amount of fat, but also the type of fat consumed may be an important risk factor in cancer development. Taking of the longer chain, highly polyunsaturated omega-3-fatty acids found in certain sea fishes may give protection against cancer. This finding has been proved by studies of populations such as Greenland Eskimos who are at low risk for cancer, including breast cancer, as they consume a considerable amounts of fat derived from sea fish.[14] Again, olive oil, which contains large amount of the monounsaturated fatty acid, tends to possess an inhibitory action on tumor growth in animals.[15] According to the study in the university of Nebraska Medical Center, Omaha, the skin tumor promotion can be inhibited by restriction of fat and carbohydrate calories in Sencar mice.[16] Again, Nutrition and Endocrinology, American Health Foundation, Valhalla, suggests that diets rich in Omega-6-polyunsaturated fatty acids stimulate the growth and metastases of transplantable mammary carcinomas in rodents, whereas fish-oil-containing diets, rich in fatty acids, suppress the growth of these mammary tumor cells.[17] From an in vitro invasion assay system, the effects of linoleic acid, an omega-6-fatty acid, and 2-omega-3-fatty acids, eicosapentaenoic acid, and decosahexaenoic acid on human breast cancer cells, it is suggested that the effects of these fatty acids are mediated through eicosanoid biosynthesis.[18] So, the factors which are related to reducing breast cancer risk rests largely in the area of dietary change, factors to be considered are presented in Table 1. Scientific evidences support the view that dietary intervention may be a very useful approach to prevent breast cancer and possibility to inhibit breast carcinoma recurrences.[19]
Table 1.
Dietary fat and breast cancer related factors

ALCOHOL AND CANCER: THE POST-REPEAL ECLIPSE IN KNOWLEDGE
National publication in the U.S.A. (1919-1933) was followed by an era in which medical scientists played an important role in minimizing the harmful effects of alcohol. Cirrhosis, cardiomyopathy, adverse fetal effects, and esophageal cancer are examples of alcohol-related health problems that are well known to all.[20,21] Chronic ethanol consumption is associated with increased incidence of cancer. It is reported that alcohol intake is to be directly related with cancers of the oral cavity, pharynx, esophagus, and larynx, where alcohol interacts synergistically with smoking to increase risk. Primary liver, rectal, pancreatic, and breast cancer also have been included with alcohol intake. The relative risks reported for these associations are weak to moderate.[22]
Studies have shown that ethanol consumption increases oxidative stress with formation of lipid peroxides and free radicals. The susceptibility of a given tissue to peroxidation is, however, a function of the overall balance between peroxidants and antioxidants defense system. The later includes both intracellular and extracellular protective factors where nutrients play an important role. Impaired nutritional status of various types of vitamins and trace elements has been found in alcoholics. Reduced levels of vitamin E have been reported in serum of alcoholics and for that reason, reduction of antioxidant capacity has been found in several tissues and may promote the generation of free radical and peroxides, DNA damage, etc., which may damage cells directly. The importance of radicals in the promotion and initiation of cancer is presently of great interest. Further investigations are required to establish the role of these reactive species in the pathogenesis of alcohol-linked disease and to access the role of nutrition with particular reference to the antioxidant defense mechanisms.[23,24]
Recently, a meta-analysis of six dietary case-control studies (one each in Argentina, Australia, Canada, and Greece and two in Italy.) investigating the association between alcohol and breast cancer risk reported that no association was seen between consumption of less than 40 g of alcohol per day and breast cancer risk. The NCI Dietary Guidelines recommended that if alcoholic beverages are consumed at all, it should be done in moderation.[25]
GLUTAMINE AND CANCER
Glutamine (α-aminoglutaric acid) found in beets[26] is the most abundant amino acid in the blood and tissues. It is essential for tumor growth and marked changes in organ glutamine metabolism are characteristic of the host with carcinoma. Numerous researches on glutamine metabolism in cancer indicate that many tumors are avid glutamine consumers, both in vivo and in vitro. In progressive tumor growth, host glutamine depletion develops and becomes a hallmark. Animal and human studies that have investigated the use of glutamine-supplemented diet in the host with cancer give suggestion that pharmacologic doses of dietary glutamine may be beneficial.[27]
