Abstract
The SPARC family of proteins represents a diverse group of proteins that modulate cell interaction with the extracellular milieu. The eight members of the SPARC protein family are modular in nature. Each shares a follistatin-like domain and an extracellular calcium binding E–F hand motif. In addition, each family member is secreted into the extracellular space. Some of the shared activities of this family include, regulation of extracellular matrix assembly and deposition, counter-adhesion, effects on extracellular protease activity, and modulation of growth factor/cytokine signaling pathways. Recently, several SPARC family members have been implicated in human disease pathogenesis. This review discusses recent advances in the understanding of the functional roles of the SPARC family of proteins in development and disease.
Keywords: SPL-1, SMOC, testican, fstl-1, Hevin, SPOCK, review
Introduction
Matricellular proteins are defined as extracellular matrix (ECM) associated proteins that do not serve structural roles in the ECM in contrast to the classical ECM proteins, collagens and laminins (Bornstein, 2000). There is a growing appreciation of the importance of matricellular proteins in processes governing both tissue development and pathogenesis of disease. SPARC (secreted protein acidic and rich in cysteine) is a prototypic collagen-binding matricellular protein that has been shown to influence a diverse array of biological functions and has been the topic of a number of recent reviews (Bradshaw, 2009, Delany and Hankenson, 2009, Rivera et al., 2011, Arnold and Brekken, 2009). SPARC is a member of a larger family of SPARC related proteins, several of which have been recently recognized for novel functional activities including some that are similar to and some that are distinct from that of SPARC. The purpose of this review is to highlight some of the topical advances in the functional characterization of the SPARC family members and to explore common and distinct functions of these family members.
The SPARC family of proteins consists of SPARC (osteonectin, BM-40) (Brekken and Sage, 2001), Hevin (SPARC-like (SPL) 1, SC1, MAST 9, RAGS-1, QR1, ECM 2) (Hambrock et al., 2003), secreted modular calcium binding protein (SMOC) 1 and 2 (SRG) (Vannahme et al., 2003, Vannahme et al., 2002), testican 1, 2, and 3 (SPARC/osteonectin, CWCV, and Kazal-like domains proteoglycans, SPOCK) (Schnepp et al., 2005, Vannahme et al., 1999, Alliel et al., 1993, Charbonnier et al., 1998), and follistatin like protein 1 (fstl-1, TSC- 36/Flik, follistatin related protein (FRP), TGF-β inducible protein) (Hambrock et al., 2004). Each SPARC family member possesses a characteristic conserved EC (E–F hand calcium binding) domain with an E–F hand motif (Figure 1). Based on sequence homologies of the EC domains, the family members can be separated into four distinct phylogenetic groups: 1) SPARC and Hevin; 2) SMOC 1 and 2; 3) testican 1, 2 and 3; and 4) Follistatin-like protein (Fstl)-1 (TSC-36/Flik) (Vannahme et al., 2003).
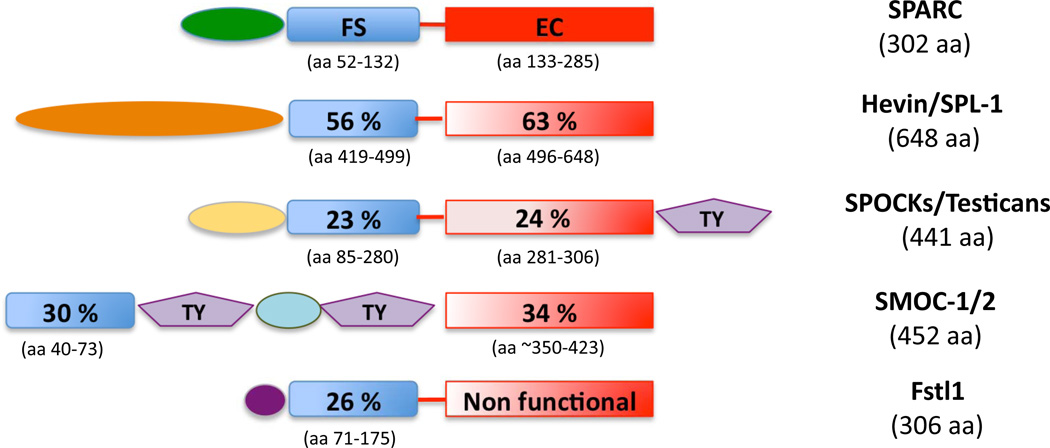
Figure 1. Schematic representation of the modular domain structure of the SPARC family of proteins.
The total amino acid number for each protein or group of proteins in shown under each protein name. The designated amino acids used to generate the percent identities (shown super-imposed on the given representative domain) for each follistatin and each EC homologous domain are shown beneath each domain. Testican 1 and SMOC-1 were used as representative for each of these sub-groups, the percent identity and amino acid numbers were found to be similar for testican 2 and 3 and SMOC-2, respectively. The EC domain of Fstl1 was shown to be non-functional and is thus labeled as such. Domains unique to given family members or sub-families are shown in green, orange, yellow, light blue, and dark purple. TY: thyroglobulin domain (purple) FS: follistatin domain (blue), EC: extracellular domain (red).
SPARC/Osteonectin/BM-40
Extracellular Matrix
The number of published reports characterizing expression and function of SPARC far exceeds those characterizing other SPARC family members. SPARC binds both fibrillar collagen and basal lamina collagen IV (Mayer et al., 1991). Phenotypic characterization of SPARC-null mice has revealed a number of aberrant pathologies associated with deficiencies in extracellular matrix (ECM) assembly and composition. For example, several connective tissues in SPARC-null mice including, dermis, heart, adipose, and periodontal ligament, were shown to contain less fibrillar collagen (Bradshaw et al., 2003b, Bradshaw et al., 2009, Bradshaw et al., 2003a, Trombetta and Bradshaw, 2010). Collagen fibril morphology in SPARC-null mice was also found to be altered and was characterized by smaller fibrils with a more uniform diameter than WT fibrils (Bradshaw et al., 2003b). In addition, the basement membrane of SPARC-null lens capsule demonstrated aberrant collagen IV staining in comparison to that of WT (Yan et al., 2002). Defects in the lens capsule resulted in early onset cataractogenesis in SPARC-null mice (Gilmour et al., 1998). Two reviews summarizing current studies on the function of SPARC in ECM assembly and fibrosis were recently published (Bradshaw, 2009).
SPARC has also been shown to regulate the activity of matrix metalloproteinases – a family of enzymes considered to be the primary mediators of ECM proteolysis and turnover. SPARC was first reported to influence MMP activity in synovial fibroblasts where exogenous SPARC induced MMP-1, MMP-3, and MMP-9 activity (Tremble et al., 1993). Regions of SPARC that induced MMP activity were mapped by peptide analysis. Interestingly, peptides representing the alpha helices of the E–F hand region were implicated in this activity (Tremble et al., 1993). The majority of subsequent studies examining effects of SPARC on MMP activity have been carried out in transformed cells and in tumor studies. For example, SPARC was found to increase activity of MT1-MMP and MMP-2 in glioma cells and increased MMP-2 activity in breast cancer cells but SPARC had no effect on MMP-2 activation in melanoma cells (McClung et al., 2007, Nischt et al., 2001, Gilles et al., 1998). Similar to effects on MMP activity, the functional role of SPARC in cancer is dependent upon tumor type and tissue environment as SPARC has been shown to both promote and inhibit different types of tumor cell activities (reviewed in (Framson and Sage, 2004)).
Growth Factor/Collagen Receptor Activity
SPARC has also been demonstrated to modulate growth factor signaling mediated by cell surface receptors including vascular endothelial growth factor (VEGF) receptor, basic fibroblast growth factor (bFGF), and transforming growth factor (TGF) β1. The influence of SPARC in the regulation of growth factor receptor activity was also recently reviewed (Rivera et al., 2011). In some cases, SPARC was found to bind directly to growth factors, such as in the case of VEGF, to modulate activity whereas in other cases, the mechanism by which SPARC influenced receptor activation was not through a direct growth factor binding mechanism (e.g. bFGF). Rivera and Brekken reported that modulation of TGF-β activity in pericytes was dependent upon SPARC binding to the TGF-β receptor endoglin that affected αv integrin activity (Rivera and Brekken, 2011). Hence, a variety of cell-dependent mechanisms of SPARC activity in the regulation of growth factor signaling have been proposed. For the most part, these activities have been shown to occur in the extracellular space and involve modulation of growth factor receptor activity either through interaction with ligand or with cell-surface receptors.
