Abstract
A higher calcium intake is still the primary recommendation for the prevention of osteoporosis, whereas vitamin D deficiency is often not addressed. To study the relative importance of dietary calcium intake and serum 25-hydroxyvitamin D [25(OH)D] status in regard to hip BMD, 4958 community-dwelling women and 5003 men ≥20 yr of age from the U.S. NHANES III population-based survey were studied. Calcium supplement users and individuals with a prior radius or hip fracture were excluded. We calculated standardized means for BMD by quartiles of sex-specific calcium intake for three 25(OH)D categories (<50, 50–74, and 75+ nM) among men and women, separately controlling for other important predictors of BMD. A higher calcium intake was significantly associated with higher BMD (p value for trend: p = 0.005) only for women with 25(OH)D status <50 nM, whereas calcium intake beyond the upper end of the lowest quartile (>566 mg/d) was not significantly associated with BMD at 25(OH)D concentrations >50 nM. Among men, there was no significant association between a higher calcium intake beyond the upper end of the lowest quartile (626 mg/d) and BMD within all 25(OH)D categories. Among both sexes, BMD increased stepwise and significantly with higher 25(OH)D concentrations (<50, 50–74, 75+ nM; p value for trend: women < 0.0001; men = 0.0001). Among men and women, 25(OH)D status seems to be the dominant predictor of BMD relative to calcium intake. Only women with 25(OH)D concentrations <50 nM seem to benefit from a higher calcium intake.
Keywords: 25-hydroxyvitamin D, dietary calcium intake, BMD, adults, men and women
INTRODUCTION
Considerable uncertainty exists regarding optimal intakes of calcium, which is reflected in markedly different recommended daily intakes among countries. For example, for adults >50 yr of age, it is 700 mg/d in the United Kingdom and 1200 mg/d in the United States.(1) These differences may be explained in part by the fact that today calcium intake recommendations do not consider the additional importance of 25-hydroxyvitamin D [25(OH)D] concentrations in regard to calcium absorption.
Recent studies have suggested that calcium absorption is enhanced with higher 25(OH)D status.(2,3) Depending on the size of this effect, calcium intake recommendations may be overestimated among individuals with higher 25(OH)D concentrations. Consistent with this, an Icelandic study suggested that, to maintain calcium metabolism, calcium intakes beyond 800 mg/d may only be needed in individuals with 25(OH)D concentrations <25 nM, whereas among individuals with higher 25(OH)D concentrations, calcium intake did not correlate with PTH concentrations.(4)
To address the relative importance of calcium intake and 25(OH)D status in regard to hip BMD, we took advantage of a large U.S. survey that included measures of BMD, 25(OH)D status, and calcium intake plus other important determinants of BMD among adults ≥20 yr of age. BMD is used to define peak bone mass in young adults,(5) and it is a strong predictor of fractures in the elderly.(6)
MATERIALS AND METHODS
Data source and subjects
The National Health and Nutrition Examination Survey III (NHANES III) was conducted between 1988 and 1994 to study the health and nutritional status of the noninstitutionalized U.S. population.(7) There were 16,573 individuals potentially eligible for BMD scans. Of these, 157 were excluded for medical reasons, and 14,646 scans were technically acceptable. Serum 25(OH)D concentrations were available for 14,083 of these individuals, 13,516 of which were either white, Mexican American, or black, and 13,432 had complete data on smoking and body mass index (BMI). Of these, 9962 had dietary calcium intake recorded with a plausible upper intake of <3500 mg/d, did not use calcium supplements, and had no prior radius or hip fracture. One woman was excluded for a 25(OH)D concentration of 400 nM. Thus, the final sample consisted of 9961 individuals (4958 women and 5003 men).
Measurement of BMD
Areal total hip BMD (bone mass per unit of area scanned) was measured using DXA on men and nonpregnant women ≥20 yr of age (Lunar DPX).(8,9)
Dietary calcium intake
Calcium intake in milligrams per day was assessed in all individuals with one 24-h dietary recall. In addition, as part of a nonrandomly selected substudy of NHANES III, ~5% of the respondents who came to the mobile exam center for measurements were invited to “repeat” the 24-h recall (n = 974) within a few weeks after their initial visit. The same computer-assisted instrument was used for this second assessment. The within-person variability in the 24-h recall will attenuate associations. For this reason, we used the deattenuation procedure correcting for measurement error based on this one repeat measurement.
The correlation between the two measurements was 0.46 among men and 0.40 among women, suggesting a moderate day-to-day variation in calcium intake. Consistently, previous studies have documented that day-to-day variation for calcium intake is moderate compared with other micronutrients.(10,11) This may be explained by relatively consistent daily dairy intakes compared with other foods.(11)
Serum concentration of 25(OH)D
Serum 25(OH)D concentrations were assayed with radioimmunoassay kits [DiaSorin, Stillwater, MN, USA; reference range for the assay is 22.5–94 nM (9–37.6 ng/ml); measures 25(OH)D2 and 25(OH)D3].(12) Serum 25(OH)D concentrations in NHANES III were higher than in those observed in the Icelandic Survey, with a small fraction <25 nM (4.2%), which is why we chose <50 nM as the lowest 25(OH)D category in this analysis. 25(OH)D concentrations of at least 75 nM have been suggested as desirable for optimal bone health(13) and fracture reduction.(14)
Other covariates
Respondents were classified as never smokers (if they had smoked <100 cigarettes in their lifetime), former smokers (≥100 lifetime cigarettes, not currently smoking), and current smokers (≥100 lifetime cigarettes, currently smoking). Smoking was studied as a potential confounder because it affects BMD and reduces calcium absorption.(15) The poverty income ratio was computed as the ratio of family income versus the poverty threshold as produced annually by the Census Bureau adjusted for changes caused by inflation. BMI is weight in kilograms divided by height in meters squared. We also adjusted for height as a potential confounder independent of BMI because of its correlation with calcium intake and BMD.(16) The variable “any estrogen use” (never, former, current) was created from the response on the use and duration of use of “birth control pills” and “estrogen or female hormone pills.” The 115 subjects with missing values were coded as missing. Estrogen use was studied as a potential confounder because it affects BMD and increases vitamin D concentrations by increasing vitamin D–binding globulin.(17)
Total calorie intake was computed from 24-h recalls and included as a potential confounder for its correlation with dietary calcium intake and association with BMD. Physical activity was calculated from the following documented activities, and their intensities were summarized by their metabolic equivalent levels (METs): walked 1 mi without stopping, swam, jogged, rode a bicycle, danced, exercised or did garden work in the last month.(18) The METs were calculated based on the frequency of each physical activity.