Glutamine, arginine, and to a lesser extent ribonucleic acid show pharmacological effect when supplied in amounts in excess of what is needed to prevent nutritional shortage. These effects are exerted primarily through the immune system and immune-enhancing diets that are embodied according to the recently developed principles of nutritional pharmacology that have been shown to reduce infectious problems by approx 75%. This findings suggest that special-type diets can be formulated that will be beneficial to cancer patients.[28]
EFFECT OF NUTRITIONAL STATUS ON THE INCIDENCE OF INFECTION IN CHILDHOOD CANCER
In children with cancer, the effect of nutritional status on infection rate was studied in the department of pediatrics, King Edward Medical Colleges, Pakistan. From this experiments, a statistically significant inverse correlation (P<0.05) was found between nutritional status and ensuing infection rate. No such correlation was found in children with solid tumors. Based on these observations, nutritional supports are recommended to children with leukemia during chemotherapy to attempt to reduce the infection rate.[29,30]
DIETARY FIBERS PROTECTIVE MECHANISMS IN NUTRITIONAL CARCINOGENESIS
The interest in defining the relation between dietary fiber and cancer can be traced to the early 1970, when Burkitt cited international epidemiologic data indicating that fiber-rich diets played a protective role in cancer of the large bowel. This study and others found that the lowest rates of colon cancer are found in African and Asian countries, where high-fiber diets are consumed and that the highest colon cancer rates are seen in Western societies, where refined carbohydrates have commonly replaced the naturally occurring fiber-rich diets and where intake of fibers is consequently decreasing.[31] The epidemiologic experimental and clinical studies conducted since Burkitt's findings suggest that the risk of colon cancer and possibly other cancers also may be lowered by taking large amount of dietary fibers and other dietary components[31] associated with high intake of grains, vegetables, and fruits.[32,33] There is inverse relation between incidence of colon cancer and the amount of fiber consumed.[34]
Actually, fibers in foods are complex carbohydrates. There are several type of fibers, but the fibers related to their mode of protective action in carcinogenesis are classified into two board types: Soluble and insoluble fibers. Soluble fibers are present in fruits, vegetables, and certain grains like oats. This type of fibers undergo metabolism in the small intestine and especially in the large intestine through bacterial enzymes, converting it to products that (i) increases stool size moderately, (ii) modify appreciably the metabolism of colon carcinogenesis like azoxymethane to yield detoxified products and thus reduce colon cancer. In contrast, insoluble fibers present in brain cereals like wheat or rice are not significantly metabolized by enzymes in the intestinal flora. The probability of colon cancer can be minimized by such fibers in different ways, i.e., (i) increase stool size substantially through different mechanisms like higher water retention, (ii) the large bulk dilutes carcinogens, specially tumor promoters such as secondary bile acids, (iii) reduce fecal pH, and (iv) accelerate the transit time of fecal substances through the intestinal tract so that carcinogens from blood have less contact with colon mucosa.[35,36]
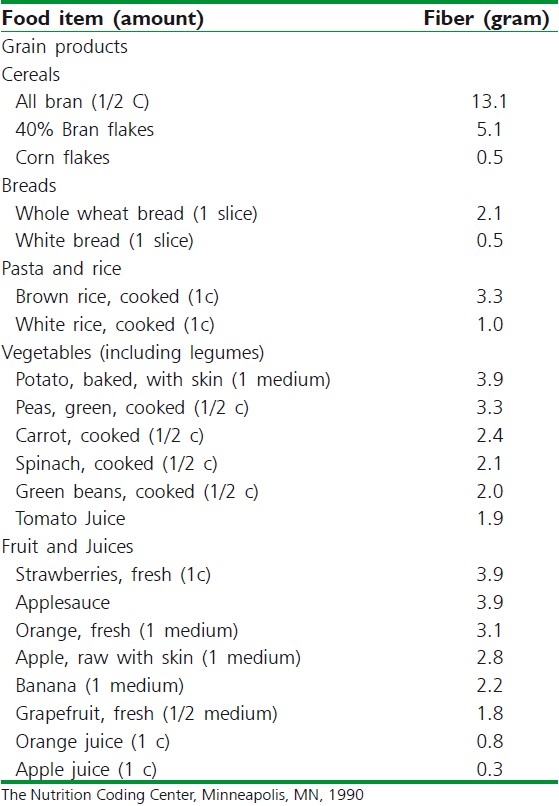
Insoluble grain fiber is more strongly associated with decreased risk of cancer than soluble grain fiber. In comparison with insoluble fibers, soluble fiber polysaccharides may enhance the development of colorectal cancer by (a) reducing the ability of insoluble fibers to adsorb hydrophobic carcinogens; (b) if soluble fiber polysaccharides are maintaining hydrophobic carcinogens in solution, then the carcinogens may come out of solution and can be deposited on the mucosal surface of the colon. These studies have important consequences for nutrition, because soluble fiber polysaccharides constitute a major part of our food.[37–39] Evidence in animal models and in human also indicates that fiber may lower the risk of breast cancer possibly through an endocrine mechanism. Based on these concepts, increased intake of wheat bran cereal fiber, to yield a daily stool in adults of about 200 g, can significantly reduce the risks of colon cancer and to a lesser but definite extent of breast cancer.[35,37,40,41] The National Cancer Institute (NCI) suggests that healthy persons take 20 to 30 g of dietary fiber per day, using a variety of food sources.[42] The fiber content of some foods in the major fiber containing food groups-grains, vegetables, and fruits are provided in Table 2.
Table 2.
