Abstract
Amoxicillin-loaded microcapsules were prepared by ionotropic gelation of sodium alginate (ALG) with chitosan (CS) in presence of calcium chloride as gastroretentive delivery system. The effect of pH, concentration of ALG, CS and calcium chloride, and drug : ALG ratio were optimized in this study for minimizing the degradation of drug in acidic environment and increasing the loading efficacy and mucoadhesive efficiency of microcapsules. The optimum condition for prepared CS-ALG microcapsules was 2%w/v ALG, 0.75%w/v CS (pH5.0), and 1.0% w/v calcium chloride. The resulting microcapsules had drug entrapment efficiency of 84% and average size of 840 mm. CS concentration significantly influenced particle size and encapsulation efficiency of CS–ALG microcapsules (P<0.05). Decrease in the drug: ALG ratio resulted in an increased release of amoxicillin in acidic media. The relative decomposition of drug after encapsulation in CS-ALG microcapsules was decreased to 20.7%, 41.9%, and 83.3% in 2, 4, and 8 hours, respectively.
Keywords: Gastroretentive delivery system, Helicobacter pylori, peptic ulcer, sodium alginate
INTRODUCTION
The drug delivery in recent time is getting highly technical with the concept of targeted drug delivery or site-specificity to increase the reliability of therapy, drug stability in the biological system including gastrointestinal tract, cost effectiveness, preventing adverse effects, and to reduce drug resistance development.[1] The main advantages are the prevention of side effects of drugs on healthy tissues and enhancement of the drug uptake by targeted cells. The basic objective of this research is to develop gastroretentive drug delivery system that meets the therapeutic needs relating to particular pathological conditions like peptic ulcer, gastritis, and Helicobacter pylori infection, along with a reduction in the systemic side effects associated with the drug. Eradication failure of H. pylori in various therapies with antibiotics like metronidazole, clarithromycin, tetracycline, amoxicillin, etc. is frequent in recent times due to antibiotic resistance, which may be due to poor drug concentration at the site of action and degradation of drug in the harsh stomach environment and a less GI transit time for conventional drug deliveries.[2] Most suitable approach includes local application of the drug on to the gastric mucosal layer with a concept of mucoadhesion that can adhere on to the bacterium, thus eradicating it completely.[3] In designing oral mucoadhesive delivery of antibiotics, natural biodegradable and bioadhesive polymers, chitosan (CS) and sodium alginate (ALG), have attracted increasing attention.
CS, a unique cationic, mucoadhesive polymer, complexes with oppositely charged ALG, an anionic polymer, to prepare CS-alginate polyelectrolyte complex (CS-ALG-PEC) delivery systems for various drugs, which may prove to be the futuristic concept in gastroretentive drug delivery.[4–5] Another advantage of this system is physical cross-linking by electrostatic interactions instead of toxic chemical cross-linking. Also, the combination of CS and antimicrobial agent (Amoxicillin) may prove to be synergistic, as CS itself exhibits antimicrobial activity.[6] Cross-linked CS-ALG complex provide a protective coating against gastric harsh environment and increase chemical stability of acid degradable moieties/drugs.[7]
Various mucoadhesive microspherical formulations were developed for antibiotics active against H. pylori using different polymers like polycarbophil and carbopol 934 P, poly (D,L-lactide)-polyethylene glycol copolymer, glyoxal-cross-linked CS, CS-coated gellan gum, gelatin, cellulose acetate butyrate-coated cholestyramine, and CS-treated ALG.[8–14] These mucoadhesive systems have increased gastroretention up to 12 hours as well as local concentration of antibacterial agents in stomach. In vivo H. pylori clearance studies using these formulations showed ~90-95% clearance with 15 mg/kg single dose of amoxicillin, while 100% clearance with 3.5 mg/kg dose twice a day for 3 days.