Statistical analyses
To minimize confounding by indication, this sample excluded individuals who used calcium supplements or had a prior radius or hip fracture (these two fractures are specifically assessed in NHANES III). To account for errors in the measurement of calcium intake, the regression calibration method was used.(19,20) In the first step, measurement error– corrected calciumintake was calculated based on one repeat 24-h recall from a 5% nonrandom subsample. We regressed the repeated measure of calcium intake on the first measure for each person, which was used as the main measurement error–corrected calcium intake variable calculated as the estimated linear regression coefficient times the measured intake plus the constant [measurement error corrected calcium intake =393 + (0.511 × measured calcium intake)].
To further address error in measuring calcium intake, we also used the multivariate regression calibration method described by Spiegelman et al.,(20) where both the point and interval estimates are corrected for measurement error, as implemented in a SAS macro. This method is referred to as the multivariate measurement error correction in Table 4.
TABLE 4.
Predicted Difference in BMD for a 300-mg Increment of Calcium Intake With and Without Measurement Error Correction
| BMD by 300-mg/d increase in calcium intake (linear variable for calcium intake) | 25(OH)D (<50 nM) | 25(OH)D (50–74 nM) | 25(OH)D (75+ nM) | |
|---|---|---|---|---|
| Women | Without error correction | |||
| Predicted difference in BMD (mg/cm2) | 0.0065 | 0.0044 | 0.0032 | |
| SE | 0.0024 | 0.0024 | 0.0025 | |
| p | 0.008* | 0.07 | 0.21 | |
| Multivariate error correction | ||||
| Predicted difference in BMD (mg/cm2) | 0.028 | 0.019 | 0.014 | |
| SE | 0.011 | 0.011 | 0.011 | |
| p | 0.01* | 0.16 | 0.22 | |
| Men | Without error correction | |||
| Predicted difference in BMD (mg/cm2) | 0.0021 | 0.0029 | −0.00032 | |
| SE | 0.0029 | 0.0021 | 0.0021 | |
| p | 0.46 | 0.17 | 0.99 | |
| Multivariate error correction | ||||
| Predicted difference in BMD (mg/cm2) | 0.008 | 0.011 | −0.00012 | |
| SE | 0.011 | 0.008 | 0.0078 | |
| p | 0.47 | 0.18 | 0.99 | |
Increment in BMD for a 300-mg/d calcium intake (with and without correction for measurement error correction) among women and men with deficient and adequate vitamin D status. All analyses controlled for age, race/ethnicity, body mass index, height, total calorie intake, estrogen use among women, physical activity, smoking, and socio-economic status. For the multivariate measurement error correction,(20) both the point and interval estimates are corrected for measurement error.
Significant.
General linear regression analyses were used to model the association between measurement error–corrected dietary calcium intake and BMD. Five covariates, 25(OH)D, sex, age, physical activity, and race/ethnicity,(9,21) were considered as potential effect modifiers. Because both measurement error–corrected calcium intake and 25(OH)D concentrations were not normally distributed, effect modification was assessed after log transformation of these variables. The p value of the interaction term from the cross-product term [log 25(OH)D × log calcium intake] was 0.005. We further studied effect modification using stratification by sex, age group (20–49 and ≥50 yr), physical activity (active/inactive), and race/ethnicity (white, black, Mexican American) within each 25(OH)D category (<50, 50–74, and 75+ nM). Only for sex was effect modification significant [p value of the interaction term: 0.01 in 25(OH)D category < 50 nM; 0.08 in 25(OH)D category 50–74 nM; 0.01 in 25(OH)D category 75+ nM]. Therefore, results are presented in six subgroups stratified by sex and 25(OH)D category. Because calcium intakes were higher in men, dietary calcium intake was divided into sex-specific quartiles. Standardized means for BMD by quartiles of dietary calcium intake within each vitamin D category were calculated using regression analysis and adjusting for the following variables: age (10-yr age categories), race/ ethnicity (white, black, Mexican American), BMI, height, total calorie intake, estrogen use among women, physical activity, smoking, and socio-economic status. The data were standardized to a person of median height and median BMI, with a median calorie intake, age 60–69 yr, of a median socio-economic status, and in the top tertile of physical activity. Among women, the data were also standardized to women not taking estrogen.
All statistical analyses were performed with SAS 9.1.
RESULTS
Table 1 shows the characteristics of participants. Men had significantly higher 25(OH)D concentrations, higher measurement error–adjusted calcium intake, higher BMD, and greater physical activity than women. Seventeen percent of all men and 27% of all women were physically inactive, defined as 0 METs, indicating that these individuals did not walk 1 mi without stopping, did not swim, jog, ride a bicycle, dance, exercise, or do garden work in the last month. Table 2 shows calcium intake by age group, sex, and race/ethnicity. Table 3 shows mean 25(OH)D concentrations by age among white, black, and Mexican-American men and women.
TABLE 1.