Interestingly, the collagen-binding pocket of SPARC was shown to share a similar structure to that of the collagen receptor discoidin domain receptor (DDR) 2, despite differences in primary amino acid sequence between these two collagenbinding proteins (Hohenester et al., 2008). Given the collagen binding site homology, it follows that SPARC and DDR2 would bind fibrillar collagens at the same site on the collagen molecule and this was found in fact to be the case (Giudici et al., 2008). Thus collagen bound by SPARC is predicted to limit collagen binding to DDR2 and diminish down–stream pathways activated by the tyrosine kinase activity of DDR2. If SPARC competes with DDR2 for collagen binding, then expression of SPARC might regulate DDR2 activity and subsequent signal transduction events. As SPARC and hevin/SPL1 share the same collagen-binding domain, collagen bound by hevin/SPL1 would also be expected to prevent DDR2 activation by collagen.
A mechanism by which SPARC might influence a number of different cell functions is through modulation of cell-surface receptor:ligand engagement – a mechanism that perhaps is shared by other SPARC family members such as the SMOC proteins (Figure 2).
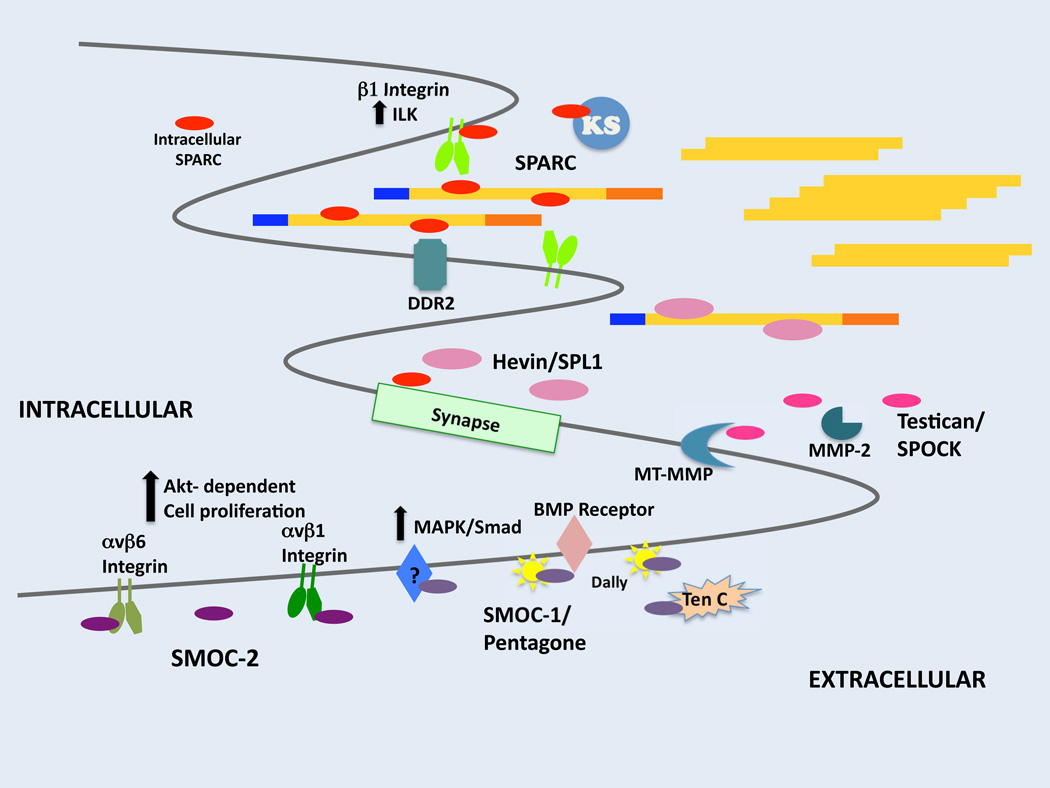
Figure 2. Diverse Functions of the SPARC Family of Proteins.
Schematic representation that highlights some of the activities attributed to each class of SPARC family members with a functional EC domain. SPARC family members are represented with ovals; red: SPARC, pink: Hevin/SPL1, magenta: Testican/SPOCK, purple: SMOC-1 and -2. Each family member is secreted and has been shown to affect some aspect of extracellular activity either through regulation of ECM assembly/degradation or through regulation of cell surface receptor/ligand interaction. In the case of Hevin/SPL-1 and SPARC this includes regulation of synapse formation via as yet unidentified factors either in the ECM and/or on cell surfaces. Collagen fibrils are shown in yellow with blue and orange rectangles representing the N and C-propeptides, respectively. KS: Killer signal (secreted by “winning” cells in Drosophila), DDR2: discoidin domain receptor 2, MT-MMP: membrane-type metalloproteinase, BMP: bone morphogenic protein, Ten C: tenascin C, MAPK: mitogen-associated protein kinase.
Survival Activity
In Drosophila melanogaster, SPARC was shown to be expressed by “loser” cells – cells proximal to “killer” or “winner” cells (Portela et al., 2010). The loser cells were induced to undergo apoptosis by a secreted factor from winner cells. SPARC expression by loser cells prolonged the life of these cells by neutralizing an unidentified secreted factor (KS, Figure 1) and preventing, at least temporarily, apoptosis of the loser cell. In mammals, lens epithelial cell survival was also promoted by SPARC (Weaver et al., 2008). Here, binding of SPARC to β1 integrin resulted in increased integrin-linked kinase (ILK) activity. Similarly, a survival activity of SPARC was observed in melanoma, where decreased expression of SPARC led to apoptosis (Fenouille et al., 2011). Whereas activation of caspase 3 was observed in SPARC depleted cells, increased expression of SPARC was associated with greater activation of Akt. Also, in leukemia cells, increased levels of intracellular SPARC were associated with survival and increased resistance to the chemotherapy agent imatinib (Fenouille et al., 2010).
However, in other cases, SPARC appeared to enhance apoptosis and has therefore been referred to as a chemo-sensitizing agent. In human colon cancer, breast and pancreatic cancer – SPARC was shown to increase apoptosis by favoring activation of caspase 8 (Rahman et al., 2011, Tang and Tai, 2007). Similar to SPARC activity in tumor progression, survival activity mediated by SPARC expression appears to be somewhat cell-type dependent and is likely influenced by other factors in the extracellular milieu and/or profiles of cell-surface receptors.
Hevin / SPARC-like protein (SLP) 1
Hevin or SPARC-like protein 1 (SLP1) is the closest family member to SPARC (Sullivan and Sage, 2004). Vertebrate hevin has ~650 amino acids encoding a protein of 71 kDa, larger than the molecular weight of SPARC (~43 kDa). Hevin shares the three primary domains contained within SPARC with an expanded N-terminal domain (Figure 1) (Sullivan and Sage, 2004). In fact, cleavage of hevin by certain proteases removes the extended N-terminal domain of hevin and generates a SPARC-like fragment (SLF). Digestion of hevin by MMP-3 results in a SLF that was shown to localize to the neovasculature of invasive glioma cells (Weaver et al., 2011). The expression of hevin is more restricted than that of SPARC with the highest levels of expression found in the nervous system. However, the expression of hevin is also found in other tissues and has been proposed to possibly function as both a tumor suppressor and as a regulator of angiogenesis (reviewed in (Sullivan and Sage, 2004)).
Extracellular Matrix
Hevin is the only other SPARC family member, in addition to SPARC, that has been shown to bind to collagens (Hambrock et al., 2003). In fact, sequence analysis demonstrates that hevin is the only other family member with the conserved collagen-binding motif defined in SPARC that supports binding to fibrillar and network collagens (Figure 2). Analysis of hevin-null mice revealed that hevin, like SPARC, influences collagen fibril architecture (Sullivan et al., 2006). The average collagen fibril diameter was decreased in hevin-null dermis, a phenotype reminiscent of collagen fibrils in SPARC-null dermis. However, whereas SPARC-null mice had significantly decreased levels of fibrillar collagen in the dermis as well as other connective tissues, overall levels of collagen were not decreased in the dermis of hevin-null mice.
