Abstract
Periodontal pockets act as a natural reservoir filled with gingival crevicular fluid for the controlled release delivery of antimicrobials directly. This article reflects the present status of nonsurgical controlled local intrapocket delivery of antimicrobials in the treatment of periodontitis. These sites have specialty in terms of anatomy, permeability, and their ability to retain a delivery system for a desired length of time. A number of antimicrobial products and the composition of the delivery systems, its use, clinical results, and their release are summarized. The goal in using an intrapocket device for the delivery of an antimicrobial agent is the achievement and maintenance of therapeutic drug concentration for the desired period of time. Novel controlled drug delivery system are capable of improving patient compliance as well as therapeutic efficacy with precise control of the rate by which a particular drug dosage is released from a delivery system without the need for frequent administration. These are considered superior drug delivery system because of low cost, greater stability, non-toxicity, biocompatibility, non-immunogenicity, and are biodegradable in nature. This review also focus on the importance and ideal features of periodontal pockets as a drug delivery platform for designing a suitable dosage form along with its potential advantage and limitations. The microbes in the periodontal pocket could destroy periodontal tissues, and a complete knowledge of these as well as an ideal treatment strategy could be helpful in treating this disease.
Keywords: Controlled release, drug delivery system, microorganisms, periodontitis
INTRODUCTION
Periodontitis, i.e., “peri” = around, “odont” = tooth, “itis” = inflammation, refers to a number of inflammatory diseases affecting the periodontium, the supporting tissues around the teeth. Periodontitis involves progressive bone loss around the teeth, leads to the loosening and subsequent loss of teeth, and is characterized by periodontal pocket formation.[1] The emergence of periodontal disease is from a pre-existing gingivitis. The inflammation of gingiva alone is termed gingivitis, and the severe inflammation of the periodontal structures with destruction of alveolar bone is called periodontal disease. Periodontitis is caused by microorganisms that adhere on the tooth's surfaces, along with an overly aggressive immune response against these microorganisms. Periodontitis is a multifactorial infection with great complexity in the mechanisms of pathogenesis.[2] Periodontal disease is one of the world's most prevalent chronic diseases. It is estimated that nearly 80% of adult Americans suffer some aspect of the disease's presence.[3] Periodontal diseases are one of the more prevalent oral diseases affecting more than 50% of Indian community.[4] Treatment of periodontal diseases has evolved appreciably in the last decade. Periodontal diseases include a group of chronic inflammatory diseases that affect the periodontal supporting tissues of teeth and encompass destructive and nondestructive diseases. Constant presence of chronic inflammation and inflammatory mediators has also been proved to be a significant risk factor.[4] Greater emphasis has been placed on nonsurgical local approaches to periodontal therapy.