Characteristics of Study Population
| Variable | All (n = 9961) | Women (n = 4958) | Men (n = 5003) |
|---|---|---|---|
| Age (yr) | |||
| Mean ± SD | 47.1 ± 18.7 | 46.6 ± 18.4 | 47.6 ± 19.0* |
| 20–29 (n; %) | 2130 (21.4%) | 1038 (20.9%) | 1093 (21.8%) |
| 30–39 (n; %) | 2061 (20.7%) | 1106 (22.3%) | 956 (19.1%) |
| 40–49 (n; %) | 1677 (16.8%) | 854 (17.2%) | 823 (16.%) |
| 50–59 (n; %) | 1126 (11.3%) | 569 (11.5%) | 558 (11.2%) |
| 60–69 (n; %) | 1380 (13.9%) | 639 (12.9%) | 741 (14.8%) |
| 70–79 (n; %) | 983 (19.9%) | 479 (9.7%) | 505 (10.1%) |
| 80+ (n; %) | 604 (6.1%) | 273 (5.5%) | 331 (6.6%) |
| Race/ethnicity (n; %) | |||
| White | 3979 (40%) | 1957 (39%) | 2026 (40%) |
| Black | 3050 (31%) | 1617 (33%) | 1433 (29%)† |
| Mexican American | 2932 (29%) | 1384 (28% | 1546 (31%)‡ |
| Total hip BMD (mg/cm2) (mean ± SD) | 0.97 ± 0.18 | 0.91 ± 0.17 | 1.02 ± 0.17† |
| Body mass index (kg/m2) (mean ± SD) | 27.3 ± 5.6 | 27.9 ± 6.5 | 26.8 ± 4.6† |
| Height (m) (mean ± SD) | 1.67 ± 0.98 | 1.60 ± 0.71 | 1.74 ± 0.75† |
| 25(OH)D concentrations (nM) | |||
| Mean ± SD | 62.6 ± 27.2 | 57.8 ± 26.8 | 67.4 ± 27.1† |
| <50 (n; %) | 3677 (37%) | 2267 (46%) | 1410 (28%) |
| 50–74 (n; %) | 3410 (34%) | 1571 (32%) | 1839 (37%) |
| 75+ (n; %) | 2874 (29%) | 1120 (22%) | 1758 (35%) |
| Physical activity (METs) | |||
| Mean ± SD | 93.3 ± 127.3 | 74.9 ± 108.6 | 111.8 ± 141.5† |
| Percent with 0 METs | 22% | 27% | 17% |
| Poverty/income ratio (mean ± SD) | 2.1 ± 1.81 | 2.0 ± 1.7 | 2.3 ± 1.9† |
| Uncorrected calcium intake (mg/d) (mean ± SD) | 749 ± 503 | 647 ± 439 | 850 ± 542† |
| Measurement error corrected calcium intake (mg/d; based on one repeat intake assessment) (mean ± SD)§ | 776 ± 257 | 724 ± 224 | 827 ± 277† |
| Any estrogen intake | |||
| Never (n; %) | 1782 (35.9%) | ||
| Former (n; %) | 2355 (47.5%) | ||
| Current (n; %) | 741 (15.0%) | ||
| Missing (n; %) | 80 (1.6%) | ||
| Smoking | |||
| Never (n; %) | 4790 (48.1%) | 2991 (60.3%) | 1799 (35.9%)† |
| Former (n; %) | 2427 (24.4%) | 800 (14.1%) | 1628 (32.5%)† |
| Current 1–10 (n; %) | 1311 (13.2%) | 564 (11.4%) | 748 (14.9%)† |
| Current 11–20 (n; %) | 973 (9.8%) | 424 (8.6%) | 549 (11.0%)† |
| Current 21+ (n; %) | 460 (4.6%) | 179 (3.6%) | 283 (5.7%)† |
p < 0.05.
p < 0.0001.
p < 0.001.
Calcium intake variable used in the analyses
TABLE 2.
Calcium Intake (Simple Measurement Error Corrected) by Age in Subgroups of the Population
| Calcium intake (mean ± SD; n) | White women (n = 1957) | White men (n = 2002) | Black women (n = 1433) | Black men (n = 1617) | Mexican-American women (n = 1548) | Mexican-American men (n = 1384) |
|---|---|---|---|---|---|---|
| Age 20–29 | 776 ± 231; 265 | 997 ± 339; 242 | 703 ± 251; 394 | 827 ± 294; 354 | 772 ± 220; 379 | 890 ± 291; 496 |
| Age 30–39 | 784 ± 232; 350 | 925 ± 302; 288 | 682 ± 233; 410 | 824 ± 291; 343 | 783 ± 260; 346 | 882 ± 273; 324 |
| Age 40–49 | 739 ± 203 296 | 842 ± 269; 293 | 650 ± 191; 307 | 745 ± 221; 249 | 751 ± 244; 251 | 841 ± 262; 281 |
| Age 50–59 | 711 ± 188; 270 | 852 ± 285; 289 | 654 ± 163; 182 | 676 ± 218; 152 | 733 ± 202; 117 | 755 ± 231; 116 |
| Age 60–69 | 751 ± 188; 256 | 829 ± 253; 329 | 632 ± 178; 185 | 697 ± 241; 198 | 715 ± 197; 198 | 741 ± 216; 214 |
| Age 70–79 | 724 ± 211; 307 | 829 ± 253; 313 | 696 ± 216; 99 | 691 ± 226; 107 | 685 ± 178; 73 | 744 ± 193; 84 |
| Age 80+ | 707 ± 197; 213 | 802 ± 212; 268 | 653 ± 182; 40 | 625 ± 115; 30 | 690 ± 213; 20 | 740 ± 228; 33 |
Among all white, Mexican-American, and black individuals, women had lower intakes than men and intake decreased with age.
TABLE 3.