Earlier studies of amoxicillin with non-specific mucoadhesive polymers like carbopol934 could not achieve the required aim of mucoadhesion in gastric environment[8] or specific sustained drug release profile which may be suitable for eradication of H. pylori.[15] Presence of CS coating on the outer surface of cationic cross-linked gellan gum beads[16] or glutaraldehyde cross-linked CS microspheres[17] resulted in sustained drug release in 0.1N HCl as well as increased mucoadhesive properties of microparticles. However, limited studies involving direct cross-linking of CS with anionic ALG have been done and rarely any study has discussed the effect of concentration of CS and ALG on gastric stability of amoxicillin, sustained release profile in gastric environment, and mucoadhesive properties of microparticulate delivery system.
In the above context, a mucoadhesive microparticulate delivery system for Anti-H. pylori agents like amoxicillin which can increase the efficiency of drug was attempted by single-step cross-linking of polycationic CS with polyanionic ALG in presence of calcium chloride. The purpose of the present study was to examine the influence of various formulation and process variables viz. pH of CS solution, surfactant concentration, calcium chloride concentration, gelation curing time, concentration of CS and ALG on the properties of microcapsules along with their influence on drug release kinetics and mucoadhesion strength.
MATERIALS AND METHODS
Materials
Amoxicillin trihydrate was provided as gift samples by Siemens Laboratories (India) Gurgaon. CS (deacetylation degree ≥90%, Mw ≤50 000) was purchased from Sigma Aldrich (USA). ALG (viscosity 5.5 ± 2cps) and sodium dioctyl sulphosuccinate (DOSS) were purchased from HiMedia laboratories (Mumbai). Potassium dihydrogen phosphate, disodium hydrogen orthophosphate, hydrochloric acid, methanol, acetic acid, and sodium hydroxide were purchased from Rankem, New Delhi. Simulated gastric fluid (SGF) without pepsin was prepared as per USP 29. Double distilled water was used in all the preparations. All other solvents and chemicals used were of analytical grade.
Methods
Preparation and optimization of CS-ALG microcapsules
CS-ALG microcapsules of amoxicillin were prepared by ionotropic gelation technique.[18] Amoxicillin trihydrate powder (2–9%w/v) was suspended thoroughly in the ALG solutions (1-3% w/v) in de-ionized water containing 0.075%w/v DOSS as surfactant by vigorous stirring for 10 minutes. The ALG-amoxicillin mixture was directly sprayed using syringe into the calcium chloride solution (1.0%w/v) containing CS (0.75–1.0% w/v) (pH adjusted to 5.0). The microcapsules were allowed to harden for 90 minutes before washing them twice with distilled water and dried at 37°C in an oven overnight and the final dried mass was recorded.
Optimization of CS-ALG microcapsules
Pre-optimization studies
0Microcapsules with 1% w/v drug concentration were prepared by ionic gelation method described above using different pH of CS solution (pH 4.5-6.0), surfactant concentration (0.05-0.125% w/v), calcium chloride concentration (0.5-3%w/v), gelation curing time (30-120 minutes), and evaluated for particle size by optical microscopy,[12] shape, and percent entrapment efficiency.[15] Parameters which gave the smaller and more uniform microcapsules with highest drug entrapment were selected for further optimization studies.
Optimization studies
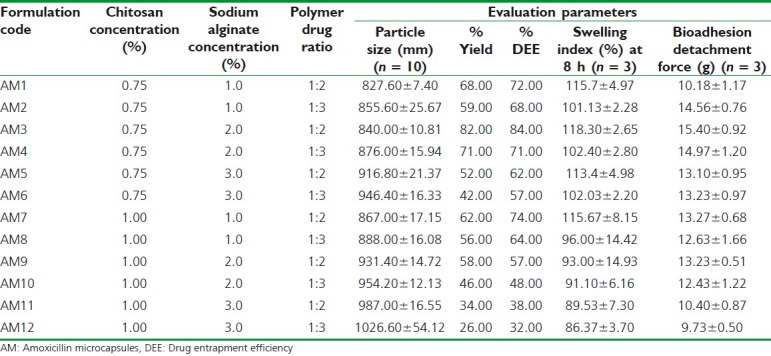
Twelve experimental runs [Table 1] were planned for the optimization of the CS-ALG microcapsules statistically with respect to the formulation parameters–concentration of the bioadhesive polymer- CS (A), concentration of the bioadhesive polymer- ALG (B), and ratio of polymer (ALG): drug ratio (C) in the formulation by evaluating the effects on the particle size (Y1), entrapment efficiency (Y2), % drug release at 4h (Y3), and bioadhesion strength (Y4).