Fiber content of selected foods
FRUIT JUICES, PROCESSED VEGETABLES JUICE, ORANGE PEEL, AND GREEN TEA IN CANCER PREVENTION
In 1933, scientist from Singapore reported that high-risk diet comprising high meat intake and a relative deficiency in fruits and vegetables (especially those of the cruciferous family) are very much relevant to colorectal and breast cancer. The active components of fruit and vegetables point to a number of micronutrients and substances which have cancer inhibitory properties. They are recently subjects of vigorous research throughout the world.[5]
Among the food material tested, vegetable flower (cooked drumstick, neem, onion), green leafy vegetables, and fruits (amla, jackfruit, pomegranate) are found to be harmless and protective against cancer and provide optimum nutrition. Among beverages (coffee, green tea), drinking of coffee is linked with cancer of bowel, pancreas, and bladder. Thus, by knowing right and protective foods, incidence of cancer can be prevented.[43,44] According to the research group of scientist in the Beckman Research Institute of the city of Hope, USA, grape juice has been found to contain compounds that inhibit aromatase/estrogen biosynthesis and thus grape juice has been suggested as potential chemopreventive agent for breast cancer.[45,46]
VITAMINS, FLAVONOIDS, AND TRACE MINERALS IN NUTRITIONAL SUPPORT OF CANCER PATIENTS: CRITICAL REAPPRAISAL
The potential of a high intake of fresh fruits and vegetables in cancer prevention is well established. Epidemiological, cohort and prospective, studies support vitamins C, E, and selenium as the active components present in these foods and antioxidant, free radical scavenging properties, and direct effects (e.g., inhibition of N-nitrosamine formation or cell-to-cell interactions)[47] are involved in protection from many types of cancer, e.g., lung, breast, mouth, skin, esophagus, stomach, etc. The role of other trace element is less clear. The modulation of immune function by vitamins and trace elements remain important and affects survival. Supplementation by these can diminish the damage induced by peroxidation.[48] There exists a combined effect of vitamin A and E in cancer prevention. The intestinal absorption of vitamin A is increased by vitamin E. This effect may be related to the prevention of vitamin A by the antioxidant properties of vitamin E. This effect may be related to the protection of vitamin A by the antioxidant properties of vitamin E. In addition, vitamin E seems to protect against various effects of hyper-vitaminosis A. In both cases, vitamin A and E may find important roles in cancer chemoprevention.[49] Vitamin E has the capacity to reduce 33% death due to cancer among the smokers, which is found in a preliminary preventive trial in Finland.[50] Preliminary research also indicates that flavonoids obtained from Enhydra fluctuans exhibit anticancer activity.[51]
PHOSPHOROUS LEVEL AND PROSTATE CANCER
Several studies support the view that higher levels of 1, 25 (OH)2D, the active metabolite of vitamin D,[52] reduce the risk of benign prostatic hyperplasia and cancer of the prostate. It is found that a low dietary intake of phosphorous has been reported to increase serum levels of 1, 25 (OH)2 D. In addition, dietary fructose (from both fruit and non-fruit sources) reduces plasma phosphate levels by 30% to 50% for more than 3 hours. All these observations support that dietary determinants of hypophosphatemia by increasing the plasma levels of 1,25 (OH)2 D could lower the risk of ageing men to develop prostatic disorders and carcinoma of prostate.[53,54]
VITAMIN D AND COLORECTAL CARCINOMA
Evidences exist regarding the role of vitamin D in colon cancer prevention.[55–57] There are several studies indicating that the protective effect of vitamin D is not strictly related to its role as a main regulator of calcium homeostasis, but rather to its direct action on colon tissue.[57] Human studies have investigated the potential role of vitamin D and calcium as protective and chemopreventive agents of colorectal carcinoma,[58,59] but more accurate and definitive evidences must come from clinical trials to establish it.
NATURAL PLANTS AS ANTIOXIDANT MAY PREVENT CANCER
Recently, a great deal of interest has been directed toward the bioactivity of natural plants as sources of antioxidants,[59,60] as the antioxidants have the free radical scavenging property. These are also related to the prevention of cancer.[61–66]
Plumbagin (2 methyl 5 hydroxy 1,4 naphthoquinone) isolated from the roots of Plumbago zeylanica (family-plumbaginaceae) has been reported for its anticancer activity. The antitumor activity of plumbagin has been evaluated against Dalton's ascetic lymphoma in Swiss albino mice.[67]
Carrot (Daucus carota linn; Umbelliferae) is widely used as a vegetable, the different part of this plant are known to possess multifarious medicinal properties. High vegetable consumption including carrots reduces the risk of breast cancer. The protective effects of carrots in case of the colon, rectum, and lungs have also been reported. The extract of the seeds of carrot showed antitumor activity, inhibiting the growth of Ehrlich ascites tumor in mice.[68]
Effects of a new mycotoxin obtained from the fungal strain Penicillium nigricans showed significant antitumor property on the growth of experimental tumor on mice.[69] Methanol extracts from Mucus pruriens and Cassia fistula fruits were found to have antineoplastic activity (60% protection) in tumor-bearing mice.[70]
RETINOIDS IN THE FIELD OF CANCER
One promising area of current research in nutrition and cancer is the possibility that micronutrients, in particular retinoids or carotenoids, may decrease the rate of epithelial cell cancers, which accounts for more than 90% of all cancer deaths and in particular lung cancer, which causes 25% of all cancer deaths in the United States.[71–73] Retinol (which is preformed vitamin A) is found in liver, egg yolks, and other animal products, fortified cereals, and vitamin pills and carotenoids (which are provitamin A) are found in deep green and orange vegetables and fruits. Deficiency of vitamin A has been found to affect the immune system at multiple sites. It affects certain subsets of T-lymphocytes, natural killers cells, cytotoxic activity, and the antibody response to bacterial polypeptides.[74] From laboratory and animal experiments, it is found that retinol and other retinoids have potent hormone like effects on cell growth and differentiation of epithelial tissues.[75,76] Most types of epithelial tissues are dependent on retinoids to control normal cell growth and differentiation. Data from numerous in vitro studies indicated that retinoids inhibit the process of malignant transformation induced in culture cells by various agents including radiation, testosterone, chemical carcinogens, etc. Retinoids can also reverse keratinization and other premalignant changes[76,77] of the epithelial cancer treated with retinoids, skin squamous-cell carcinoma, and cervical cancers, the most positive response on treatment with 13-cis-retinoic acid [Figure 2].[78] Retinoic acid has shown both a pathogenic and therapeutic role in the treatment of promyelocytic leukemia.[49,79]
Figure 2.