Assays of collagen fibrillogenesis performed in vitro showed an enhancement of collagen fibril formation by hevin, in contrast to the activity of SPARC which acted to delay collagen fibrillogenesis in vitro (Giudici et al., 2008). In addition, decreases in amounts of decorin were found in the absence of hevin by immunofluorescence. Media conditioned by hevin-null dermal fibroblasts contained less decorin than that of wild-type cells and levels were restored by the addition of recombinant hevin. However, expression of mRNA encoding decorin was unaffected by hevin. Perhaps hevin acts to stabilize decorin in the extracellular space, although a direct binding of hevin to decorin was not detected in vitro. Nonetheless, the authors of this study concluded that changes in collagenous ECM in hevin-null skin likely arose from decorin-independent effects on collagen fibrillogenesis coupled with decorin-dependent regulation of collagen fibril assembly (Sullivan et al., 2006). These changes in dermal ECM in hevin-null mice resulted in measurable increases in the elastic modulus of the skin but no detectable differences in tensile strength in comparison to the skin of wild-type mice.
Counter-Adhesion
Hevin–null animals also demonstrated increased fibrovascular invasion into an implanted polyvinyl alcohol sponge and accelerated excisional wound closure at 4 days after wounding versus wild-type mice (Sullivan et al., 2008). Likely contributing to increased invasion and wound closure was the increase in Rac-1-dependent migration detected in hevin-null cells, an activity diminished by the addition of recombinant hevin. Similar to SPARC activity, hevin was also shown in these studies to be counter-adhesive to wild-type dermal fibroblasts (Sullivan et al., 2008). However, in contrast to SPARC, hevin did not influence rates of cell proliferation.
Hevin in the Nervous System
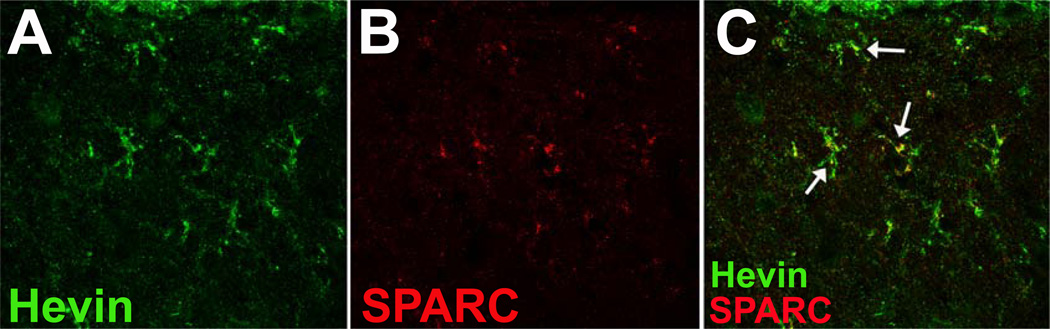
As mentioned above, high levels of hevin are expressed in the nervous system. An appreciation of the importance of astrocyte expression of matricellular proteins in the regulation of synapse formation and turnover is emerging (recently reviewed by (Eroglu, 2009)). Astrocytes in the CNS express both hevin and SPARC, although hevin is more robustly produced in comparison with SPARC (Kucukdereli et al., 2011). Whereas hevin was found to have synaptogenic activity in vitro, SPARC was found to inhibit hevin-dependent synapse formation in retinal ganglion cell culture. In vivo, retinal ganglion cells synapse at the superior colliculus during development. Hevin-null mice were found to have reduced numbers of synapses at this site in the brain whereas SPARC-null mice had greater numbers of synapses (Kucukdereli et al., 2011). These data are consistent with a mechanism by which astrocyte expression of hevin facilitates synapse formation while expression of SPARC serves to diminish the activity of hevin-dependent synaptogenesis. Perhaps, hevin binds to extracellular of cell surface factors via its SLF domain and is also able to bind a second factor, via the unique N-terminal domain, that strengthens synapse formation. SPARC would then compete with hevin for binding to the first ligand but would not engage the second ligand that interacts with the unique hevin N-terminal domain. Hence, SPARC acts to compete with hevin for the initial interaction but does not strengthen synapse formation due to the lack of the hevin N-terminal domain. In fact, co-localization of SPARC and hevin was demonstrated in the developing superior colliculus (Figure 3), supporting the idea that these matricellular proteins function together to regulate synapse formation in the developing CNS.
Figure 3. SPARC and Hevin Co-Localization in the Developing Central Nervous System.
At post-natal day 15, Hevin (panel A, Green) and SPARC (panel B, Red) demonstrated co-localization (Panel C, merged A and B, arrows) in sagittal sections from mouse superior colliculus. Reproduced from (Kucukdereli et al., 2011).
Furthermore, Weaver et al. showed that hevin is cleaved both in vitro and in vivo, specifically in brain tissue, by the protease ADAMTS4 to generate a SLF (Weaver et al., 2010). Brain tissue from ADAMTS4 null mice displayed similar phenotypic differences in Purkinje cell phenotype to those of hevin-null cerebellum. These results implied that cleavage of hevin by ADAMTS4 was critical for normal brain development (Weaver et al., 2010). The SLF generated by ADAMTS4 from native hevin is predicted to compete with hevin in a similar way to that of SPARC. Hence, the SLF generated by protease digestion might also compete with hevin to regulate synaptogenic activity. Perhaps modulation of hevin activity by protease digestion, in combination with expression of SPARC, is a mechanism by which hevin can influence CNS development and synaptic rearrangement.
Secreted Modular Calcium Binding-1 (SMOC-1)
Extracellular Matrix
SMOC-1 contains an EC domain common to the SPARC family members with an additional follistatin-like domain, two thyroglobulin-like domains, and a novel domain (Figure 1) (Vannahme et al., 2002). SMOC-1 was originally found to localize preferentially with basement membranes but has also been found in association with other types of ECMs as well (Gersdorff et al., 2006, Vannahme et al., 2002). Neither SMOC-1 nor 2 are predicted to bind collagen (Vannahme et al., 2002). Recently SMOC-1 was shown to bind another matricellular protein, tenascin C in addition to the serum proteins, fibulin-1 and C-reactive protein (Novinec et al., 2008, Brellier et al., 2011). Elevated expression of SMOC-1 was found in brain tumors and, in glioma cells, SMOC-1 was found to counteract the chemotactic activity of tenascin C (Brellier et al., 2011).
SMOC as a Regulator of BMP Signaling
Drosophila melanogaster
A SMOC homologue expressed in Drosophila, pentagone/magu, was recently identified and characterized. Pentagone/magu was shown to function as an inhibitor of decapentaplegic (the Drosophila homologue of BMP) in flies (Vuilleumier et al., 2010). In this system, pentagone affected the extracellular gradient of decapentaplegic (Dpp) required for wing development. Pentagone/magu helped to establish the Dpp (BMP) gradient in drosophila, an activity dependent upon the glypican, Dally. Dally was pulled down bound to pentagone but pentagone did not bind directly to Dpp suggesting that pentagone worked in association with Dally to establish the Dpp gradient (Vuilleumier et al., 2010). In the absence of pentagone, severe contraction of the BMP activity gradient resulted in patterning and growth defects. Pentagone was therefore required to mediate the long-range distribution of Dpp. The authors of this study proposed that the Drosophila homologue of SMOC might function to control extracellular levels of decapentaplegic by regulating cellular uptake of Dally/Dpp (Vuilleumier et al., 2010).
Pentagone/magu was also identified by another group as a Drosophila gene that, when moderately over-expressed, increased longevity and fecundity, but was maternal-effect lethal when robustly over-expressed (Zheng et al., 2011). While BMP signaling is also important in germ line stem cells, here a gradient of BMP is not required, as signaling occurs between neighboring cells. These results suggest perhaps multiple tissue-dependent roles of Pentagone/magu in BMP signaling in Drosophila.
Xenopus laevis
The homologue of SMOC-1 expressed in Xenopus laevis, XSMOC-1, was also recently shown to inhibit BMP signaling. XSMOC-1 inhibition of BMP occurred in the presence of constitutively active BMP receptor and was thus predicted to function downstream of BMP receptor engagement (Thomas et al., 2009). Therefore, XSMOC-1 did not appear to function to block BMP from binding its receptor, a mechanism used by a number of other BMP antagonists. In fact, an abrupt arrest in Xenopus development at the onset of neurulation was observed with XSMOC-1 depletion – this loss of function phenotype abrogated postgastrulation development – a phenotype similar to that of simultaneous loss of function of three ligand-binding BMP antagonists. Gain of function studies with XSMOC-1 produced similar outcomes to over-expression of established BMP antagonists. Thomas et al. (2009) also showed that XSMOC-1 induced MAP kinase phosphorylation of the Smad linker region, a mechanism previously shown to inhibit BMP-mediated Smad activation. Thus, in Xenopus, SMOC-1 is predicted to facilitate activation of MAP kinase through an, as yet, undefined mechanism thereby reducing BMP signaling during development.