The treatment of periodontitis mainly focuses on the reduction of the total bacterial count. Conventional treatment methods include surgical and non-surgical management which includes mechanical scaling, root planning,[5] often accompanied by antimicrobials.[6] These treatment modalities are aimed at eliminating the entire bacterial flora; there should also be prolonged drug–microbial contact.[7] In contrast to rectal, vaginal, sublingual, and nasal drug delivery for controlled release, the sub-gingival route has rich blood supply and it is relatively permeable. The rationale for use of antimicrobials in periodontitis is based on the fact that bacteria are the primary cause of periodontal diseases and thus treatment should be directed toward controlling the bacterial flora.[8] Local delivery of antimicrobials has been investigated for the possibility of overcoming the limitations of conventional therapy.[8] The goal of nonsurgical antimicrobial therapy is to return the tissues to a state of health that can be easily maintained by the client through periodontal debridement procedures.[9] The appearance of periodontal pockets is the first clinical manifestation of periodontal disease which offers a favorable medium for bacterial colonization. The deeper periodontal pockets can be diagnosed by clinical examination using periodontal probe in combination with X-ray imaging and microbiological techniques for a precise analysis of the infectious agents.[10] The failure to find the right causative pathogens results in therapeutic frustration, changing the course of the studies in periodontology. Disease activity in periodontal disease may range from slow, chronic, progressive destruction to brief and acute episodic bursts with varying intensity and duration [Table 1].[10,12,13] The periodontal route provides an excellent route for large, hydrophilic, and unstable proteins, oligonucleotides, and polysaccharides, as well as conventional small drug molecules.[10]
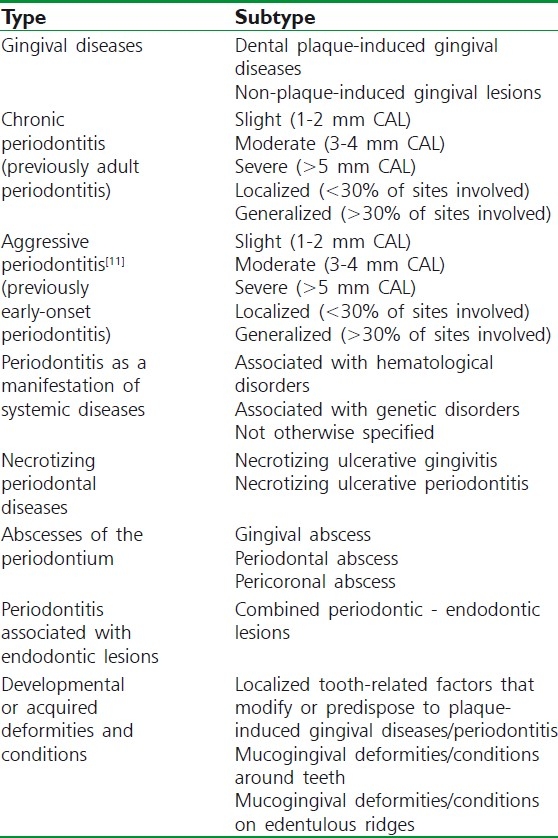
Table 1.
Classification of periodontal disease
POTENTIAL ADVANTAGE OF LOCAL ANTIMICROBIAL DRUG DELIVERY INTO PERIODONTAL POCKET
It can show improve patient acceptance and compliance.
This route is more possible for direct access to target diseases/various periodontal diseases.
This may reduce oral healthcare treatment cost.
It offers avoidance of GI tract with problems of oral drug administration.
It can serve as a reliable route for drug administration in very ill patient who are not able to swallow.
It can provide rapid absorption due to rich blood supply in comparison with transdermal.
It bypasses the first pass metabolism by the liver.
It can offer increase therapeutic efficacy of the drug.
It offers close proximity to blood flow.
This route is safe and convenient route.
It can produce longer duration of action.
It offers noninvasive, painless, and simple application.
LIMITATIONS
This route is not feasible for local irritants.
The drug and other excipients used in the formulation processing either erythema, itching, or local arrhythmia cannot be delivered by this route.
Dose is limited because of relatively small area.
Presystemic metabolism may occur by the enzymes like peptidase and esterase.
This route is not feasible for peptide delivery due to peptidase.
This route understood the needs for high-potency drugs.
It should be devoid of irritancy or a sensitization.
Manufacturing cost of the patches or devices should be taken in consideration.
PERIODONTAL PATHOGEN
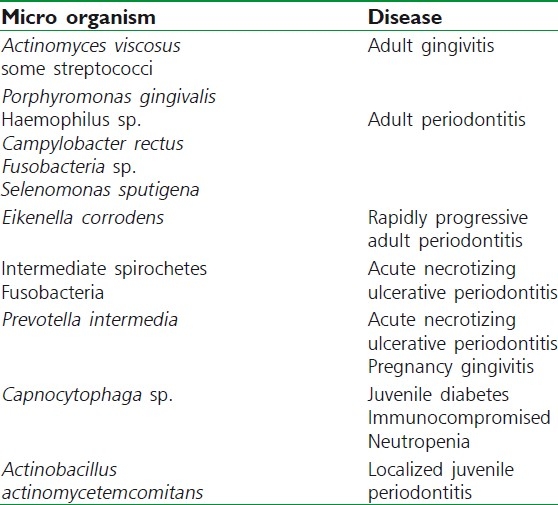
The microorganisms could produce disease directly, by invasion on the tissues, or indirectly by bacterial enzymes and toxins [Table 2].
Table 2.
Microbes and their specific disease
SIGN AND SYMPTOMS
Loose and sensitive teeth
Receding gums or longer appearing teeth [Figure 1].