25(OH)D Concentrations in Subgroups of the Population
| Variable (mean ± SD; n) | White women (n = 1957) | White men (n = 2002) | Black women (n = 1433) | Black men (n = 1617) | Mexican-American women (n = 1548) | Mexican-American men (n = 1384) |
|---|---|---|---|---|---|---|
| 25(OH)D concentrations | ||||||
| Age 20–29 | 90.4 ± 34.9; 265 | 86.6 ± 27.5; 242 | 44.1 ± 18.1; 394 | 51.6 ± 20.4; 354 | 57.4 ± 22.8; 379 | 69.3 ± 21.6; 496 |
| Age 30–39 | 77.8 ± 29.8; 350 | 86.2 ± 30.6; 288 | 42.0 ± 18.6; 410 | 50.1 ± 23.0; 343 | 54.1 ± 20.4; 346 | 66.8 ± 23.3; 324 |
| Age 40–49 | 79.5 ± 27.3; 296 | 78.1 ± 25.4; 293 | 42.3 ± 19.7; 307 | 51.0 ± 19.5; 249 | 52.7 ± 20.9; 251 | 62.1 ± 23.1; 281 |
| Age 50–59 | 65.8 ± 26.4; 270 | 77.8 ± 27.2; 289 | 47.6 ± 20.0; 182 | 50.2 ± 20.7; 152 | 53.3 ± 20.6; 117 | 61.9 ± 22.0; 116 |
| Age 60–69 | 66.1 ± 23.1; 256 | 78.8 ± 26.2; 329 | 48.1 ± 20.5; 185 | 56.5 ± 23.4; 198 | 54.0 ± 23.4; 198 | 63.4 ± 24.6; 214 |
| Age 70–79 | 65.6 ± 25.1; 307 | 77.8 ± 25.0; 313 | 49.6 ± 22.5; 99 | 54.8 ± 21.0; 107 | 53.7 ± 21.8; 73 | 63.9 ± 24.4; 84 |
| Age 80+ | 58.7 ± 22.8; 213 | 70.8 ± 22.0; 268 | 44.2 ± 17.2; 40 | 52.1 ± 20.4; 30 | 50.9 ± 21.7; 20 | 63.3 ± 24.1; 33 |
| Percent individuals with 75+ nM 25(OH)D | ||||||
| Age 20–29 | 65.7% | 64.9% | 5.6% | 14.7% | 20.6% | 36.7% |
| Age 30–39 | 50.3% | 58.3% | 5.4% | 11.7% | 13.6% | 33.6% |
| Age 40–49 | 38.2% | 51.9% | 5.5% | 10.4% | 13.6% | 27.1% |
| Age 50–59 | 33.3% | 50.5% | 8.8% | 12.5% | 18.0% | 27.6% |
| Age 60–69 | 34.8% | 50.2% | 11.4% | 20.2% | 15.7% | 30.8% |
| Age 70–79 | 30.0% | 52.4% | 14.1% | 16.8% | 19.2% | 23.8% |
| Age 80+ | 20.7% | 40.7% | 2.5% | 16.7% | 20.0% | 24.2% |
| 25(OH)D concentrations by physical activity | ||||||
| Active | 74.3 ± 29.3; 1562 | 80.4 ± 26.7; 1781 | 45.2 ± 19.6; 1165 | 52.3 ± 21.2; 1214 | 55.4 ± 22.1; 886 | 66.3 ± 22.9; 1166 |
| Inactive | 59.0 ± 25.6; 395 | 71.7 ± 26.0; 241 | 42.4 ± 18.8; 452 | 49.6 ± 22.8; 219 | 53.3 ± 21.1; 498 | 63.7 ± 23.6; 382 |
| Percent inactive | 20% | 12% | 32% | 14% | 32% | 28% |
The percent of individuals with adequate 25(OH)D concentrations of at least 75 nM was highest among white individuals and lowest among blacks. Nonetheless, the adequate 25(OH)D concentration segment of the white population diminished with age, especially among white women. Inactive individuals of all ethnic groups had lower 25(OH)D concentrations.
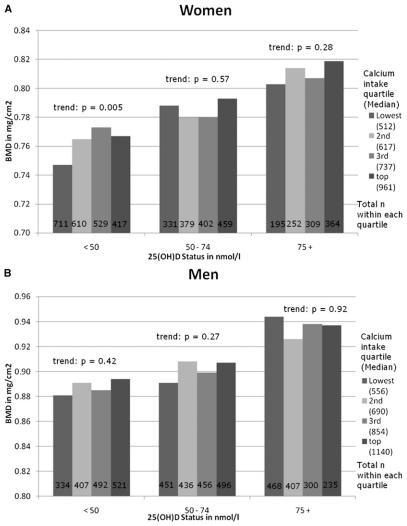
We found that the association between calcium intake and total hip BMD was significantly modified by sex and 25(OH)D concentration but not by age, physical activity, or race/ethnicity. Thus, results are presented for six subgroups determined by sex and three concentrations of 25(OH)D. These multivariate analyses are shown in Fig. 1A for women and Fig. 1B for men. The bars show standardized means of total hip BMD by quartiles of measurement error corrected calcium intake in three 25(OH)D categories (<50, 50–74, and 75+ nM). Among women, only at 25(OH)D concentrations <50 nM was a higher calcium intake significantly associated with higher BMD (p value for trend: p = 0.005). Among women with 25(OH)D concentrations >50 nM, a calcium intake above the lowest quartile (>566 mg/d) was not associated with higher BMD. Among men, there was no significant association between a higher calcium intake and BMD within any 25(OH)D category.
FIG. 1.
Total hip BMD by calcium intake (corrected for measurement error) in individuals with a 25(OH)D status <50, 50–74, or 75+ nM. (A) Values for the measurement error corrected calcium intake in quartiles among women: lowest ≤566 mg/d; second = 567–671 mg/d; third = 672–825 mg/d; top = 826–2143 mg/d. p value for trend across categories of 25(OH)D concentrations was <0.0001 while controlling for calcium intake, age (10-yr age categories), race/ethnicity (white, black, Mexican American), body mass index, height, total calorie intake, estrogen use among women, physical activity, smoking, and socio-economic status. (B) Values for the measurement error–corrected calcium intake quartiles among men: lowest ≤626 mg/d; second = 627–761 mg/d; third = 762–962 mg/d; top = 963–2152 mg/d. p value for trend across categories of 25(OH)D concentrations was 0.0001 while controlling for calcium intake, age (10-yr age categories), race/ethnicity (white, black, Mexican American), body mass index, height, total calorie intake, physical activity, smoking, and socioeconomic status.
To address the potential influence of menopausal status on calcium absorption among women, we performed subgroup analyses among women<50, 50–69, and ≥70 yr of age. Among women ≥50 yr of age, we excluded women who reported estrogen therapy (n = 178). In the younger women and women ≥70 yr of age, women in the lowest 25(OH)D category seemed to benefit from a higher calcium intake, whereas women with higher 25(OH)D levels did not. However, among women 50–69 yr of age, a higher calcium intake was not associated with BMD at any 25(OH)D category, whereas independent of calcium intake, BMD increased stepwise with each higher 25(OH)D status category, similar to our results for all men and women (data not shown).
