Abstract
Furosemide is a powerful diuretic and antihypertensive drug which has low bioavailability due to hepatic first pass metabolism and has a short half-life of 2 hours. To overcome the above drawback, the present study was carried out to formulate and evaluate sustained release (SR) pellets of furosemide for oral administration prepared by extrusion/spheronization. Drug Coat L-100 was used within the pellet core along with microcrystalline cellulose as the diluent and concentration of selected binder was optimized to be 1.2%. The formulation was prepared with drug to polymer ratio 1:3. It was optimized using Design of Experiments by employing a 32 central composite design that was used to systematically optimize the process parameters combined with response surface methodology. Dissolution studies were carried out with USP apparatus Type I (basket type) in both simulated gastric and intestinal pH. The statistical technique, i.e., the two-tailed paired t test and one-way ANOVA of in vitro data has proposed that there was very significant (P≤0.05) difference in dissolution profile of furosemide SR pellets when compared with pure drug and commercial product. Validation of the process optimization study indicated an extremely high degree of prognostic ability. The study effectively undertook the development of optimized process parameters of pelletization of furosemide pellets with tremendous SR characteristics.
Keywords: Central composite design, extrusion/spheronization, furosemide, statistical analysis, Taguchi orthogonal array design
INTRODUCTION
Conventional medication systems that necessitate multi-dose therapy are not without problems. With a view to triumph over these problems, the modern trend in pharmaceutical research is to design and develop new formulations, thereby boosting the therapeutic efficacy of existing drugs. Moreover, the impetus for research into drug delivery can be attributed to the exorbitant cost and large development period involved in “new drug development” with concomitant recognition of the therapeutic advantages of controlled drug delivery.[1]
Sustained release (SR) dosage forms are designed to achieve a prolonged therapeutic effect by continuously releasing the medication over an extended period of time after administration of a single dose. However, this period is measured in hours and critically depends on the residence time of the dosage form in the gastrointestinal (GI) tract.[2]
Being a multiparticulate solid dosage form, pellets offer numerous advantages when compared with tablets as classical “single-unit” dosage forms.[3] Physiological advantages are endorsed to more even distribution of pellets in GI tract, while technological advantages (good sphericity, smooth surface properties, narrow size distribution, and low friability) ensure a drug content uniformity with minimized risk of dose “dumping.” In addition, pellets offer better flexibility in dosing and drug release design.
Extrusion/spheronization is a well-established technique for the production of pellets. Microcrystalline cellulose (MCC) is now the most widely used excipient for pellets prepared through extrusion/spheronization because it has good binding properties and provides the necessary plasticity to the wet mass, which ensures successful extrusion/spheronization.[4] However, MCC pellets do not disintegrate and drug release occurs via diffusion through an insoluble inert matrix. If pellets are used for sustained drug delivery, pellet disintegration is not required.
Pellets are defined as spherical, free-flowing granules with a narrow size distribution, typically varying between 500 to 1 500 μm for pharmaceutical applications. The curiosity in pellets as dosage forms has been escalating continuously, since their multiparticulate nature offers some momentous pharmacological as well as technological advantages over conventional single-unit solid dosage forms. The homogeneous dispersion of a drug into small dosage units trim down the risk of high local drug concentration. Furthermore, drug absorption is maximized and peak plasma fluctuations are reduced.[5,6]
The implementation of Design of Experiments (DoE) optimization techniques invariably encompasses use of experimental designs and generation of mathematical equations and graphic outcomes, thus depicting an inclusive picture of variation of the product/process response (s) as a function of input variable.[7–9] Response surface methodology (RSM) is a rapid technique used to empirically derive a functional relationship between an experimental response and a set of input variables in the development and optimization of drug delivery systems. RSM trim down the number of experimental runs that are necessary to establish a mathematical trend in the experimental design region allow to determine the optimum level of experimental factors required for a given response.[10]
Experimental design technique such as Taguchi OA design was employed to demarcate the effect of each of these parameters and to optimize these variables on the response variables. In this work, central composite design (CCD) was used simultaneously to study the effect of the two process parameters involved in the preparation of pellets by extrusion/spheronization against three response variables. CCD is a response surface design which provides information on direct effects, pair-wise interaction effects, and curvilinear variable effects and is widely used for formulation and process optimization in the field of pharmaceutics.[11,12]
Furosemide 5-(aminosulfonyl)-4-chloro-2-[(furanylmethyl) amino] benzoic acid is a diuretic and antihypertensive drug was selected and belongs to biopharmaceutical classification system class IV. Furosemide is absorbed mostly in the stomach and upper part of the small intestine, possibly due to its weak acidic properties and is characterized by a short half-life of 1.5 to 2 hours.[13] Water-insoluble polymer Drug coat L-100 [Figure 1] was used within the pellet core. Drug coat L-100 is an anionic co-polymer of methacrylic acid and methyl methacrylate. The use of Drug coat L-100 employed inside the pellet core has not been reported for sustaining the drug release.
Figure 1.
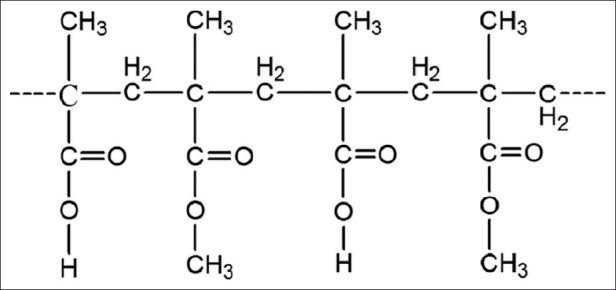
Chemical structure of the drug coat L-100
The first aim of the present study was to investigate whether it is possible to manufacture stronger and more uniform pellets by using an extrusion/spheronization technique with Drug Coat L-100. To develop the product as commercial product, the prerequisite is to scale up the blue-collar technique to pilot plant. The second aim was to acquire a comprehensible understanding the influence of process variables which powerfully affect the characteristics of the resultant SR pellets on percentage yield, pellets size, as well as on the in vitro drug release were studied in order to acquire an optimal formulation by using CCD combined with RSM.