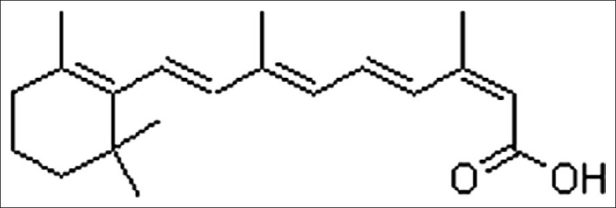
Chemical structure of 13-cis-retinoic acid[52]
Even retinoids are documented to reduce cancer risks; the transport and storage of retinoids in the body present a major drawback to their possible use as prophylactic agents by the general population. Because excess unbound retinol is stored in the liver, long-term high intake can lead to hepatotoxicity or other symptoms of hypervitaminosis A.[9] But combination therapy with chemotherapeutic agents can reduce these side effects as well as improve the efficacy and a lower retinoid dose is required, e.g., animal studies have indicated that combination therapy using trans RA or 9-cis-RA with tamoxifen provides chemoprevention of mammary carcinoma. Again, retinoids are given on combination therapy with interferon in skin cancer to supply sufficient chemopreventive potential.[78]
CAROTENOIDS FOR CANCER CHEMOPREVENTION
In laboratory and animal studies, it is seen that some beta-carotene [Figure 3] is converted directly to retinol in the body so that ingestion of large amounts of beta-carotene could reduce cancer risk indirectly by preventing retinoid deficiency.[9,80] However, maximum dietary carotenoids are absorbed directly from the gastrointestinal tract without undergoing transformation to retinol. Two intriguing properties of beta-carotene are very much relevant to its potential for chemoprevention of cancer; (i) It has the ability to trap certain organic free radicals,[81] (ii) It has the capacity to deactivate excited molecules, particularly excited of singlet oxygen,[82] which is generated as a byproduct of many normal metabolite processes. There have been experiments performed to mice and rats, where highly significant protective effects of diets containing large amounts of beta-carotene (e.g., 90 mg/kg) have been reported.[83] In human beings, overall, the epidemiological studies of beta-carotene are remarkably consistent in suggesting a protective effect on cancer risk. To date, more than 85 questionnaire studies of intake of fruits and vegetables rich in beta-carotene in relation to risk for cancer at 16 different sites were conducted in more than a dozen locations within the United States and 20 other countries. The strongest and most consistent evidence of a protective effect of high intake of carotene-rich foods comes from studies of lung cancer, most of which have suggested beneficial effects[84–86] [Table 3]. Studies of beta-carotene and cancer at other parts of the body are less consistent. There have been found inverse relation between dietary carotene and cancer risk on studies of cancer of the esophagus.[87] Studies of colorectal cancer and prostate cancer, breast cancer, cervix,[88] and ovary have been largely supportive of a protective effect of beta-carotene intake, though most do not achieve statistical significance.
Figure 3.
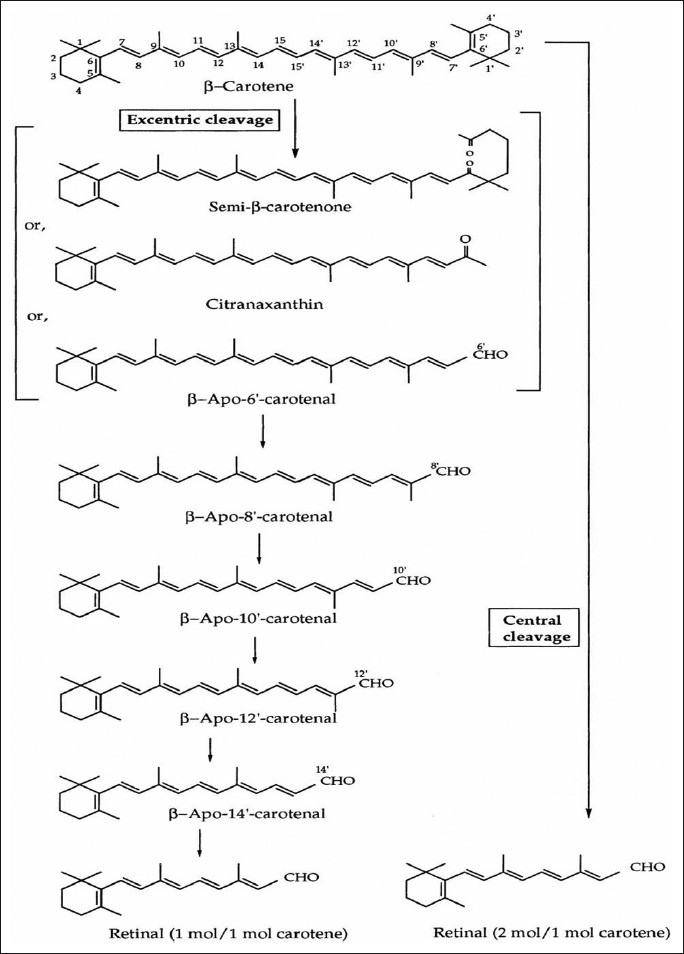
Bioconversion of β-carotene in the body[52]
Table 3.
Questionnaire studies of beta-carotene intake and lung cancer
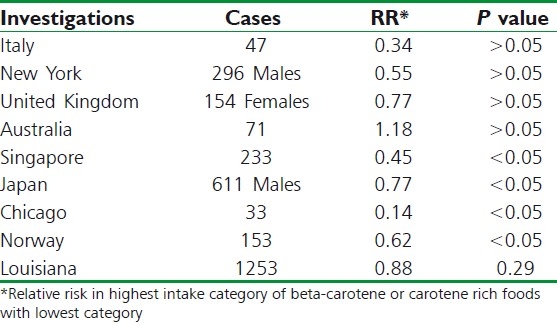
If beta-carotene reduces risk for cancer, one main advantage as a potential chemopreventive agent among large populations is its metabolic properties. In contrast with retinol, dietary intake of beta-carotene appears to be directly related to blood levels.[89] Again, excess carotene is stored in adipose tissues rather than in the liver; so, if high doses for long periods are taken, it does not seem to cause toxic manifestations.[90]
CONCLUSION
Diet is one the most important factor for the formation and prevention of cancer. Thus, in part to reduce cancer risk, two dietary goals have to be achieved for the year 2000: (i) the caloric contribution of fat should not be more than 30% of total caloric intake which can be achieved by reducing present fat intake by 18%; (ii) The consumption of carbohydrate and fiber-containing foods should be doubled by increasing fruit and vegetable consumption to five servings per day. If alcoholic beverages are consumed anyway, according to NCI guidelines, it should be taken in moderate dose (less than 40 g per day). Retinoids, carotenoids, vitamin C, and vitamin E should be taken in optimum amount. In order to control the malignant tumor, the above mentioned diet has been adopted in America for last 20 years. This type of food habits should be adopted in our country; instead of high intake of meal, fruits and vegetable rich diets should be taken. More cost-effective and timelier methods of dietary surveillance of target population must be developed to support the development and evaluation of more effective interventions. The more recent advances in molecular and cellular biology that underlie understanding multi-stage carcinogenesis are providing further support for new research strategies to prevent and treat cancer.