Table 4 shows calcium intake as a continuous variable predicting BMD in each of the six subgroups. We first examined continuous calcium intake without measurement error correction and then the multivariate error correction including an inflation of the SE. The results from both continuous calcium intake analyses were consistent with the main analyses by quartiles of calcium intake (Figs. 1A and 1B).
DISCUSSION
Our study examined the relative importance of dietary calcium intake and 25(OH)D serum concentrations with respect to total hip BMD among a large sample of community- dwelling individuals ≥20 yr of age. Among women with low 25(OH)D concentrations, there was a statistically significant positive association between calcium intake and BMD, whereas among women with 25(OH)D concentrations >50 nM, a higher calcium intake was not appreciably associated with BMD. Among men, calcium intake was not associated with BMD in all three 25(OH)D categories.
Calcium is a structural component of bone, and calcium supplementation may improve BMD and reduce fractures by suppressing PTH secretion and thus reducing bone resorption.(22) In a 2004 meta-analysis of randomized controlled trials (RCTs), calcium supplementation of 500–2000 mg/d in postmenopausal women provided a modest benefit on BMD: 2.05% difference in total body BMD, 1.66% for lumbar spine BMD, and 1.64% for hip BMD.(23,24) However, the effects of calcium supplementation on BMD seem to represent a one-time increment that does not continue to accrue with time,(25,26) and the implications for fracture risk prevention of such small differences are unclear.
Observational studies of the association between dietary calcium intake and hip fracture risk were summarized in a recent meta-analysis.(27) For seven studies including 170,991 women with 2954 hip fractures, there was no association between total calcium intake and hip fracture risk (pooled RR per 300 mg of total calcium = 1.01; 95% CI: 0.97, 1.05). Similarly, in five studies with 68,606 men and 214 hip fractures, there was no significant benefit of a higher calcium intake (pooled RR per 300 mg of total calcium = 0.92; 95% CI: 0.82, 1.03).(26) For randomized trials of calcium supplementation alone, five studies (5666 primarily postmenopausal women plus 1074 men) with 814 nonvertebral fractures were pooled. Comparing calcium supplementation (800–1600 mg/d) with placebo, the pooled RR was 0.92 (95% CI 0.81, 1.05). Based on four studies with separate results for hip fracture (6504 individuals with 139 hip fractures), the pooled RR comparing calcium with placebo was 1.64 (95% CI: 1.02, 2.64).(28)
One potential explanation for the lack of an observed protective effect in the most recent meta-analysis is that participants in the trials already were consuming “enough” calcium, which would explain the neutral effect on nonvertebral fractures. Additionally, it is possible that 25(OH)D concentrations were high enough so that additional calcium supplementation had no effect on fracture reduction. In fact, baseline 25(OH)D concentrations were >50 nM in four of five trials, and mean baseline concentration across all five trials was 61.5 nM.(25,28–31)
Regarding the suggested adverse effect of calcium supplementation on hip fracture risk in the most recent metaanalysis on calcium alone,(28) it is possible that among the frail individuals at risk for hip fractures, other deficiencies need correction along with adequate calcium intake.(32) Calcium carbonate or citrate supplements, as used in the trials pooled for the meta-analysis, can reduce phosphate absorption,(33) which may be detrimental because a balanced calcium–phosphate ratio is needed for bone mineralization.(34) Each increase in calcium intake by 500 mg/d decreases phosphorus absorption by 166 mg,(33) so a calcium supplement of 1000 mg may shift an elderly person on a relatively low phosphorus intake caused by a low protein intake into phosphate deficiency.(33,35) This could augment bone resorption(33,36,37) and thus increase fracture risk.
Our analyses suggested that, for women, a calcium intake beyond 566 mg/d, the upper end of the lowest calcium intake quartile, may be sufficient if 25(OH)D concentrations are at least 50 nM. This may be explained by a calcium- sparing effect of higher 25(OH)D concentrations.(2) Thus, individuals with higher 25(OH)D concentrations may need less calcium to absorb the basic amount needed for bone mineralization. However, in this national survey, 45.7% of women had 25(OH)D concentrations <50 nM. In this group of women, a higher calcium intake beyond 566 mg/d may be warranted, or, these women could be supplemented with vitamin D to shift them to 25(OH)D concentration >50 nM or best >75 nM.
Mean dietary calcium intakes were significantly higher among men, which may explain why men at any 25(OH)D serum concentration had no additional benefit of a higher dietary calcium intake beyond 626 mg/d (upper end of the lowest intake quartile).
Calcium absorption efficacy declines with age and at menopause.(38,39) This may in part be explained by declining 25(OH)D levels with age.(2,40) Additionally, at menopause, loss of estrogen triggers bone loss that in turn results in a subtle increase in serum ionized calcium, which suppresses PTH, lowers 1,25-dihydroxyvitamin D production, and lowers calcium absorption.(41–43) Consistent with earlier findings by Dawson-Hughes et al.,(43) we found no correlation between higher calcium intake and BMD among women in the age range of early menopause (50– 69), even among women in the lowest 25(OH)D category.
Our findings of a primary role of 25(OH)D serum concentrations relative to calcium intake in all women independent of age and menopausal status are consistent with those from Khosla et al.,(44) where 25(OH)D serum concentration was the strongest inverse correlate of PTH among premenopausal and postmenopausal women with or without estrogen replacement therapy, whereas calcium intake was a weak inverse correlate of PTH in all subgroups.
