Abstract
Earlier ethnopharmacological records divulged the traditional usages of mangrove Aegiceras corniculatum (Linn.) Blanco distributed in coastal and estuarine areas of Southeast India. Excluding scientific knowledge of A. corniculatum against diabetes an upgrowing endocrinal disorder, our present study evaluated the effect on alloxan-induced diabetic rats. Diabetes was induced in adult rats of the Wistar strain by intraperitoneal injection of alloxan monohydrate. The experimental rats were administered with leaf suspension of A. corniculatum post orally using an intragastric tube. On completion of the 60-day treatment, a range of biochemical parameters were tested including liver hexokinase, glucose-6phosphatase and fructose 1, 6 bisphosphatase in the liver of control and allaxon-diabetic rats. As a result, A. corniculatum leaf suspension showed moderate reduction in blood glucose (from 382 ± 34 to 105 ± 35), glycosylated hemoglobin, a decrease in the activities of glucose-6 phosphatase and fructose 1, 6-bisphosphatase, and an increase activity of liver hexokinase achieved through the oral administration of extract on 100 mg/kg. The present findings support promising results in terms of antidiabetic activities establishing its candidacy for further purification of individual compound in order to understand their mechanism of action.
Keywords: Alloxan, antidiabetic, black mangrove, gluconeogenic enzymes, hexokinase
INTRODUCTION
Diabetes is increasing in a panic way all over the world and is relied on the use of traditional medicine which are largely derived from plants. About 200 million in the world are affected and 2.5 to 7% of the people are diagnosed by the most common endocrine disease diabetes.[1] The pathogenesis of insulin-dependent diabetes mellitus involves environmental causes that may activate autoimmune mechanisms on genetically susceptible individuals, leading to progressive loss of pancreatic islet β-cells resulting in insulin deficiency.[2] Non-insulin-dependent diabetes mellitus is associated with impaired insulin secretion, obesity, insulin resistance, and hereditary disposition in individuals over 40 years of age.[3] However, the increasing causes of commercially available anti-hyperglycemic drugs and insulin sensitizers, such as long-term adverse effects on blood vessels, nerves, and other organ systems, demand the discovery of new alternatives mainly insulin substitutes. The findings of insulin substitutes from the coastal medicinal plants with hypoglycemic effect have been a recent trend in marine pharmacological research.
Traditionally more than 100 numbers of mangroves and mangrove-associated plant used for the treatment of diabetes have been reported, but only a very few number of plants are evaluated and reported scientifically.[4] The antidiabetic effect of leaves of mangrove plants Rhizophora mucronata and Ceriops decandra had been documented and the gut perfusion studies on Long Evans rats reported the mode of action of Rhizophora mucronata leaves’ hypoglycemic conditions.[5–7] Recently, the medicinal value of mangroves and associated plants persist to provide priceless therapeutic agents, both in modern medicines and in traditional systems.[8] Traditional applications of the black mangrove Aegiceras corniculatum (Linn.) Blanco has been selected for the present study, which belongs to the myrsinaceae family distributed in coastal and estuarine areas of India. Also, the ethnopharmacological consequence pointed out the study plant traditionally used for the treatment of rheumatism, painful arthritis, inflammation, asthma antioxidant, free radical scavenging, anti-inflammatory, antinociceptive, diabetes, and hepatoprotective actions.[9] However, there are no scientific reports regarding the effects of this plant on Diabetes mellitus. In consequence, we tried to establish mangrove floral therapy as antidiabetic drug instead of chemical drug. Hence, in the present study, we evaluated the effects of an ethanolic extract of Aegiceras corniculatum leaves (ACEt) in alloxan-induced diabetic rats for the potential use in the long-term treatment of diabetes.
MATERIALS AND METHODS
Chemicals and Drugs
Alloxan-monohydrate was obtained from Sigma-Aldrich Company (St. Louis, Missouri, U.S.A.). The other experimental chemicals used were analytical grade purchased from HiMedia (Mumbai, India).
Plant Material
Leaves of A. corniculatum were collected from Pichavaram mangrove forest (Tamil Nadu) Southeast coast of India. The specimen was botanically certified and a voucher specimen (AUCASMB02) deposited in the Herbarium of Centre of Advanced Study in Marine Biology, Faculty of Marine Sciences, Annamalai University, India. Mature leaves were separated manually from the aerial part of the plant. Then, the leaves were dried and minced with a grinder into a powder in preparation for extraction.