ACKNOWLEDGEMENT
The authors greatly acknowledge Dr. R. M. Dubey, Vice Chancellor, IFTM University, Lodhipur Rajput, Moradabad, for providing necessary computer facilities.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Kesteloof H. Nutrition and health: An epidemiological approach. Verh K Acad Geneeskd Belg. 1993;55:399–421. [PubMed] [Google Scholar]
- 2.Prentice RL, Sheppard L. Dietary fat and cancer: Rejoinder and discussion of research strategies. Cancer Causes Control. 1998;2:53–8. doi: 10.1007/BF00052360. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez Castillo CP, James WP. A metabolic unit for studies on human nutrition. Arch Latinoam Nutr. 1993;43:277–85. [PubMed] [Google Scholar]
- 4.Clayson DB. Food safety: Are human activities really worse than nature's? Regul Toxicol Pharmacol. 1993;17:145–56. doi: 10.1006/rtph.1993.1014. [DOI] [PubMed] [Google Scholar]
- 5.Lee HP. Diet and cancer: A short review. Ann Acad Med Singapore. 1993;22:355–9. [PubMed] [Google Scholar]
- 6.Ovesen L, Hannibal J, Mortensen EL. The interrelationship of weight loss, dietary intake, and quality of life in ambulatory patients with cancer of the lung, breast, and ovary. Nutr Cancer. 1993;19:159–67. doi: 10.1080/01635589309514246. [DOI] [PubMed] [Google Scholar]
- 7.Bal DG, Foerster SB. Dietary strategies for cancer prevention. Cancer. 1993;72:1005–10. doi: 10.1002/1097-0142(19930801)72:3+<1005::aid-cncr2820721310>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 8.Serra Majem L, La Vecchia C, Ribas Barba L, Prieto Ramos F, Lucchini F, Ramon JM, et al. Changes in diet and mortality from selected cancers in southern Mediterranean countries 1960-1989. Eur J Clin Nutr. 1993;47:825–34. [PubMed] [Google Scholar]
- 9.Vincent T, De Vita J, Hellman Samuel, Rosenberg Steven A. Cancer: Principles and Practice of Onchology. Philadelphia: JB Lippincott Co; 1993. [Google Scholar]
- 10.Hursting SD, Thornquist M, Henderson MM. Types of dietary fat and the incidence of cancer at five sites. Prev Med. 1990;19:242–53. doi: 10.1016/0091-7435(90)90025-f. [DOI] [PubMed] [Google Scholar]
- 11.Ganz PA, Schag AC. Nutrition and breast cancer. Oncology Huntingt. 1993;7:71–5. [PubMed] [Google Scholar]
- 12.Muir CS, Waterhouse J, Mack TM, Powel J, Whelan S. In Cancer incidence in five continents. V. Lyon, France: WHO International Agency for Research in Cancer; 1987. [Google Scholar]
- 13.Ferraroni M, Gerber M, Decarli A, Richardson S, Marubini E, Crastes de Paulet P, et al. HDL-cholesterol and breast cancer: A joint study in northern Italy and southern France. Int J Epidemiol. 1993;22:772–80. doi: 10.1093/ije/22.5.772. [DOI] [PubMed] [Google Scholar]
- 14.Kromann N, Green A. Epidemiological studies in the Upernavik district, Greenland. Incidence of some chronic diseases 1950-1974. Acta Med Scand. 1980;208:401–6. [PubMed] [Google Scholar]
- 15.Carvoll KK. Summation: Which fat/how much fat–animals. Prev Med. 1987;16:510–5. doi: 10.1016/0091-7435(87)90065-x. [DOI] [PubMed] [Google Scholar]
- 16.Birt DF, Pinch HJ, Barnett I, Phan A, Dimitroff K. Inhibition of skin tumor promotion by restriction of fat and carbohydrate calories in SENCAR mice. Cancer Res. 1993;53:27–31. [PubMed] [Google Scholar]
- 17.Rose DP, Cannoly JM, Rayburn J. Effect of diets containing different levels of linoleic acid on human breast cancer growth and lung metastasis in nude mice. Cancer Inst. 1993;85:1743–7. [PubMed] [Google Scholar]
- 18.Cannolly JM, Rose DP. Effects of fatty acids on invasion through reconstituted basement membrane (‘Matrigel’) by a human breast cancer cell line. Cancer Lett. 1993;75:137–42. doi: 10.1016/0304-3835(93)90198-i. [DOI] [PubMed] [Google Scholar]
- 19.Rose DP, Cannolly JM. Dietary prevention of breast cancer. Med Oncol Tumor Pharmacother. 1990;7:121–30. doi: 10.1007/BF02988539. [DOI] [PubMed] [Google Scholar]
- 20.Katcher BS. The post-repeal eclipse in knowledge about the harmful effects of alcohol. Addiction. 1993;88:729–44. doi: 10.1111/j.1360-0443.1993.tb02088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franceschi S. Role of nutrition in the aetiology of oesophageal cancer in developed countries. Endoscopy. 1993;25:613–6. doi: 10.1055/s-2007-1010416. [DOI] [PubMed] [Google Scholar]
- 22.National Academy of Sciences, National Research Council, Commission of Life Sciences, Food and Nutrition Board, Diet and Health: Implication for reducing chronic disease risk. Washington DC: National Academy Press; 1989. [Google Scholar]