Table 1.
Composition and evaluation of amoxicillin microcapsules
Swelling study
Accurately weighed (50 mg) amoxicillin-loaded microcapsules (W0) were placed separately in Petridish containing 50 ml of 0.1N HCl in an incubator maintained at 37 ± 0.5°C. The percent swelling index was calculated by reweighing (Wt) the microcapsules at 4 hours and 8 hours, using the following formula:[19]
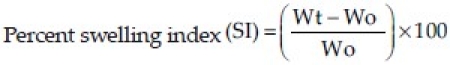
Bioadhesion detachment force study
The modified balance method was used to study the bioadhesion of microcapsules to the mucosal membrane.[20] 10 mg amoxicillin microcapsules were placed between the two sections (area - 2 × 4 cm2) of pig stomach mucosal tissues (from the antrum region), fixed onto each glass slide using cyanoacrylic glue and were allowed to stay at 37.0°C for 10 minutes. Bioadhesion detachment force or bioadhesion strength (g) was determined in triplicate from the minimal weights that detached the two slides.
In vitro drug release studies
Dissolution studies of amoxicillin-loaded microcapsules equivalent to 50 mg of amoxicillin were carried out by USP29 type-II tablet dissolution test apparatus (M/s Electrolab India Ltd., Mumbai) at 50 rpm and 37 ± 0.5°C, using 900 ml of 0.1N HCl (pH 1.2) as the dissolution medium. An aliquot of sample (5 ml) was withdrawn periodically, replaced with equivalent volume of dissolution medium. Samples, filtered through Whatman filter paper (0.45 μm), were analyzed spectrophotometrically at 272.6 nm. Drug release data obtained during in vitro dissolution studies were analyzed for release kinetics using zero order, first order, and Higuchi model equations and fitted into Korsmeyer-Peppas model for evaluation of release mechanism from microcapsules.[21]
Stability of amoxicillin in simulated gastric fluid
Amoxicillin-loaded microcapsules equivalent to 25 mg of amoxicillin were placed in nine conical flasks containing 100 ml of SGF, maintained at 37°C at 50 rpm in a shaking incubator. After 2, 4, and 8 hours, aliquot of sample (5 ml) was withdrawn from different flasks and analyzed spectrophotometrically at 272.6 nm for drug released from microcapsules (X1). The remaining microcapsules in the flasks were also recovered quantitatively, washed twice with double distilled water, and air dried. The dried microcapsules were grinded in a mortar, ultrasonicated after dispersing in 50 ml of distilled water until complete release of amoxicillin, and the released drug content was analyzed spectrophotometrically (λmax.-272.1 nm) (X2) to calculate percent degradation of drug using the formula:
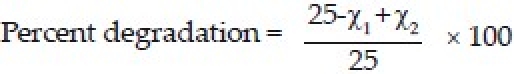
The similar study was carried out for plain drug to check the percent degradation in SGF.
Statistical Analysis
Experimental results were expressed as mean ± SD. Student's t-test and one-way analysis of variance (ANOVA) were applied to check significant differences in drug release, mucoadhesive properties, and particle size determination for different formulations. Differences were considered to be statistically significant at P<0.05.
RESULTS AND DISCUSSION
Formulation and Optimization of Amoxicillin Microcapsules
On the basis of pre-optimization studies, following parameters were used in optimization study:
-
a)
pH of CS solution-5.0
-
b)
Concentration of CaCl2,- 1.0%w/v
-
c)
Concentration of DOSS-0.075%w/v
-
d)
Gelation time (GCT) – 90 minutes.