Halitosis, or bad breath, and a persistent metallic taste in the mouth
Gingival recession, resulting in apparent lengthening of teeth.
Deep pockets between the teeth and the gum
Red or swollen gums
Tender or bleeding gums
Painful chewing.[14]
Figure 1.
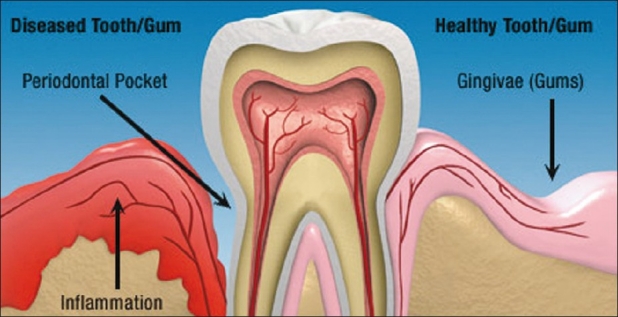
Structure showing healthy/infected tooth
PATHOLOGY
Gram-negative tooth-associated microbial biofilms initiate chronic periodontitis which produce a host response, leads to bone and soft tissue destruction. As a result of endotoxin derived from periodontal pathogens, several osteoclast-related mediators target the destruction of alveolar bone and supporting connective tissue including periodontal ligament [Figure 2]. Most of this aggressive tissue destruction is of matrix metalloproteinases, cathepsins, and other osteoclast-derived enzymes.[14]
Figure 2.
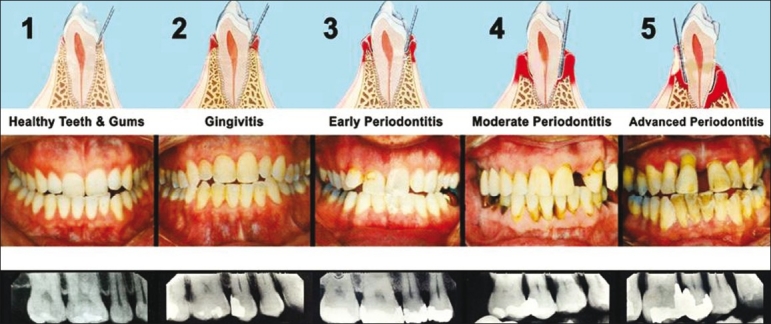
Progress of periodontal disease stage
MICROBIOLOGY
The disease is associated with a variable microbial pattern.
Anaerobic species of bacteria–Porphyromonas gingivalis, Bacteroides forsythus, Treponema denticola, Prevotella intermedia, Fusobacterium nucleatum, Eubacterium sp.—have all been implicated in chronic periodontitis.
Microaerophile bacteria—Actinomyces actinomycetemcomitans, Campylobacter rectus, and Eikenella corrodens also may play a role in chronic periodontitis.[15]
CONTROLLED RELEASE LOCAL DELIVERY DEVICES
Reservoir devices
The device consist of a dialysis tube (5 mm long and 0.2 mm wide) having a core of drug solution, which is inserted in the periodontal pocket for one week. Reservoir devices that lack rate control include hollow fibers, gels, and dialysis tubing.[16] These systems are marginally classified as sustained release devices as they cause several problems including irritation of the pocket, premature loss from the pocket, and rapid release of chemotherapeutic agents.
Monolithic devices
In the monolithic-controlled release devices, the drug is dispersed in a solid polymer matrix. Examples include acrylic strips and ethylene vinyl acetate (EVA) fibers. Acrylic strips are typically 0-2 mm thick. Drug release occurs over a period of 10 to 14 days (14 days drug release is considered to be advantageous, not a demerit). Other monolithic devices include strips made of [Table 3][18] ethyl cellulose, poly ethylene glycol, Hydroxy propyl methyl cellulose, and cross-linked collagen films.[19] Bioabsorbable, biodegradable materials can be left in situ which eliminates the risk of disturbing a site after therapy. The release from monolithic devices depends on diffusion of drug through matrix.[18]
Table 3.
Polymers used in the oral cavity

The two basic types are:
Non-erodible monolithic devices: Where the polymer maintains its integrity as the drug is lost.
Erodible monolithic devices: Which breaks up as the drug is released.
The monolithic devices can be prepared conveniently by using simple polymer fabrication techniques.