Human Disease
Three separate groups have recently identified SMOC-1 mutations associated with individuals with Warrdenburg Anopthalmia Syndrome (Okada et al., 2011, Rainger et al., 2011, Abouzeid et al., 2011). Eye malformations, most often bilateral anophthalmia and post-axial oligosyndactyly, are characteristic manifestations of this rare syndrome. Two independent transgenic mice targeting SMOC-1 have also been generated (Okada et al., 2011, Rainger et al., 2011). One line with complete abrogation of expression did not survive past 3 weeks of age (Okada et al., 2011, Vannahme et al., 2003). Another line with 10% expression of WT SMOC-1 levels died at birth (Rainger et al., 2011). Both mice had significant eye and limb abnormalities, including syndactyly and hypoplasia of optic nerves. Similarly, knockdown of SMOC-1 by morpholino targeting in zebrafish resulted in micropthalmia (Abouzeid et al., 2011). As BMP signaling pathways are essential for eye and limb development, the mechanism underlying this syndrome likely involves SMOC-1 regulation of BMP activity, however further characterization will shed more light on these interactions in mice and in human disease.
SMOC-2
Like SMOC-1, the domain structure of SMOC-2 consists of two thyroglobulin-like domains, a follistatin-like domain, a novel domain and the E–F hand calcium-binding domain common to the SPARC family (Vannahme et al., 2003). SMOC-2 is widely expressed with the highest levels of SMOC-2 mRNA detected in heart, muscle, spleen, and ovary – a pattern that shares some similarities and some differences with that of SMOC-1 (Vannahme et al., 2003). Expression of both SMOC-1 and SMOC-2 were found during development of the fetal gonad and reproductive tract suggesting that these proteins might function to regulate differentiation of these tissues (Pazin and Albrecht, 2009). Maier et al. (2008) also showed that SMOC-2 expression was more frequently found at non-basement membrane sites in comparison with the predominant basement membrane pattern of SMOC-1. In addition, SMOC-2 promoted attachment of epidermal cells but not fibroblasts. Integrins αvβ6 and αvβ1 were identified as important cell receptors for SMOC-2 on epidermal cells (Maier et al., 2008).
SMOC-2 in Angiogenesis
SMOC-2 acted to promote angiogenic activity in human umbilical chord endothelial cells (HUVECs) where SMOC-2 expression was localized to the extracellular periphery of the cell membrane (Rocnik et al., 2006). In addition, over-expression of SMOC-2 in HUVECs stimulated DNA synthesis in a dose-dependent manner whereas decreased expression of SMOC-2 diminished proliferation. SMOC-2 activity was synergistic with basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) suggesting that SMOC-2 enhanced the angiogenic affect of these growth factors (Rocnik et al., 2006). The angiogenic promoting activity of SMOC-2 compares with that of SPARC in that both SMOC-2 and SPARC promote angiogenesis induced by VEGF. However, in contrast to SMOC-2, SPARC was shown to inhibit bFGF-driven angiogenesis (Rivera et al., 2011).
In mouse fibroblasts, SMOC-2 promoted proliferation, through increased cyclin D1 expression that was dependent upon integrin linked kinase (ILK) activity (Liu et al., 2009a). Changes in SMOC-2 expression did not effect PDGF receptor activation indicating that SMOC-2 did not affect PDGF binding to its receptor. The authors of this study concluded that SMOC-2 acted to maintain ILK activation during the G1 phase of the cell cycle and suggested a novel function for SMOC-2 in the regulation of cell cycle progression (Liu et al., 2009a). As mentioned above, SPARC has also been implicated in ILK activation in lens epithelial cells.
SMOC-2 was recently identified as a transcriptional target of aryl-hydrocarbon receptor signaling, an important pathway in cellular response to pollutants (Liu et al., 2009a). Exposure to pollutants that stimulated Ahr signaling repressed SMOC-2 expression. These results support a role of decreased SMOC-2 expression to limit or diminish cell growth and proliferation in response to pollutant exposure.
Human Disease
SMOC-2 has been identified as a potential candidate gene implicated in generalized vitiligo. Birlea et al. found a SMOC-2 gene variant when investigating genetic origins of vitiligo in an isolated Romanian community with a higher incidence of vitiligo than other European Caucasian groups (Birlea et al., 2010). However, the SMOC-2 variant associated with the Romanian community was not found in individuals with vitiligo in a study of Jordanian Arabs (Alkhateeb et al., 2010).
Testican/SPOCK
There are three testican proteins encoded by separate genes, testican-1, -2 and -3, of which testican-1 is the best characterized (Alliel et al., 1993, Schnepp et al., 2005). Testicans contain a follistatin domain, one thyropin domain, and a calcium-binding E–F hand domain (Figure 1) (Schnepp et al., 2005). Testican-1 is a proteoglycan originally found as a component of seminal fluid and was hence named testican (Alliel et al., 1993). Although expressed in testicles and other tissues in humans, the highest levels of expression of testican-1 were found in brain (Edgell et al., 2004). In fact, in mice, testican-1 was detected only in brain (Bonnet et al., 1996).
Regulation of Protease Activity
Purified testican-1 was shown to inhibit the activity of the protease cathepsin L but not cathepsin B in vitro (Meh et al., 2005). This activity was mediated by the thyroglobulin domain – a domain that has been associated previously with cysteine protease inhibitors (Mihelic and Turk, 2007). In astrocytic tumors, testican-1 inhibited the protease activity of membrane-type metalloproteinases (MT1-MMP and MT2-MMP) (Nakada et al., 2001). This activity was originally identified for testican-3 and predicted by sequence homology to also be relevant for testican-1. The N-terminal region of testican 3 encoding the follistatin domain and the EC domain were found to confer inhibition of MT-MMP activity. In addition to MT-MMP activity, levels of active MMP-2 were also reduced in the presence of testicans 1 and 3, likely through a decrease in enzymatic activation of proMMP-2 by MT1-MMP. Furthermore, testican-2 was shown to counter the inhibitory action of testican-1 (and -3) and was thereby permissive for MTI-MMP mediated cell migration (Nakada et al., 2003). In adult T-cell leukemia, testican-3 expression was also associated with decreases in MMP-2 and MT1-MMP expression. In these cells, expression of testican-3 was regulated by activating transcriptional factor 3 (Kamioka et al., 2009).
In contrast, in a mouse model of bacterial eye infection, increased testican-1 expression was associated with increased MMP-2 activity (Berger et al., 2011). A reduction in testican expression by siRNA resulted in decreased MMP-2 levels whereas application of recombinant testican resulted in increased MMP-2 activity. The authors of this study suggested that levels of TIMP-2 might influence MT1-MMP substrate specificity and MMP-2 activation during eye infection. Thus testican -1 was also implicated in the regulation of MMP-2 activity in this model although the complex environment of the infected eyes likely introduced additional layers of regulation including levels of TIMP-2.
Although mechanisms by which SPARC affects MMP activity are not currently well defined, the capacity of SPARC to influence these protease activities shares functional overlap with testicans 1, 2, and 3 suggesting that common structural features of these SPARC family members might interact with MMPs or molecules that regulate MMP activity such as tissue-inhibitors of metalloproteinases (TIMPs). Notably, regions that comprise one α helix of the conserved E–F hand motif of SPARC were implicated in the induction of MMP activity by initial peptide studies. On the other hand, testicans appear to interact directly with specific MMPs to diminish activity via sites in the N-terminal portion of the protein containing the follistatin and EC domains. Future experiments to explore specific structure function interaction between SPARC family members and MMPs and TIMPs are needed to address specific mechanisms of action.
Testican 1-Null Mice
Abrogation of expression of testican-1 in mice did not reveal any demonstrable phenotypic differences during development or in adult mice (Roll et al., 2006). Histological examination of the brains of testican-1 null mice (the site of highest testican-1 expression) exhibited no significant differences from that of wild-type mice. Although increases in levels of testican-2 and -3 were not detected in the absence of testican-1, compensation was likely conferred for the lack of testican-1 by these related family members (Roll et al., 2006). Of note, abrogation of many matricellular proteins, have resulted in relatively mild phenotypes that are more pronounced in response to injury or challenge. Whether testican-1 null mice might exhibit phenotypic differences in response to pathological insult remains to be tested.