MATERIALS AND METHODS
Materials
Furosemide was received as gift sample from Micro labs, Bangalore, India. Drug coat L-100 Poly (methacrylic acid, methyl methacrylate) was received as a gift sample from Vikram Thermo (India) Ltd. Gujarat. This polymer is indigenous and the SR properties for this polymer were not yet reported. MCC was acquired from S.D fine-chem Limited, Mumbai. PVP K-30 was supplied by Himedia Laboratories Pvt. Ltd, Mumbai. All excipients used to prepare pellets were of standard pharmaceutical grade and all chemicals used were of analytical grade.
Methods
Screening for influential factors
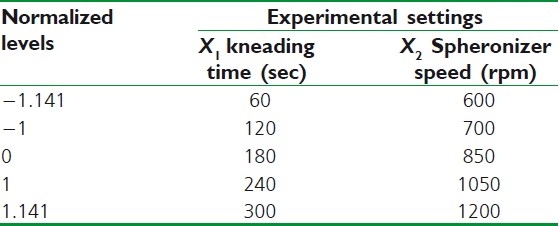
During initials studies, it is imperative to scrutinize the possible process variables of the system under study to know their influence on the quality of the product. A process variable identified as a factor increases the chance of success, while a process variable that is not a factor has no consequences. The screening study was performed using a Taguchi OA design for finding the relevant main parameters of the extrusion/spheronization process. Two process parameters were scrutinized through a design matrix of four experiments. The studied parameters were as follows: (1) kneading time (2) extruder speed (3) spheronizer speed and (4) spheronizer time. Based on preliminary experiments, the extreme levels of each factor were set. Symbols, coded and actual level of variables of the screening study are shown in Table 1. The significant parameters of the extrusion/spheronization were determined using the bar plot which illustrates the persuade of every process parameter on the response variables.
Table 1.
Experimental conditions for the screening study

Experimental Design
Central composite design
CCD has widely been used for fitting a second-order model and to require a minimum number of experiments to be performed. The independent process variables including kneading time (X1) (processing time after the liquid addition) and speed of the spheronizer (X2) were defined as factors, percentage yield (Y1), pellets size (Y2) and dissolution rate of pellets (Y3) were used separately as the responses in the CCD. A polynomial equation, with six coefficients to estimate, was produced for the account of the measured responses as function of the process variables.
The data obtained for the three responses in each trial were fitted to classical second-order polynomial model. The mathematical model was expressed as follows,
Second-order polynomial model:
y = b0 + b1x1 + b2x2 + b3x12 + b4x22 + b5x1x2
Where, y is the measured response, b0 is an intercept and b1–b5 are the regression coefficients, x1, x2 represents the main effect, x12, x22 the quadratic effect, and x1x2 is the interaction effect. The effects were evaluated statistically at 0.1 level (α = 1.141). Data were analyzed by nonlinear estimation using Design Expert® software 6.0. Against the two factors, graphs of surface responses were plotted with the response variation.
The process variables with their relative experimental values are reported in Table 2. Pellets were produced according to different levels of the factors [Table 2]. Each experiment was executed in triplicate.
Table 2.
Factors and levels of the circumscribed central composite design
Preparation of Drug-loaded Pellet
Pellets were prepared by laboratory scale extrusion/spheronization process by following steps:
Preparation of wet mass
The wet mass was produced by dry mixing the powders for 5 minutes in a blender mixer (Hobart, London, UK). A powder mixture constituted 20% furosemide, 40% MCC as diluent, and 40% Drug coat L-100 as SR polymer used inside the pellet core. Purified water was used as the binder liquid containing PVP K-30 at a concentration of 1.2%. A precisely determined amount of binder liquid (55 ml/100 g) was then added to the powder mixture and the mass was kneaded for a preset period of time after the liquid addition. The pellets were prepared under standardized operating conditions.
Extrusion
The wet powder mass was immediately extruded at 80 rpm through a radial screen extruder (Model S 250, Umang Pharmascitech, Mumbai, India), supplied with a 1 mm aperture screen.
Spheronization
A radial plate spheronizer with a plate diameter of 45.0 cm was used (model S250, Umang Pharmascitech, Mumbai, India). The friction plate speed in the spheronizer was varied between 6001200 rpm, respectively. The extrudate was spheronized for 10 minutes. The wet pellets were dried in a hot air oven at 40°C for 24 hours and then stored in sealed bags.
Characterization of Pellets
The pellets of the different formulations were characterized for the following properties:
Size
Pellet size was evaluated with a Motic BA300 microscope (China) connected to a video camera. Size was estimated by motic images plus 2.0 software for a total of 100 pellets per lot. The size data were best fitted by a normal distribution.[14] In addition, photomicrographs of pellets were obtained with a scanning electron microscope (Jeol JSM 5600 LV, Jeol, Tokyo, Japan).