Melt fabrication technique
Films can be produced by extrusion in to thin film. Another process is known as calendaring, where the polymer is squeezed between heated rollers to form film.[18]
Solution casting
The polymer is dissolved in a suitable solvent to form a viscous solution, which is then spread on flat non-adhesive surface and the solvent is allowed to evaporate. The resultant film is peeled from the surface.
Polymerisation in situ
A liquid polymer or pre-polymerized inside a suitable mould.
RATIONALE FOR LOCAL INTRAPOCKET DELIVERY OF ANTIBACTERIAL DRUGS
For an antimicrobial agent to be successful, the pathogen must be known and it should be susceptible to the drug and not readily develop resistance for an adequate period of time. The periodontal pocket provides a natural reservoir bathed by gingival crevicular (I could not find any mistake in this word. Gingival crevicular fluid (GCF) is an inflammatory exudate that can be collected at the gingival margin or within the gingival crevice) fluid which is easily accessible for the insertion of a delivery device. The GCF provides a leaching medium for the release of a drug from the solid dosage form and for its distribution throughout the pocket. Moreover, the periodontal diseases are localized to the immediate environment of the pocket, enabling the periodontal pocket a natural site for treatment with local sustained-release delivery systems.[20] The sustained-release dosage forms maximize the therapeutic effect of antimicrobials by maintaining a constant plasma drug concentration over MIC for a prolonged period of time in a controlled manner.[16]
LOCALLY DELIVERED ANTIMICROBIAL PRODUCTS
Intrapocket devices can be divided into two broad categories, degradable and non-degradable devices.[21] Non-degradable devices have the advantage that the therapist controls the removal of the device and hence possess greater control over the time of exposure of the pocket environment to the drug. The degradable devices have the great advantage of requiring the patient to pay only a single visit to the therapist for inserting the device which minimizes patient non-compliance.[22]
The following medications are the most often prescribed.[23]
Actisite®
A commercially available delivery system (Actisite®, Corporation, Palo Alto, CA),[19,24] based on Alza a monolithic EVA fiber, 23 cm long and 0.5 mm diameter that delivers 12.7 mg tetracycline hydrochloride (J.M. Goodson, D.W. Holborow, R.L. Dunn, P.E. Hogan, S.L., 1983,1985, 1991 a,b,c; Tonetti et al., 1990; Heijl et al., 1991). Actisite was the first commercially available controlled-release antimicrobial product and was introduced in 1994.
The drug was selected based on its documented safety and efficacy against many human anaerobic periodontal floras.[25] The fiber, which appears as a slender cord, is designed to be applied to completely fill the pocket. It is applied either continuously in layers or circumferentially to completely fill the pocket and remains there with the assistance of a supplied cyanoacrylate adhesive for 7 to 10 days. The principle is that the local tissue concentration of active ingredients can be enhanced by incorporating drug as a controlled release delivery system to be placed directly into the periodontal pocket. The fiber is non-biodegradable and must be removed at the end of therapy. In comparison with an oral dose of tetracycline of 250 mg in adults, which yields 1 mcg/ml level in the serum and concentrates to approximately 4 mcg/ml in the GCF, Actisite, employing 12.7 mg of tetracycline, achieves an initial concentration level of 1 590 mcg/ml in the periodontal pocket. This level is sustained at a mean concentration of approximately 1 300 mcg/ml for a 7-day period, with mean concentrations of 43 mcg/ml in the superficial portions of the soft tissue wall. Serum concentrations are less than 0.1 mcg/ml. The range of MIC is from 0.5 to 32 mcg/ml. The MIC for Actinobacillus actinomycetemcomitans species is at approximately 32 mcg/ml.[26–28]
Atridox®
Atridox® (doxycycline hyclate) 10% is a biodegradable formulation for subgingival controlled release containing 10% by weight of doxycycline hyclate and a vehicle, each in a separate plastic syringe. One syringe contains 42.5 mg of doxycycline; the other contains 450 mg of the ATRIGEL Delivery System, a flowable polymeric formulation that is a combination of 36.7% poly- DL-lactide dissolved in 63.3% N-methyl-2-pyrrolidone. The contents of the two syringes are mixed. It is the only locally applied antibiotic gel that is placed gently below the gum line into periodontal pockets where bacteria thrive and cause infection. Atridox flows to the bottom of pockets and fills even the smallest spaces between teeth and gums. When the gel is applied, it hardens upon contact with oral fluids (saliva) to a wax-like substance, and the antibiotic is slowly released into the surrounding infected tooth pocket for 21 days. In clinical studies, Atridox has been shown to improve the health of gums and to help stop the advancement of gum disease.