Preparation of the Extract
The ACEt was prepared by cold maceration of 250 g of the shade-dried leaf powder in 500 ml of 80% ethanol for 2 days. This process was repeated three times. The extract was filtered, concentrated, dried in yield 1.7%, and the residue stored in a refrigerator at 3-7°C for use in subsequent experiments.
Experimental Animals
About 200 to 250 g weight of male albino rats purchased from the Central Animal House, Rajah Muthiah Medical College (RMMCH), Annamalai University were used in this study. The animals were fed on pellet diet (Hindustan Lever, India) water ad libitum and maintained at Central Animal House, RMMCH. All studies were conducted in accordance with the National Institute of Health's Guide for the Care and Use of Laboratory Animals and the study was approved by the Institutional Animal Ethical Committee of Rajah Muthiah Medical College and Hospital (Prop. No. 534, Reg.No. 160/1999/CPCSEA), Annamalai University, Tamil Nadu, India.
Acute Toxicity Studies
Enthusiastic adult albino male rats, be malnourished overnight, were divided into four groups (n = 6) and were orally fed with the ACEt in increasing dose levels of 50, 100, 250, 500, and 1 000 mg/kg body weight. The behavioral, neurological, and autonomic profiles of rats were observed continuously for 2 hours and after a period of 24 and 72 hours for any lethality or death.[10]
Experimental Design
The rats were intraperitoneally injected by alloxan monohydrate (150 mg/kg body weight) dissolved in freshly prepared citrate buffer (0.1M, pH 4.5) to induce diabetes after an overnight fast.[11] Since alloxan is capable of producing fatal hypoglycemia as a result of massive pancreatic insulin release, rats were treated with 30% glucose solution orally at different time intervals after six hours of alloxan induction, and 5% glucose solution was kept in bottles in their cages for the next 24 hour to prevent hypoglycemia. Hyperglycemia was confirmed by the elevated glucose levels (250 to 375 mg/dl) in plasma, determined at 72 hours and then on day 7 after injection. The rats found with permanent diabetes were used for the antidiabetic study. Animals were divided into six groups of six rats (namely a-f) each. The extract was administered for 60 days. Feed and water were provided ad libitum to the animals. The plant extract (ACEt) or glibenclamide were dissolved in water and administered post orally using an intragastric tube on the treatment of 60 days.
Group I: Normal control rats treated with saline daily for 60 days
Group II: Normal rats treated with (100 mg/kg) of ACEt
Group III: Diabetic control rats
Group IV: Diabetic rats treated with (25 mg/kg) of ACEt
Group V: Diabetic rats treated with (50 mg/kg) of ACEt
Group VI: Diabetic rats treated with (100 mg/kg) of ACEt
Group VII: Diabetic rats treated with glibenclamide (600 μg/ kg)
Effect on Fasting Blood Glucose and Glycosylated Hemoglobin
Day 7 of induction was designated as day 1 for extract administration in diabetic rats. Fasting plasma glucose was estimated on days 1, 5, and 12 of extract administration. Blood was collected from rats by tail-puncture method. About 0.50 ml of blood was collected from each rat into a vial containing heparin (35 units/ml of blood) solution. The effects of ACEt on normal and diabetic rats were determined by measuring fasting blood glucose level that was estimated by Trinder using a reagent kit.[12] Glycosylated hemoglobin (HbA1c) was estimated by Drabkin and Austin.[13]
Assay for Carbohydrate Metabolic Enzymes
After 60 days of treatment, the rats were anesthetized by intramuscular Ketamine (24 mg/kg/body) injection and sacrificed by cervical decapitation. The liver was dissected out and washed with ice-cold saline immediately to remove blood. The hepatic Hexokinase (EC2.7.1.1), Glucose-6-phosphatase (EC 3.1.3.9), and Fructose-1, 6-bisphosphatase (EC3.1.3.11) are the key enzymes in glucose homeostasis that were estimated to determine the effect of ACEt toward the cure and management of diabetes.[14–16] For these experiments, 1 g of fresh/frozen liver was chopped and homogenized in ice-cold sucrose (15 ml, 250 mM) with a Potter-Elvehjem homogenizer for 2minutes, centrifuged at 10 000 rev./min for 30 minutes, and the pellet was discarded and the supernatant was used as the source for the biochemical estimation of enzymes. Also, the protein content of the supernatant was determined by the method of Lowry et al.[17]
Statistical Analysis
Values were represented as mean ± SD. Data were analyzed using analysis of variance and group means were compared with Duncan's multiple range tests.