Flow properties
Particle size distribution
The pellet size and pellet size distribution were estimated by sieve analysis (Test sieve shaker, Retsch VE1000 shaker, Germany) and optical microscope. Each batch of the pellets was sieved before the subsequent test in order to remove the lumps larger than 1 000 μm. About 20 g of the sample was sieved using a set of standard sieves (1 600, 1 250, 1 000, 800, 710, 630, 500, and 400 μm) trembling at an amplitude of 2 mm for 5 minutes. The fraction remaining on each screen was weighed and expressed as percentage of the total weight and medium diameter of pellets was obtained according to the cumulative size distribution. In optical microscopy method, particle size of pellets was determined by using 10 X objective lens. A clean glass slide was taken and small drop of glycerine was added and spread over the slide. Small quantities of pellets were then placed over the slide and particle size of about 100 pellets was determined. All results presented were the mean of three determinations. Pellets ranging between 700 and 850 μm in size were selected for in vitro dissolution test so that the effect of particle size on dissolution rate is excluded.
Angle of repose
The static angle of repose was measured according to the standard reported method. Angle of repose (θ°) was calculated from the standard trigonometric relationship.[15]
Volume, density, and compressibility
50 g sample was put into a 250-ml graduated cylinder of a volume and density apparatus (Electrolab TAB density Tester, USP, Model ETD 1020, Mumbai). Bulk density, tapped density, and Carr's percent compressibility were calculated.[16,17]
Friability
The friability of the SR pellets was assayed with a Roche friabilator for 10 minutes at 25 rpm. For each assay, 20 g of pellets was mixed with 30 g of glass beads.[17]
Assay of the drug content
The samples were assayed by a UV spectroscopic method according to the Indian Pharmacopoeial 2007 norms. SR pellets equivalent to 40 mg (one dose) of the drug were weighed and grounded to fine powder and dissolved in a 0.1M NaOH. After 2 hours of stirring with magnetic stirrer, the sample was filtered through a 0.45-μm membrane and the samples were analyzed spectrophotometrically at a wavelength of 271 nm (Model UV-1700, UV-Visible spectrophotometer, Shimadzu, Kyoto, Japan).
In vitro Release Studies
In vitro release studies were carried out in Electrolab TDT-06PL Dissolution tester USP apparatus Type I (rotating basket) at a speed of 75 rpm in 900 ml of dissolution medium. Initially for 2 hours, the study was carried out in simulated gastric fluid pH (1.2). Furthermore, the study was continued up to 7 hours in simulated intestinal fluid pH (6.8). The temperature was retained at 37.0 ± 0.5°C. 5 ml of samples were withdrawn at predetermined intervals and were analyzed by UV spectrophotometer at 271 nm (Model UV-1700, UV-Visible spectrophotometer, Shimadzu, Kyoto, Japan). Based on the drug content and dissolution studies, the optimized formulation was further subjected for Differential scanning calorimetry (DSC), X-ray diffraction, and Scanning electron microscopy (SEM) analysis. All experiments were carried out in triplicate.
Statistical Analysis
The in vitro data were further statistically analyzed by employing two-tail paired t test and one way ANOVA test using GraphPad InStat® software (Version 3.01). Statistically significant difference was considered when (P≤0.05) for paired samples.
Differential Scanning Calorimetry
Differential scanning calorimetric (DSC) analysis was used to characterize the thermal behavior of furosemide, physical mixture, and best optimized formulation. DSC thermograms were acquired using an automatic thermal analyzer system (Pyris 6 DSC, Perkin-Elmer, USA). Temperature calibration was performed using Indium Calibration Reference Standard (transition point: 156.60°C) as a standard. Samples were crimped in standard aluminium pans and heated from 40 to 300°C at a heating rate of 5°C/minute under constant purging of dry nitrogen at 30 ml/minute. An empty pan was used as a reference and sealed in the same way as the sample.
Powder X-ray Diffraction
Powder X-ray diffraction (PXRD) was performed using a Rigaku D/max-2500PC diffractometer (Rigaku, Tokyo, Japan) with monochromatic Cu Kα radiation (λ = 1.5407 Å), voltage 50 kV, current 100 mA, and 2θ over a 2-45° range. Diffraction patterns of pure furosemide, physical mixture, and best optimized formulation were determined.
Scanning Electron Microscopy
SEM (Jeol JSM 5600 LV, Jeol, Tokyo, Japan) was used to visualize the surface morphology of optimized formulation. Pellets were coated with platinum sputter coater 208 HR (Cressington Scientific Instruments Ltd., Watford, UK) to assure conductivity.
Accelerated Stability Studies
Stability testing is an integral part of formulation development. It provides evidence on how the quality of a drug substance or drug product varies with time under the influence of a variety of environmental factors. It establishes a re-test period for the drug substance or a shelf-life for the drug product and is used to recommend storage condition. Stability studies were carried out for 6 months under the storage conditions of 40 ± 2°C/75% ± 5% RH as per ICH guideline (Q1(A)R2) (International conference on Harmonization).
RESULTS AND DISCUSSION
SR formulations of furosemide were designed and developed. The pure drug with MCC as spheronizer aid and Drug Coat L-100 used inside a pellet core as SR polymer were the composition of the pellets prepared by extrusion/spheronization. The type and amount of granulation liquid was selected by investigating water, isopropyl alcohol, combination of water and isopropyl alcohol in different ratios. After several trials with different batches of pellets, purified water was selected as wet massing liquid.
The optimum level of granulating fluid is that level which results in maximum pellet roundness in the targeted particle size range. In preliminary work, every formulation was granulated using a series of water levels. The optimum level was identified as that level which produced nearly round particles whose average size is similar to the aperture of the extrusion screen. Binder selection was carried out using PVP K30 and Sodium CMC. PVP K30 was selected based on its capability to produce spherical-shaped pellets. Concentration of selected binder was optimized to be 1.2%.