The effect of pH is an important parameter which controls particle size of microcapsules. Alginate has the property of shrinking at low pH and getting dissolved in higher pH, whereas CS dissolves in low pH and is insoluble in higher pH ranges.[4] CS precipitates on addition of ALG solution pH 7 as the majority of amine groups undergo deprotonation and hence do not participate in ionic interactions.[22] The optimum pH was found to be 5.0 for ALG where maximum interaction between both polymeric groups occurred electrostatically, resulting in highest drug entrapment and small and uniform size of microcapsules.
By increasing the concentration of CaCl2 from 0.50 to 1.0%w/v, drug entrapment increased with smaller particle size and smooth surface. Beyond 1.0%w/v concentration of CaCl2, drug entrapment was decreased with increase in particle size which may be due to more gelation of ALG.
By increasing the concentration of DOSS (surfactant), the greater interfacial area is formed due to lowering of interfacial tension, hence the particle size decreased. Based on highest entrapment efficiency, the 0.075%w/v. concentration of DOSS was selected.
With increase in gel curing time (GCT) up to 90 minutes, entrapment efficiency of microcapsule increased which may be due to strengthening of matrix system.
Effect on particle size
During optimization studies, particle size of various microcapsule formulations was determined by optical microscopy as shown in Table 1. The results showed increase in particle size with increase in CS, ALG, and drug concentration. Increase in both polymers—CS and ALG concentration lead to PEC between carboxyl groups of ALG and the amino groups of CS, leading to increased size.[22] Increase in ratio of CS: ALG from 1:1, there is sharp increase in particle size. Formulation AM3 showed lowest particle size of 840 μm. It was found that the particle size distribution of each formulation was within a narrow range, but the mean particle size was slightly different among the formulations.
Effect on drug entrapment efficiency
The drug entrapment increased by increasing ALG concentration from 1 to 2% w/v, which may be attributed to stable gel complex in presence of CS and Ca++. Further increase in concentration of ALG resulted in decreased drug entrapment efficiency (DEE). CS and drug concentration influenced negatively on DEE because at higher concentrations, CS led to the formation of aggregates upon addition of ALG.[23] The viscosity of ALG solution above 3% w/v concentration was high enough to be used in the experiment. The optimum concentration for ALG was found to be 2% w/v based on uniformity and size of microcapsules.
Swelling studies
Results of swelling studies as in Figure 1 showed the decreasing trend with increase in CS concentration at different time intervals. At lower CS concentration, increase in concentration of ALG from 1.0% to 2.0% resulted in increased swelling. Formulation-AM3 showed highest swelling index at 8 hours (118.3%).
Figure 1.
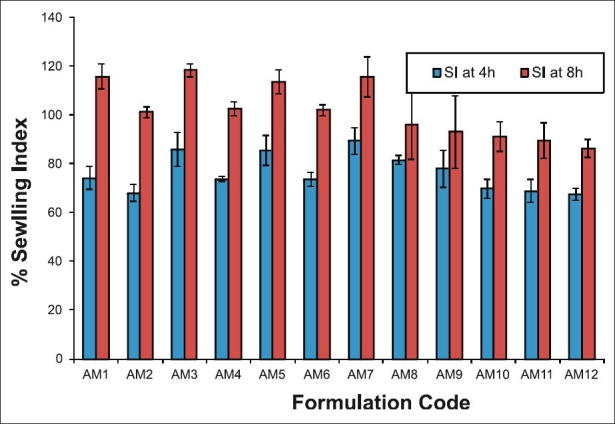
Swelling index profile of amoxicillin microcapsules at 37°C in 0.1N HCl Mean value±SD (n = 3)
Increase in ALG concentration at high value of CS resulted in lowering of swelling index, which may be due to reversal of shrinkage of ALG at lower pH as well as erosion of CS matrix in acidic environment.[4] Amoxicillin is more soluble in acidic media and hence results in decreased swelling of polymer with increase in its concentration.
Swelling increases with time because polymer gradually absorbs water due to its hydrophilicity. The outermost layer of the polymer hydrates, swells, and a gel barrier is formed at the outer surface.