Human Disease
Of interest, the gene encoding SPOCK (testican) was recently identified in a genome-wide association analysis of genes involved in the determination of age at menarche (Liu et al., 2009b). Age of menarche has been found to be a key determinant in a number of women’s health issues and therefore the identification of factors that regulate this process is of significant interest. The SPOCK gene was found to have seven single nucleotide polymorphisms (SNPs) positively associated with age at menarche. The authors speculated that the capacity of SPOCK to influence MMP-2 activity might be relevant in age at menarche as MMP-2 has been shown to be a critical component in endometrial menstrual breakdown. However, whether the effects of SPOCK, and hence SNPs in SPOCK, have a primary role in reproductive tissues and/or brain function, for example, remains to be determined. Whereas the regulatory function of SPOCK in MMP-2 activation provides a potential link worthy of future exploration in this area of research, the cellular basis of SPOCK in age of menarche requires a great deal of further study.
Fstl-1 (Follistatin like protein-1)/TSC-36
Although the sequence of Fstl-1 encodes a putative E-C domain like other members of the SPARC family, the calcium binding domain in fstl-1 did not undergo characteristic structural changes upon calcium addition or depletion and therefore the E–F hand of fstl-1 was not considered to be a functional calcium binding domain (Hambrock et al., 2004).
Fstl-1 in Autoimmune Disease
Increased expression of fstl-1 was found in a mouse model of collagen-induced arthritis (Miyamae et al., 2006). In fact, over-expression of fstl-1 in the feet of mice, in the absence of arthritis inducing factors, was found to be sufficient to induce severe paw swelling and arthritis (Miyamae et al., 2006). Thus fstl-1was identified as a novel pro-inflammatory protein. The fstl-1 response was tied to interferon-γ expression and neutralization of fstl-1 reduced collagen-induced arthritis in mice (Clutter et al., 2009). Recently, evidence that fstl-1 might be a useful bio-marker of disease activity in juvenile rheumatoid arthritis was put forth (Wilson et al., 2010). Likewise, fslt-1 was found to be elevated in the serum of individuals with rheumatoid arthritis and systemic autoimmune diseases (Li et al., 2011a). Furthermore, a correlation with elevated fstl-1 in the serum of heart failure patients with associated left ventricular hypertrophy have provided impetus to explore fstl-1 as a marker of chronic systolic heart failure as well (El-Armouche et al., 2011).
Fstl-1 as a Regulator of BMP Signaling
Experiments that assessed functional roles of predicted homologues of fstl-1 in Drosophila, chick, and zebra fish strongly suggested a function of fstl-1 in the regulation of BMP signaling. Generation of fstl-1 null mice revealed a perinatal lethality associated with abrogated expression of this SPARC family member (Sylva et al., 2011). Newborn mice exhibited respiratory distress and multiple defects in lung development. Fstl-1 null mice also displayed numerous skeletal malformations including limb patterning of the axial skeleton. The phenotype of the fstl-1 null mouse was consistent with a critical role for fstl-1 in BMP signaling (Sylva et al., 2011). Geng et al. (2011) went on to show that fstl-1 interacted directly with BMP4 and resulted in abrogated Smad activation by BMP4 and inhibition of BMP4-dependent surfactant expression. These results are consistent with fstl-1 acting as a BMP4 antagonist critical for lung and skeletal development in mice.
Hence, Fstl-1 shares functional overlap with SMOC-1 and 2 in terms of regulation of BMP signaling. As the EC domain was not found to be functional in fstl-1, possibly the Ca2+ -binding EC domain is not structurally required for modulation of BMP signaling. The shared follistatin domain likely mediates the regulation of BMP signaling as follistatin is known to bind activin, a member of the TGF-β super family (that includes BMPs 2–7). In the case of the SMOC proteins, a mechanism by which direct binding of SMOCs to BMP family members was not found to be the primary mechanism of regulation of BMP gradient formation.
Fstl-1 in the Nervous System
Fstl-1 is also expressed by neurons of the dorsal root ganglion. (Li et al., 2011b) identified Fstl-1 as a novel, critical factor in sensory synapse transmission. Fstl-1 bound directly to the α1 subunit of Na+,K+-ATPase to activate ATPase activity. Fstl-1-dependent activation of the Na+,K+-ATPase was proposed to act as a primary regulator of homoeostatic regulation of somatic sensation. Targeted deletion of fstl-1 in dorsal root ganglion gave rise to mice with a hypersensitivity to noxious stimuli (Li et al., 2011b). Hence, fstl-1, like hevin, is another SPARC family member with critical functions in the nervous system.
Conclusion
Recent developments in diverse areas of research have contributed to the functional characterization of the cellular role of the SPARC family of proteins. A summary of activities highlighting known functions in three broad categories is shown in Table 1 and shown schematically in Figure 2. Whereas the SMOCs and fstl-1 have been strongly implicated in the regulation of BMP signaling, SPARC and hevin/SPL-1 regulate both ECM assembly and cooperate to regulate synaptogenesis in the central nervous system. Fstl-1 functions in the peripheral nervous system as well, to regulate the activity of sensory neurons. Interestingly, most SPARC family members including SPARC have been shown to have high levels of expression in reproductive tissues although the function of these proteins at this site remains unclear. SPARC and testicans have both been implicated in the regulation of cathepsin and MMP activity. However, testicans have been shown to interact directly with both types of proteases whereas the effects of SPARC on protease activity appear to be indirect and result from induction of activity likely through activation of cellular pathways.
Table 1.
Functional Activities Associated with SPARC Family Proteins
| Protein | ECM Activity | Growth Factor Activity |
Cell Adhesion |
|---|---|---|---|
| SPARC | Binds collagens, Enhances collagen Deposition, effects collagen fibril morphology (Bradshaw, 2009*) | Effects VEGF, TGF-β1, and bFGF activity (Rivera et al., 2011*) | Counter-adhesive (Lane and Sage, 1990) |
| Hevin/SPL1 | Binds collagens, effects collagen fibril morphology (Sullivan et al., 2006) | Undetermined | Counter-adhesive (Brekken et al., 2004) |
| SMOC-1 | Binds fibulin-1 (Novinec et al., 2008) and tenascin C (Brellier et al., 2011) | With Dally, establishes gradient of Dpp (Vuilleumier et al., 2010), inhibits BMP signaling (Thomas et al., 2009). | Undetermined |
| SMOC-2 | Undetermined | Enhances VEGF and bFGF-dependent angiogenesis (Liu et al., 2008, Rocnik et al., 2006) | Promotes attachment of epidermal cells (Maier et al., 2008) |
| Testicans /SPOCKs | Effects Cathepsin (Mihelic and Turk, 2007*), and matrix metalloproteinase activity (Mihelic and Turk, 2007, Nishida et al., 2008) | Undetermined | Testican-1: Counter-adhesive (Marr and Edgell, 2003). Testican-2: inhibits neurite outgrowth (Schnepp et al., 2005) |
| Fstl 1 | Undetermined | Mediator of inflammatory responses, (Miyamae et al., 2006) inhibits BMP-4. (Geng et al., 2011) | Undetermined |
Reviews cited for brevity, please see references therein.
A general theme common to most matricellular proteins is modulation of cellular interaction with extracellular cues and each of the SPARC family of proteins appears to function at this level in diverse manners (Figure 2). SPARC is clearly a critical factor in collagen assembly and deposition however only hevin appears to effect similar processes and, in addition, appears to serve novel functions in the central nervous system. We have proposed that SPARC might function to diminish or direct collagen binding to cell surface receptors to facilitate procollagen processing and subsequent deposition to the ECM (Bradshaw, 2009). That SMOC-1 also appears to limit or direct BMP interactions with cells to establish a gradient of BMP in Drosophila suggests a similar type of activity for SMOC-1.
Perhaps the SPARC family of proteins acts to modulate interactions of extracellular proteins with cell surfaces to direct factors to their appropriate extracellular site. Certainly we have much to learn and to explore in the biology of the SPARC family of proteins – an endeavor that will likely give important insight into mechanisms that regulate both development and disease.