Dose of vitamin D supplementation needed for serum 25(OH)D correction could be estimated based on a dose– response calculation proposed by Heaney,(45) where a 1.0 nM increase in 25(OH)D level can be expected with l μg vitamin D3/d (40 IU) at low starting levels and a 0.6 nM 25(OH)D increase can be expected per l μg vitamin D3/d at higher starting levels (i.e., >70 nM). A similar observation has been reported for vitamin D2 in one study,(46) whereas some studies suggested less efficacy in raising 25(OH)D levels with daily(47) or intermittent D2.(48) Studies in older persons showed that 25(OH)D concentrations could be increased by ~40–65 nM to mean concentrations of 75–100 nM with 800–1000 IU of vitamin D per day.(32,49–52) Higher intakes may, however, be needed among individuals with low starting concentrations of 25(OH)D(53) and those who are overweight or obese,(54) because effects of supplementation depend on starting concentrations and body size.
Consistent with the literature,(55,56) in this large sample of adult individuals, 25(OH)D concentrations decreased dramatically with age, darker skin tone, female sex, and inactivity. Among individuals 20–29 yr of age, 34% of white women, 35% of white men, 79% of Mexican-American women, 63% of Mexican-American men, 94% of black women, and 85% of black men had 25(OH)D concentrations <75 nM. Among individuals ≥80 yr of age, 79% of white women, 59% of white men, 80% of Mexican-American women, 76% of Mexican- American men, 94% of black women, and 85% of black men had 25(OH)D concentrations <75 nM.
The strengths of the study are its large sample size that provided enough power to perform stratification by 25(OH)D category and sex. One limitation is the cross-sectional design, which prevents one from assigning a causal relationship between vitamin D concentrations or calcium intake and BMD. However, a causal relationship between both calcium and vitamin D supplementation has been shown in several randomized controlled trials.(24,25,32,49) Furthermore, our results pertain to total hip BMD and may not reflect the relation of calcium intake or vitamin D status to the entire skeleton or other endpoints for which the optimal intake of calcium or vitamin D intake may well be substantially different.
An advantage of the cross-sectional design is that this is more likely to represent the long-term effects of calcium intake and 25(OH)D serum concentrations, as opposed to a short-term intervention. Furthermore, results were consistent, independent of how calcium intake was analyzed (with or without measurement error correction, in quartiles or as a continuous variable).
In conclusion, our study adds support to the concept that the correction of 25(OH)D status is more important than increasing dietary calcium intake beyond 566 mg/d among women and 626 mg/d among men for better hip BMD. A higher calcium intake beyond 566 mg/d may only be important among women with 25(OH)D concentrations <50 nM, whereas among women with higher 25(OH)D concentrations, hip BMD is not correlated with calcium intake. Among men, the need for a calcium intake beyond 625mg/d in respect to total hip BMD is not supported by our results, whereas higher 25(OH)D concentrations were associated with higher BMD among men and women. Within this sample of U.S. adults, a large fraction of younger and older adults was below the desirable 25(OH)D threshold of at least 75 nM, whereas calcium intakes seemed to be adequate in the majority of individuals with respect to hip BMD.
ACKNOWLEDGMENTS
This study was supported by grants from the International Foundation for the Promotion of Nutrition Research and Nutrition Education (ISFE); the Swiss Foundation for Nutrition Research (SFEFS); and the Swiss National Foundations (SNF Professorship grant).
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.Feskanich D, Willett WC, Stampfer MJ, Colditz GA. Milk, dietary calcium, and bone fractures in women: A 12-year prospective study. Am J Public Health. 1997;87:992–997. doi: 10.2105/ajph.87.6.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25- hydroxyvitamin D. J Am Coll Nutr. 2003;22:142–146. doi: 10.1080/07315724.2003.10719287. [DOI] [PubMed] [Google Scholar]
- 3.Heaney RP, Barger-Lux MJ, Dowell MS, Chen TC, Holick MF. Calcium absorptive effects of vitamin D and its major metabolites. J Clin Endocrinol Metab. 1997;82:4111–4116. doi: 10.1210/jcem.82.12.4412. [DOI] [PubMed] [Google Scholar]
- 4.Steingrimsdottir L, Gunnarsson O, Indridason OS, Franzson L, Sigurdsson G. Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA. 2005;294:2336–2341. doi: 10.1001/jama.294.18.2336. [DOI] [PubMed] [Google Scholar]
- 5.Loro ML, Sayre J, Roe TF, Goran MI, Kaufman FR, Gilsanz V. Early identification of children predisposed to low peak bone mass and osteoporosis later in life. J Clin Endocrinol Metab. 2000;85:3908–3918. doi: 10.1210/jcem.85.10.6887. [DOI] [PubMed] [Google Scholar]
- 6.Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, Ensrud KE, Cauley J, Black D, Vogt TM. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 7.National Center for Health Statistics. Plan and operation of the Third National Health and Nutrition Examination Survey 1988-1994. Vital Health Stat. 1996;1:94–1308. [PubMed] [Google Scholar]
- 8.Wahner HW, Looker A, Dunn WL, Walters LC, Hauser MF, Novak C. Quality control of bone densitometry in a national health survey (NHANES III) using three mobile examination centers. PG - 951-60. J Bone Miner Res. 1994;9:951–960. doi: 10.1002/jbmr.5650090621. [DOI] [PubMed] [Google Scholar]