Bioadhesion detachment force study
All formulations have showed sufficiently high bioadhesion strength (>8 g); however, bioadhesion strength of formulation-AM3 was observed highest. On contact with hydrated mucous of pig stomach tissue, hydrogels swell rapidly, resulting in reduced glass transition temperature and increased uncoiling of polymer chains. The uncoiled polymeric chains interact electrostatically and tend to increase the adhesion and interpenetration with mucin.[24] Poor swelling of ALG in acidic environment may be a reason for further decrease in mucoadhesion strength of microcapsules at higher concentration of ALG and CS. Also, this may be due to decrease in net positive charge (amino groups) on CS after interaction with anionic ALG, leading to poor interaction with sialic acid and other anionic groups present on mucin.[25]
In vitro drug release studies
The presence of ALG in the microcapsules resulted in controlled drug release in acidic environment; hence a suitable polymer for control drug delivery as reported earlier.[14] This can be attributed to poor swelling of ALG as well as low erosion of cross-linked CS in presence of acidic environment. Increase in CS and ALG resulted in strong PEC in presence of calcium ions, leading to highest stable gel matrix with reduced porosity.[5] Hence, poor drug release profile was obtained with increase in concentration of CS and ALG beyond formulation AM3. At high concentration of CS and ALG, the drug release was decreased to as low as ~65% in case of formulation AM12. At low pH, the alginates do not swell, but a reversal of shrinkage takes place and the contents are not released.[5]
The in vitro drug release studies were performed using 0.1N HCl as dissolution medium to evaluate the feasibility of bioadhesive formulation for sustained release of drug in gastric environment. For eradication of H. pylori, antibiotics must be released near the antrum region of stomach for mucosal penetration in controlled manner. The pre-optimized release profile considered for formulation was ~35%, 60%, 80%, and ~95% drug in 1, 4, 8, and 10 hours, respectively. Hence, the formulation with high similarity factor with theoretical release profile was selected as optimized formulation. The dissolution profile of various bioadhesive microcapsules of amoxicillin are tabulated as Table 2 and graphically represented as Figures 2 and 3.
Table 2.
Dissolution test profile of amoxicillin microcapsules (n = 3)
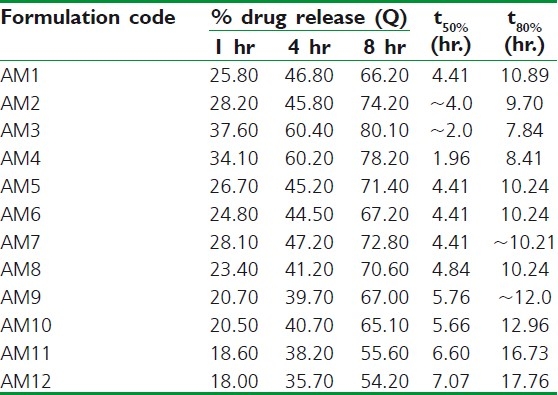
Figure 2.
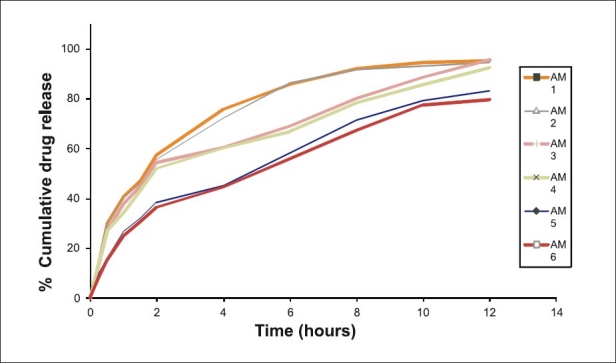
Dissolution rate studies of amoxicillin microcapsules (AM1-AM6) in SGF without pepsin (n=3)
Figure 3.