Doxycycline is a broad-spectrum, semi-synthetic tetracycline that is bacteriostatic through inhibition of bacterial protein synthesis due to disruption of transfer ribonucleic acid (RNA) and messenger RNA at ribosomal sites.[29] In vitro testing has indicated that several of the periodontal pathogens, including P. gingivalis and Fusobacterium nucleatum, are susceptible to doxycycline at concentrations of 6 mcg/ml.[30] In a clinical pharmacokinetic study, on placement of Atridox, doxycycline levels in GCF peaked at 1 500 mcg/ml in 2 hours. Levels remained greater than 1 000 mcg through 18 hours. On day 7, the concentration of doxycycline remained well above the MIC for periodontal pathogens. Serum levels never exceeded 0.1 mcg/ml. On the other hand, subjects receiving systemic doxycycline had GCF levels peak at 2.5 mcg/ml at 12 hours.[31]
In one study that evaluated the effect of Atridox on microflora, 45 subjects with adult periodontitis were treated with a single treatment of Atridox.[32] Concentration remained at least 100 times above MIC for periodontal pathogens through day 7, with effective penetration of the biofilms reported. Levels of aerobic and anaerobic bacteria were significantly reduced. All post-treatment samples showed statistically significant differences in the total cultivable anaerobic counts recovered for the Atridox-treated group relative to baseline over the 6-month treatment. During these studies, no overgrowth of drug-resistant organisms was observed. Many studies have additionally shown that the adjunctive use of Atridox in combination with scaling and root planning (SRP) shows significant clinical improvement in pocket depth reduction, clinical attachment gains, and bleeding upon probing.[33]
Arestin®
Arestin®, a controlled-release product containing the antibiotic, minocycline hydrochloride 1 mg, in a bioresourceable polymer, poly (glycolide-co-dl-lactide), or PGLA produced in a microencapsulation process. Minocycline is a member of the tetracycline class of antibiotics and has a broad spectrum of activity.[34] The drug exerts its antibacterial action by inhibiting protein synthesis. It is a prescription antibiotic approved by the Food and Drug Administration (FDA). It is used together with SRP procedures performed by a trained dental professional for the treatment of periodontal (gum) disease. Arestin shows activity against organisms such as P. gingivalis, Prevotella intermedia, F. nucleatum, Eikenella corrodens, and A. Actinomycetemcomitans.[27] Once inserted into the periodontal pocket, these microspheres adhere to the walls of the pocket. GCF hydrolyzes the polymer, causing water-filled channels to form inside the microspheres. These areas provide for the encapsulated antibiotic to be released.[27] Over a 2-week period, the minocycline diffuse from the microspheres as they are hydrolyzed. At day 14, a level of 340 mcg/ml has still been found in the pocket. Eventually, the microspheres themselves are completely fragmented and bioresorbed.[35] Arestin has the clinical advantage of ease of application. One drawback is that it is a single-use product.
PerioChip®
PerioChip® (chlorhexidine gluconate) is a small, orange-brown, rectangular chip (rounded at one end) for insertion into periodontal pockets. The PerioChip® is a gelatin matrix containing a controlled-release antiseptic called chlorhexidine cross-linked with glutaraldehyde. It also contains glycerine and purified water. Once the chip is implanted, it biodegrades and releases its bacteria-killing medication directly into the pockets enlarged by plaque. There, the antiseptic begins to kill off the microbes, and the gum pockets generally become shallower and the gums less inflamed. A healing process can then begin, preventing potential gum and bone loss.