RESULTS
Acute Toxicity Studies
On treatment of enthusiastic adult albino male rats with A. corniculatum ethanolic extract, we could not find any lethality or toxic reactions while using different dose concentrations used in the present study, until the end of the study period.
Effect on Fasting Blood Glucose and Glycosylated Hemoglobin
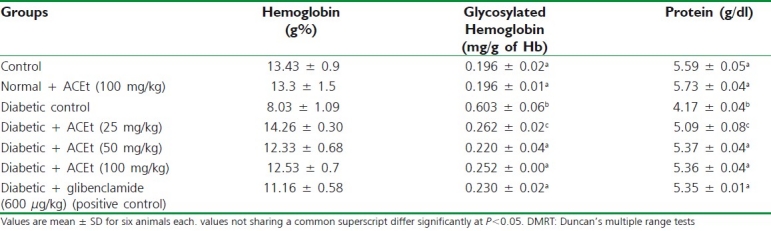
Changes in blood glucose and body weight in diabetic control and on treatment of diabetic rats with A. corniculatum, glibenclamide are presented in Table 1. The results reveal a momentous increase in blood glucose and substantial reduction in body weight in diabetic rats when compared with control rats. Table 2 elucidated the effects of A. corniculatum and glibenclamide on hemoglobin, HbA1c, and serum protein, in control and alloxan-diabetic rats. There is a considerable reduction in hemoglobin and serum protein while HbA1c appreciably increased in diabetic rats when compared with control rats. Rats administrated orally with a leave extract of A. corniculatum (25, 50, and 100 mg/kg body weight) intensively attained the value to near normal.
Table 1.
Changes in blood glucose and body weight in control and alloxan diabetic rats treated with Aegiceras corniculatum and glibenclamide
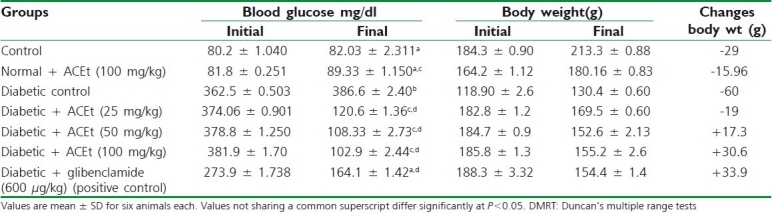
Table 2.
Effect of Aegiceras corniculatum on hemoglobin, glycosylated hemoglobin and protein, in control, and alloxan diabetic rats
Effect on Carbohydrate Metabolic Enzymes
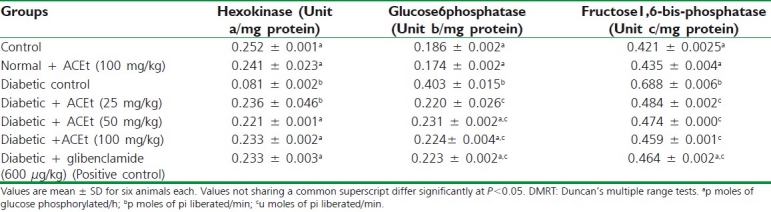
Effects on the administration of A. corniculatum and glibenclamide on hepatic hexokinase, glucose-6-phosphatase, and fructose-1, 6-bisphosphatase of liver are presented in Table 3. The activity of hepatic hexokinase is appreciably decreased while glucose-6-phosphatase and fructose-1, 6-bisphosphatase are significantly elevated in alloxan-treated diabetic rats as compared with normal rats. There is an increased activity of hexokinase and decreased the activities of glucose 6-phosphatase and fructose- 1, 6- bisphosphatase in the administration of A. corniculatum (25, 50, and 100 mg of body weight) and glibenclamide compared with diabetic rats. There was no statistical significance in parameters estimated in normal animals. The antidiabetic activity was not found to be dose dependent as there was no significant difference between the 25, 50, and 100 mg/kg extract-treated groups.