- 9.Looker AC, Johnston CC, Jr, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Lindsay RL. Prevalence of low femoral bone density in older U.S. women from NHANES III. J Bone Miner Res. 1995;10:796–802. doi: 10.1002/jbmr.5650100517. [DOI] [PubMed] [Google Scholar]
- 10.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 11.Willett CW. Nutritional Epidemiology. 2nd ed. Oxford University Press; New York NY, USA: 1998. [Google Scholar]
- 12.Gunter EW, Lewis BL, Konkikowski SM. Laboratory Methods Used for the Third National Health and Nutrition Examination Survey (NHANES II), 1988-1994 (CD-ROM) Centers for Disease Control and Prevention; Hyattsville, MD, USA: 1996. [Google Scholar]
- 13.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 14.Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: A meta-analysis of randomized controlled trials. JAMA. 2005;293:2257–2264. doi: 10.1001/jama.293.18.2257. [DOI] [PubMed] [Google Scholar]
- 15.Krall EA, Dawson-Hughes B. Smoking increases bone loss and decreases intestinal calcium absorption. J Bone Miner Res. 1999;14:215–220. doi: 10.1359/jbmr.1999.14.2.215. [DOI] [PubMed] [Google Scholar]
- 16.Barger-Lux MJ, Heaney RP. Calcium absorptive efficiency is positively related to body size. J Clin Endocrinol Metab. 2005;90:5118–5120. doi: 10.1210/jc.2005-0636. [DOI] [PubMed] [Google Scholar]
- 17.Krall EA, Dawson-Hughes B. Oestrogen effects on calcitriol levels in post-menopausal women: A comparison of oral versus transdermal administration. Clin Endocrinol (Oxf) 1995;43:219–224. doi: 10.1111/j.1365-2265.1995.tb01918.x. [DOI] [PubMed] [Google Scholar]
- 18.Nelson KM, Reiber G, Boyko EJ. Diet and exercise among adults with type 2 diabetes: Findings from the third national health and nutrition examination survey (NHANES III) Diabetes Care. 2002;25:1722–1728. doi: 10.2337/diacare.25.10.1722. [DOI] [PubMed] [Google Scholar]
- 19.Rosner B. Measurement Error Methods Fundamentals of Biostatistics. 5th ed. PWS-Kent; Boston, MA, USA: 2000. [Google Scholar]
- 20.Spiegelman D, McDermott A, Rosner B. Regression calibration method for correcting measurement-error bias in nutritional epidemiology. Am J Clin Nutr. 1997;65(Suppl):1179S–1186S. doi: 10.1093/ajcn/65.4.1179S. [DOI] [PubMed] [Google Scholar]
- 21.Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30:771–777. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 22.Blumsohn A, Herrington K, Hannon RA, Shao P, Eyre DR, Eastell R. The effect of calcium supplementation on the circadian rhythm of bone resorption. J Clin Endocrinol Metab. 1994;79:730–735. doi: 10.1210/jcem.79.3.8077353. [DOI] [PubMed] [Google Scholar]
- 23.Shea B, Wells G, Cranney A, Zytaruk N, Robinson V, Griffith L, Ortiz Z, Peterson J, Adachi J, Tugwell P, Guyatt G. Meta-analyses of therapies for postmenopausal osteoporosis. VII. Meta-analysis of calcium supplementation for the prevention of postmenopausal osteoporosis. Endocr Rev. 2002;23:552–559. doi: 10.1210/er.2001-7002. [DOI] [PubMed] [Google Scholar]
- 24.Shea B, Wells G, Cranney A, Zytaruk N, Robinson V, Griffith L, Hamel C, Ortiz Z, Peterson J, Adachi J, Tugwell P, Guyatt G. Calcium supplementation on bone loss in postmenopausal women. Cochrane Database Syst Rev. 2004;1:CD004526. doi: 10.1002/14651858.CD004526.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Reid IR, Mason B, Horne A, Ames R, Reid HE, Bava U, Bolland MJ, Gamble GD. Randomized controlled trial of calcium in healthy older women. Am J Med. 2006;119:777–785. doi: 10.1016/j.amjmed.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 26.Bischoff-Ferrari HA, Rees JR, Grau MV, Barry E, Gui J, Baron JA. Effect of calcium supplementation on fracture risk: A double-blind randomized controlled trial. Am J Clin Nutr. 2008;87:1945–1951. doi: 10.1093/ajcn/87.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bischoff-Ferrari HA, Dawson-Hughes B, Baron JA, Burckhardt P, Li R, Spiegelman D, Specker B, Orav JE, Wong JB, Staehelin HB, O’Reilly E, Kiel DP, Willett WC. Calcium intake and hip fracture risk in men and women: A meta-analysis of prospective cohort studies and randomized controlled trials. Am J Clin Nutr. 2007;86:1780–1790. doi: 10.1093/ajcn/86.5.1780. [DOI] [PubMed] [Google Scholar]
- 28.Bischoff-Ferrari HA, Dawson-Hughes B, Baron JA, Staehelin HB, Willett CW. Calcium intake and risk of hip fracture in men and women: A meta-analysis of prospective cohort studies and randomized controlled trials. Am J Clin Nutr. 2007;86:1780–1790. doi: 10.1093/ajcn/86.5.1780. [DOI] [PubMed] [Google Scholar]
- 29.Riggs BL, O’Fallon WM, Muhs J, O’Connor MK, Kumar R, Melton LJ., 3rd Long-term effects of calcium supplementation on serum parathyroid hormone level, bone turnover, and bone loss in elderly women. J Bone Miner Res. 1998;13:168–174. doi: 10.1359/jbmr.1998.13.2.168. [DOI] [PubMed] [Google Scholar]
- 30.Prince RL, Devine A, Dhaliwal SS, Dick IM. Effects of calcium supplementation on clinical fracture and bone structure: Results of a 5-year, double-blind, placebo-controlled trial in elderly women. Arch Intern Med. 2006;166:869–875. doi: 10.1001/archinte.166.8.869. [DOI] [PubMed] [Google Scholar]
- 31.Grant AM, Avenell A, Campbell MK, McDonald AM, MacLennan GS, McPherson GC, Anderson FH, Cooper C, Francis RM, Donaldson C, Gillespie WJ, Robinson CM, Torgerson DJ, Wallace WA. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): A randomised placebo-controlled trial. Lancet. 2005;365:1621–1628. doi: 10.1016/S0140-6736(05)63013-9. [DOI] [PubMed] [Google Scholar]