Chlorhexidine gluconate is a long-chain molecule with a positive charge that is attracted to the negatively charged surface of the biologic membranes of bacterial and epithelial cells. Chlorhexidine has a nonspecific mechanism of action against bacteria. It attaches to the cell walls, disrupting them and entering the cell. This disrupts the cytoplasm, which flows out of the ruptured cell resulting in bacterial death.[26,28] It is reported to be self-retentive and to biodegrade over the subsequent 7- to 10-day period.[27,31] In vitro studies of the release of chlorhexidine from its carrier showed that 40% of the chlorhexidine was released within 24 hours, with the remainder being released in the subsequent 7 to 10 days. The mean concentration for the 7-day period was 125 mcg/ml, in contrast to a level of 1 450 mcg/ml at 4 hours and 480 mcg/ml at 3 days.[27,36] Studies have shown suppression of the pocket flora for up to 11 weeks following treatment with Periochip.[37]
Periostat®
Periostat® is a systemically delivered collagenase inhibitor consisting of a 20-mg capsule of doxycycline hyclate for oral administration. This is the first FDA-approved systemic drug for host modulation as an adjunct to SRP in the treatment of periodontitis. Periostat, administered BID, reduced the elevated collagenase activity in the gingival fluid of patients with adult periodontitis. Each tablet contains 23 mg of doxycycline hyclate equivalent to 20 mg of doxycycline.[38,39]
A randomized, multi-center, double blind study was performed to compare the efficacy of SRP plus placebo to SRP plus Periostat administered BID. That study revealed statistically significant pocket depth reduction with adjunctive use of Periostat at 3, 6, and 9 months post-initial therapy (for initial depth >7 mm, 1.20 vs 1.68 mm, at depths 4-6 mm, 0.69 vs 0.95 mm) and gain of clinical attachment (for initial depths >7 mm, 1.17 mm vs 1.35 mm, at depths 4-6 mm, 0.86 vs 1.03 mm). Mean changes in pocket depth and attachment level across large numbers of patients and tooth sites were small, and may not reflect the magnitude of change that may occur in an individual patient or tooth site. In a 3-month follow-up study, where patients received no additional therapy, pocket depth reductions and clinical attachment level gains observed following 9 months adjunctive Periostat were maintained.[38,39]
REGULATORY ASPECTS
All periodontal products shall comply with all the regulatory requirements in terms of safety and efficacy. All the standard testing in accordance with pharmacology, toxicology should be properly conducted and documented. In the design of clinical protocols, two aspects must be defined; first, the relevant information for the active ingredient, excipients, dose, stability and second, the various evaluation parameters.
CONCLUSION
New formulations and novel-controlled delivery system will continue to be explored. One of the most persistent problems faced by the formulation technologist is that many drugs do not reach the target areas in the therapeutic concentrations intended. By the development of novel drug delivery systems, a more site-specified and controlled drug delivery systems was made possible, thereby dose and side effects can be reduced which ensures better patient compliance. The site-specific delivery of drug can thus overcome the disadvantages faced during systemic administration of antimicrobials for periodontitis, where the drug gets diluted many times before it reaches the site of action. In conclusion, hence antimicrobial drug as site-specific dental formulations into periodontal pocket proves to be a viable alternative to conventional periodontal therapy. Obviously, its clinical use in the fast-growing field is promising.[40]
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontal. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Page RC. The etiology and pathogenesis of periodontitis. Compend Contin Educ Dent. 2002;23:11–4. [PubMed] [Google Scholar]
- 3.Gum disease. Medline Plus website. [Last Cited on 2011 Nov 13]. Available from: http://www.nlm.nih.gov/medlineplus/gumdisease.html .