Table 3.
Effect of Aegiceras corniculatum on the activities of hepatic enzymes in control and experimental animals
DISCUSSION
Mangroves and associated plants provide a wide domain for therapeutic application in recent years, most yet to be explored. The leaves of A. corniculatum are reportedly rich in flavonoids with proven anti-inflammatory and antioxidant property.[18] Evaluation of physiological and toxic effects, solvent used for extraction, route of administration, and acute or chronic effect of A. corniculatum leave extract are quite diversified, which is encouraged by delineating the beneficial applications of the study plant and confines various assessments.
Alloxan is an oxygenated pyrimidine derivative which selectively destroys insulin-secreting beta-cells in the experimental animals, which results in alloxan diabetes.[19] In the present investigation, blood sugar level increased as expected in alloxan-injected animals, since alloxan causes a massive reduction in insulin release, by the destruction of the beta cells of the islets of Langerhans and inducing hyperglycemia.[20]
Oral administration of A. corniculatum (25, 50, and 100 mg/kg body weight) resulted in a significant reduction in the blood glucose and improvement in body weight. The decrease in body weight in diabetic rats clearly confirms a degradation of structural proteins due to diabetes. The structural proteins are known to contribute for the body weight.[21] The deficiency of the anabolic hormone insulin decreased protein synthesis in all tissues in alloxan-induced diabetic rats. Structural proteins are important constituents of the body and are required for the body's structure and proper function. The rate of structural protein synthesis affects organ protein mass, body size, and amino acid requirements. The ability of the A. corniculatum to protect from maximum body weight loss seems to be due to its ability to reduce hyperglycemia.
Within six to eight weeks, the blood sugar level of the patients had been determined by the amount of HbA1c in the red blood cells. Normal human red cells contain 3.4 to 5.8% HbA1c; during diabetes, the level has been increased within the red blood cells, which reflects the average level of glucose to which the cell has been exposed during the life cycle.[22] In the present study, diabetic rats have high blood glucose level due to the influences of level that was high in red blood cells. The sugar levels decreased immensely in A. corniculatum-administered diabetic rats.
The intracellular glucose has been utilized by insulin in several ways. The increased level of insulin influences the activity of gluconeogenic enzymes that results in the initiation of hepatic glycolysis. All types of cells contain hexokinase. Hexokinase D or glucokinase is more specific for glucose and differ with other forms of hexokinase in kinetic and regulatory properties, which has been found in hepatocytes.[23] Hexokinase plays a central role in the maintenance of glucose homeostasis, it catalyzes the conversion of glucose to glucose-6-phosphate. Also, hexokinase is an important regulator of glucose storage and disposal in the liver.[24] In the present study, the hexokinase activity was decreased in alloxan-diabetic rats which may be due to insulin deficiency (insulin stimulates and activates glucokinase). Treatment with A. corniculatum or glibenclamide elevated the activity of more effective therapeutic compounds in mangroves.