The amount and concentration of binder affected the appearance of the resulting pellets. Increasing the volume of binder solution augmented the mean size of pellets but dwindle the yield in the desirable pellet size range. The exploit of an overindulgence amount of binder gave rod-shaped pellets and increased the hardness of the pellets. Due to the influence of the amount of granulating liquid on the processability of the wet mass, the volume of granulating liquid was further optimized to achieve accurate wetting of mass.
An increase of spheronization speed has been already reported as beneficial for pellet sphericity. This was arbitrated in terms of a high batch yield and a high percentage of pellets obtained with size ranging from 700 to 850 μm. At higher water concentrations, agglomeration occurred, whereas using a sub-optimal amount of water resulted in dumbbells. The significant interaction between water and spheronization speed indicated that a low spheronization speed and water level decreased the sphericity of the end product.
Screening Study
During screening studies, Taguchi OA design was used to study the effect of most influential process parameters on the response variables. Four process parameters were chosen for the study and pellets were prepared and evaluated for percentage yield, particle size, and in vitro dissolution profile. The significant parameters of the extrusion/spheronization were determined using the bar plot [Figure 2], which illustrates the influence of every process parameter on the response variables.
Figure 2.
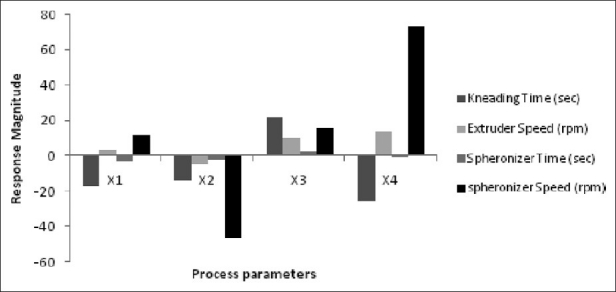
Influence of process parameters on the response magnitude
Each process parameters in the studied model was characterized by a P value (the smaller the P value, the greater the influence of the concerned parameter on the model). From the information given by the bar plot, we could infer that the kneading time and spheronizer speed were positively significant parameters and that, by contrast, the extruder speed and spheronizer time were negatively significant. Finally, the extruder speed and spheronizer time were considered as not significant at all and these were neglected for the optimization step.
To optimize the influence of Drug Coat L-100 to MCC ratio, an in vitro drug release studies were carried out (data not shown). We have attempted four different ratios of Drug Coat L-100 to MCC (1:1, 1:2, 2:1, and 2:2). Of these, 2:1 was the preeminent optimized ratio. Because the proportion of the more lipophilic polymer Drug coat L-100 had the most significant effect on drug release as compared with MCC, higher proportion gave SR and have a momentous effect on the rate of drug release till 9 hours.
Optimization of the Formulation and Pellets Characterization
Furosemide SR pellets were prepared according to the circumscribed CCD. Table 3 gives an overview of the characteristics of these pellets. Both independent variables, i.e., kneading time and spheronizing speed had a statistically significant effect on pellet sphericity.
Table 3.
Experimental plan and observed response values of SR pellets
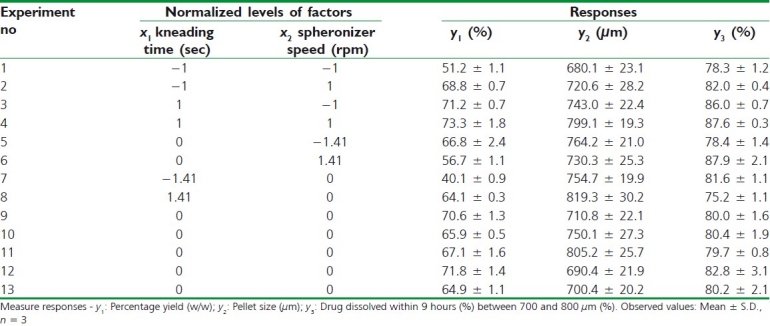
As the speed of the spheronizer was increased, the pellets became more spherical, rounder, and uniform size. However, at moderate to high level of spheronizer speed, pellet roundness was observed. There are factors in the pelletization process that cannot be controlled and these influence the results.
Figures 3a to 5a depict the 3-dimensional response surface plots for the studied response properties, viz., % yield, pellet size, and drug dissolution, while [Figure 3b to 5b] depict the corresponding contour plots which are extremely useful to study the interaction effects of the independent variables on the responses.
Figure 3.
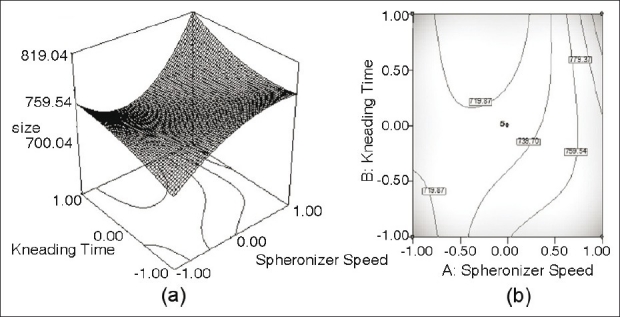
(a) Response surface plot showing the influence of Kneading time and Spheronizer speed on the value of pellet size of furosemide SR formulation; (b) the corresponding contour plot
Figure 5.