- 32.Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, Delmas PD, Meunier PJ. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327:1637–1642. doi: 10.1056/NEJM199212033272305. [DOI] [PubMed] [Google Scholar]
- 33.Heaney RP, Nordin BE. Calcium effects on phosphorus absorption: Implications for the prevention and co-therapy of osteoporosis. J Am Coll Nutr. 2002;21:239–244. doi: 10.1080/07315724.2002.10719216. [DOI] [PubMed] [Google Scholar]
- 34.Chen TC, Persons K, Liu WW, Chen ML, Holick MF. The antiproliferative and differentiative activities of 1,25-dihydroxyvitamin D3 are potentiated by epidermal growth factor and attenuated by insulin in cultured human keratinocytes. J Invest Dermatol. 1995;104:113–117. doi: 10.1111/1523-1747.ep12613601. [DOI] [PubMed] [Google Scholar]
- 35.Heaney RP. Phosphorus nutrition and the treatment of osteoporosis. Mayo Clin Proc. 2004;79:91–97. doi: 10.4065/79.1.91. [DOI] [PubMed] [Google Scholar]
- 36.Raisz LG, Niemann I. Effect of phosphate, calcium and magnesium on bone resorption and hormonal responses in tissue culture. Endocrinology. 1969;85:446–452. doi: 10.1210/endo-85-3-446. [DOI] [PubMed] [Google Scholar]
- 37.Lotz M. The diagnostic importance of hypophosphatemia. Med Times. 1968;96:1166–1168. [PubMed] [Google Scholar]
- 38.Nordin BE, Need AG, Morris HA, O’Loughlin PD, Horowitz M. Effect of age on calcium absorption in postmenopausal women. Am J Clin Nutr. 2004;80:998–1002. doi: 10.1093/ajcn/80.4.998. [DOI] [PubMed] [Google Scholar]
- 39.Heaney RP, Recker RR, Stegman MR, Moy AJ. Calcium absorption in women: Relationships to calcium intake, estrogen status, and age. J Bone Miner Res. 1989;4:469–475. doi: 10.1002/jbmr.5650040404. [DOI] [PubMed] [Google Scholar]
- 40.Barger-Lux MJ, Heaney RP, Lanspa SJ, Healy JC, DeLuca HF. An investigation of sources of variation in calcium absorption efficiency. J Clin Endocrinol Metab. 1995;80:406–411. doi: 10.1210/jcem.80.2.7852497. [DOI] [PubMed] [Google Scholar]
- 41.Dick IM, Devine A, Beilby J, Prince RL. Effects of endogenous estrogen on renal calcium and phosphate handling in elderly women. Am J Physiol Endocrinol Metab. 2005;288:E430–435. doi: 10.1152/ajpendo.00140.2004. [DOI] [PubMed] [Google Scholar]
- 42.Van Cromphaut SJ, Rummens K, Stockmans I, Van Herck E, Dijcks FA, Ederveen AG, Carmeliet P, Verhaeghe J, Bouillon R, Carmeliet G. Intestinal calcium transporter genes are upregulated by estrogens and the reproductive cycle through vitamin D receptor-independent mechanisms. J Bone Miner Res. 2003;18:1725–1736. doi: 10.1359/jbmr.2003.18.10.1725. [DOI] [PubMed] [Google Scholar]
- 43.Dawson-Hughes B, Dallal GE, Krall EA, Sadowski L, Sahyoun N, Tannenbaum S. A controlled trial of the effect of calcium supplementation on bone density in postmenopausal women. N Engl J Med. 1990;323:878–883. doi: 10.1056/NEJM199009273231305. [DOI] [PubMed] [Google Scholar]
- 44.Khosla S, Atkinson EJ, Melton LJ, III, Riggs BL. Effects of age and estrogen status on serum parathyroid hormonolevels and biochemical markers of bone turnover in women: A population-based study. J Clin Endocrinol Metab. 1997;82:1522–1527. doi: 10.1210/jcem.82.5.3946. [DOI] [PubMed] [Google Scholar]
- 45.Heaney RP. The vitamin D requirement in health and disease. J Steroid Biochem Mol Biol. 2005;15:13–19. doi: 10.1016/j.jsbmb.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 46.Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, Reitz R, Salameh W, Ameri A, Tannenbaum AD. Vitamin D2 Is as Effective as Vitamin D3 in Maintaining Circulating Concentrations of 25-Hydroxyvitamin D. J Clin Endocrinol Metab. 2008;93:677–681. doi: 10.1210/jc.2007-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trang HM, Cole DE, Rubin LA, Pierratos A, Siu S, Vieth R. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr. 1998;68:854–858. doi: 10.1093/ajcn/68.4.854. [DOI] [PubMed] [Google Scholar]
- 48.Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89:5387–5391. doi: 10.1210/jc.2004-0360. [DOI] [PubMed] [Google Scholar]
- 49.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337:670–676. doi: 10.1056/NEJM199709043371003. [DOI] [PubMed] [Google Scholar]
- 50.Tangpricha V, Pearce EN, Chen TC, Holick MF. Vitamin D insufficiency among free-living healthy young adults. Am J Med. 2002;112:659–662. doi: 10.1016/s0002-9343(02)01091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barger-Lux MJ, Heaney RP, Dowell S, Chen TC, Holick MF. Vitamin D and its major metabolites: Serum levels after graded oral dosing in healthy men. Osteoporos Int. 1998;8:222–230. doi: 10.1007/s001980050058. [DOI] [PubMed] [Google Scholar]
- 52.Dawson-Hughes B. Impact of vitamin D and calcium on bone and mineral metabolism in older adults. In: Holick MF, editor. Biologic Effects of Light 2001. Kluwer Academic Publishers; Boston, MA, USA: 2002. pp. 175–183. [Google Scholar]
- 53.Heaney RP. Barriers to optimizing vitamin D3 intake for the elderly. J Nutr. 2006;136:1123–1125. doi: 10.1093/jn/136.4.1123. [DOI] [PubMed] [Google Scholar]
- 54.Parikh SJ, Edelman M, Uwaifo GI, Freedman RJ, Semega-Janneh M, Reynolds J, Yanovski JA. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab. 2004;89:1196–1199. doi: 10.1210/jc.2003-031398. [DOI] [PubMed] [Google Scholar]
- 55.Holick MF. Environmental factors that influence the cutaneous production of vitamin D. Am J Clin Nutr. 1995;61(suppl):638S–645S. doi: 10.1093/ajcn/61.3.638S. [DOI] [PubMed] [Google Scholar]
- 56.Chen TC, Chimeh F, Lu Z, Mathieu J, Person KS, Zhang A, Kohn N, Martinello S, Berkowitz R, Holick MF. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys. 2007;460:213–217. doi: 10.1016/j.abb.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]