CONCLUSION
In conclusion, this is the first report on black mangrove A. corniculatum on antidiabetic activity in experimental model. Further bioactive compound isolation, clinical trials, hematological parameters, and drug development will be promising the development of antidiabetic products from mangroves with unidentified level of side effects.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Seghrouchni JE, Drai J, Bannier P, Riviere I, Calmard J, Garacia Origiazzi A. Oxidative stress parameters in type I, type II and insulin-treated type 2 diabetes mellitus: Insulin treatment efficiency. Clin Chim Acta. 2002;32:189–96. doi: 10.1016/s0009-8981(02)00099-2. [DOI] [PubMed] [Google Scholar]
- 2.Harrison LC, Honeyman MC. Cow's milk and type 1 diabetes: the real debate is about mucosal immune function. Diabetes. 1999;48:1501–7. doi: 10.2337/diabetes.48.8.1501. [DOI] [PubMed] [Google Scholar]
- 3.Zimmet P, Dowse G, Finch C, Serjeantson S, King H. The epidemiology and natural history of NIDDM-lessons from the South Pacific. Diabetes Metab Rev. 1990;6:91–124. doi: 10.1002/dmr.5610060203. [DOI] [PubMed] [Google Scholar]
- 4.Bandaranayake WM. Bioactivities, bioactive compounds and chemical constituents of mangrove plants. Wetlands Ecol Manage. 2002;10:421–52. [Google Scholar]
- 5.Ramanathan T, Hariharan B, Ganesan K. Antidiabetic activity of a coastal mangrove leaves of Rhizophora mucronata. Int J Plant Res Plant Arch. 2008;8:931–3. [Google Scholar]
- 6.Nabeel MA, Kathiresan K, Manivannan S. Antidiabetic activity of the mangrove species Ceriops decandra in alloxan-induced diabetic rats. J Diabetes. 2010;2:97–103. doi: 10.1111/j.1753-0407.2010.00068.x. [DOI] [PubMed] [Google Scholar]
- 7.Gaffar MU, Morshed MA, Azim Uddin, Saurov Roy, Hannan JMA. Study the efficacy of Rhizophora mucornata Poir. leaves for diabetes therapy in Long Evans rats. I J Biomol Biomed. 2011;1:20–6. [Google Scholar]
- 8.Kathiresan K, Ramanathan T. Medicinal Plants of Parangipettai Coast. Tamil Nadu, India: Annamalai University; 1997. p. 72. [Google Scholar]
- 9.Roome T, Dar A, Ali S, Naqvi S, Choudhary MI. A study on antioxidant, free radical scavenging, anti-inflammatory and hepatoprotective actions of Aegiceras corniculatum (stem) extracts. J Ethnopharmacol. 2008;118:514–21. doi: 10.1016/j.jep.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 10.Ecobichon DJ. The Basis of toxicology Testing. New York: CRC Press; 1997. p. 43. [Google Scholar]
- 11.Katsumata K, Katsumata Y, Ozawa T, Katsumata K. Po-tentiating effects of combined usage of three sulfo-nylurea drugs on the occurrence of alloxan diabetic rats. Horm Metab Res. 1999;25:125. doi: 10.1055/s-2007-1002058. [DOI] [PubMed] [Google Scholar]
- 12.Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem. 1969;6:24–7. [Google Scholar]
- 13.Darbkin DL, Austin JM. Spectrophotometric constants for common haemoglobin derivatives in human, dog and rabbit blood. J Biol Chem. 1932;98:719–33. [Google Scholar]
- 14.Brandstrup N, Kirk JE, Bruni C. Determination of hexokinase in tissues. J Gerontol. 1957;12:166–71. doi: 10.1093/geronj/12.2.166. [DOI] [PubMed] [Google Scholar]
- 15.Baginski ES, Foa PP, Zak B. In: Methods of Enzymatic Analysis. Vol. 2. New York: Academic Press; 1974. pp. 876–80. [Google Scholar]
- 16.McGilvery RW. Fructose-1,6-diphosphatase from liver. Methods Enzymol. 1955;2:543–6. [Google Scholar]
- 17.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 18.Banerjee D, Chakrabarti S, Hazra AK, Banerjee S, Ray J, Mukherjee B. Antioxidant activity and total phenolics of some mangroves in Sundarbans. Afr J Biotechnol. 2008;7:805–10. [Google Scholar]
- 19.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetol. 2008;51:216–26. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 20.Bouwens L, Rooman I. Regulation of Pancreatic Beta-Cell Mass. Physiol Rev. 2005;85:1255–70. doi: 10.1152/physrev.00025.2004. [DOI] [PubMed] [Google Scholar]
- 21.Rajkumar L, Govindarajulu P. Increased degradation of dermal collagen in diabetic rats. Indian J Exp Biol. 1991;29:1081–3. [PubMed] [Google Scholar]
- 22.Paulsen EP. Experience in sulfonylurea therapy. J Metabolism. 1973;22:381–5. doi: 10.1016/0026-0495(73)90195-9. [DOI] [PubMed] [Google Scholar]
- 23.Lehninger AL, Nelson DL, Michael M. In: Principles of Biochemistry. 2ns Ed. New Delhi: CBS Publishers Distributors>; 1993. p. 406. [Google Scholar]
- 24.Ghosh MN. In: Fundamentals of Experimental Pharmacology. Calcutta: Scientific Book Agency; 1984. p. 153. [Google Scholar]