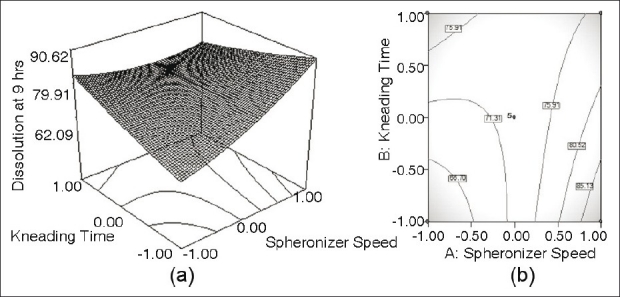
(a) Response surface plot showing the influence of Kneading time and Spheronizer speed on the value of dissolution at 9 hours of furosemide SR formulation; (b) the corresponding contour plot
Figure 3a shows a nonlinear trend in the values of size markedly increasing with the augmentation of kneading time. With spheronizer speed, the values of pellet size tend to rise non-linearly, followed by asymptote at low level of kneading time. The same is evident from the corresponding contour plot [Figure 3b], showing somewhat declining nonlinear contour lines.
Figure 4a reveal a sharp decline in the value of % yield at low level of both the parameters, i.e., kneading time and spheronizer speed, the influence of spheronizer speed being much more pronounced. Nonlinear descending contour lines in Figure 4b further shows that the variation in % yield is a complex function of the process parameter levels, the effect of kneading time being less prominent.
Figure 4.
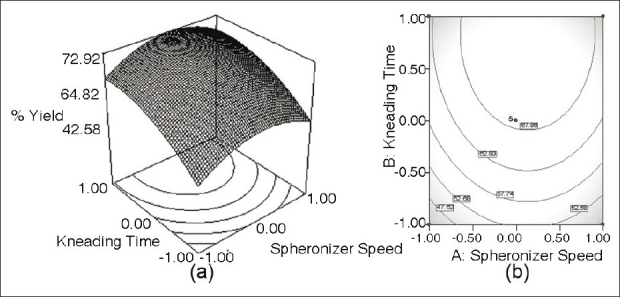
(a) Response surface plot showing the influence of Kneading time and Spheronizer speed on the value of percentage yield between 700 and 800 μm of furosemide SR formulation; (b) the corresponding contour plot
It was found that extending kneading time increased the particle size, where the pellet size grew from 743 μm to (experiment no. 3) to 799 μm (experiment no. 4) in Table 3. Generally speaking, prolonging kneading time and spheronizer speed also increased the pellet size and percentage yield in the 680-800 μm size fractions (experiment nos. 1-4). The observed positive effect of kneading time is assumed to be the result of the fact that extended kneading period offers extra colliding probabilities which are indispensable for the granule growth.
Another reason for this may be enhanced wetting of the wet mass throughout the pelletization process. On the other hand, the influence of kneading time at high levels was more pronounced than that at low levels. Consequently, the liquid kept inside pellets would be squeezed out to the surfaces, which may make the mass wet and adhesive and led to the fast growth of particles. As shown in Figure 3a, a high value of the speed provoked the formation of large pellets. In Figure 4a, high percentage yield of desired size fraction was observed when the spheronizer speed was around 1050 rpm.
Figure 5a shows a nearly linear ascending pattern for the values of dissolution at 9 hours, as the level of either kneading time or spheronizer speed is increased. The changes in the values of drug dissolution were caused by the variation of the process parameter. Figure 5a shows that about 85.521% ± 1.3 drug was released after 9 hours (Y2) when both the parameters, kneading time and spheronizer speed, are at highest level. Maximum drug dissolved is observable at the highest levels of process parameter, viz., kneading time and spheronizer speed. The corresponding contour plot [Figure 5b] also demonstrates an unambiguous, nearly linear trend at the high levels of kneading time and high levels of spheronizer speed.
Table 4 shows the content of the drug in the SR pellet formulations was determined. The drug content in the pellets was ranging from 85.2 ± 1.2% to 95.4% ± 0.8, indicating the uniformity in drug content. The angle of repose and flow rates of all the experimental formulations were carried out. It was observed that the SR pellets had minimum angle of repose and good circularity as well as high density and high flow rate according to fixed funnel method. The results of bulk density, tapped density, and compressibility index are shown in Table 4. The compactness of pellets should be taken into consideration not only for technological purposes, but also because the majority of them are typically encapsulated in two-piece hard shell capsules and consequently for these fixed volume dosage forms, the density will determine the fill weight. The bulk density of all the formulations was within the range from 0.76 to 0.88 g/ml. The result of compressibility index was acceptable, which is below 15%, indicating excellent flow properties.
Table 4.
Evaluation parameters of the sustained release pellets
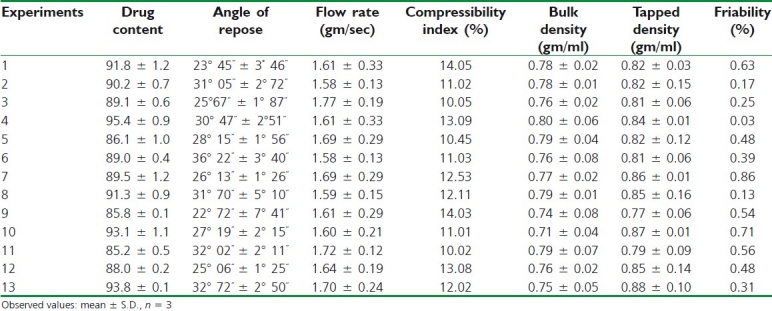
The friability test depicts that less than 1% weight loss in Table 4 occurred in all the experiments, which might be due to large surface area of pellets and presence of few cracks on the surface of pellets. Friability decreases along with augment in uniformity of pellets size and shape. The results shows that prepared pellets are less friable and within the limit according to pharmacopeial 2007 norms.