- 4.Agarwal V, Khatri M, Singh G, Gupta G, Marya CM, Kumar V. Prevalence of periodontal diseases in India. J Oral Health Comm Dent. 2010;4(Spl):7–16. [Google Scholar]
- 5.Cobb CM. Microbes, inflammation, scaling and root planning and the periodontal condition. J Dent Hyg. 2008;82:4–9. [PubMed] [Google Scholar]
- 6.Haffajee AD, Socransky SS, Gunsolley JC. Systemic anti-infective periodontal therapy. Ann J Periodontol. 2003;8:115–81. doi: 10.1902/annals.2003.8.1.115. [DOI] [PubMed] [Google Scholar]
- 7.Ebersole JL, Taubman MA. Protective nature of host responses in periodontal diseases. Periodontol 2000. 1994;5:112–41. doi: 10.1111/j.1600-0757.1994.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 8.Hanes PJ, Purvis JP. Local anti-infective therapy: Pharmacological agents. A systematic review. Ann Periodontol. 2003;8:79–98. doi: 10.1902/annals.2003.8.1.79. [DOI] [PubMed] [Google Scholar]
- 9.Rams T, Slots J. Local delivery of antimicrobial agents in the periodontal pocket. Periodontol 2000. 1996;10:139–59. doi: 10.1111/j.1600-0757.1996.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 10.Bascones-Martinez A, Matesanz-Perez P, Scribano-Bermejo M, Gonzá-lez-Moles MA, Bascones-Ilundain J, Meurman JH. Periodontal disease and diabetes-Review of the literature. Med Oral Patol Oral Cir Bucal. 2011;16:e722–9. doi: 10.4317/medoral.17032. [DOI] [PubMed] [Google Scholar]
- 11.Tihomir G, Kamen N. Rational antibiotic therapy in the complex treatment of patients with generalized Periodontitis. J IMAB. 2009;2:10–3. [Google Scholar]
- 12.Armitage GC. Diagnosis of periodontal diseases. J Periodontol. 2003;74:1237–47. doi: 10.1902/jop.2003.74.8.1237. [DOI] [PubMed] [Google Scholar]
- 13.Wiebe CB, Putnins EE. The periodontal disease classification system of the American Academy of Periodontology-An update. J Can Dent Assoc. 2000;66:594–7. [PubMed] [Google Scholar]
- 14.Sorsa T, Tjäderhane L, Konttinen YT, Lauhio A, Salo T, Lee HM, et al. Matrix metalloproteinases: Contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann Med. 2006;38:306–321. doi: 10.1080/07853890600800103. [DOI] [PubMed] [Google Scholar]
- 15.Loesche WJ, Grossman NS. Periodontal disease as a specific, albeit chronic, infection: Diagnosis and treatment. Clin Microbiol Rev. 2001;14:727–52. doi: 10.1128/CMR.14.4.727-752.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodson JM. Controlled drug delivery. A new means of treatment of dental diseases. (35-6).Compend Cont Educ Dent. 1985;6:27–32. [PubMed] [Google Scholar]
- 17.Mohammed GA. Formulation of chitosan-based ciprofloxacin and diclofenac film for periodontitis therapy. Trop J Pharma Res. 2009;8:33–41. [Google Scholar]
- 18.Eley DD. Theory of rolling plastics. I. Calculation of roll pressure. J Polym Sci. 1946;1:529–34. [Google Scholar]
- 19.Lindhe J, Heijl L, Goodson JM, Socransky SS. Local tetracycline delivery using hollow fibre devices in periodontal therapy. J Clin Periodontol. 1979;6:141–9. doi: 10.1111/j.1600-051x.1979.tb02193.x. [DOI] [PubMed] [Google Scholar]
- 20.Jorgens S. Efficient antimicrobial treatment in periodontal maintenance care. J Am Dent Assoc. 2000;131:1293–304. doi: 10.14219/jada.archive.2000.0383. [DOI] [PubMed] [Google Scholar]
- 21.Rams TE, Slots J. Local delivery of antimicrobial agents in the periodontal pocket. J Periodontal. 1996;10:139–59. doi: 10.1111/j.1600-0757.1996.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 22.Nagaraju R, Jacob M, Udupa N, Verma BR. Biodegrable dental implants of ciprofloxacin BCD complexes in the treatment of periodontitis. Indian J Exp Biol. 1999;37:305–7. [PubMed] [Google Scholar]
- 23.Kornman KS. Controlled-release local delivery antimicrobials in periodontics: Prospects for the future. J Periodontol. 1993;64:782–91. doi: 10.1902/jop.1993.64.8s.782. [DOI] [PubMed] [Google Scholar]