- 23.Bjomeboe A, Bjomeboe GE. Antioxidant status and alcohol-related diseases. Alcohol Alcohol. 1993;28:111–6. [PubMed] [Google Scholar]
- 24.Tripathi KD. Essentials of Medical Pharmacology. New Delhi: Jaypee Brother Medical Publishers Pvt. Ltd; 1999. [Google Scholar]
- 25.Howe G, Rohan T, Decarli A. The association between alcohol and breast cancer risk: Evidence from the combined analysis of six dietary case-control studies. Int J Cancer. 1991;47:707–10. doi: 10.1002/ijc.2910470514. [DOI] [PubMed] [Google Scholar]
- 26.Morrison RT, Boyd RN. Organic Chemistry. New Delhi: Prentice Hall of India; 2000. [Google Scholar]
- 27.Souba WW. Glutamine and cancer. Ann Surg. 1993;218:715–28. doi: 10.1097/00000658-199312000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexander JW. Immunoenhancement via enteral nutrition. Arch Surg. 1993;128:1242–5. doi: 10.1001/archsurg.1993.01420230070011. [DOI] [PubMed] [Google Scholar]
- 29.Taj MM, Peason AD, Mumford DB, Price L. Effect of nutritional status on the incidence of infection in childhood cancer. Pediatr Hematol Oncol. 1993;10:283–7. doi: 10.3109/08880019309029498. [DOI] [PubMed] [Google Scholar]
- 30.Wallin E, Bremberg S, Haglund B, Holm LE. Cancer prevention in schools: Design and pilot testing of a nutritional curriculum for mid-adolescents. J Cancer Educ. 1993;8:144–50. doi: 10.1080/08858199309528222. [DOI] [PubMed] [Google Scholar]
- 31.Satyanarayana U. Biochemistry. Calcutta: Books and Allied Pvt. Ltd; 1999. [Google Scholar]
- 32.Trock B, Lanza E, Greenwald P. Dietary fiber, vegetables, and colon cancer: Critical review and meta-analyses of the epidemiologic evidence. J Natl Cancer Inst. 1990;82:650–61. doi: 10.1093/jnci/82.8.650. [DOI] [PubMed] [Google Scholar]
- 33.McIntosh GH. Colon cancer: Dietary modifications required for a balanced protective diet. Prev Med. 1993;22:767–74. doi: 10.1006/pmed.1993.1070. [DOI] [PubMed] [Google Scholar]
- 34.Greenwald P, Lanza E, Eddy GA. Dietary fiber in the reduction of colon cancer risk. J AM Diet Assoc. 1987;87:1178–88. [PubMed] [Google Scholar]
- 35.Weisburger JH, Reddy BS, Rose DP, Cohen LA, Kendall ME, Wynder EL. Protective mechanisms of dietary fibers in nutritional carcinogenesis. Basic Life Sci. 1993;61:45–63. doi: 10.1007/978-1-4615-2984-2_4. [DOI] [PubMed] [Google Scholar]
- 36.Cummings JH, Biingham SA. Dietary fibre, fermentation and large bowel cancer. Cancer Surv. 1987;6:601–21. [PubMed] [Google Scholar]
- 37.Freudenheim JL, Graham S, Horvath PJ, Marshall JR, Haughey BP, Wilkinson G. Risks associated with source of fiber and fiber components in cancer of the colon and rectum. Cancer Res. 1990;50:3295–300. [PubMed] [Google Scholar]
- 38.Harris PJ, Roberton AM, Watson ME, Triggs CM, Ferguson LR. The effects of soluble-fiber polysaccharides on the adsorption of a hydrophobic carcinogen to an insoluble dietary fiber. Nutr Cancer. 1993;19:43–54. doi: 10.1080/01635589309514235. [DOI] [PubMed] [Google Scholar]
- 39.Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–5. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 40.Shankar S, Lanza E. Dietary fiber and cancer prevention. Hematol Oncol Clin North Am. 1991;5:25–41. [PubMed] [Google Scholar]
- 41.Clifford C, Kramer B. Diet as risk and therapy for cancer. Med Clin North Am. 1993;77:725–44. doi: 10.1016/s0025-7125(16)30220-6. [DOI] [PubMed] [Google Scholar]
- 42.Lanza E, Jones DY, Block G, Kessler L. Dietary fiber intake in the US population. Am J Clin Nutr. 1987;46:790–7. doi: 10.1093/ajcn/46.5.790. [DOI] [PubMed] [Google Scholar]
- 43.Pai RR, Raghuveer CV, Jayasree A, Kini H. Sex cord-stromal tumours of the ovary. A clinicopathological spectrum. Indian J Nutr Diet. 2000;43:113–21. [PubMed] [Google Scholar]
- 44.Ohnishi S, Aoyama H, Shiga J, Itai Y, Moriyama T, Ishikawa T, et al. Establishment of a new cell line from a woodchuck hepatocellular carcinoma. Hepatology. 1988;8:104–7. doi: 10.1002/hep.1840080121. [DOI] [PubMed] [Google Scholar]
- 45.Chen S, Sun XZ, Kao YC, Kwon A, Zhou D, Eng E. Suppression of breast cancer cell growth with grape juice. Pharm Biol. 1998;36:53–61. [Google Scholar]
- 46.Ikken Y, Cambero I, Marin ML, Martinez A, Haza AI, Morales P. Protective effect of broccoli, onion, carrot, and licorice extracts against cytotoxicity of N-nitrosamines evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. J Agri food Chem. 1998;46:585–9. doi: 10.1021/jf970636i. [DOI] [PubMed] [Google Scholar]