In vitro Drug Release Studies
In vitro drug release studies are valuable tools to judge quality and stability of SR dosage forms and are often used to predict in vivo performance. From the results reported in Table 2, it can be perceived that SR pellets released around 85.521% of the drug in dissolution medium in a SR manner till 9 hours. After 9 hours, the furosemide was entirely released into the dissolution medium. Pellets showed excellent gastric protection for 2 hours and displayed a more SR profile in alkaline medium. This might be attributed to the existence of the polymer. In this study, it may be elucidated by the fact that the pellets attained sufficient wetting as they resided at the bottom of the testing cup throughout the entire test. The proportion of the more lipophilic polymer Drug coat L-100 had the most significant effect on drug release; higher proportions gave SR and have a momentous effect on the rate of drug release till 9 hours.
The model equation generated to fit the data and reflected the influence of process parameters on percentage yield is as follow:
y1 = 75.56 – 3.22x1 – 2.55x2 – 0.39x12 + 2.49 x22
(r2 = 0.9527, p < α = 0.1)
With respect to the pellet size characteristics, both responses, y2 (pellet size) and y3 (drug dissolved), were affected remarkably by the investigated process variables. The equations generated are as follow:
y2 = 893.52 + 34.239x1 + 49.193x2 - 52.165x12-26.658x22
(r2 = 0.9263, p < α = 0.1)
y3 = 83.92 + 2.894x1 + 1.845x2 - 4.067x21
(r2 = 0.8992, p < α = 0.1)
Mathematical Modeling of Release Kinetics
To study the release mechanism, different dissolution models were applied to the in vitro release profiles of the 13 different formulations. The in vitro drug release data were fitted to zero-order model and Higuchi model [Table 5] and using the following set of equations:
Table 5.
Dissolution model study by fitting in vitro release study

Zero-order kinetic model
Mo - Mt = kot
Higuchi model
Mt = K√t
Where, Mo, Mt, and M1 correspond to the drug amount taken at time equal to zero, dissolved at a particular time, t, and at infinite time, respectively. Various other terms, viz. k, ko, refer to the release kinetic constants obtained from the linear curves of zero-order and Higuchi model, respectively.
Validation of Model Optimization Selection of Optimum Formulation
Upon assessment of the observed responses with those of the predictable ones in Table 6, the prediction error varied between –4.1 and 3.2%. In order to calculate the optimization capability of the models engendered according to the results of the circumscribed CCD, SR pellets were prepared using the optimal process variable settings. In vitro dissolution and yield comparisons of the results obtained from prepared pellets with that predicted by models are shown in Table 7.
Table 6.
Comparison of the experimental results with the predicted responses
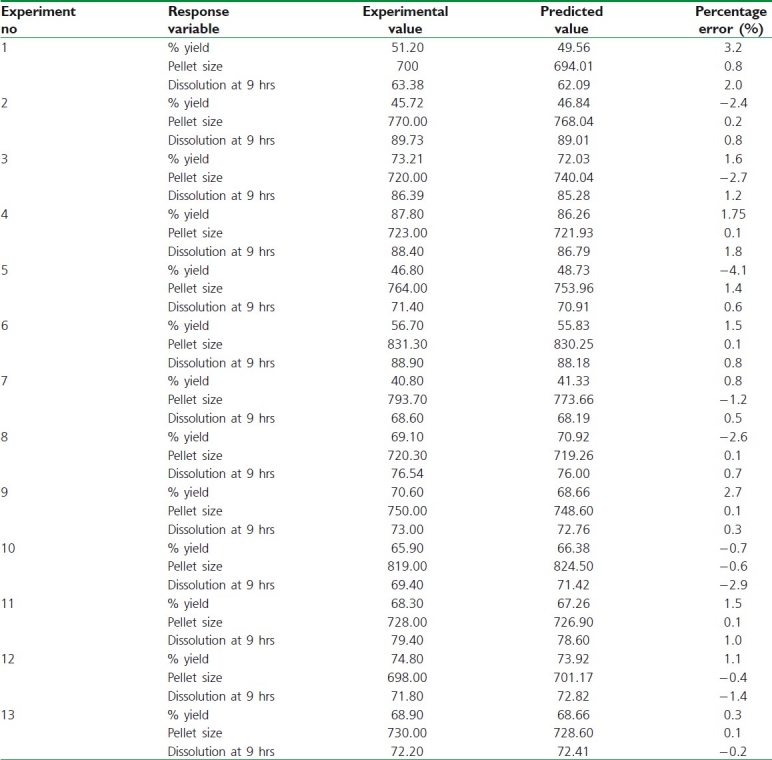
Table 7.
Result of experiments for confirming optimization capability

The results demonstrated good agreement on product properties with theoretical predictions. The in vitro drug release was also evaluated from pellets which were prepared with the optimal settings after storage in 40 ± 2°C/75% ± 5% RH for 6 months.
Figure 6 reveals dissolution comparisons of initial pellets with those stored for 6 months. It can be perceived that dissolution rate of the drug after storage for 6 months appeared to be much similar to that obtained at initial time, which indicated that pellets were stable under the storage condition. Figure 7 illustrates the SEM of optimized SR pellets and stored for 6 months. Figure 8 depicts that pellets were spherical in shape and the size was ranging from 700 to 850 μm.
Figure 6.
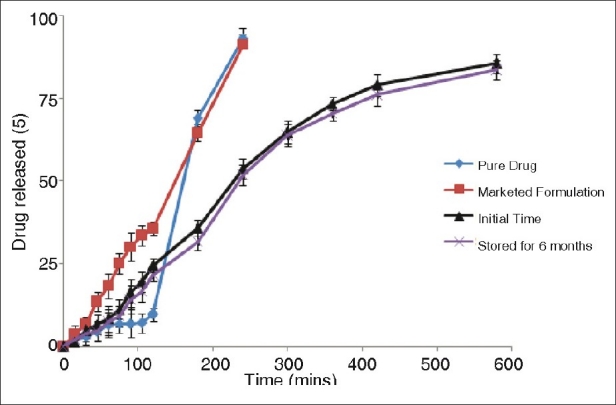
Comparison of dissolution profiles of pure drug, marketed formulation with initial pellets and that stored for 6 months as per the experimental design (n=3). The kneading time and spheronizer speed used for preparing pellets was 280 seconds and 1 050 rpm. The crossbars indicate ± 1 SD
Figure 7.