- 24.Tonetti M, Cugini MA, Goodson JM. Zero order delivery with periodontal placement of tetracycline-loaded ethylene vinyl acetate fibres. J Periodontal Res. 1990;25:243–9. doi: 10.1111/j.1600-0765.1990.tb00911.x. [DOI] [PubMed] [Google Scholar]
- 25.Minabe M. Intra-pocket antibiotic therapy using reabsorbable and non-resorbable slow-release devices containing tetracycline. Periodontal Clin Investig. 2000;22:14–21. [PubMed] [Google Scholar]
- 26.De Graaff J, van Winkelhoff AJ, Goen RJ. The role of Actinobacillus actinomycetemcomitans in periodontal disease. Infection. 1989;17:269–71. doi: 10.1007/BF01639538. [DOI] [PubMed] [Google Scholar]
- 27.Slots J, Rams TE. Antibiotics in periodontal therapy: advantages and disadvantages. J Clin Periodontol. 1990;17:479–93. doi: 10.1111/j.1365-2710.1992.tb01220.x. [DOI] [PubMed] [Google Scholar]
- 28.Kornman KS. Controlled-release local delivery antimicrobials in periodontics: Prospects for the future. J Periodontol. 1993;64:782–91. doi: 10.1902/jop.1993.64.8s.782. [DOI] [PubMed] [Google Scholar]
- 29.Nagaraju R, Udupa N, Arshad S, Verma BR. Controlled release biodegradable implants of doxycycline and its clinical efficacy. Antiseptic. 2001;98:165–9. [Google Scholar]
- 30.Garrett S, Johnson L, Drisko CH, Adams DF, Bandt C, Beiswanger B, et al. Two multi-center studies evaluating locally delivered doxycycline hyclate, placebo control, oral hygiene, and scaling and root planing in the treatment of periodontitis. J Periodontol. 1999;70:490–503. doi: 10.1902/jop.1999.70.5.490. [DOI] [PubMed] [Google Scholar]
- 31.Stoller NH, Johnson LR, Trapnell S, Harrold CQ, Garrett S. The pharmacokinetic profile of a biodegradable controlled-release delivery system containing doxycycline compared to systemically delivered doxycycline in gingival crevicular fluid, saliva and serum. J Periodontol. 1998;69:1085–91. doi: 10.1902/jop.1998.69.10.1085. [DOI] [PubMed] [Google Scholar]
- 32.Walker CB, Godowski KC, Borden L, Lennon J, Nangó S, Stone C, et al. The effects of sustained release doxycycline on the anaerobic flora and antibiotic-resistant patterns in subgingival plaque and saliva. J Periodontol. 2000;71:768–74. doi: 10.1902/jop.2000.71.5.768. [DOI] [PubMed] [Google Scholar]
- 33.Treatment of plaque-induced gingivitis, chronic periodontitis, and other clinical conditions. J Periodontol. 2001;72:1790–800. doi: 10.1902/jop.2001.72.12.1790. [DOI] [PubMed] [Google Scholar]
- 34.Yeom H. Clinical and microbiological effects of minocycline loaded microcapsules in adult peridontitis. J Periodontal. 1997;68:1102–9. doi: 10.1902/jop.1997.68.11.1102. [DOI] [PubMed] [Google Scholar]
- 35.Williams RC, Paquette DW, Offenbacher S. Treatment of periodontitis by local administration of minocycline microspheres: A controlled trial. J Periodontol. 2001;72:1535–44. doi: 10.1902/jop.2001.72.11.1535. [DOI] [PubMed] [Google Scholar]
- 36.Ciancio SG. Local delivery of chlorhexidine. Compend Contin Educ Dent. 1999;20:427–32. [PubMed] [Google Scholar]
- 37.Paquette DW, Ryan ME, Wilder RS. Locally delivered antimicrobials: Clinical evidence and relevance. J Dent Hyg. 2008;82:10–5. [PubMed] [Google Scholar]
- 38.Caton JG, Blieden T, Adams DF, Crout RJ, Hefti A, Killoy WJ, et al. Subantimicrobial doxycycline therapy for periodontitis. J Dent Res. 1997;76:177. [Google Scholar]
- 39.Caton J, Ciancio S, Crout R, Hefti A, Polson A. Adjunctive use of subantimicrobial doxycycline therapy for periodontitis. J Dent Res. 1998;77:1001. [Google Scholar]
- 40.Mastiholimath VS, Dandagi PM, Gadad AP, Patil MB, Manvi FV, Chandur VK. Formulation and evaluation of ornidazole dental implants for periodontitis. Indian J Pharm Sci. 2006;68:68–71. [Google Scholar]