- 47.Xu GP, Song PJ, Reed PI. Effects of fruit juices, processed vegetable juice, orange peel and green tea on endogenous formation of N-nitrosoproline in subjects from a high-risk area for gastric cancer in Moping County, China. Eur J Cancer Prev. 1993;2:327–35. doi: 10.1097/00008469-199307000-00007. [DOI] [PubMed] [Google Scholar]
- 48.Stahelin HB. Critical reappraisal of vitamins and trace minerals in nutritional support of cancer patients. Support Care Cancer. 1993;1:295–7. doi: 10.1007/BF00364966. [DOI] [PubMed] [Google Scholar]
- 49.Hardman JG, Limbard LE. Goodman and Gilman's The Pharmacological Basis of Therapeutics. USA: Mc Graw Hill Health Professions Division; 1996. pp. 1582–6. [Google Scholar]
- 50.Lang L. New Health Reform Law to Benefit 30 Million Women. Gastroenterol. 2010;139:1069–428. [Google Scholar]
- 51.Sanigrahi S, Mazumber UK, Mondal A, Pal DK, Mishra SL, Roy S. Flavonoids of Enhydra fluctuans exhibit anticancer activity ehrlich's ascites carcinoma in mice. Nat Prod Comm. 2010;5:1230–42. [PubMed] [Google Scholar]
- 52.Lehninger AL, Nelsol DL, Cox MM. Principles of Biochemistry. Delhi: CBS Publishers and Distributors; 1993. [Google Scholar]
- 53.Kapur S. Phosphorus balance and prostate cancer. Indian J of Exp Biol. 1999;37:623–6. [PubMed] [Google Scholar]
- 54.Hallfrisch J, Ellwood K, Michaelis OE, Reiser S, Prather KS. Plasma fructose, uric acid, and inorganic phosphorus responses of hyperinsulinemic men fed fructose. J Am Coll Nutr. 1986;5:61–8. doi: 10.1080/07315724.1986.10720113. [DOI] [PubMed] [Google Scholar]
- 55.Niv Y. New tools for better staging of colorectal cancer-therapeutic applications. Cancer. 1999;36:865–6. [PubMed] [Google Scholar]
- 56.La Vecchia C, Braga C, Begri E, Francesschi S, Russo A, Conti E, et al. Intake of selected micronutrients and risk of colorectal cancer. Int J Cancer. 1997;73:525–30. doi: 10.1002/(sici)1097-0215(19971114)73:4<525::aid-ijc12>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 57.White E, Shannon JS, Patterson RE. Relationship between vitamin and calcium supplement use and colon cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:769–74. [PubMed] [Google Scholar]
- 58.Chritakos S, Rayal Pandya M, Werney RP, Yang W. Genomic mechanisms involved in the pleiotropic actions of 1,25-dihydroxyvitamin D3. Biochem J. 1996;316:361–71. doi: 10.1042/bj3160361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cros HS, Bajna E, Bises G, Genser D, Kallay E, Potzi R. Vitamin D receptor and cytokeratin expression may be progression indicators in human colon cancer. Anticancer Res. 1996;16:2333–7. [PubMed] [Google Scholar]
- 60.Pal DK, Mitra S. A preliminary study on the in-vitro antioxidant activity of the stems of Opuntia vulgaris. J Adv Pharm Tech Res. 2010;1:268–72. [PMC free article] [PubMed] [Google Scholar]
- 61.Pal DK, Halder P, Bhuniya A. A study on the in-vitro antioxidant activity of aerial parts of Celsia coromandeliane Vahl and bark of Mesua ferrea Linn. Pharmacologyonline. 2009;3:200–3. [Google Scholar]
- 62.Pal DK, Samanta K, Maity P. Evaluation of antioxidant activity of aerial parts of Hygrophila difformis. Asian J Chem. 2010;22:2459–61. [Google Scholar]
- 63.Pal DK, Kumar M, Chakraborty P, Kumar S. Evaluation of antioxidant activity of aerial parts of Cynodon dactylon. Asian J Chem. 2008;20:2479–81. [Google Scholar]
- 64.Pal DK, Kumar S, Chakraborty P, Kumar M. A study on the antioxidant activity of Semecarpus anacardium L. f. nuts. J Nat Rem. 2008;8:160–3. [Google Scholar]
- 65.Pal DK, Nimse SB. Screening of the antioxidant activity of Hydrilla verticillata Plant. Asian J Chem. 2006;18:3004–8. [Google Scholar]
- 66.Pal DK, Dutta S. Evaluation of the Antioxidant activity of the roots and Rhizomes of Cyperus rotundus L. Indian J Pharm Sci. 2006;68:256–8. [Google Scholar]
- 67.Kavimani S, Ilango R, Madheswaran M, Jayakar B, Gupta M, Majumdar UK. Antitumour activity of plumbagin against dalton's ascitic lymphoma. Indian J Pharm Sci. 1996;58:194–6. [Google Scholar]
- 68.Mazumder PK, Gupta M. Effect of the seed extract of carrot (Daucus carota Linn.) on the growth of Ehrlich ascites tumour in mice. Phytother Res. 1998;12:584–5. [Google Scholar]