SEM of optimized SR pellets and stored for 6 months
Figure 8.

Size of the pellets in the size fraction of 700 to 850 μm
Analysis of Data
The in vitro release profile of pure drug, optimized formulation, and marketed formulation was statistically analyzed by two-tailed paired t test and one way ANOVA indicated a very significant difference (P≤0.05) in dissolution profile of optimized formulation when compared with pure drug and marketed formulation.
Differential Scanning Calorimetric Analysis
DSC thermograms of the drug and the optimized formulation were recorded, in order to determine the thermal changes of polymers and drug before and after preparation. The characteristic endothermic peak of the drug at 230°C was observed [Figure 9]. Nevertheless, no related peak was observed for all the samples. Optimized formulation showed that drug peak intensity was reduced further, compared with physical mixture, and shifted toward slightly higher temperature at 236.7°C, which indicates that such an effect may be observed in the presence of moisture content. So, these facts authenticate that furosemide presented a needle crystallographic habit as shown in Figure 10.
Figure 9.
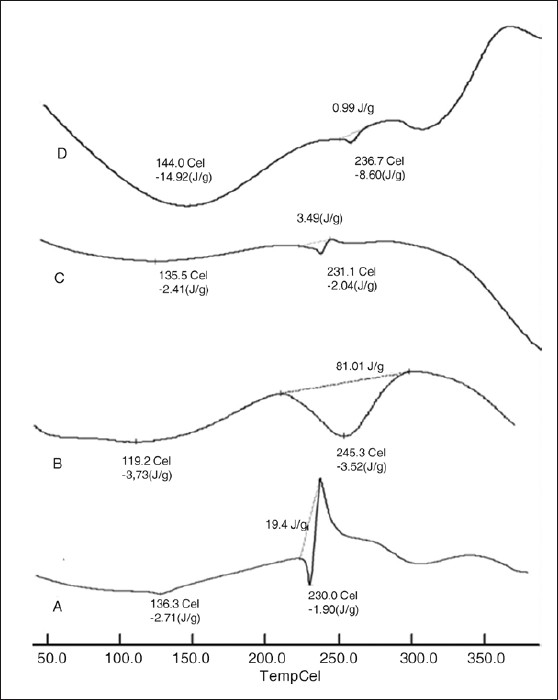
Differential scanning thermo gram of (a) pure drug, (b) drug coat L-100, (c) physical mixture, and (d) optimized formulation
Figure 10.
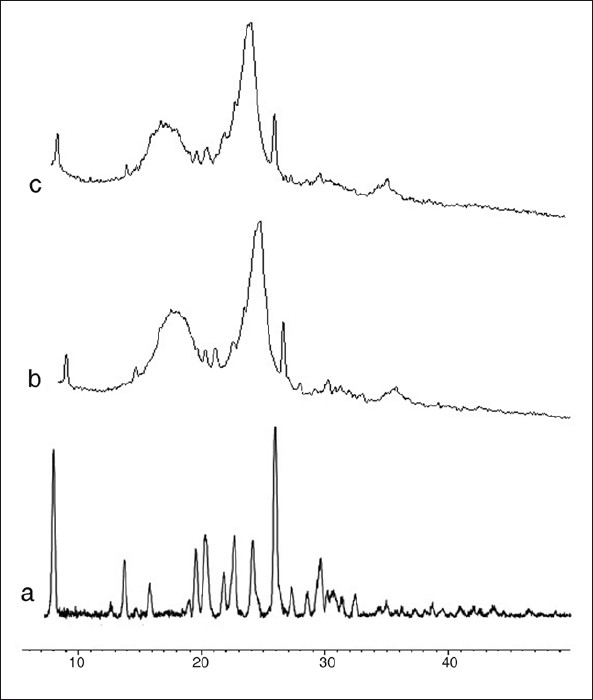
Powder X-ray diffractometry of (a) pure drug, (b) physical mixture, and (c) optimized formulation
Powder X-ray diffraction
X-ray diffraction patterns illustrated that original furosemide was crystalline in nature [Figure 10]. The X-ray pattern of pure furosemide revealed a drug fingerprint with intense and sharp peaks, indicating its crystalline nature as demonstrated by sharp peaks observed at 2, 12, 14, 17, 18, 19, 21, 23, 25, 26, 28, and 29°. A reduction in crystallinity was observed in optimized formulation spectrum, indicating that the crystallinity of the drug was reduced to greater extent. All the principal peaks from pure furosemide were present in their physical mixture and optimized formulation with lower intensity and broadened. No new peaks could be observed, suggesting the absence of interaction between the furosemide and the Drug Coat L-100 suggested that either the quality of the crystals was reduced, or a change into the amorphous form.
Scanning electron microscopy
SEM studies were carried out on final optimized formulation to determine surface morphology and intactness of the drug was observed. SR pellets were uniform size and spherical in shape with small porous and little rough surface was observed in optimized formulation [Figure 7]. The rough surface is caused due to rapid loss of moisture from the wet mass with the high liquid content that results in a porous surface structure. Pellets originating from wet mass with a higher liquid content that are processed by granulation-spheronization tend to have a rough surface.