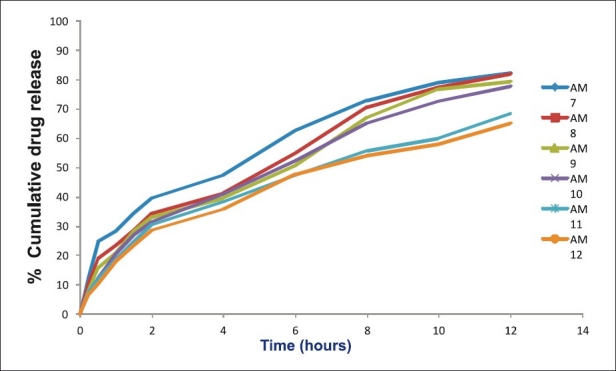
Dissolution rate studies of amoxicillin microcapsules (AM7-AM12) in SGF without pepsin (n=3)
At low concentration of ALG and CS (AM1-2), the release of drug was faster than the expected release profile which may be due to low ionic interaction and hence faster solubilization of microcapsules in acidic environment due to erosion of CS matrix.[26] Also, it may be attributed to reduction in polymer viscosity and hence increased drug diffusion through gel matrix.[27] The release profile of AM3 was more similar to theoretical release profile (f2~87%).
Drug Release Kinetics
The drug release profile of CS-ALG microcapsule formulations was evaluated for various release kinetic equations and the characteristic parameters are tabulated as Table 3.
Table 3.
Drug release kinetics of amoxicillin microcapsules (n=3)
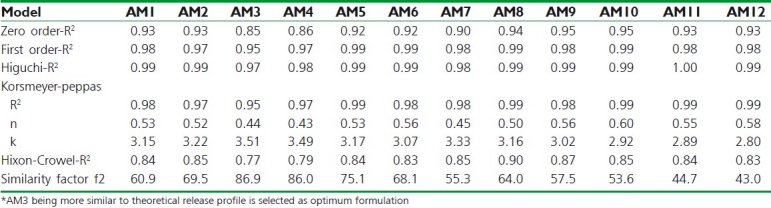
The value of n (Korsmeyer-Peppas model) varies between 0.43 and 0.60, delineating non-Fickian (anomalous) release behavior. The release mechanism from the microcapsule formulations refers to a combination of both diffusion- and erosion-controlled drug release, which is attributed to the rapid hydration, swelling, and erosion of polymeric blend. This may be attributed to low binding of CS and its erosion from the surface resulting in small pores in microcapsules.[5] Based on highest value of R2 in release kinetics study, all the formulations followed Higuchi model of release (R2 between 0.968-0.995).
Stability of amoxicillin in simulated gastric fluid
The purpose of current research work was not only to control the release profile of amoxicillin, but also to improve the stability of amoxicillin in presence of gastric fluid. In order to examine the stability of amoxicillin in stomach, SGF without pepsin was used as a medium for determination of relative decomposition of amoxicillin over the period of 8 hours. Decomposition of amoxicillin in microcapsules was found less than that for the pure drug [Figure 4]. The % degradation of amoxicillin was decreased from 90.9% to 7.6% at 8 hour by encapsulating in microcapsules. The relative decomposition of drug after encapsulation in CS-ALG microcapsules was decreased to 20.7%, 41.9%, and 83.3% in 2, 4, and 8 hours, respectively, and hence microencapsulation improved the stability of drug in stomach.
Figure 4.
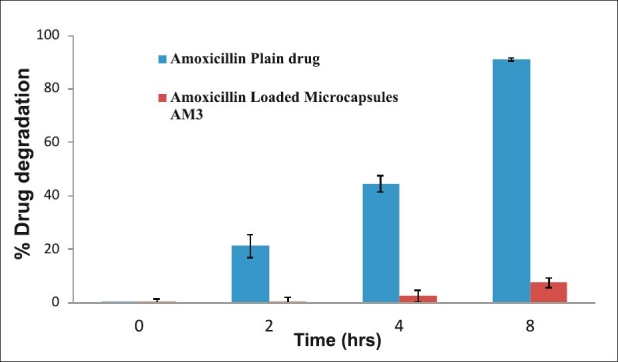
In vitro stability studies of amoxicillin plain drug/microcapsules in SGF without pepsin (n=3)
CONCLUSION
In order to overcome the problems associated with non-specific mucoadhesive polymers and stability of drug in acidic environment, a gastric mucoadhesive delivery system of amoxicillin was developed which provided controlled drug release in the gastric environment. The present research optimized the polymeric concentration, pH, and mixing ratio of polymers, drug:polymer ratio to formulate highly mucoadhesive microcapsules of amoxicillin using natural mucoadhesive polymers—CS and ALG. The optimized formulation AM3 was composed of 0.75%w/v CS, 2%w/v ALG, 0.075%w/v DOSS, 1%w/v calcium chloride with 840 μm size, highest drug entrapment (84%), and highest bioadhesive strength. The release profile of optimum formulation followed Higuchi model (r2>98%) and the release mechanism as per power law was Fickian diffusion (n = 0.43). The in vitro mucoadhesion studies confirmed the gastric retention of prepared microcapsules up to 8 hours, proving its efficacy as stomach-specific delivery of antibiotics. The gastric stability of amoxicillin in AM3 was 92% even after 8 hours.