Acknowledgements
This was supported in part by the NIH, NIHLB through 094517 (ADB), and a Merit Award from the Veteran’s Administration to ADB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abouzeid H, Boisset G, Favez T, Youssef M, Marzouk I, Shakankiry N, Bayoumi N, Descombes P, Agosti C, Munier FL, Schorderet DF. Mutations in the SPARC-related modular calcium-binding protein 1 gene, SMOC1, cause waardenburg anophthalmia syndrome. Am J Hum Genet. 2011;88:92–98. doi: 10.1016/j.ajhg.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhateeb A, Al-Dain Marzouka N, Qarqaz F. SMOC2 gene variant and the risk of vitiligo in Jordanian Arabs. Eur J Dermatol. 2010;20:701–704. doi: 10.1684/ejd.2010.1095. [DOI] [PubMed] [Google Scholar]
- Alliel PM, Perin JP, Jolles P, Bonnet FJ. Testican, a multidomain testicular proteoglycan resembling modulators of cell social behaviour. Eur J Biochem. 1993;214:347–350. doi: 10.1111/j.1432-1033.1993.tb17930.x. [DOI] [PubMed] [Google Scholar]
- Arnold SA, Brekken RA. SPARC: a matricellular regulator of tumorigenesis. J Cell Commun Signal. 2009 doi: 10.1007/s12079-009-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger EA, McClellan SA, Barrett RP, Hazlett LD. Testican-1 promotes resistance against Pseudomonas aeruginosa-induced keratitis through regulation of MMP-2 expression and activation. Invest Ophthalmol Vis Sci. 2011;52:5339–5346. doi: 10.1167/iovs.10-6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birlea SA, Gowan K, Fain PR, Spritz RA. Genome-wide association study of generalized vitiligo in an isolated European founder population identifies SMOC2, in close proximity to IDDM8. J Invest Dermatol. 2010;130:798–803. doi: 10.1038/jid.2009.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet F, Perin JP, Charbonnier F, Camuzat A, Roussel G, Nussbaum JL, Alliel PM. Structure and cellular distribution of mouse brain testican. Association with the postsynaptic area of hippocampus pyramidal cells. J Biol Chem. 1996;271:4373–4380. doi: 10.1074/jbc.271.8.4373. [DOI] [PubMed] [Google Scholar]
- Bornstein P. Matricellular proteins: an overview. Matrix Biol. 2000;19:555–556. doi: 10.1016/s0945-053x(00)00103-7. [DOI] [PubMed] [Google Scholar]
- Bradshaw AD. The role of SPARC in extracellular matrix assembly. J Cell Commun Signal. 2009;3:239–246. doi: 10.1007/s12079-009-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD, Baicu CF, Rentz TJ, Van Laer AO, Boggs J, Lacy JM, Zile MR. Pressure overload-induced alterations in fibrillar collagen content and myocardial diastolic function: role of secreted protein acidic and rich in cysteine (SPARC) in post-synthetic procollagen processing. Circulation. 2009;119:269–280. doi: 10.1161/CIRCULATIONAHA.108.773424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD, Graves DC, Motamed K, Sage EH. SPARC-null mice exhibit increased adiposity without significant differences in overall body weight. Proc Natl Acad Sci U S A. 2003a;100:6045–6050. doi: 10.1073/pnas.1030790100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD, Puolakkainen P, Dasgupta J, Davidson JM, Wight TN, Sage EH. SPARC-null mice display abnormalities in the dermis characterized by decreased collagen fibril diameter and reduced tensile strength. J Invest Dermatol. 2003b;120:949–955. doi: 10.1046/j.1523-1747.2003.12241.x. [DOI] [PubMed] [Google Scholar]
- Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix communication. Matrix Biol. 2001;19:816–827. doi: 10.1016/s0945-053x(00)00133-5. [DOI] [PubMed] [Google Scholar]
- Brekken RA, Sullivan MM, Workman G, Bradshaw AD, Carbon J, Siadak A, Murri C, Framson PE, Sage EH. Expression and characterization of murine hevin (SC1), a member of the SPARC family of matricellular proteins. J Histochem Cytochem. 2004;52:735–748. doi: 10.1369/jhc.3A6245.2004. [DOI] [PubMed] [Google Scholar]
- Brellier F, Ruggiero S, Zwolanek D, Martina E, Hess D, Brown-Luedi M, Hartmann U, Koch M, Merlo A, Lino M, Chiquet-Ehrismann R. SMOC1 is a tenascin-C interacting protein over-expressed in brain tumors. Matrix Biol. 2011;30:225–233. doi: 10.1016/j.matbio.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Charbonnier F, Perin JP, Mattei MG, Camuzat A, Bonnet F, Gressin L, Alliel PM. Genomic organization of the human SPOCK gene and its chromosomal localization to 5q31. Genomics. 1998;48:377–380. doi: 10.1006/geno.1997.5199. [DOI] [PubMed] [Google Scholar]
- Clutter SD, Wilson DC, Marinov AD, Hirsch R. Follistatin-like protein 1 promotes arthritis by up-regulating IFN-gamma. J Immunol. 2009;182:234–239. doi: 10.4049/jimmunol.182.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delany AM, Hankenson KD. Thrombospondin-2 and SPARC/osteonectin are critical regulators of bone remodeling. J Cell Commun Signal. 2009;3:227–238. doi: 10.1007/s12079-009-0076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgell CJ, BaSalamah MA, Marr HS. Testican-1: a differentially expressed proteoglycan with protease inhibiting activities. Int Rev Cytol. 2004;236:101–122. doi: 10.1016/S0074-7696(04)36003-1. [DOI] [PubMed] [Google Scholar]
- El-Armouche A, Ouchi N, Tanaka K, Doros G, Wittkopper K, Schulze T, Eschenhagen T, Walsh K, Sam F. Follistatin-like 1 in Chronic Systolic Heart Failure: A Marker of Left Ventricular Remodeling. Circ Heart Fail. 2011 doi: 10.1161/CIRCHEARTFAILURE.110.960625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu C. The role of astrocyte-secreted matricellular proteins in central nervous system development and function. J Cell Commun Signal. 2009;3:167–176. doi: 10.1007/s12079-009-0078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenouille N, Puissant A, Dufies M, Robert G, Jacquel A, Ohanna M, Deckert M, Pasquet JM, Mahon FX, Cassuto JP, Raynaud S, Tartare-Deckert S, Auberger P. Persistent activation of the Fyn/ERK kinase signaling axis mediates imatinib resistance in chronic myelogenous leukemia cells through upregulation of intracellular SPARC. Cancer Res. 2010;70:9659–9670. doi: 10.1158/0008-5472.CAN-10-2034. [DOI] [PubMed] [Google Scholar]
- Framson PE, Sage EH. SPARC and tumor growth: where the seed meets the soil? J Cell Biochem. 2004:679–690. doi: 10.1002/jcb.20091. [DOI] [PubMed] [Google Scholar]
- Geng Y, Dong Y, Yu M, Zhang L, Yan X, Sun J, Qiao L, Geng H, Nakajima M, Furuichi T, Ikegawa S, Gao X, Chen YG, Jiang D, Ning W. Follistatin-like 1 (Fstl1) is a bone morphogenetic protein (BMP) 4 signaling antagonist in controlling mouse lung development. Proc Natl Acad Sci U S A. 2011;108:7058–7063. doi: 10.1073/pnas.1007293108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersdorff N, Muller M, Schall A, Miosge N. Secreted modular calcium-binding protein-1 localization during mouse embryogenesis. Histochem Cell Biol. 2006;126:705–712. doi: 10.1007/s00418-006-0200-7. [DOI] [PubMed] [Google Scholar]
- Gilles C, Bassuk JA, Pulyaeva H, Sage EH, Foidart JM, Thompson EW. SPARC/osteonectin induces matrix metalloproteinase 2 activation in human breast cancer cell lines. Cancer Res. 1998;58:5529–5536. [PubMed] [Google Scholar]