- 69.Gupta M, Mazumder UK, Ray MR, Mukherjee DK. Inhibition of experimental murine tumors by MT81, a new mycotoxin from Penicillium nigricans. Neoplasma. 1997;44:329–33. [PubMed] [Google Scholar]
- 70.Gupta M, Mazumder UK, Chakraborti S, Bhattacharya S, Rath N, Bhawal SR. Antiepileptic and anticancer activity of some indigenous plants. Indian J Physiol and Allied Sci. 1997;51:53–6. [Google Scholar]
- 71.Cairns J. The Leeuwenhoek Lecture, 1978. Bacteria as proper subjects for cancer research. Proc R Soc Lond B Biol Sci. 1980;208:121–33. doi: 10.1098/rspb.1980.0046. [DOI] [PubMed] [Google Scholar]
- 72.Doll R, Peto R. The causes of cancer: Quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66:1192–308. [PubMed] [Google Scholar]
- 73.Hennekens CH. Vitamin A analogues in cancer chemoprevention. Important Adv Oncol. 1986:23–5. [PubMed] [Google Scholar]
- 74.Parthasarathy A. IAP Textbook of Paediatrics. New Delhi: Jaypee Brothers Medical Publishers; 2007. pp. 53–58. [Google Scholar]
- 75.Wolback SB, Howe PR. Tissue changes following deprivation of fat-soluble a vitamin. J Exp Med. 1925;42:753–77. doi: 10.1084/jem.42.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sporn MB, Dunlop NM, Newton DL, Henderson WR. Relationships between structure and activity of retinoids. Nature. 1976;263:110–3. doi: 10.1038/263110a0. [DOI] [PubMed] [Google Scholar]
- 77.Dickens MS, Sarof S. Carcinogen-protein complexes in mammary gland after administration of 3-methylcholanthrene. Proc Am Assoc Cancer Res. 1977;79:713–9. doi: 10.1016/0006-291x(77)91170-6. [DOI] [PubMed] [Google Scholar]
- 78.Manfred EW. Berger's Medicinal Chemistry and Drug Discovery. Vol. 3. Hoboken, NJ, San Francisco, Oxford: Willey Interscience Publication; 1996. pp. 591–4. [Google Scholar]
- 79.Rang HP, Dale MM, Ritter JM. Pharmacology. Edinburg, London, Madrid, Melbourne, New York, Tokyo: Churchill Livingstone; 1995. [Google Scholar]
- 80.Peto R, Doll R, Buckley JD, Sporn MB. Can dietary beta-carotene materially reduce human cancer rates? Nature. 1981;290:201–8. doi: 10.1038/290201a0. [DOI] [PubMed] [Google Scholar]
- 81.Krinsky NI, Deneke SM. Interaction of oxygen and oxy-radicals with carotenoids. J Natl Cancer Inst. 1982;69:205–10. [PubMed] [Google Scholar]
- 82.Lederman DM, Cumming RD, Petschek HE, Levine PH, Krinsky NI. The effect of temperature on the interaction of platelets and leukocytes with materials exposed to flowing blood. Pure App Chem. 1978;24:557–60. [PubMed] [Google Scholar]
- 83.Seifter E, Padawer J, Rettura G, Goodwin P, Levenson SM. Cancer control: X-ray induced C3HBA tumor regression and prevention of its regrowth by beta-carotene or vitamin A. Prog Clin Biol Res. 1983;130:237–47. [PubMed] [Google Scholar]
- 84.Marchand L, Yoshizawa CN, Kolonel LN, Hnkin JH, Goodman MT. Vegetable consumption and lung cancer risk: A population-based case-control study in Hawaii. J Natl Cancer Inst. 1989;81:1158–64. doi: 10.1093/jnci/81.15.1158. [DOI] [PubMed] [Google Scholar]
- 85.Harris RW, Key TJ, Silcocks PB, Bull D, Wald NJ. A case-control study of dietary carotene in men with lung cancer and in men with other epithelial cancers. Nutr Cancer. 1991;15:63–8. doi: 10.1080/01635589109514113. [DOI] [PubMed] [Google Scholar]
- 86.Fontham ET, Pickle LW, Haenszel W, Correa P, Lin Y, Falk RT. Dietary vitamins A and C and lung cancer risk in Louisiana. Cancer. 1988;62:2267–73. doi: 10.1002/1097-0142(19881115)62:10<2267::aid-cncr2820621033>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 87.Graham S, Marshall J, Haughey B, Brasure J, Freudenheim J, Zielezny M, et al. Nutritional epidemiology of cancer of the esophagus. Am J Epidemiol. 1990;131:454–67. doi: 10.1093/oxfordjournals.aje.a115520. [DOI] [PubMed] [Google Scholar]
- 88.Huda SN, Hossain AM, Islam K, Akhter PS, Sarma SK, Mahmud Z, et al. Plasma level of antioxidant nutrients (retinol and alphatocopherol) in cases with different grades of cervical carcinoma. Bangladesh Med Res Counc Bull. 1993;19:79–85. [PubMed] [Google Scholar]
- 89.Willett WC, Stampfer MJ, Underwood BA, Taylor JO, Hennekens CH. Vitamins A, E, and carotene: Effects of supplementation on their plasma levels. Am J Clin Nutr. 1983;38:559–66. doi: 10.1093/ajcn/38.4.559. [DOI] [PubMed] [Google Scholar]
- 90.Neiman C, Obbink HJ. The biochemistry and pathology of hypervitaminosis A. Vitam Horm. 1954;12:69–99. doi: 10.1016/s0083-6729(08)61009-2. [DOI] [PubMed] [Google Scholar]