ACKNOWLEDGEMENT
The authors are grateful to Prof. R. S. R. Murthy for kind guidance in the final outcome of this manuscript and Mr. Parveen Garg, Chairman, ISF College of Pharmacy, Moga, for providing facilities and financial assistance during the project. We also acknowledge Siemens Laboratories (Gurgaon, India) for generous gift samples of drug.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Juan MI, Konecny MH, Espuelas S, Campanero MA, Arbos P. Bioadhesive properties of gantrez nanoparticles. Molecules. 2005;10:126–45. doi: 10.3390/10010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vakil N, Megraud F. Eradication therapy for Helicobacter pylori. Gastroenterology. 2007;133:985–1001. doi: 10.1053/j.gastro.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Lai SK, Wang YY, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliver Rev. 2009;61:158–71. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Honary S, Maleki M, Karami M. The effect of chitosan molecular weight on the properties of alginate/chitosan microparticles containing prednisolone. Trop J Pharm Res. 2009;8:53–61. [Google Scholar]
- 5.Takka S, Gürel A. Evaluation of chitosan/alginate beads using experimental design: Formulation and in vitro characterization. AAPS Pharm Sci Tech. 2010;11:460–6. doi: 10.1208/s12249-010-9406-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerc J, Opara J. A new amoxicillin/clavulanate therapeutic system: Preparation, in vitro and pharmacokinetic evaluation. Int J Pharm. 2007;335:106–13. doi: 10.1016/j.ijpharm.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Li XY, Chenb XG, Suna ZW, Parkc HJ, Cha DS. Preparation of alginate/chitosan/carboxy methyl chitosan complex microcapsules and application in Lactobacillus casei ATCC 393. Carbohydrate Polymers. 2011;83:1479–85. [Google Scholar]
- 8.Cuna M, Alonso MJ, Torres D. Preparation and in vivo evaluation of mucoadhesive microparticles containing amoxycillin-resin complexes for drug delivery to the gastric mucosa. Eur J Pharm Biopharm. 2001;51:199–205. doi: 10.1016/s0939-6411(01)00124-2. [DOI] [PubMed] [Google Scholar]
- 9.Ren JM, Zou QM, Wang FK, He Q, Chen W, Zen WK. PELA microspheres loaded H. pylori lysates and their mucosal immune response. World J Gastroenterol. 2002;8:1098–102. doi: 10.3748/wjg.v8.i6.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hejazi R, Amiji M. Stomach-specific anti-H. pylori therapy Part III: Effect of chitosan microspheres crosslinking on the gastric residence and local tetracycline concentrations in fasted gerbils. Int J Pharm. 2004;272:99–108. doi: 10.1016/j.ijpharm.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Rajinikanth PS, Mishra B. Preparation and In vitro characterization of gellan based floating beads of acetohydroxamic acid for eradication of H. pylori. Acta Pharm. 2007;57:413–27. doi: 10.2478/v10007-007-0033-5. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Tauchi Y, Deguchi Y, Morimoto K, Tabata Y, Ikada Y. Positively charged gelatin microspheres as gastric mucoadhesive drug delivery system for eradication of H. pylori. Drug Deliv. 2000;7:237–43. doi: 10.1080/107175400455173. [DOI] [PubMed] [Google Scholar]
- 13.Umamaheshwari RB, Jain S, Jain NK. A new approach in gastroretentive drug delivery system using Cholestyramine. Drug Deliv. 2003;10:151–60. doi: 10.1080/713840399. [DOI] [PubMed] [Google Scholar]