Stability Studies
Stability studies of the optimized formulation under accelerated storage conditions did not expose any degradation of the drug and alter in the in vitro release profiles of the optimized formulation after storage for 6 months.
CONCLUSION
A statistical model was developed by simultaneously studying the effect of various process parameters using experimental design to predict the release properties of the drug from the delivery system. By using DoEs, the screening study confirmed that a few experimental parameters could influence the studied process, namely: The kneading time and spheronizer speed. The pragmatic responses for the optimum formulation were in close concurrence with the predicted values, demonstrating the excellent predictability of the optimization procedure. The polymer Drug Coat L-100 used imparts a conspicuous effect on release mechanism. This polymer studied in this work can be used in pharmaceuticals to sustain the release of drug from the dosage form. The result obtained indicated promising future for the exploit of this polymer Drug Coat L-100 in pelletization.
ACKNOWLEDGEMENTS
Authors are grateful to Prof. B.G Shivananda, Principal, Al-Ameen College of Pharmacy, Bangalore, for his continuous support. Authors are thankful to Micro Labs Ltd. for providing the gift sample of furosemide. Authors are also thankful to Vikram Thermo (India) Ltd for providing the gift sample of Drug Coat L-100 (methacrylic acid polymer).
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Gandhi R, Kaul CL, Panchagnula R. Extrusion and spheronization in the development of oral controlled-release dosage forms. Pharm Sci Technolo Today. 1999;2:160–70. doi: 10.1016/s1461-5347(99)00136-4. [DOI] [PubMed] [Google Scholar]
- 2.Remington . The science and practice of pharmacy. New Delhi: Wolters Kluwer; 2005. [Google Scholar]
- 3.Erkoboni DF. Extrusion/spheronization in: Pharmaceutical Extrusion Technology. New York: Marcel Dekker; 2003. [Google Scholar]
- 4.Harris MR, Ghebre-Sellasie I. Formulation variables in: Pharmaceutical Pelletization Technology. New York: Marcel Dekker; 1989. [Google Scholar]
- 5.Ott AD, Thommes M, Remon JP, Kleinebudde P, Vervaet C. Production of pellets via extrusion–spheronisation without the incorporation of microcrystalline cellulose. Eur J Pharm Biopharm. 2009;71:38–46. doi: 10.1016/j.ejpb.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Schilling SU, Shah NH, Malick AW, McGinity JW. Properties of melt extruded enteric matrix pellets. Eur J Pharm Biopharm. 2010;74:352–61. doi: 10.1016/j.ejpb.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Dayal P, Pillay V, Babu RJ, Singh M. Box-Behnken experimental design in the development of a nasal drug delivery system of model drug hydroxyurea: Characterization of viscosity, in vitro drug release, droplet size and dynamic surface tension. AAPS Pharm Sci Tech. 2005;6:E573–85. doi: 10.1208/pt060472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh B, Chakkal SK, Ahuja N. Formulation and optimization of controlled release mucoadhesive tablets of atenolol using response surface methodology. AAPS Pharm Sci Tech. 2006;7:E1–9. doi: 10.1208/pt070103. [DOI] [PubMed] [Google Scholar]
- 9.Singh SK, Reddy IK, Khan MA. Optimization and characterization of controlled release pellets coated with experimental latex: II. Cationic drug. Int J Pharm. 1996;141:179–95. [Google Scholar]
- 10.Gotti R, Furlanetto S, Andrisano V, Cavrini V, Pinzauti S. Design of experiments for capillary electrophoretic enantioresolution of salbutamol using dermatan sulphate. J Chromatogr. 2000;875:411–22. doi: 10.1016/s0021-9673(99)01303-5. [DOI] [PubMed] [Google Scholar]
- 11.Lafuente CS, Furlanetto S, Arevalo MF, Fuentes JA, Rabasco AM, Faucci MT, et al. Didanosine extended-release matrix tablets: Optimization of formulation variables using statistical experimental design. Int J Pharm. 2002;237:107–18. doi: 10.1016/s0378-5173(02)00028-5. [DOI] [PubMed] [Google Scholar]
- 12.Krogars K, Heinamaki J, Vesalahti J, Marvola M, Antikainen O, Yliruusi J. Extusion-spheronization of pH-sensitive polymeric matrix pellets for possible drug delivery. Int J Pharm. 2000;199:187–94. doi: 10.1016/s0378-5173(00)00382-3. [DOI] [PubMed] [Google Scholar]
- 13.Klausner EA, Lavy E, Stepensky D, Cserepes E, Barta M, Friedman M, et al. Furosemide pharmacokinetics and pharmacodynamics following gastro retentive dosage form administration to healthy volunteers. J Clin Pharmacol. 2003;43:711–20. [PubMed] [Google Scholar]
- 14.Exner HE, Link E. Image analysis for characterization of size and shape distribution of lead powders. Powder Metall Int. 1977;9:131–3. [Google Scholar]
- 15.Singh R, Poddar SS, Chivate A. Sintering of wax for controlling release from pellets. AAPS Pharm Sci Tech. 2007;8:E175–83. doi: 10.1208/pt0803074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke GM, Newton JM, Short MB. Comparative gastrointestinal transit of pellet systems of varying density. Int J Pharm. 1995;114:1–11. [Google Scholar]
- 17.Souto C, Rodrıguez A, Parajes S, Pacheco RM. A comparative study of the utility of two superdisintegrants in microcrystalline cellulose pellets prepared by extrusion–spheronization. Eur J Pharm Biopharm. 2005;61:94–9. doi: 10.1016/j.ejpb.2005.04.003. [DOI] [PubMed] [Google Scholar]