- Gilmour DT, Lyon GJ, Carlton MB, Sanes JR, Cunningham JM, Anderson JR, Hogan BL, Evans MJ, Colledge WH. Mice deficient for the secreted glycoprotein SPARC/osteonectin/BM40 develop normally but show severe age-onset cataract formation and disruption of the lens. Embo J. 1998;17:1860–1870. doi: 10.1093/emboj/17.7.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudici C, Raynal N, Wiedemann H, Cabral WA, Marini JC, Timpl R, Bachinger HP, Farndale RW, Sasaki T, Tenni R. Mapping of SPARC/BM-40/osteonectin-binding sites on fibrillar collagens. J Biol Chem. 2008;283:19551–19560. doi: 10.1074/jbc.M710001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambrock HO, Kaufmann B, Muller S, Hanisch FG, Nose K, Paulsson M, Maurer P, Hartmann U. Structural characterization of TSC-36/Flik: analysis of two charge isoforms. J Biol Chem. 2004;279:11727–11735. doi: 10.1074/jbc.M309318200. [DOI] [PubMed] [Google Scholar]
- Hambrock HO, Nitsche DP, Hansen U, Bruckner P, Paulsson M, Maurer P, Hartmann U. SC1/hevin. An extracellular calcium-modulated protein that binds collagen I. J Biol Chem. 2003;278:11351–11358. doi: 10.1074/jbc.M212291200. [DOI] [PubMed] [Google Scholar]
- Hohenester E, Sasaki T, Giudici C, Farndale RW, Bachinger HP. Structural basis of sequence-specific collagen recognition by SPARC. Proc Natl Acad Sci U S A. 2008;105:18273–18277. doi: 10.1073/pnas.0808452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamioka M, Imamura J, Komatsu N, Daibata M, Sugiura T. Testican 3 expression in adult T-cell leukemia. Leuk Res. 2009;33:913–918. doi: 10.1016/j.leukres.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Kucukdereli H, Allen NJ, Lee AT, Feng A, Ozlu MI, Conatser LM, Chakraborty C, Workman G, Weaver M, Sage EH, Barres BA, Eroglu C. Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc Natl Acad Sci U S A. 2011;108:E440–E449. doi: 10.1073/pnas.1104977108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane TF, Sage EH. Functional mapping of SPARC: peptides from two distinct Ca+(+)-binding sites modulate cell shape. J Cell Biol. 1990;111:3065–3076. doi: 10.1083/jcb.111.6.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Wang Y, Xu N, Wei Q, Wu M, Li X, Zheng P, Sun S, Jin Y, Zhang G, Liao R, Zhang P. Follistatin-like protein 1 is elevated in systemic autoimmune diseases and correlated with disease activity in patients with rheumatoid arthritis. Arthritis Res Ther. 2011a;13:R17. doi: 10.1186/ar3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li KC, Zhang FX, Li CL, Wang F, Yu MY, Zhong YQ, Zhang KH, Lu YJ, Wang Q, Ma XL, Yao JR, Wang JY, Lin LB, Han M, Zhang YQ, Kuner R, Xiao HS, Bao L, Gao X, Zhang X. Follistatin-like 1 suppresses sensory afferent transmission by activating Na+,K+-ATPase. Neuron. 2011b;69:974–987. doi: 10.1016/j.neuron.2011.01.022. [DOI] [PubMed] [Google Scholar]
- Liu P, Lu J, Cardoso WV, Vaziri C. The SPARC-related Factor SMOC-2 Promotes Growth Factor-induced Cyclin D1 Expression and DNA Synthesis via Integrin-linked Kinase. Mol Biol Cell. 2008;19:248–261. doi: 10.1091/mbc.E07-05-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Pazin DE, Merson RR, Albrecht KH, Vaziri C. The developmentally-regulated Smoc2 gene is repressed by Aryl-hydrocarbon receptor (Ahr) signaling. Gene. 2009a;433:72–80. doi: 10.1016/j.gene.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YZ, Guo YF, Wang L, Tan LJ, Liu XG, Pei YF, Yan H, Xiong DH, Deng FY, Yu N, Zhang YP, Zhang L, Lei SF, Chen XD, Liu HB, Zhu XZ, Levy S, Papasian CJ, Drees BM, Hamilton JJ, Recker RR, Deng HW. Genome-wide association analyses identify SPOCK as a key novel gene underlying age at menarche. PLoS Genet. 2009b;5 doi: 10.1371/journal.pgen.1000420. e1000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier S, Paulsson M, Hartmann U. The widely expressed extracellular matrix protein SMOC-2 promotes keratinocyte attachment and migration. Exp Cell Res. 2008;314:2477–2487. doi: 10.1016/j.yexcr.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Marr HS, Edgell CJ. Testican-1 inhibits attachment of Neuro-2a cells. Matrix Biol. 2003;22:259–266. doi: 10.1016/s0945-053x(03)00036-2. [DOI] [PubMed] [Google Scholar]
- Mayer U, Aumailley M, Mann K, Timpl R, Engel J. Calcium-dependent binding of basement membrane protein BM-40 (osteonectin, SPARC) to basement membrane collagen type IV. Eur J Biochem. 1991;198:141–150. doi: 10.1111/j.1432-1033.1991.tb15996.x. [DOI] [PubMed] [Google Scholar]
- McClung HM, Thomas SL, Osenkowski P, Toth M, Menon P, Raz A, Fridman R, Rempel SA. SPARC upregulates MT1-MMP expression, MMP-2 activation, and the secretion and cleavage of galectin-3 in U87MG glioma cells. Neurosci Lett. 2007;419:172–177. doi: 10.1016/j.neulet.2007.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meh P, Pavsic M, Turk V, Baici A, Lenarcic B. Dual concentration-dependent activity of thyroglobulin type-1 domain of testican: specific inhibitor and substrate of cathepsin L. Biol Chem. 2005;386:75–83. doi: 10.1515/BC.2005.010. [DOI] [PubMed] [Google Scholar]
- Mihelic M, Turk D. Two decades of thyroglobulin type-1 domain research. Biol Chem. 2007;388:1123–1130. doi: 10.1515/BC.2007.155. [DOI] [PubMed] [Google Scholar]
- Miyamae T, Marinov AD, Sowders D, Wilson DC, Devlin J, Boudreau R, Robbins P, Hirsch R. Follistatin-like protein-1 is a novel proinflammatory molecule. J Immunol. 2006;177:4758–4762. doi: 10.4049/jimmunol.177.7.4758. [DOI] [PubMed] [Google Scholar]
- Nakada M, Miyamori H, Yamashita J, Sato H. Testican 2 abrogates inhibition of membrane-type matrix metalloproteinases by other testican family proteins. Cancer Res. 2003;63:3364–3369. [PubMed] [Google Scholar]
- Nakada M, Yamada A, Takino T, Miyamori H, Takahashi T, Yamashita J, Sato H. Suppression of membrane-type 1 matrix metalloproteinase (MMP)-mediated MMP-2 activation and tumor invasion by testican 3 and its splicing variant gene product, N-Tes. Cancer Res. 2001;61:8896–8902. [PubMed] [Google Scholar]
- Nischt R, Wallich M, Reibetanz M, Baumann P, Krieg T, Mauch C. BM-40 and MMP-2 expression are not coregulated in human melanoma cell lines. Cancer Lett. 2001;162:223–230. doi: 10.1016/s0304-3835(00)00659-5. [DOI] [PubMed] [Google Scholar]
- Nishida Y, Miyamori H, Thompson EW, Takino T, Endo Y, Sato H. Activation of matrix metalloproteinase-2 (MMP-2) by membrane type 1 matrix metalloproteinase through an artificial receptor for proMMP-2 generates active MMP-2. Cancer Res. 2008;68:9096–9104. doi: 10.1158/0008-5472.CAN-08-2522. [DOI] [PubMed] [Google Scholar]