- 14.Ishak AH, Gehanne AS, Mortada ND. Preparation, in vitro and in vivo evaluation of stomach-specific metronidazole-loaded alginate beads as local anti-Helicobacter pylori therapy. J Control Release. 2007;119:207–14. doi: 10.1016/j.jconrel.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Liu Z, Lu W, Qian L, Zhang X, Zeng P, Pan J. In vitro and in vivo studies on mucoadhesive microspheres of amoxicillin. J Control Release. 2005;102:135–44. doi: 10.1016/j.jconrel.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 16.Narkar M, Sher P, Pawar A. Stomach-specific controlled release gellan beads of acid-soluble drug prepared by ionotropic gelation method. AAPS Pharm sci tech. 2010;11:267–77. doi: 10.1208/s12249-010-9384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel JK, Patel MM. Stomach specific anti-Helicobacter pylori therapy: Preparation and evaluation of amoxicillin-loaded chitosan mucoadhesive microspheres. Curr Drug Deliv. 2007;4:41–50. doi: 10.2174/156720107779314811. [DOI] [PubMed] [Google Scholar]
- 18.Wittaya-Areekul S, Kruenate J, Prahsarn C. Preparation and in vitro evaluation of mucoadhesive properties of alginate/chitosan microparticles containing prednisolone. Int J Pharm. 2006;312:113–8. doi: 10.1016/j.ijpharm.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Patel VM, Prajapati BG, Patel HV, Patel KM. Mucoadhesive bilayer tablets of propranolol hydrochloride. AAPS Pharm Sci Tech. 2007;8:1–12. doi: 10.1208/pt0803077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerec M, Bogataj M, Mugerle B, Gaš perlin M, Mrhar A. Mucoadhesion on pig vesical mucosa: Influence of polycarbophil/calcium interactions. Int J Pharm. 2002;241:135–43. doi: 10.1016/s0378-5173(02)00231-4. [DOI] [PubMed] [Google Scholar]
- 21.Jagdale SC, Agavekar AJ, Pandya SV, Kuchekar BS, Chabukswar AR. Formulation and evaluation of gastroretentive drug delivery system of propranolol hydrochloride. AAPS PharmSciTech. 2009;10:1071–9. doi: 10.1208/s12249-009-9300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Douglas KL, Tabriziani M. Effect of experimental parameters on the formation of alginate–chitosan nanoparticles and evaluation of their potential application as DNA carrier. J Biomater Sci Polym. 2005;16:43–56. doi: 10.1163/1568562052843339. [DOI] [PubMed] [Google Scholar]
- 23.Motwari SK, Chopra S, Talegaonkar S, Kohli K, Ahmad FJ, Khar RK. Chitosan–sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: Formulation, optimization and in vitro characterisation. Eur J Pharm Biopharm. 2008;68:513–25. doi: 10.1016/j.ejpb.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed AA, El-Gazayerly ON, El-Gendy NA, Ali AA. Floating tablet of trimetazidine dihydrochloride: An approach for extended release with zero-order kinetics. AAPS Pharm sci tech. 2010;11:1058–67. doi: 10.1208/s12249-010-9468-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehr CM, Bouwstra JA, Schacht EH, Junginger HE. In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int J Pharm. 1992;78:43–48. [Google Scholar]
- 26.Mi FL, Sung HW, Shyu SS. Drug release from chitosan–alginate complex microcapsules reinforced by a naturally occurring cross-linking agent. Carbohy dr. Polym. 2002;48:61–72. [Google Scholar]
- 27.Longer MA, Ching HS, Robinson JR. Bioadhesive polymers as platform for oral control drug delivery III- oral delivery of chlorthiazide using bioadhesive polymer. J Pharm Sci. 1985;74:406–10. doi: 10.1002/jps.2600740408. [DOI] [PubMed] [Google Scholar]