- Novinec M, Kovacic L, Skrlj N, Turk V, Lenarcic B. Recombinant human SMOCs produced by in vitro refolding: calcium-binding properties and interactions with serum proteins. Protein Expr Purif. 2008;62:75–82. doi: 10.1016/j.pep.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Okada I, Hamanoue H, Terada K, Tohma T, Megarbane A, Chouery E, Abou-Ghoch J, Jalkh N, Cogulu O, Ozkinay F, Horie K, Takeda J, Furuichi T, Ikegawa S, Nishiyama K, Miyatake S, Nishimura A, Mizuguchi T, Niikawa N, Hirahara F, Kaname T, Yoshiura K, Tsurusaki Y, Doi H, Miyake N, Furukawa T, Matsumoto N, Saitsu H. SMOC1 is essential for ocular and limb development in humans and mice. Am J Hum Genet. 2011;88:30–41. doi: 10.1016/j.ajhg.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazin DE, Albrecht KH. Developmental expression of Smoc1 and Smoc2 suggests potential roles in fetal gonad and reproductive tract differentiation. Dev Dyn. 2009;238:2877–2890. doi: 10.1002/dvdy.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portela M, Casas-Tinto S, Rhiner C, Lopez-Gay JM, Dominguez O, Soldini D, Moreno E. Drosophila SPARC is a self-protective signal expressed by loser cells during cell competition. Dev Cell. 2010;19:562–573. doi: 10.1016/j.devcel.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Rahman M, Chan AP, Tai IT. A Peptide of SPARC Interferes with the Interaction between Caspase8 and Bcl2 to Resensitize Chemoresistant Tumors and Enhance Their Regression In Vivo. PLoS One. 2011;6:e26390. doi: 10.1371/journal.pone.0026390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainger J, van Beusekom E, Ramsay JK, McKie L, Al-Gazali L, Pallotta R, Saponari A, Branney P, Fisher M, Morrison H, Bicknell L, Gautier P, Perry P, Sokhi K, Sexton D, Bardakjian TM, Schneider AS, Elcioglu N, Ozkinay F, Koenig R, Megarbane A, Semerci CN, Khan A, Zafar S, Hennekam R, Sousa SB, Ramos L, Garavelli L, Furga AS, Wischmeijer A, Jackson IJ, Gillessen-Kaesbach G, Brunner HG, Wieczorek D, van Bokhoven H, Fitzpatrick DR. Loss of the BMP antagonist, SMOC-1, causes Ophthalmo-acromelic (Waardenburg Anophthalmia) syndrome in humans and mice. PLoS Genet. 2011;7 doi: 10.1371/journal.pgen.1002114. e1002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera LB, Bradshaw AD, Brekken RA. The regulatory function of SPARC in vascular biology. Cell Mol Life Sci. 2011;68:3165–3173. doi: 10.1007/s00018-011-0781-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera LB, Brekken RA. SPARC promotes pericyte recruitment via inhibition of endoglin-dependent TGF-beta1 activity. J Cell Biol. 2011;193:1305–1319. doi: 10.1083/jcb.201011143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocnik EF, Liu P, Sato K, Walsh K, Vaziri C. The novel SPARC family member SMOC-2 potentiates angiogenic growth factor activity. J Biol Chem. 2006;281:22855–22864. doi: 10.1074/jbc.M513463200. [DOI] [PubMed] [Google Scholar]
- Roll S, Seul J, Paulsson M, Hartmann U. Testican-1 is dispensable for mouse development. Matrix Biol. 2006;25:373–381. doi: 10.1016/j.matbio.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Schnepp A, Komp Lindgren P, Hulsmann H, Kroger S, Paulsson M, Hartmann U. Mouse testican-2. Expression, glycosylation, and effects on neurite outgrowth. J Biol Chem. 2005;280:11274–11280. doi: 10.1074/jbc.M414276200. [DOI] [PubMed] [Google Scholar]
- Sullivan MM, Barker TH, Funk SE, Karchin A, Seo NS, Hook M, Sanders J, Starcher B, Wight TN, Puolakkainen P, Sage EH. Matricellular hevin regulates decorin production and collagen assembly. J Biol Chem. 2006;281:27621–27632. doi: 10.1074/jbc.M510507200. [DOI] [PubMed] [Google Scholar]
- Sullivan MM, Puolakkainen PA, Barker TH, Funk SE, Sage EH. Altered tissue repair in hevin-null mice: inhibition of fibroblast migration by a matricellular SPARC homolog. Wound Repair Regen. 2008;16:310–319. doi: 10.1111/j.1524-475X.2008.00370.x. [DOI] [PubMed] [Google Scholar]
- Sullivan MM, Sage EH. Hevin/SC1, a matricellular glycoprotein and potential tumor-suppressor of the SPARC/BM-40/Osteonectin family. Int J Biochem Cell Biol. 2004;36:991–996. doi: 10.1016/j.biocel.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Sylva M, Li VS, Buffing AA, van Es JH, van den Born M, van der Velden S, Gunst Q, Koolstra JH, Moorman AF, Clevers H, van den Hoff MJ. The BMP Antagonist Follistatin-Like 1 Is Required for Skeletal and Lung Organogenesis. PLoS One. 2011;6:e22616. doi: 10.1371/journal.pone.0022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang MJ, Tai IT. A novel interaction between procaspase 8 and SPARC enhances apoptosis and potentiates chemotherapy sensitivity in colorectal cancers. J Biol Chem. 2007;282:34457–34467. doi: 10.1074/jbc.M704459200. [DOI] [PubMed] [Google Scholar]
- Thomas JT, Canelos P, Luyten FP, Moos M., Jr Xenopus SMOC-1 Inhibits bone morphogenetic protein signaling downstream of receptor binding and is essential for postgastrulation development in Xenopus. J Biol Chem. 2009;284:18994–19005. doi: 10.1074/jbc.M807759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremble PM, Lane TF, Sage EH, Werb Z. SPARC, a secreted protein associated with morphogenesis and tissue remodeling, induces expression of metalloproteinases in fibroblasts through a novel extracellular matrix-dependent pathway. J Cell Biol. 1993;121:1433–1444. doi: 10.1083/jcb.121.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombetta JM, Bradshaw AD. SPARC/osteonectin functions to maintain homeostasis of the collagenous extracellular matrix in the periodontal ligament. J Histochem Cytochem. 2010;58:871–879. doi: 10.1369/jhc.2010.956144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannahme C, Gosling S, Paulsson M, Maurer P, Hartmann U. Characterization of SMOC-2, a modular extracellular calcium-binding protein. Biochem J. 2003;373:805–814. doi: 10.1042/BJ20030532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannahme C, Schubel S, Herud M, Gosling S, Hulsmann H, Paulsson M, Hartmann U, Maurer P. Molecular cloning of testican-2: defining a novel calcium-binding proteoglycan family expressed in brain. J Neurochem. 1999;73:12–20. doi: 10.1046/j.1471-4159.1999.0730012.x. [DOI] [PubMed] [Google Scholar]
- Vannahme C, Smyth N, Miosge N, Gosling S, Frie C, Paulsson M, Maurer P, Hartmann U. Characterization of SMOC-1, a novel modular calcium-binding protein in basement membranes. J Biol Chem. 2002;277:37977–37986. doi: 10.1074/jbc.M203830200. [DOI] [PubMed] [Google Scholar]
- Vuilleumier R, Springhorn A, Patterson L, Koidl S, Hammerschmidt M, Affolter M, Pyrowolakis G. Control of Dpp morphogen signalling by a secreted feedback regulator. Nat Cell Biol. 2010;12:611–617. doi: 10.1038/ncb2064. [DOI] [PubMed] [Google Scholar]
- Weaver M, Workman G, Schultz CR, Lemke N, Rempel SA, Sage EH. Proteolysis of the matricellular protein hevin by matrix metalloproteinase-3 produces a SPARC-like fragment (SLF) associated with neovasculature in a murine glioma model. J Cell Biochem. 2011 doi: 10.1002/jcb.23235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver MS, Workman G, Cardo-Vila M, Arap W, Pasqualini R, Sage EH. Processing of the matricellular protein hevin in mouse brain is dependent on ADAMTS4. J Biol Chem. 2010;285:5868–5877. doi: 10.1074/jbc.M109.070318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver MS, Workman G, Sage EH. The copper binding domain of SPARC mediates cell survival in vitro via interaction with integrin beta1 and activation of integrin-linked kinase. J Biol Chem. 2008;283:22826–22837. doi: 10.1074/jbc.M706563200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DC, Marinov AD, Blair HC, Bushnell DS, Thompson SD, Chaly Y, Hirsch R. Follistatin-like protein 1 is a mesenchyme-derived inflammatory protein and may represent a biomarker for systemic-onset juvenile rheumatoid arthritis. Arthritis Rheum. 2010;62:2510–2516. doi: 10.1002/art.27485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Clark JI, Wight TN, Sage EH. Alterations in the lens capsule contribute to cataractogenesis in SPARC-null mice. J Cell Sci. 2002;115:2747–2756. doi: 10.1242/jcs.115.13.2747. [DOI] [PubMed] [Google Scholar]
- Zheng Q, Wang Y, Vargas E, Dinardo S. magu is required for germline stem cell self-renewal through BMP signaling in the Drosophila testis. Dev Biol. 2011;357:202–210. doi: 10.1016/j.ydbio.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]